Introduction
The Lakshadweep archipelago consists of 36 islands, 10 of them inhabited, and five submerged reefs, the only coral atolls in India (Ghosh, Reference Ghosh1991). The islands are scattered over a large stretch of the Arabian Sea, off the south-west coast of India (Figure 1). They are all characterized by the presence of a large lagoon with numerous reef patches on the western side, and steep slopes and rich coral reefs on the eastern side.

Fig. 1. Lakshadweep Islands (black rectangle) near the west coast of southern India (top) and magnified image of Agatti Island (below) with collection sites of new species – Pseudoceros galaxea sp. nov. (red dot); Pseudoceros bipurpurea sp. nov. (yellow dot). Source: Google Earth.
Polyclads of the suborder Cotylea, Lang, Reference Lang1884 are characterized by the presence of a ventral sucker-like organ called a cotyl which is posterior to the female gonopore (Lang, Reference Lang1884). Velasquez et al. (Reference Velasquez, Bolaños and Benayahu2018) indicated the presence of around 380 cotylean species worldwide, with highest diversity in the Caribbean and the Indo–Pacific regions. The genus Pseudoceros is the most populous genus under the suborder Cotylea with a total of 138 species worldwide (1 species, Jie et al., Reference Jie, Gan, Cgen and Kuo2016; 1 species, Cuadrado et al., Reference Cuadrado, Moro and Noreña2017; 135 species, Tyler et al., Reference Tyler, Artois, Schilling, Hooge and Bush2006–2020; 1 species, Ramos-Sánchez et al., Reference Ramos-Sanchez, Bahia and Bastida-Zavala2020). Because of homogeneity in reproductive structures, some authors suggested that the specific identification relies on the colour and colouration pattern of the Pseudoceros species (Prudhoe, Reference Prudhoe1989; Newman & Cannon, Reference Newman and Cannon1994, Reference Newman and Cannon1996, Reference Newman and Cannon1997, Reference Newman and Cannon1998). This criterion of identification based on colours was further supported by Goggin & Newman (Reference Goggin and Newman1996) and Litvaitis & Newman (Reference Litvaitis and Newman2001) based on ribosomal nucleotide sequences. However, when species share relatively homogeneous external features such as colour or colouration patterns, internal characters such as orientation of seminal vesicle, morphology of the prostatic vesicle, depth of the male atrium, shape and size of the stylet, distance between sucker and female gonopore (Bolaños et al., Reference Bolanos, Quiroga and Litvaitis2007; Marquina et al., Reference Marquina, Aguado and Noreña2015) and musculature of the penile sheath (Ramos-Sánchez et al., Reference Ramos-Sanchez, Bahia and Bastida-Zavala2020) can be considered.
The first survey in Lakshadweep archipelago dated from the beginning of the 20th century (Ladilaw, Reference Laidlaw and Gardiner1902) and was carried out in Minicoy Island. The only available additional information about the polyclad fauna of these islands are reports from Kavaratti Island and Agatti Island (Apte & Pitale, Reference Apte and Pitale2011; Dixit et al., Reference Dixit, Bayyana, Manjebrayakat, Saravanane and Sudhakar2019, Reference Dixit, Bayyana, Manjebrayakat, Saravanane and Sudhakar2021). This paper is a result of a continuous survey being undertaken at Agatti Island by CMLRE to assess the biodiversity of polyclads and describe two pseudocerotid flatworms. With this study, the number of polyclads reported from this archipelago will increase to 29 species. The present contribution not only adds new information to the biodiversity of polyclads in these unique ecosystems but also provides an updated checklist of polyclads inhabiting Indian waters (Table 1).
Table 1. Checklist of polyclads reported from Indian coasts
AN, Andaman and Nicobar Islands; GOM, Gulf of Mannar; LK, Lakshadweep Islands; MH, Maharashtra.
Materials and methods
Specimens were collected during scuba diving in subtidal and by hand picking in intertidal areas of Agatti Island (Figure 1) and photographed using an Olympus TG-5 camera with underwater housing. Animals were fixed on frozen 10% formalin buffered with seawater (modified methodology of Newman & Cannon, Reference Newman and Cannon2003). Key external morphological characters such as cerebral eyes, tentacular eyes, marginal eyes, pseudotentacles, dorsal and ventral surface were photographed with a stereo microscope (Leica, M80) and measurements such as total length and width of the specimen, distance between mouth, gonopores and sucker were made. The portions with reproductive structures were dissected out, serially sectioned (7–8 μm), stained with haematoxylin and eosin and photographed with a LEICA DMi8 inverted microscope. The holotypes and paratype of the newly described species are deposited at the Referral Centre at Centre for Marine Living Resources and Ecology (CMLRE), Kochi, India.
