Introduction
Regarding global biodiversity, Brazil is considered an important hotspot area due to the wide variety of environments it contains, whose characteristics can determine the establishment or exclusion of certain organisms (Mantelatto et al., Reference Mantelatto, Bernardo, Silva, Bernardes, Cobo and Fransozo2016). On the south-eastern Brazilian coast, specifically in the Ubatuba region, there are numerous bays that facilitate the establishment of decapod species by providing shelter/protection and food resources (Furlan et al., Reference Furlan, Castilho, Fernandes-Goes, Fransozo, Bertini and Costa2013).
The high occurrence of these individuals in the Ubatuba region has led to its exploitation, mainly by shrimp fishing (Castilho et al., Reference Castilho, Bauer, Freire, Fransozo, Costa, Grabowski and Fransozo2015; Costa et al., Reference Costa, Bochini, Simões, Lopes, Sancinetti, Castilho and Fransozo2016; Fransozo et al., Reference Fransozo, Sousa, Rodrigues, Telles, Fransozo and Negreiros-Fransozo2016). The ecological impacts caused by this activity can destroy benthic communities (Fransozo et al., Reference Fransozo, Sousa, Rodrigues, Telles, Fransozo and Negreiros-Fransozo2016) through unwanted, frequent and massive capture of accompanying fauna; reduction of habitat complexity (by sediment homogenization); changes in species abundance and distribution, and even the reduction of the asymptotic size of individuals (Kaiser et al., Reference Kaiser, Collie, Hall, Jennings and Poiner2002).
In an attempt to minimize these impacts and ensure the conservation and sustainable use of marine resources, the Ubatuba region has been included in a Marine Protection Area (MPA – Cunhambebe Sector) since 8 October 2008, created by the Ministry of the Environment (decree number 53.525). Fishing in this region is only allowed for subsistence of traditional communities, amateurs, and as a leisure activity, while commercial fishing is illegal. In this sense, studies focused on comparing this environment before and after the MPA was implemented are important to determine whether such protection measures are sufficient for conservation.
In the Ubatuba region, spider crabs of the genus Libinia Leach, 1815, mainly Libinia ferreirae Brito Capello, 1871 and Libinia spinosa Guérin, 1832, are frequently caught as by-catch (Bertini et al., Reference Bertini, Fransozo and Melo2004, Reference Bertini, Fransozo and Negreiros-Fransozo2010a; Braga et al., Reference Braga, Fransozo, Bertini and Fumis2005; Fransozo et al., Reference Fransozo, Sousa, Rodrigues, Telles, Fransozo and Negreiros-Fransozo2016; Mantelatto et al., Reference Mantelatto, Bernardo, Silva, Bernardes, Cobo and Fransozo2016). The species L. ferreirae may have a symbiotic relationship with the jellyfish Lychnorhiza lucerna Haeckel, 1880 during their larval and juvenile phases (Nogueira Jr & Haddad, Reference Nogueira and Haddad2005; Gonçalves et al., Reference Gonçalves, Bolla, Negreiros-Fransozo and Castilho2016a, Reference Gonçalves, Wolf, Costa and Castilho2016b). The association of crabs with jellyfish occurs when the latter go near the ocean floor or when they are dragged by marine currents in contact with the benthic environment (Corrington, Reference Corrington1927; Colombo et al., Reference Colombo, Mianzan and Madirolas2003; Towanda & Thuesen, Reference Towanda and Thuesen2006). Additionally, the megalopa stage could move down and up (Queiroga, Reference Queiroga1998) and thus reach the jellyfish. Larval and juvenile individuals may be associated with L. lucerna because the latter provide a protected environment during the most vulnerable period of their lives (Nogueira Jr & Haddad, Reference Nogueira and Haddad2005; Gonçalves et al., Reference Gonçalves, Grabowski, Bochini, Costa and Castilho2017). Interactions between L. ferreirae and individuals of different trophic levels highlight the important ecological functions that this crab has in the marine environment. Therefore, it is essential to understand its population dynamics, since these data can generate new information and, consequently, improve mitigating measures that preserve this species.
The population dynamics of crustaceans can be addressed through several aspects, e.g. frequency distribution of individuals in size classes, reproductive period, recruitment, differential distribution of demographic groups and sex ratio (Garcia & Mantelatto, Reference Garcia and Mantelatto2001; Chan & Williams, Reference Chan and Williams2004; Andrade et al., Reference Andrade, Bertini, Fransozo, Teixeira, Barros-Alves and Fransozo2014, Reference Andrade, Costa, Castilho, Frameschi, Sancinetti and Fransozo2017; Silva et al., Reference Silva, Andrade, Fransozo, Freire and Fransozo2018; Bernardes et al., Reference Bernardes, Martins, Rodrigues, Bernardo, Sousa, Bertini and Fransozo2019; Sousa et al., Reference Sousa, Bernardes, Silva, Bertini, Taddei and Fransozo2019).
