INTRODUCTION
Species are the fundamental unit of biodiversity (Mayr, Reference Mayr1982), therefore proper species delimitation and identification are important prerequisites to population genetic, physiological and ecological studies (Wiens, Reference Wiens2007; Butlin et al., Reference Butlin, Bridle and Schluter2009). The interaction between host and parasite has profound consequences for biology. Conflict between interaction partners is known to result in rapid changes to phenotypes, and is thought to be the driving force of phenomena such as the evolution of sex and speciation (Jaenike, Reference Jaenike1978; Hamilton, Reference Hamilton1980).
Ghost shrimps or mud shrimps, representatives of the infraorders Axiidea and Gebiidea, are among the most common benthic macro-invertebrates in littoral zones of the Persian Gulf and Gulf of Oman (Sepahvand et al., Reference Sepahvand, Sari, Salehi, Nabavi and Ghorbanzadeh2013). These shrimps are adapted to a burrowing lifestyle and their burrows can also be occupied by a variety of organisms, including copepods (Dworschak et al., Reference Dworschak, Felder, Tudge, Schram and von Vaupel Klein2012).
According to Huys & Boxshall (Reference Huys and Boxshall1991), nearly half of all known copepod species have lived in close association with other phyla at least since the lower Cretaceous period. Clausidiid copepods are external associates of marine invertebrates and Clausidium species inhabit the burrow of Callianassid crustacea. According to Sepahvand et al. (Reference Sepahvand, Sari, Salehi, Nabavi and Ghorbanzadeh2013) two out of 11 species of burrowing shrimps recorded from the littoral zone of Iran have associated copepods from the genus Clausidium. Although these clausidiid copepods are relatively rarely recorded because of the cryptic lifestyle of their hosts, a total of 14 species of Clausidium have been described so far (Walter & Boxshall, Reference Walter and Boxshall2017). This genus has seven species recorded from the Atlantic Ocean and two species from the Pacific Ocean. The remaining two species, Clausididum travancorense Pillai, Reference Pillai1959 and Clausidium chelatum Pillai, Reference Pillai1959 have been found in the Indian Ocean (Pillai, Reference Pillai1959).
The present study, investigating Clausidium copepods of Iranian coastal waters of the Gulf of Oman, describes two new species associated with the ghost shrimps Neocallichirus natalensis (Barnard, Reference Barnard1947) and Corallianassa martensi (Miers, Reference Miers1884). These two species extend the group distribution to the north-west Indian Ocean and represent the first records of the genus in Iran.
MATERIALS AND METHODS
Sampling was carried out at two localities along the Iranian coast of the Gulf of Oman (Figure 1), with material collected from the large chelipeds of the ghost shrimp in the Gulf of Oman. At the sampling site, the copepods were relaxed with drops of Menthol 1.5% added to the seawater and separated from the host by filtration through a 63 µm mesh size net. The collected specimens were transferred to 75% ethanol. Whole specimens were temporarily mounted on slides with glycerin, and adhesive plastic discs were used to support the coverslip. Specimens were dissected under a Leica MZ12 stereomicroscope (Leica, Wetzlar, Germany). Dissected parts were mounted on slides using glycerin as mounting medium, and preparations were sealed with transparent nail varnish. The material was studied with a Leica DMR differential interference contrast microscope (Leica, Wetzlar, Germany) equipped with a drawing tube.

Fig. 1. The type localities of Clausidium makranensis (yellow circle) and Clausidium sarii (green circle).