Results
SYSTEMATICS
Order POLYCLADIDA Lang, Reference Lang1881
Suborder COTYLEA Lang, Reference Lang1884
Family PSEUDOCEROTIDAE Lang, Reference Lang1884
Genus Pseudoceros Lang, Reference Lang1884
Pseudoceros bipurpurea sp. nov. Dixit
(Figures 2–4)

Fig. 2. Pseudoceros bipurpurea sp. nov. (A) Holotype (live); (B) cerebral and tentacular eyes (preserved specimen); (C) ventral surface (preserved specimen); (D) dorsal surface (preserved specimen). ce, cerebral eyes; fp, female gonopore; m, mouth; mp, male gonopore; p, pharynx; su, sucker; te, tentacular eyes.

Fig. 3. Pseudoceros bipurpurea sp. nov. Sagittal sections (A) showing arrangement of reproductive structures; (B) female reproductive system; (C) and (D) male reproductive system; (E) stylet (magnified). cg, cement glands; cp, cement pouch; ed, ejaculatory duct; fa, female atrium; fp, female gonopore; ma, male atrium; mp, male gonopore; ov, oviduct; pd, prostatic duct; pp, penis papilla; ps, penis sheath; pv, prostatic vesicle; st, stylet; sv, seminal vesicle; va, vagina.

Fig. 4. Pseudoceros bipurpurea sp. nov. Diagrammatic reconstruction of male and female reproductive system. cg, cement glands; cp, cement pouch; ed, ejaculatory duct; fa, female atrium; fp, female gonopore; in, intestine; ma, male atrium; mp, male gonopore; ov, oviduct; p, pharynx; pd, prostatic duct; pp, penis papilla; ps, penis sheath; pv, prostatic vesicle; st, stylet; sv, seminal vesicle; va, vagina.
Type material
Holotype: One specimen, 16 × 9 mm, serial sectioned and mounted on 43 slides. Solar Point, Agatti Island, Lakshadweep, India (10°50′28′′N 72°11′30′′E); sandy substratum; water depth: 32 m; (CMLRE IO/DV/POY/00028); Coll. S. Dixit, 6 May 2019.
Etymology
The specific name bipurpurea is a compound Latin noun meaning ‘two purple’ refers to the characteristic dorsal colour pattern, two (bi) purple (purpura in Latin) regions of dense purple spots surrounding the orange median line.
Diagnosis
Background body colour cream with an orange median band surrounded by dense patches of purple spots without touching the margin (Figure 2A). These dense purple spots tend to disperse and broaden toward posterior end. Marginal rim blue.
Description
Live: Body oval and margin without ruffles. Background body colour cream with an orange median band. The median band is surrounded by dense purple spots (Figure 2A). These purple spots tend to disperse and broaden toward posterior end encircling the orange median band. The median band and purple spots start at the same point below cerebral eyes cluster and end without touching the posterior end. Marginal rim blue (Figure 2A). Pseudotentacles are simple folding of the anterior margin with same colour pattern as margin. Cerebral eyes dense and more or less forming a circular cluster (Figure 2A, B), tentacular eyes hard to locate due to the blue colour of the pseudotentacles and only visible in the preserved specimen (Figure 2B). The ventral surface is of cream colour.
Preserved: Specimen whitish and colour lost after fixation (Figure 2B, D). Orange median band discoloured and purple spots turned light brown showing the spotted pattern more clearly. Male and female gonopores are 1 mm apart, while female gonopore and sucker are 1.3 mm apart (Figure 2C). Pharynx ruffled with eight folds and male gonopore is situated very near to last pair of pharyngeal folds (Figure 2C). Pharynx is 3.2 mm long and distance between mouth and male gonopore is 2.3 mm. Cerebral eye cluster with about 60−70 eyes. Tentacular eyes arranged in two scattered lines (Figure 2B).