Most decapod species in tropical regions present two types of reproduction: continuous, which occurs all year round (Costa & Negreiro-Fransozo, Reference Costa and Negreiros-Fransozo1998; Reigada & Negreiros-Fransozo, Reference Reigada and Negreiros-Fransozo2000) and continuous with peaks in some periods (Bertini et al., Reference Bertini, Teixeira, Fransozo and Fransozo2010b; Castilho et al., Reference Castilho, Bauer, Freire, Fransozo, Costa, Grabowski and Fransozo2015; Frameschi et al., Reference Frameschi, Andrade, Alencar, Teixeira, Fransozo, Fernandes-Góes and Fransozo2015). In temperate regions, reproductive cycles are frequently seasonal (Lancaster, Reference Lancaster1990; Gherardi & Cassidy, Reference Gherardi and Cassidy1995; Terossi et al., Reference Terossi, Wehrtmann and Mantelatto2010). However, Bauer (Reference Bauer1992) stated that there is no general model to explain how long the reproductive periods of marine crustaceans last. Different species have distinct phylogenetic histories, peculiar reproductive capacities and restrictions imposed by body size. Moreover, at similar latitudes, there are variations in habitat characteristics and even other biotic and abiotic parameters that can influence reproductive patterns.
Temperature, salinity, sediment texture, occurrence of water masses, food availability and even the specific physiological needs of each life cycle phase can also modulate the ontogenetic distribution of brachyurans (Andrade et al., Reference Andrade, Fransozo, Bertini, Negreiros-Fransozo and López-Greco2015; Silva et al., Reference Silva, Andrade, Fransozo, Freire and Fransozo2018; Theuerkauff et al., Reference Theuerkauff, Rivera-Ingraham, Roques, Azzopardi, Bertini, Lejeune, Farcy, Lignot and Sucré2018; Bernardo et al., Reference Bernardo, Bernardes, Silva, Sousa, Taddei and Fransozo2020). In this sense, we tested the hypothesis that there is differential occupation between the demographic groups of L. ferreirae in space and time.
Furthermore, given the importance of studies about population characteristics, especially those conducted before the MPA was established, we decided to investigate the population dynamics of L. ferreirae in the Ubatuba region, focusing on the frequency distribution of individuals in size classes, sex ratio and effect of environmental variables (temperature, salinity, texture and organic matter content in the sediment) on reproduction and recruitment.
Materials and methods
Sampling and study area
We captured crabs monthly from January 1998 to December 2000. A total of 480 trawl sets were performed in three bays at varying depths. Trawling was done on a fishing boat outfitted with double-rig nets, each area was trawled for 30 min and covered a total of 18,000 m2 per trawl. From 1998–1999, the Ubatumirim (UBM), Ubatuba (UBA) and Mar Virado (MV) Bays were sampled once a month. In each bay, six sampling stations were established: three in areas sheltered from the waves (5, 7.5 and 10 m deep), and three in exposed areas (10, 15 and 20 m deep) (Figure 1). The stations (except the 7.5 and 10 m depths) were positioned along transects that were parallel to the coastline. These stations were selected according to the following characteristics: their position relative to the bay's mouth, the presence of rocky shores or beaches along the bay's perimeter, freshwater inflow, proximity to offshore water, depth, and sediment texture. Similarly, the collections of 2000 were only carried out in Ubatuba Bay, at the 5, 10, 15 and 20 m sample stations.

Fig. 1. Map of the Ubatuba region, in the north-eastern coast of São Paulo state, Brazil, showing the three bays (Ubatumirim, Ubatuba and Mar Virado), and their respective sampling stations. Figure adapted from Andrade et al. (Reference Andrade, Bertini, Fransozo, Teixeira, Barros-Alves and Fransozo2014).
At each station we collected bottom and surface water samples using a Nansen bottle, and measured salinity and temperature (°C) with an optical refractometer and a mercury thermometer, respectively. Sediment samples were collected with a Van Veen grab, from which we obtained sediment texture and organic matter content. Depth was measured with an echobathymeter connected to a Global Positioning System (GPS). Immediately after collection, we put the sediment samples into labelled plastic bags and froze them to minimize organic matter decomposition until further analyses.
In the laboratory, the sediment was dried at 70°C for 72 h in an oven. For the grain size composition analysis, two 50 g sub-samples for each sampling station were separated, treated with 250 ml of NaOH solution (0.2 mol 1−¹) and stirred for 5 min to release silt and clay particles. Afterwards, sub-samples were rinsed in a 0.063-mm sieve. The grain size was classified according to the Wentworth (Reference Wentworth1922) scale: >2 mm (gravel); 2.0–1.0 mm (very coarse sand); 1.0–0.5 mm (coarse sand); 0.5–0.25 mm (medium sand); 0.25–0.125 mm (fine sand); 0.125–0.063 mm (very fine sand), and smaller particles were classified as silt and clay.