Total body length was measured from the anterior margin of the rostrum to the posterior margin of the caudal rami. The descriptive terminology follows Huys et al. (Reference Huys, Gee, Moore, Hamond, Kermack, Barnes and Crothers1996). Abbreviations used in the text are: ae, aesthetasc; P1–P5, legs 1–5; exp and enp, exopod and endopod respectively; exp (enp)-1 (−2, −3), proximal (middle, distal) segments of a ramus. The type material is deposited in the collection of the Zoological Museum, University of Tehran (ZUTC). For confocal laser scanning microscopy (CLSM), selected material was stained with 1:1 solution of Congo red and acid fuchsin overnight. Whole specimens and dissected parts were mounted on slides with glycerin following the procedure described by Michels & Büntzow (Reference Michels and Buntzow2010). The material was scanned using a Leica TCS SP5 (Leica, Wetzlar, Germany) equipped with a Leica DM5000 B upright microscope (Leica, Wetzlar, Germany) and three visible-light lasers (DPSS 10 mW 561 nm; HeNe 10 mW 633 nm; Ar 100 mW 458 nm, 476 nm, 488 nm and 514 nm), combined with the software LAS AF 2.2.1. Leica Application Suite Advanced Fluorescence (Leica, Wetzlar, Germany). To obtain a three-dimensional representation from selected body parts, the data produced during the CLSM scanning was processed with the free software Drishti (http://anusf.anu.edu.au/Vizlab/drishti/). Final plates were composed and adjusted for contrast and brightness using the software Adobe Photoshop CS4 (Adobe Systems, San José, USA).
RESULTS
SYSTEMATICS
Order Poecilostomatoida Thorell, Reference Thorell1859
Family Clausidiidae Embleton, Reference Embleton1901
Genus Clausidium Kossmann, Reference Kossmann1874
Clausidium makranensis Sepahvand & Kihara sp. nov.
(Figures 2, 3 & 6–8)

Fig. 2. Clausidium makranensis sp. nov. Holotype ZUTC 5916. (A) antennule; (B) maxilla; (C) maxilliped; (D) labrum; (E) mandible; (F) antenna; (G) caudal ramus; (H) maxillule. Scale bar A: 100 µm; B–H: 25 µm.

Fig. 3. Clausidium makranensis sp. nov. Holotype ZUTC 5916. (A) Leg 1; (B) Leg 2; (C) Leg 3; (D) Leg 4; (E) Leg 5. The square symbol indicates the adhesive fringe, the asterisk indicates the long seta of end-2. Scale bar A–C: 100 µm; D–E: 75 µm.

Fig. 4. Clausidium sarii sp. nov. Holotype ZUTC 5915. (A) maxillule; (B) maxilla; (C) labrum; (D) mandible; (E) maxilliped; (F) antennule; (G) rostrum; (H) antenna. Scale bar A and F: 100 µm; B–H: 50 µm.

Fig. 5. Clausidium sarii sp. nov. Holotype ZUTC 5915. (A) Leg 1; (B) Leg 2; (C) caudal rami; (D) Leg 5; (E) Leg 3; (F) Leg 4. The square symbol indicates the adhesive fringe, the asterisk indicates the small naked subterminal seta. Scale bar A, B, E, F: 100 µm; C, D: 50 µm.

Fig. 6. Clausidium makranensis sp. nov. Holotype ZUTC 5916. Confocal laser scanning microscopy images. (A) habitus, dorsal; (B) habitus, ventral. Scale bars: A–B = 400 µm.