Reproductive system: The male copulatory apparatus consists of seminal vesicle, free prostatic vesicle, penis papilla armed with a stylet housed in male atrium which opens to the outside via male gonopore. An elongated seminal vesicle (670 μm long and 280 μm high) is present (Figures 3A & 4). An oval and thick-walled prostatic vesicle (192 long × 89 μm high) is present anterior to seminal vesicle (Figures 3C & 4). Prostatic vesicle has smooth glandular lining, 12–15 μm high (Figure 3C). Prostatic and ejaculatory duct enter separately in penis papilla and join before stylet (Figures 3D & 4). Male atrium conical (Figure 3C) housing conical penis papilla (130 μm) armed with an elongated stylet (241 μm) (Figure 3C, E) opening to the exterior via male gonopore (Figure 3C).
Female copulatory apparatus consists of oviduct, vagina, cement pouch, cement glands, female atrium and female gonopore (Figures 3B & 4). The common oviduct enters in vagina which then leads to a short female atrium via cement pouch. Numerous cement glands are scattered in parenchyma tissue from oviduct to seminal vesicle surrounding the vagina. Female atrium opens to exterior via female gonopore (Figures 3B & 4).
Taxonomic remarks
Presence of smooth dorsal surface, ruffled pharynx, cerebral and tentacular eyes, marginal tentacles formed by upfolding of anterior margin, centrally located sucker behind female gonopore, male copulatory apparatus single and just behind pharyngeal cavity, free prostatic vesicle and penis papilla armed with stylet place this new species within the family Pseudocerotidae and in the genus Pseudoceros.
Pseudoceros bipurpurea sp. nov. differs from all other Pseudoceros species by its colour pattern on dorsal surface. In terms of background colour and marginal rim (cream background and blue rim) the newly described species here matches with Pseudoceros indicus Newman & Schupp, Reference Newman and Schupp2002, P. gamblei Laidlaw, Reference Laidlaw and Gardiner1902, P. rubrotentaculatus Kaburaki, Reference Kaburaki1923 and P. concinnus (Collingwood, Reference Collingwood1876) but differs in terms of colour pattern on dorsum. Several species of Pseudoceros display median stripes (either one or more than one). A species which possesses a single median stripe of matching colour (orange) on dorsum is Pseudoceros galatheensis Dixit, Raghunathan & Chandra, 2017 (thin orange median stripe) but it has light blue background colour which grows darker towards margins which differentiates it with newly described species here. However, there are a few species with median stripe bordered or surrounded by other stripes of different colour which include Pseudoceros bifurcus Prudhoe, Reference Prudhoe1989 (white median stripe outlined with dark burgundy), Pseudoceros monostichos Newman & Cannon, Reference Newman and Cannon1994 (black brown median line bordered by white and light brown), Pseudoceros susanae Newman & Anderson, Reference Newman and Anderson1997 (white median stripe surrounded by two orange stripes) and Pseudoceros tristriatus (three orange stripes on dorsum). Pseudoceros bipurpurea sp. nov. differs from all above-mentioned species by presence of an orange median stripe surrounded by dense purple spots. As no other valid species under the genus Pseudoceros is comparable with the newly described species in terms of colouration and pattern, we establish Pseudoceros bipurpurea sp. nov. as a new species to science.
Pseudoceros galaxea sp. nov. Dixit
(Figures 5–7)

Fig. 5. Pseudoceros galaxea sp. nov. (A) Holotype (live); (B) pseudotentacles and cerebral eyes (preserved specimen); (C) ventral surface (preserved specimen). ce, cerebral eyes; fp, female gonopore; m, mouth; mp, male gonopore; p, pharynx; pt, pseudotentacles; su, sucker.