Grain diameter was expressed in phi (φ) values (calculated from the formula phi = −log2d, where d = grain diameter in mm), and the following classes were obtained: −2 ≤ phi < −1 (gravel), −1 ≤ phi < 0 (very coarse sand), 0 ≤ phi < 1 (coarse sand), 1 ≤ phi < 2 (medium sand), 2 ≤ phi < 3 (fine sand), 3 ≤ phi < 4 (very fine sand), and phi ≥ 4 (silt and clay). From the cumulative distribution curves of these classes, the 16th, 50th and 84th percentiles were extracted and the mean diameter (md) was calculated using the formula: MD = (phi16 + phi50 + phi84)/3 (Suguio, Reference Suguio1973). The three most quantitatively important sediments were defined according to Magliocca & Kutner (Reference Magliocca and Kutner1965): Class A corresponds to sediments with medium sand, coarse sand, very coarse sand and gravel; class B is fine sand and very fine sand and class C is silt and clay.
The organic-matter content of the sediment at each sampling station was estimated as the difference between the initial and ash-free dry weights of three subsamples (10 g each) incinerated in porcelain crucibles at 500°C for 3 h (Hakanson & Jansson, Reference Hakanson and Jansson1983).
Biological data
The crabs were identified to species (Melo, Reference Melo1996) and sex was determined by the shape of the abdomen (a thin shape for males and an oval shape for females) and the number of pleopods (two pairs for males and four pairs for females). The carapace width (CW) was measured with a 0.1 mm precision calliper.
Individuals were classified as juveniles or adults based on the examination of secondary sexual characters such as pleopod morphology, free abdomen (i.e. the abdomen does not adhere to the thoracic sternites), convex abdomen (forming an incubator chamber) in the females, and distinct cheliped development in adult males when compared with juvenile males. Such changes associated with sexual maturity are similar to those described for the epialtid Epialtus brasiliensis Dana, 1852 and Acanthonyx scutiformis (Dana, 1851) (Negreiros-Fransozo et al., Reference Negreiros-Fransozo, Fransozo and Reigada1994; Teixeira et al., Reference Teixeira, Fransozo, Cobo and Hiyodo2009). The identification of juvenile specimens based on the abdominal condition (sealed or not) has been widely used for Portunoidea (e.g. Taissoun, Reference Taissoun1969; Williams, Reference Williams1974; Silva et al., Reference Silva, Taddei, Bertini, Andrade, Teixeira and Fransozo2017; Sousa et al., Reference Sousa, Bernardes, Silva, Bertini, Taddei and Fransozo2019) and is useful for the representatives of the superfamily Majoidea. Four demographic groups were used in the analyses: juvenile males and females (J), adult males (AM), non-ovigerous adult females (AF) and ovigerous females (OF).
All crabs were submitted to a macroscopic examination of gonads, and the stages of gonadal development were determined based on the shape, colour and size of the ovaries, testicles and vas deferens (Choy, Reference Choy1988; Abelló, Reference Abelló1989). Four stages of gonadal development were established for males and females based on gonad size, shape and colour: (1) RU: rudimentary (males: uncoloured filamentous vas deferens; females: whitish, thin filamentous ovary); (3) ED: developing (males: small, white gonads, being smaller than the hepatopancreas; females: beginning of maturation, small yellow ovaries); (4) DE: developed (males: largest gonadal development size, white gonads; females: bright yellow ovaries filling almost the entire thoracic cavity) (Choy, Reference Choy1988; Abelló, Reference Abelló1989). Females carrying embryos (fertilized eggs) adhered to the pleopods (ovigerous females – OF) were described in posterior analyses.
Data analyses
Bottom temperature and salinity (TS) diagrams were created to show the influence of the water masses in the study regions (Tomczak, Reference Tomczak1999).
Tests for homoscedasticity (Levene tests) and normality (Shapiro–Wilk tests) were first performed as prerequisites for the statistical test (P = 0.05) (Zar, Reference Zar1999). The variance analysis (ANOVA) and a posteriori Tukey test (P < 0.05) were used to compare surface and bottom temperature values (ST and BT, respectively), bottom salinity (BS), percentage of organic matter in sediment (OM) and phi among the sampling stations and seasons (summer from January to March; autumn from April to June; winter from July to September; and spring from October to December). All environmental factors were also compared between protected and exposed areas through Student's t-test (P < 0.05).
Modal values were determined for each CW frequency using the software PEAKFIT (Automatic Peak Fitting Detection and Fitting, Method I-Residual, no Data Smoothing).
The CW of adult males and females was compared using the Student's t-test for two independent samples of parametric data. The Kolmogorov–Smirnov test was used to compare the size frequency distribution of males and females. Possible deviations from the expected 1:1 sex ratio in each size class were verified by the binomial test (Wilson & Hardy, Reference Wilson, Hardy and Hardy2002).
A contingency table with the abundance of demographic groups per season and bathymetric zone was created using a correspondence analysis (CA). To clearly visualize the correlation of abundance of each demographic group per season and bathymetric zone, each datapoint was plotted proportionally to the abundance in the contingency table.
The reproductive period was estimated based on the monthly frequency of DE females in relation to the total number of females. Similarly, recruitment was expressed by the monthly frequency of juveniles in relation to the total number of individuals.