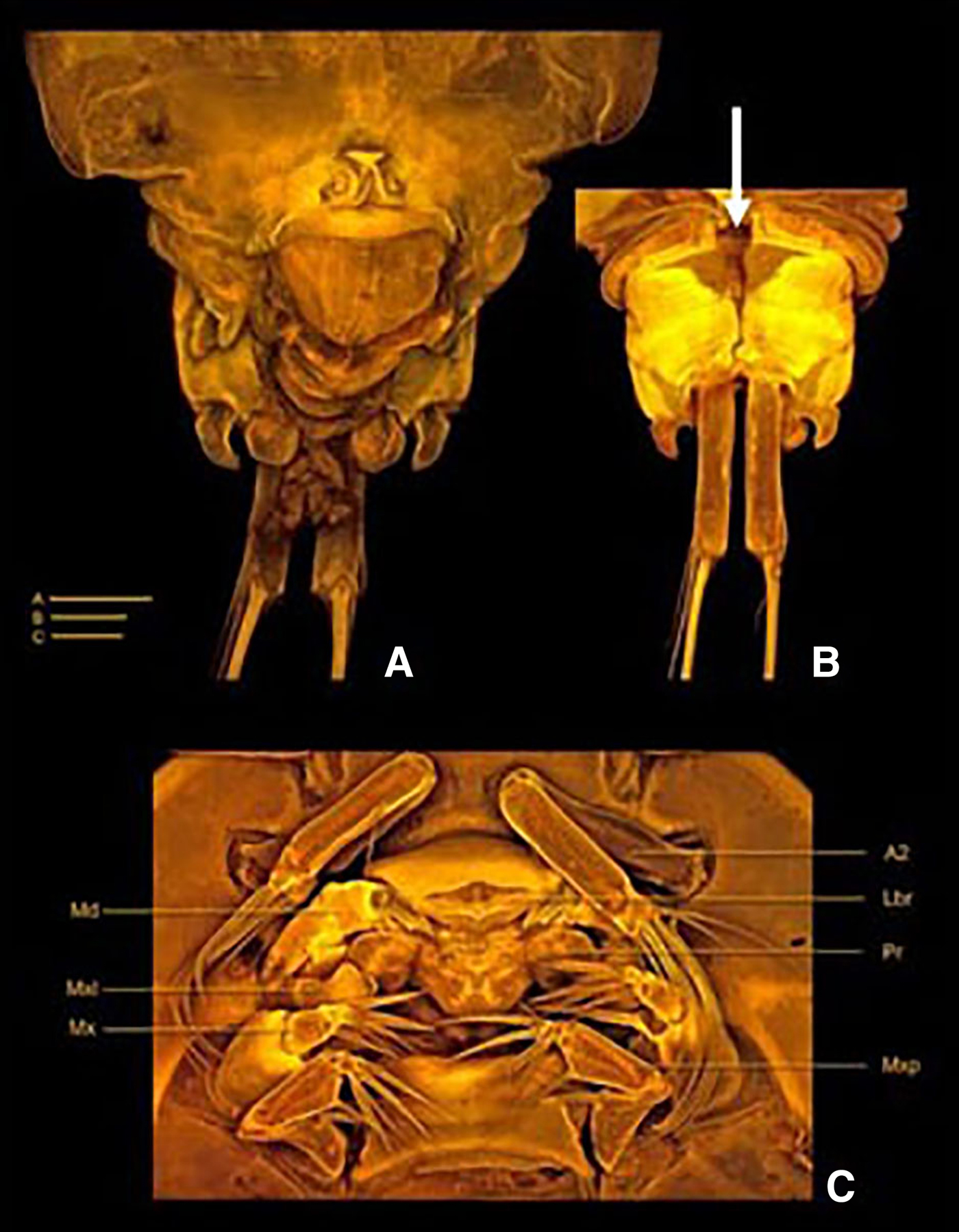
Fig. 7. Clausidium makranensis sp. nov. Holotype ZUTC 5916. Confocal laser scanning microscopy images. (A) anal somite, dorsal; (B) anal somite and caudal rami, ventral; (C) oral region. A–C = 50 µm. A2: antenna; Lbr: labrum; Md: mandible; Pr: prosomite; Mxl: maxillule; Mx: maxilla; Mxp: maxilliped. Arrow shows the medial pore.

Fig. 8. Clausidium makranensis sp. nov. Holotype ZUTC 5916. Three-dimensional representation (Drishti software) based on confocal laser scanning microscopy images. (A) urosome, dorsal; (B) urosome, lateral; (C) Leg 1. Scale bars: A–B = 200 µm; C = 100 µm.
TYPE MATERIAL
Holotype: adult female dissected on 25 slides (ZUTC 5916) deposited at the Zoological Museum, University of Tehran Crustacean collection, Tehran, Iran.
Paratype: adult female dissected on 25 slides (ZUTC 5917) deposited at the Zoological Museum, University of Tehran Crustacean Collection, Tehran, Iran. Material collected from the type locality by Vahid Sepahvand.
Type locality: Chabahar, Tis (25°21′25″N 60°36′17″E) from the Gulf of Oman, Iran (Figure 1). Specimen collected from the large chelipeds of the ghost shrimp Corallianassa martensi (Miers, Reference Miers1884).
DESCRIPTION
Body oval (Figure 6A, B), dorsoventrally compressed, total length 1.65 mm. Prosome (Figure 6A, B) 1.8 times longer than urosome. Maximum width measured at posterior margin of second pedigerous somite. First pedigerous somite fused with cephalosome. Body prosomites with minute integumental pits, sensilla and numerous pores distributed as illustrated in Figures. Epimera of second and third pedigerous somites expanded posteriorly. Fourth pedigerous somite trapezoid in form, longer than the two anterior somites combined and tapering towards distal end. Posterior margin of fourth pedigerous somite smooth. Urosome (Figure 8A, B) 3-segmented, distinctly narrower than prosome. Urosome comprising fifth pedigerous somite, genital double-somite and anal somite, segmentation not clear in dorsal view. Somite bearing P5 (Figure 6B) 2 times wider than long in ventral view and with P5 arising ventrolaterally. Genital double-somite (Figures 7A, B & 8A, B) rectangular, 1.4 times longer than wide. Genital apertures (Figure 7A, B) near the proximal third, located dorsolaterally on each side. Presence of dorsal medial pore (as illustrated in Figure 7B). Egg sacs dorsolaterally located on each side, extending beyond the distal margin of the caudal rami and each sac containing 25–27 eggs. Anal somite (Figure 7A, B) well developed, formed by second to fourth abdominal somites fused in one single segment; irregular in shape, incised medially, with protuberances and intricate folders dorsally and along lateral margins, distal outer corners with hook-like projections as illustrated in Figures. Caudal rami (Figures 2G & 7B) about four times longer than wide, and armed with six setae. Seta I absent, setae II and III pinnate; setae IV and V strongly developed and geniculate (seta V 2 times longer than seta IV); seta VI small; seta VII located at inner posterior corner, both naked. Rostrum (Figure 6B) completely incorporated into cephalothorax.
Antennule (Figure 2A) 7-segmented. Segment 2 longest, with long seta inserted on inner distal corner and almost reaching the tip of segment 4. Aesthetascs inconspicuous. Segment 7 with apical acrothek consisting of aesthetasc and 2 setae. Armature formula: I-[5], II-[16], III-[6], IV-[5], V-[5], VI-[3 ], VII-[8]. Antenna (Figures 2F & 7C) 4-segmented. Coxobasis elongated, with rows of spinules along inner margin, with single seta on inner distal corner. Endopod 3-segmented; segment 1 with naked seta along the inner