Then, the collinearity between the environmental variables was verified, as recommended by Zuur et al. (Reference Zuur, Ieno and Elphick2010). The relationship between the abundance of demographic groups and environmental parameters (BT, BS, OM and PHI) was evaluated with a Redundancy Analysis (RDA). The RDA is a multivariate statistical test that measures how strong the association between groups of variables is. The environmental parameters were included in the first group, and the abundance of juvenile males and females (J), adult males (AM), non-ovigerous adult females (AF) and ovigerous females (OF), were included in the second group. The RDA produces final coordination scores that summarize the linear relationship between the explanatory and response variables. Only environmental variables with coefficients greater than the module of ± 0.4 were considered biologically significant (Rakocinski et al., Reference Rakocinski, Lyczkowski-Shultz and Richardson1996). The CA and RDA were performed using the ‘ca’ and ‘vegan’ packages (Nenadic & Greenacre, Reference Nenadic and Greenacre2007; Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'hara, Simpson, Solymos, Henry, Stevens, Szoecs and Wagner2013; R Development Core Team, 2020).
Results
The sampling areas exposed to wave action (10, 15 and 20 m) showed lower values of BT (T = −5.254; df = 430; P < 0.001; Figure 2). The mean BT in the exposed area was 22.2 ± 2.8°C. The highest variations among BT and ST values in the Ubatuba region occurred in summer and spring (Figure 2). During the autumn and winter there was no variation of BT and ST related to increased depth (Figure 2).
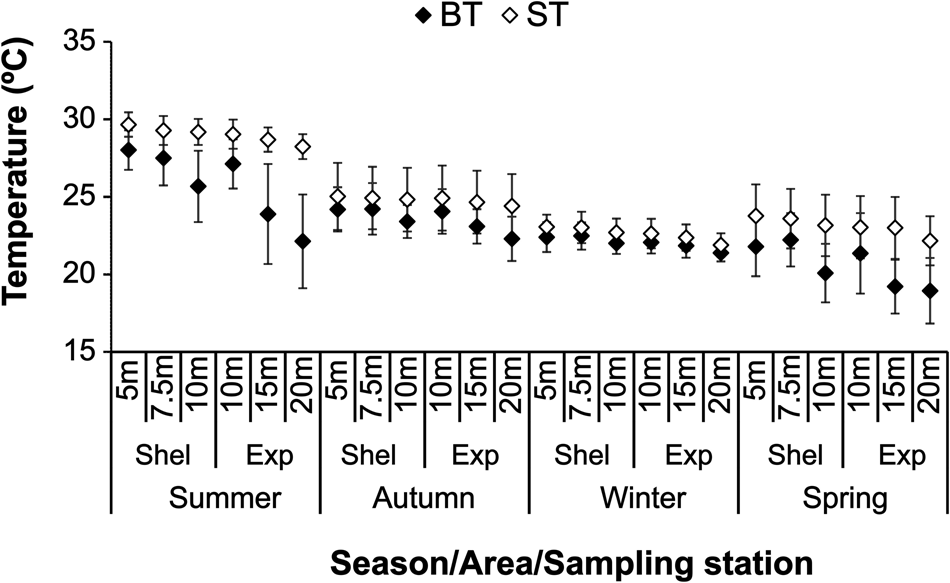
Fig. 2. Bottom and surface temperature, seasonal variation in each sampling stations and areas from 1998 to 2000 (BT, bottom temperature; Exp, exposed area; Shel, sheltered area; ST, surface temperature).
The area exposed to wave action presented the highest BS values (T = 3.5456; df = 430; P < 0.001; Figure 3). The BS mean in exposed areas was 34.6 ± 1.4‰. Seasonally, the highest values of BS occurred in the summer (34.9 ± 0.9‰) and autumn (35.4 ± 0.8‰) (ANOVA; F = 23.95; df = 3; P < 0.001; Figure 3). Spatially comparing all sampling areas, the highest values of BS (ANOVA; F = 4. 553; df = 5; P < 0.001), occurred at the 20 m deep site (35.1 ± 1.2‰; Figure 3).

Fig. 3. Bottom salinity seasonal variation in each sampling stations and areas from 1998 to 2000 (Exp, exposed area; Shel, sheltered area).
In the study region, there were three water masses, which are typical in the region. The Coastal Water (CW) (T ≥ 20°C; S ≤ 36) predominated throughout the year; while South Atlantic Central Water (SACW) (T ≤ 18°C; S ≤ 36) and Tropical Water (TW) (T ≥ 20°C; S ≥ 36) presented high influence in the spring and autumn, respectively (Figure 4).

Fig. 4. Diagram showing the seasonal variation of water temperature and salinity from January 1998 to December 2000, in Ubatuba region, south-eastern coast of Brazil (CW, Coastal Water; TW, Tropical Water; SACW, South Atlantic Central Water).
The features of the sediment (Phi and OM) differed among the sampled areas (Phi – F = 90.64; df = 5; P < 0.001; OM – F = 13.35; df = 5; P < 0.001). In all study periods, the lowest mean OM (3.3%) (P < 0.001) was at 20 m depth, with a mean Phi value equal to 3.0 (Figure 5).

Fig. 5. Granulometric fractions, organic matter content and the mean of phi for each transect (grain-size classes (%): A, medium sand, coarse sand, very coarse sand and gravel; B, fine sand and very fine sand; C, silt + clay; %OM, percentage of organic matter content).
A total of 222 individuals of Libinia ferreirae were captured, comprising 123 J (males and females), 43 AM, 25 AF and 31 OF. The CW of adult males and adult females (AF and OF) was not significantly different (T = −0.8561; df = 153; P = 0.393).
The mean CW of each demographic group, along with standard deviation and range, are shown in Table 1. The size-class frequency distribution was polymodal for both sexes (Figure 6). The highest abundance of J occurred in the fourth (22–|28 mm CW) and fifth size class (28–|34 mm CW). Adult males and females were more abundant in the eighth size class (46–|52 mm CW).

Fig. 6. Libinia ferreirae Brito Capello, 1871. Frequency distribution in size classes for males and females with indication of modal peaks in the Ubatuba region, São Paulo northern coast, Brazil (*, modal peaks).
Table 1. Libinia ferreirae Brito Capello, 1871

Number of individuals (N), size ranges, means and standard deviations of carapace width for each demographic group.
N, number; SD, standard deviations.
In the total population, the sex ratio (M: F) was 1:1.6 (P < 0.05); among size classes, a female-biased sex ratio was only seen in the 10–|16 mm CW (Table 2). The frequency per size class was not significantly different between males and females (dmax = 0.122; df = 2; P = 0.08).
Table 2. Libinia ferreirae Brito Capello, 1871

Distribution of individuals in size classes and demographic group.
*, statistical difference (P < 0.05).
Most of the sampled population was composed of J, concentrated in the shallower sample areas, such as 5 and 7.5 m of depth. The adults (males and females, mainly ovigerous) had a high association with deeper areas, especially at 15 m (Figure 7).

Fig. 7. Libinia ferreirae Brito Capello, 1871. Correspondence analysis (CA) of the abundance of demographic groups in the sampling stations sampled in the Ubatuba region. At the bottom the statistical summary of Pearson's χ2 test for randomness of the observed association (AF, adult females; AM, adult males; Exp, exposed; J, juveniles; OF, ovigerous females; Shel, sheltered).
Both the reproductive and recruitment periods were continuous with peaks (Figure 8). Reproductive males and females were found in all seasons. Even though all demographic groups were found during the entire survey, except J, the temporal distribution based on the CA analysis (Figure 9), indicated a relationship between J and spring, and between female adults (AF and OF) and summer and autumn.

Fig. 8. Libinia ferreirae Brito Capello, 1871. Seasonal variation in the proportion of individuals in different gonadal stages in the Ubatuba region: (A) males; (B) females (DE, developed gonads; ED, developing gonads; IM, immatures; OF, ovigerous females; RU, rudimentary gonads).

Fig. 9. Libinia ferreirae Brito Capello, 1871. Correspondence analysis (CA) of the abundance of demographic groups in the seasons in 1998 and 2000 sampled in the Ubatuba region (AF – adult females; AM, adult males; J, juveniles; OF, ovigerous females).
According to the RDA axis 1, which explained 94% of the variance, the abundance of J, AM and AF was inversely proportional with BT and BS, while OF had a proportional relationship with BT and BS (Figure 10).

Fig. 10. Libinia ferreirae Brito Capello, 1871. Results from the redundancy analysis (RDA): ordination of the first two canonical axes, with environmental variable data and demographic categories abundance in the Ubatuba region. Coefficients greater than the module of ± 0.4 were considered ecologically relevant (Rakocinski et al., Reference Rakocinski, Lyczkowski-Shultz and Richardson1996) and are shown in the plot (BT, bottom temperature; BS, bottom salinity; AF, adult females; AM, adult males; J, juveniles; OF, ovigerous females).
Discussion
The results of this study revealed the absence of size sexual dimorphism among adults of Libinia ferreirae and a sex ratio favouring females, even though most brachyuran crab species have sex ratios close to 1:1 (Hartnoll, Reference Hartnoll1978).
Sexual dimorphism with larger males than females seems to be a common pattern for crustaceans, including brachyurans and crayfishes (Miazaki et al., Reference Miazaki, Simões, Castilho and Costa2019; Sousa et al., Reference Sousa, Bernardes, Silva, Bertini, Taddei and Fransozo2019; Bernardo et al., Reference Bernardo, Bernardes, Silva, Sousa, Taddei and Fransozo2020; Hamasaki et al., Reference Hamasaki, Osabe, Nishimoto, Dan and Kitada2020). According to Hartnoll (Reference Hartnoll1969), males are larger because they tend to protect pre- and post-moulting females with the copulatory embrace, representing an adaptation to ensure reproductive success. However, the average size of L. ferreirae males and females did not differ significantly herein.