margin; segment 2 with 4 setae (1 bipinnate, 1 unipinnate and 2 naked); segment 3 with 6 naked setae. Labrum (Figures 2D & 7C) 2 times wider than long; distal area with row of long denticles. Metastomal area ornamented as in Figure 8C. Mandible (Figures 2E & 7C) well developed. Armed with 3 elements, 1 toothed projection, 1 naked seta and 1 flat structure covered with long spinules. Maxillule (Figures 2H & 7C) slightly bilobed, with 1 lateral seta. Outer lobe with 4 setae (3 pinnate and 1 naked). Inner lobe with 3 pinnate setae. Maxilla (Figures 2B & 7C) 2-segmented. Syncoxa with 2 pinnate spines, both accompanied basally with stout spine with spinules on distal edge. Basis with large spinous and bifid process, bearing 3 pinnate elements (2 spines and 1 seta), lateral margin with 1 pinnate spine and 1 pinnate seta. Maxilliped (Figures 2C & 7C) 4-segmented. Syncoxa with 2 pinnate setae along inner margin. Basis with 2 naked setae. Endopod 2-segmented; first segment unarmed; second segment bearing 2 naked lateral setae, 3 stout distal spines and 1 naked inner seta. P1 (Figures 3A & 8C) biramous, both rami 3-segmented and modified for prehension. Coxa and basis fused forming well-developed segment with 1 naked seta on outer corner near exopod insertion; large blade-like seta on inner corner, with lines and apex as shown in Figures 3A & 8C. Exp-1 with row of small spinules and outer seta. Exp-2 with rows of small spinules and denticles, and outer seta. Exp-3 with rows of denticles and 3 outer setae (proximal and distal ones reduced), 2 apical pinnate spines and 2 inner naked setae. Enp-1 with 1 stout curved process with adhesive areas along the distal margin (marked with square in Figure 3A). Enp-2 with a minute seta (marked with asterisk in Figure 3A). Enp-3 elongated, irregular segment ending in a lobe armed with 1 seta and 2 sucking discs; proximal sucking disc 2 times larger than distal one. P2–P4 (Figure 3B–D) biramous, with both rami 3-segmented. Coxae with inner pinnate seta. Basis with pinnate seta on outer distal corner and row of setules along inner margin; extremely elongated in P4 (longer than exopod or endopod). Exopod outer spines serrate. Endopod outer apical spines serrate, sucking discs on distal outer edge of enp-1 and proximal and subterminal outer edges of enp-3. Armature formula of P2–P4 as follows (Roman numerals representing spines, Arabic numerals representing setae):

P5 (Figures 3E & 6B) uniramous, 2-segmented and located obliquely on somite. Protopod with 1 outer seta; free exopodal segment with 3 serrate spines and 1 small seta. P6 (Figure 8B) consisting of 2 setae.
Male: Unknown.
Etymology. The species name makranensis refers to Makran, ancient Persian word to designate the area along the coast of the Gulf of Oman referring to the provenance of the material.
Colour: Body orangish and appendage translucent when alive.
Clausidium sarii Sepahvand & Kihara sp. nov.
(Figures 4, 5 & 9–11)
Type materials: Holotype, female and dissected on 25 slides (ZUTC 5915), deposited at the Zoological Museum, University of Tehran Crustacean collection, Tehran, Iran. Material collected from the type locality by Vahid Sepahvand.
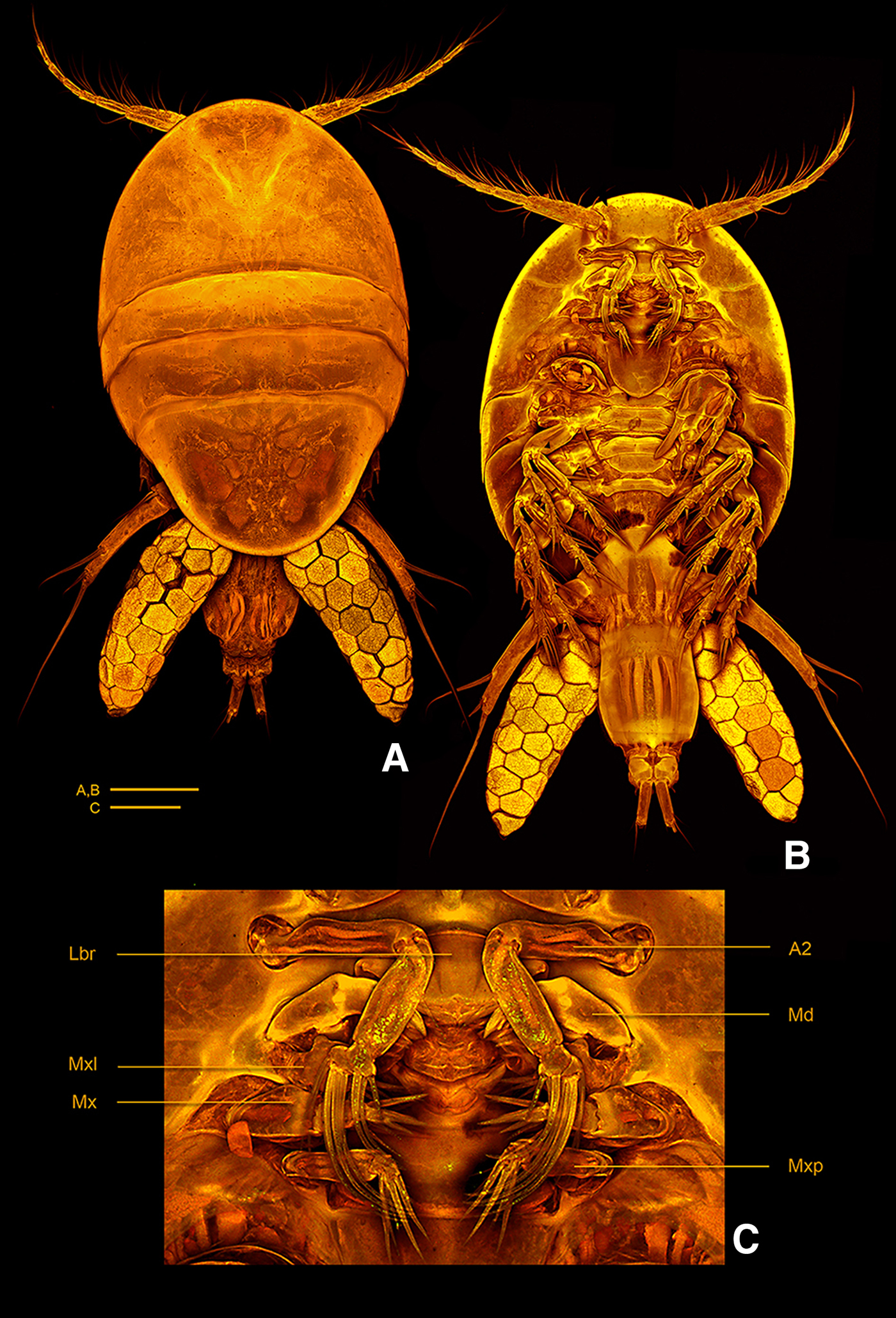
Fig. 9. Clausidium sarii sp. nov. confocal laser scanning microscopy images.(A) habitus, dorsal; (B) habitus, ventral; (C) oral region. Scale bars: A–B = 200 µm; C = 50 µm. A2: antenna; Lbr: labrum; Md: mandible; Mxl: maxillule; Mx: maxilla; Mxp: maxilliped.

Fig. 10. Clausidium sarii sp. nov. Holotype ZUTC 5915. Confocal laser scanning microscopy images. (A) anal somite and caudal rami, dorsal; (B) anal somite and caudal rami, ventral. Scale bars: A–B = 25 µm.

Fig. 11. Clausidium sarii sp. nov. Holotype ZUTC 5915. Three-dimensional representation (Drishti software) based on confocal laser scanning microscopy images. (A) urosome, dorsal; (B) urosome, ventral; (C) Leg 1. Scale bars: A–B = 200 µm; C = 100 µm.
Type locality: Djod (Figure 1) (25°26′58″N, 59°30′28″E) from the Gulf of Oman, Iran. Specimen collected from the large chelipeds of the ghost shrimp Neocallichirus natalensis (Barnard, Reference Barnard1947).
DESCRIPTION
Body oval (Figure 9A, B), dorsoventrally compressed and total length 1.42 mm. Prosome (Figure 9A, B) 2 times longer than urosome. Maximum width measured at posterior margin of second pedigerous somite. First pedigerous somite fused with cephalosome. Body prosomites with minute integumental pits, sensilla and numerous pores distributed as illustrated in Figure 9A, B. Epimera of second and third pedigerous somites expanded posteriorly. Fourth pedigerous somite longer than the two anterior somites combined and tapering towards distal end. Posterior margin of fourth pedigerous somite rounded. Urosome (Figures 9B & 11A, B) 3-segmented, distinctly narrower than prosome. Urosome comprising fifth pedigerous somite, genital double-somite and anal somite, segmentation not clear in dorsal view. Somite bearing P5 (Figure 9B) as wider as long in ventral view and with P5 arising ventrolaterally. Genital double-somite (Figure 11A, B) rectangular, 1.3 times longer than wide, slightly depressed on distal half. Genital apertures near the proximal third, located dorsolaterally on each side. Egg sacs dorsolaterally located on each side, reaching the distal margin of the caudal rami and each sac containing 24–27 eggs.