Sexual dimorphism seems to be a variable characteristic within Libinia. Studies about the population characteristics of Libinia dubia H. Milne Edwards, 1834 (Able et al., Reference Able, Landau and O'Brien1999) and L. ferreirae (Gonçalves et al., Reference Gonçalves, Bolla, Negreiros-Fransozo and Castilho2016a) showed that there was no difference in size between the sexes for the former species (Able et al., Reference Able, Landau and O'Brien1999), while females were larger than males in the latter (Gonçalves et al., Reference Gonçalves, Bolla, Negreiros-Fransozo and Castilho2016a). On the other hand, males of L. emarginata Leach, 1815 and L. spinosa were significantly larger than females (Able et al., Reference Able, Landau and O'Brien1999; Sal Moyano et al., Reference Sal Moyano, Gavio and Maggi2011). The sex ratio for Libinia also seems to be variable, e.g. adults in the present study had a proportion of 0.8♂:1♀ (Table 2); L. dubia 1.3♂:1♀ (Able et al., Reference Able, Landau and O'Brien1999); L. ferreirae from Cananéia 0.5♂:1♀ (Gonçalves et al., Reference Gonçalves, Bolla, Negreiros-Fransozo and Castilho2016a); L. emarginata 2.5♂:1♀ (Able et al., Reference Able, Landau and O'Brien1999) and L. spinosa 1.5♂:1♀ (Sal Moyano et al., Reference Sal Moyano, Gavio and Maggi2011). These results suggest that dimorphism related to size and sex ratio may be closely related, with males tending to be larger than females in populations where they are more abundant.
Similarly, there was no sexual dimorphism found for Achelous spinimanus (Latreille, 1819) in Macaé, on the coast of Rio de Janeiro (Andrade et al., Reference Andrade, Costa, Castilho, Frameschi, Sancinetti and Fransozo2017) and A. spinicarpus (Stimpson, 1871) in the region of Ubatuba and Caraguatatuba (Silva et al., Reference Silva, Taddei, Bertini, Andrade, Teixeira and Fransozo2017), with all these studies showing female-biased sex ratios. In addition, some authors have pointed out that trawling (as used in the present study) is the most reliable collection method for characterizing population aspects (Able et al., Reference Able, Landau and O'Brien1999).
The frequency distribution by size revealed that both sexes in the population of L. ferreirae from the Ubatuba region have polymodal distributions. This type of classification indicates the presence of two or more age groups, or that these differences in frequency are related to differential migration, mortality and/or birth rates (Diaz & Conde, Reference Díaz and Conde1989). In the sampled population, most peaks could be associated with the differential displacement of the demographic groups, since juveniles of L. ferreirae can live in association with jellyfish L. lucerna (Gonçalves et al., Reference Gonçalves, Grabowski, Bochini, Costa and Castilho2017), while brachyuran OFs migrate to deeper areas to optimize larval dispersion and survival (Abelló, Reference Abelló1989; Andrade et al., Reference Andrade, Bertini, Fransozo, Teixeira, Barros-Alves and Fransozo2014; Bernardes et al., Reference Bernardes, Martins, Rodrigues, Bernardo, Sousa, Bertini and Fransozo2019).
This symbiotic relationship between L. ferreirae and jellyfish may also explain the lower CW values of juveniles from Cananéia (Gonçalves et al., Reference Gonçalves, Grabowski, Bochini, Costa and Castilho2017), compared with the same group in the present study. During trawls in Cananéia, jellyfish were examined for the presence of crabs (Gonçalves et al., Reference Gonçalves, Grabowski, Bochini, Costa and Castilho2017), consequently there were a lot of small individuals. Due to the low number of jellyfishes in the Ubatuba region (Gonçalves et al., Reference Gonçalves, Wolf, Costa and Castilho2016b), they were not sampled. However, despite the absence of L. lucerna, the studied area also seems to favour species recruitment, since juveniles corresponded to around 60% of the crabs sampled.
In Cananéia, from February 2013 to January 2014, the average size of adults was also lower (AM = 37.8 mm; AF = 42.1) (Gonçalves et al., Reference Gonçalves, Bolla, Negreiros-Fransozo and Castilho2016a) when compared with adults in the Ubatuba region (AM – 55.3 ± 8.6 44.0; AF – 53.6 ± 5.4). The larger size of the individuals could be associated with the fact that the sampling was performed at a time when there was less intense fishing activity (1998–2000). The number of productive units (fishermen and vessels) in São Paulo State in 2013 was double that of 1998 (Instituto de Pesca, São Paulo), consequently, the populations of species associated with by-catch, such as L. ferreirae, are impacted by this activity as mortality rates tend to be higher in adults and the sooner individuals reach maturity, the greater their reproductive gain. Therefore, fishing pressure can cause individuals to reach maturity at smaller sizes, which can reduce fecundity and reproduction rates (Keunecke et al., Reference Keunecke, D'Incao, Verani and Vianna2012).
In addition to fishing activity, there are other factors that can change the size of individuals, e.g. temperature. Several rules have been proposed from numerous observations and comparisons of specimens from different latitudes (Vernberg, Reference Vernberg1962; Blackburn et al., Reference Blackburn, Gaston and Loder1999). One such rule is that of Jame, which suggests that the smallest individuals of a species are generally found in geographic areas with higher temperatures, i.e. in regions with lower latitudes. Conversely, larger specimens will be collected in colder regions, at higher latitudes (Blackburn et al., Reference Blackburn, Gaston and Loder1999). This probably occurs because lower temperatures require lower metabolic rates, which leads to lower energy expenditure, thus facilitating the accumulation of reserves and the acquisition of larger body sizes. In addition, colder waters tend to have higher productivity, which would also support larger body sizes.