Anal somite (Figure 10A, B) well developed, formed by second to fourth abdominal somites fused in one single segment; irregular in shape, incised medially, with protuberances and intricate folders dorsally and along lateral margins, distal outer corners with elongated hook-like projections as illustrated in Figure 10A, B. Caudal ramus (Figures 5C & 10B) about 4 times longer than wide, and armed with 6 setae. Seta I absent, setae II and III naked; setae IV and V strongly developed and geniculate; setae VI small and naked; setae VII located at inner posterior corner. Rostrum (Figure 9B) incorporated into cephalothorax, with broad posteroventral margin. Antennule (Figure 4F) 7-segmented. Segment 2 longest, with long seta inserted on inner distal corner and almost reaching the tip of segment 4. Aesthetascs inconspicuous. Segment 7 with apical acrothek consisting of aesthetasc and 2 setae. Armature formula: I-[5], II-[15], III-[5], IV-[4], V-[5], VI-[3], VII-[8]. Antenna (Figures 4H & 9C) 4-segmented. Coxobasis elongated, with single seta naked on inner distal corner. Endopod 3-segmented; segment 1 with a naked spine along inner margin; segment 2 with 2 pectinate spines and 2 naked setae; Segment 3 with 6 apical setae (3 pectinate and 3 naked). Labrum (Figures 4C & 9C) 2 times wider than long; distal area with row of long denticles. Metastomal area ornamented as in Figure. Mandible (Figures 4D & 9C) well developed. Armed with 3 elements, 1 toothed projection, 1 spinulose seta, and 1 flat structure covered with long spinules. Maxillule (Figures 4A & 9C) unsegmented and slightly bilobed, Outer lobe with 4 pinnate setae. Inner lobe 3 setae (2 pinnate and 1 naked). Maxilla (Figures 4B & 9C) 2-segmented. Syncoxa with 2 pinnate setae and 1 pinnate spine, both accompanied basally with stout spine with spinules on distal edge. Basis with large spinous and bifid process, bearing 3 elements (2 pinnate spines and 1 naked seta). Maxilliped (Figures 4E & 9C) 4-segmented. Syncoxa with 2 pinnate setae along inner margin. Basis with 2 pinnate setae. Endopod 2-segmented; first segment unarmed; second segment bearing 3 naked setae and 3 stout spine. P1 (Figures 5A & 11C) biramous and modified for prehension. Coxa and basis fused forming well-developed segment with 1 naked seta on outer corner near exopod insertion; large blade-like seta on inner corner, with lines and apex as shown in Figure 8A. Exopod 2-segmented. Exp-1 with 1 strong seta and 1 reduced seta. Exp-3 with 3 outer setae (proximal and distal ones reduced), 2 apical pectinate setae and 2 inner setae (1 pinnate seta and 1 naked seta). Endopod 3-segmented. Enp-1 with 1 stout curved process with adhesive areas along the distal margin (marked with square in Figure 5A). Enp-2 short, wide and unarmed. Enp-3 elongated, irregular segment ending in a lobe armed with 1 seta and 2 sucking discs; proximal sucking disc 2.2 times larger than distal one. P2–P4 (Figures 5B & 8E, F) biramous, with both rami 3-segmented. Coxae with inner pinnate seta. Basis with pinnate seta on outer distal corner and row of setules along inner and outer margins. Exopod outer spines serrate. Endopod outer/outer apical spines serrate, sucking discs on distal outer edge of enp-1 and proximal and subterminal outer edges of enp-3. Armature formula of P2–P4 as follows (Roman numerals representing spines, Arabic numerals representing setae):

P5 (Figures 5D & 9B) uniramous, 2-segmented and located obliquely on somite. Protopod with 1 outer seta; free exopodal segment elongated, with 2 pinnate setae along the outer margin, 1 small naked subterminal seta (marked with asterisk in Figure 5D) and 1 long pinnate apical seta. P6 (Figure 11A, B) consisting of 2 setae.
Male: Unknown.
Colour: Body reddish when alive and appendage translucent.
Distribution: Known only from the type locality: Gulf of Oman North West of Indian Ocean.
Etymology: The new species is named in honour of Prof. Dr Alireza Sari (University of Tehran) in gratitude of his significant efforts to gain recognition for biodiversity of the Persian Gulf and Gulf of Oman.
DISCUSSION
Allocation of two new species and differentiation from congeners:
The two new species Clausidium makranensis sp. nov. and Clausidium sarii sp. nov. are assigned to Clausidium on account of the presence of sucking discs on endopods of legs 1 to 4, oval and flattened body, prosome comprising cephalothorax and 3 free pedigerous somites, urosome comprising 5 or 6 segmented, 7-segmented antennule, 4-segmented antenna, biramous P1–4, of which first pair highly modified, and uniramus and 2 segmented fifth leg. No male specimens of the two new species (C. makranensis sp. nov. and C. sarii sp. nov.) were available for examination in spite of our sampling efforts.
Clausidium makranensis sp. nov. most resembles Clausidium travancovernse Pillai, Reference Pillai1959, from the Indian Ocean, with shared characters in the antenna, maxillule, mandible and armature of P2–P5, but is distinguishable from the latter by having 2 spines on the syncoxa of the maxillae (vs 1 spine and 2 pinnate setae), the distal part of endopod-2 of the maxillipeds with 3 spines plus 1 seta (vs 2 pinnate setae and 1 spine), the blade-like process of P1 with an acute tip (vs a blunt tip), and the anal somite armed with 1 hook on each side(vs such hooks being absent).
Clausidium sarii further shares with C. macranensis the armature of P2–P5, maxilla and mandible, but distinguished from the latter by the free exopodal segment of p5, which is elongated about 5 times longer than wide and with 3 serrate spines (vs 3 times longer than wide with 4 serrate spines), the blade-like process of P1 with a blunt tip (vs acute tip), the syncoxa of the maxillae with 1 spine and 2 pinnate setae(vs 3 spines and 1 seta), the genital double somite swollen medially with a fine curved process on each side of the posterior end in C. macranensis (vs such structure being absent).
It should be noted that during this and the previous works we found that Clausidium species are host specific. It is advisable to examine the recent character for better and easy recognition of Clausidium copepods. Early researchers studying Clausidium assumed that some species were symbiotic and associates of ghost shrimps (Corsetti & Strasser, Reference Corsetti and Strasser2003; Kihara & Rocha, Reference Kihara and Rocha2013), although some species were thought to be parasitic (Kossmann, Reference Kossmann1874; Wilson, Reference Wilson1937; Pearse, Reference Pearse1947; Humes, Reference Humes1949; Pillai, Reference Pillai1959). This complex interaction is a potentially important aspect of ghost shrimp biology. Because of the influential role that ghost shrimps play in aquatic systems (Berkenbusch & Rowden, Reference Berkenbusch and Rowden2003) Clausidium copepods may have indirect effects on local communities and ecosystem processes via their direct effects on ghost shrimps.
ACKNOWLEDGEMENTS
We are very grateful to Professor Dr Pedro Martinez Arbizu from the Senckenberg am Meer, German Center for Marine Biodiversity Research, Wilhelmshaven for help and encouragement during this study. His hospitality during molecular and CLSM study in his lab to Vahid Sepahvand is gratefully acknowledged. The authors express their gratitude to Dr Abdolvahab Maghsoudlou from Iranian National Institute for Oceanography and Atmospheric Science for his cooperation in this study. Thanks are due to Professor Alireza Sarii and Dr Reza Naderloo from the University of Tehran for their help. Special thanks are due to Dr Christopher Tudge from the American University and Smithsonian Institution, Washington DC for reading of the manuscript and his helpful comments. Sampling would have been impossible without the help of Mr Abdolmohammad Baziar from the Iranian National Institute for Oceanography and Atmospheric Science (INIOAS).