When comparing the size variation of Libinia OFs from Argentina with that of the present study (45.7–63.2 mm CW), we note that Jame's rule did not apply. In Mar del Plata, Argentina (38°S 57°33′W), OF size ranged from 40.0–56.0 mm CW (Sal Moyano et al., Reference Sal Moyano, Gavio and Maggi2011) and in Cracker Bay, Patagonia-Argentina (42°56′S 64°21′W) this variation was 41.9–62.7 mm CW (González-Pisani & López Greco, Reference González-Pisani and López Greco2014). These results could be consequences of countergradient variation, which is based on the fact that individuals of a given species can reproduce and grow at the same temperatures, regardless of latitude, simply by restricting these physiological activities to the appropriate portion of the temperature cycle. In this case, the duration of the growing season (instead of the temperatures at which growth occurs) would decrease with increasing latitude (Conover & Present, Reference Conover and Present1990). The authors also propose that if the growth rates of organisms in environments with short growing periods exceed those of environments with long growing periods, the duration of the growth phase will be compensated at all temperatures that allow development. This has been evidenced by several marine invertebrates (Levinton, Reference Levinton1983; Lonsdale & Levinton, Reference Lonsdale and Levinton1985), including another Majoidea: Chionoecetes opilio (O. Fabricius, 1788) (Burmeister & Sainte-Marie, Reference Burmeister and Sainte-Marie2010).
Countergradient variation was also observed for jellyfish that have a symbiotic relationship with Libinia spp. When comparing the diameter of jellyfish at different latitudes, such as Mar Chiquita, Argentina (37°45′S 57°26′W) (Zamponi, Reference Zamponi2002); Rio de La Plata, Argentina/Uruguay (35°S 57°W) (Sal Moyano et al., Reference Sal Moyano, Schiariti, Giberto, Briz, Gavio and Mianzan2012); Punta del Este, Uruguay (34°58′S 54°57′W) (Vaz Ferreira, Reference Vaz Ferreira1972); Paraná, Brasil (25°55′S 48°35′W) (Nogueira Jr & Haddad, Reference Nogueira and Haddad2005); Cananéia (25°04′S 47°50′W) (Gonçalves et al., Reference Gonçalves, Wolf, Costa and Castilho2016b); Macaé (22°22′S 41°46′W) (Gonçalves et al., Reference Gonçalves, Wolf, Costa and Castilho2016b) and in Paraíba (06°58′S 34°51′W) (Baeza et al., Reference Baeza, Barros-Alves, Lucena, Lima and Alves2017), we noticed that the average diameter of jellyfish was similar at all localities.
The sex ratio favouring females in the present study could be associated with the fact that Majoidea females do not require periodic copulations, since they can store sperm and spawn several times with just one sperm mass from a single copulation (González-Gurriarán et al., Reference González-Gurriarán, Fernández and Muiño1998). Cananéia also presented the highest amount of L. ferreirae females in the population (Gonçalves et al., Reference Gonçalves, Grabowski, Bochini, Costa and Castilho2017), with these authors highlighting differential occupation of habitats between the sexes as a justification for such a result.
In the Ubatuba region, there was differential occupation between juveniles and adults of L. ferreirae throughout the study period. The grouping of immatures in the shallowest sites, especially at 5 and 7.5 m depth, could be associated with the fact that these sites have greater availability of calcium-rich food, such as shell fragments (Almeida et al., Reference Almeida, Fransozo, Teixeira, Hiroki, Furlan and Bertini2012). Moulted crabs are often found on hard substratum in shallow waters where they feed on calcium-rich organisms such as mussels and barnacles (Karlsson & Christiansen, Reference Karlsson and Christiansen1996). In addition, the 7.5 m deep sampling area is naturally excluded from fishing, likely contributing to the abundance of individuals. The habitat complexity in areas less impacted by fishing is typically preserved, which favours the establishment of new individuals (Kaiser et al., Reference Kaiser, Collie, Hall, Jennings and Poiner2002).
The inversely proportional abundance of juveniles with BT and SF values may be related to peak recruitment during SACW entrance (T ≤ 18°C; S ≤ 36) in the spring. This water mass is characterized by low values of salinity and temperature, besides being responsible for the enrichment of water in coastal regions, and increasing primary productivity (Castro-Filho et al., Reference Castro-Filho, Miranda and Myao1987). Subsequently, the high phytoplankton density sustains a large abundance of Salpas (Tunicata, Salpidae) and other invertebrate groups, whose carcasses and fecal pellets transfer organic matter to the sediment, benefiting benthic organisms (Pires-Vanin et al., Reference Pires-Vanin, Rossi-Wongtschowski, Aidar, Mesquita, Soares, Katsuragawa and Matsuura1993). In this sense, the physicochemical characteristics of SACW are probably the real modulators of L. ferreirae recruitment in our study region. Other studies have also pointed to the higher supply of food as a modulating factor for the abundance of L. ferreirae (Gonçalves et al., Reference Gonçalves, Grabowski, Bochini, Costa and Castilho2017) and other brachyurans, such as L. spinosa (Braga et al., Reference Braga, Fransozo, Bertini and Fumis2007), H. pudibundus (Bernardes et al., Reference Bernardes, Martins, Rodrigues, Bernardo, Sousa, Bertini and Fransozo2019) and A. spinimanus (Sousa et al., Reference Sousa, Bernardes, Bernardo, Taddei, Teixeira, Costa and Fransozo2020).
The higher abundance of adults, mainly AF and OF, at greater depths may have occurred because of the behavioural pattern of crustaceans with planktotrophic larval stages. In places far from the coast, larval dispersion and survival are optimized since larval dispersion is facilitated by ocean currents in deeper regions (Andrade et al., Reference Andrade, Bertini, Fransozo, Teixeira, Barros-Alves and Fransozo2014) and because environmental conditions are stable, physiological stress in larvae is reduced (Abelló, Reference Abelló1989). Bernardes et al. (Reference Bernardes, Martins, Rodrigues, Bernardo, Sousa, Bertini and Fransozo2019) suggested that the OFs of H. pudibundus are generally found in deeper areas due to the fact that salinity is higher, which favours embryonic development during incubation. Additionally, these authors suggested that this group moves away from the coast because their larvae float more easily in higher salinities.
Herein, a continuous reproductive period was found for L. ferreirae. Extensive reproductive periods are characterized by various spawning events throughout the year (Giese, Reference Giese1959). When analysing L. emarginata and other Majoidea as Chionoecetes opilio and Maja squinado (Herbst, 1788), it has been found that OFs can also copulate, since their gonads are already developed and they can store new sperm masses, with females being able to spawn up to four times in a row (Hinsch, Reference Hinsch1968; Elner & Beninger, Reference Elner and Beninger1995; González-Gurriarán et al., Reference González-Gurriarán, Fernández and Muiño1998). The same pattern was found in Cananéia for this species (Gonçalves et al., Reference Gonçalves, Grabowski, Bochini, Costa and Castilho2017), with continuous reproduction being a common feature of tropical and subtropical marine brachyurans such as: L. spinosa (Braga et al., Reference Braga, Fransozo, Bertini and Fumis2007), Persephona mediterranea (Herbst, 1794) (Bertini et al., Reference Bertini, Teixeira, Fransozo and Fransozo2010b), C. ornatus (Andrade et al., Reference Andrade, Bertini, Fransozo, Teixeira, Barros-Alves and Fransozo2014), Arenaeus cribarius (Lamarck, 1818) (Silva et al., Reference Silva, Andrade, Fransozo, Freire and Fransozo2018) and H. pudibundus (Miazaki et al., Reference Miazaki, Simões, Castilho and Costa2019).
However, in Cracker Bay, Patagonia-Argentina (42°56′S 64°21′W), individuals from the same genus reproduce seasonally, mainly in periods with higher temperatures and food availability (González-Pisani, Reference González-Pisani2011). These results corroborate the latitudinal effect paradigm, which states that reproduction can be continuous in tropical regions (lower latitudes) due to constant environmental conditions that favour gonadal development, feeding and spawning, while in temperate regions at higher latitudes, reproduction is generally restricted to a few months due to resource limitation and temperature variation (Bauer, Reference Bauer1992). Thus, we can infer that the reproductive pattern of Libinia is related to latitude, i.e. its metabolism could depend on the region where it is, affecting its reproductive characteristics.
We believe that the OFs of L. ferreirae peaked in the autumn in order to maximize their reproductive success, as there is higher food availability during this season. During this time of the year, the SACW usually recedes and there is intense suspension of sediments, causing a carriage with particulate organic matter generated in the previous period and, although crabs feed on bivalves and gastropods, they also consume the organic matter in the sediment (Gonçalves et al., Reference Gonçalves, Negreiros-Fransozo, Fransozo and Castilho2020). The mechanisms used by L. ferreirae to expand reproductive success in the present study were probably successful since there was ‘effective spawning’ in the region. This term refers to situations in which there is a peak in the number of juveniles soon after a peak in the abundance of reproductive individuals, making it possible to prove that reproductive individuals in a given period generated offspring in the following months (Crocos & Van der Velde, Reference Crocos and Van Der Velde1995).
Some of the characteristics of the population observed herein indicate that L. ferreirae uses the Ubatuba region throughout its entire life cycle. These features include continuous reproduction, high abundance of immature individuals and the presence of individuals at all stages of gonadal development. Therefore, direct and indirect changes in the region should be monitored to prevent damaging stocks of this species and others that are caught as by-catch. Furthermore, this study provides a basis in which to compare current data and also attests to the effectiveness of conservation strategies implemented in 2008–2009 for this species.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315420001289
Acknowledgements
We are thankful to the NEBECC co-workers for their help during the fieldwork.
Financial support
We are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo for providing financial support (FAPESP: #94/4878–8, 97/12108–6, 97/12106–3, 97/12107–0 and 98/3134–6), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Research Scholarships #302528/2015-6 AF and 310199/2018-2 GMT).