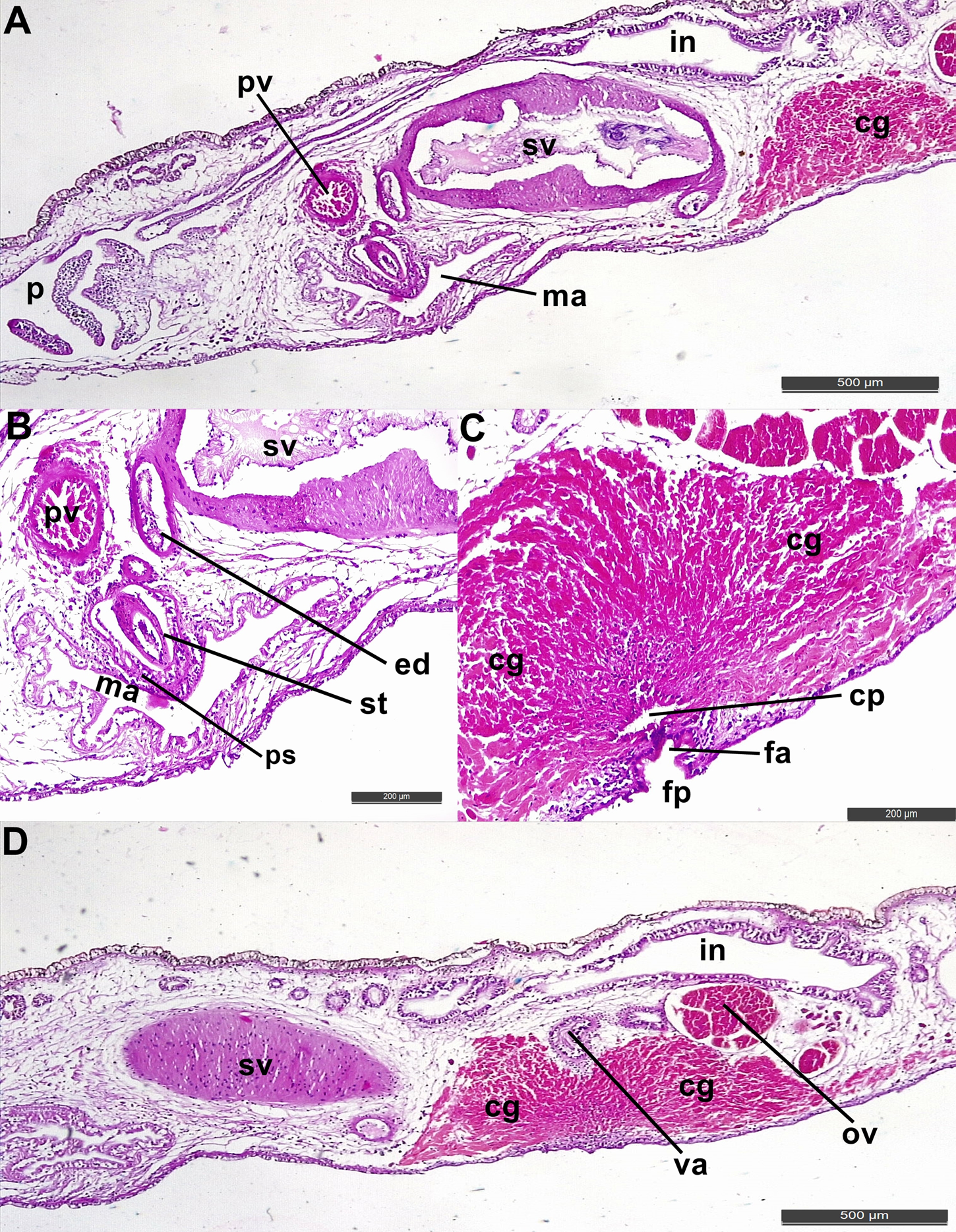
Fig. 6. Pseudoceros galaxea sp. nov. Sagittal sections (A) male reproductive structures, cement glands and pharynx; (B) male reproductive system (magnified); (C) female reproductive system showing cement glands, cement pouch and female atrium; (D) female reproductive structures showing vagina and oviduct. cg, cement glands; cp, cement pouch; ed, ejaculatory duct; fa, female atrium; fp, female gonopore; in, intestine; ma, male atrium; ov, oviduct; p, pharynx; ps, penis sheath; pv, prostatic vesicle; st, stylet; sv, seminal vesicle.

Fig. 7. Pseudoceros galaxea sp. nov. Diagrammatic reconstruction of male and female reproductive system. cg, cement glands; cp, cement pouch; ed, ejaculatory duct; fa, female atrium; fp, female gonopore; in, intestine; ma, male atrium; ov, oviduct; pd, prostatic duct; pp, penis papilla; ps, penis sheath; pv, prostatic vesicle; st, stylet; sv, seminal vesicle; va, vagina.
Type material
Holotype: One specimen, 28 × 20 mm, serial sectioned and mounted on 22 slides. Intertidal area near CMLRE field research station, Agatti Island, Lakshadweep, India (10°50′38′′N 72°11′17′′E) (CMLRE IO/IT/POY/00029); Coll. S. Dixit, 7 May 2019.
Paratype: One specimen, 16 × 12 mm. Location and collection details same as holotype (CMLRE IO/IT/POY/00030).
Etymology
The specific name galaxea is the Latin genitive form of galaxiās (galaxy), which means ‘belong to the galaxy’ and refers to the colour pattern resembling the stars in a galaxy.
Diagnosis
Background body colour chocolate brown with numerous small white to cream dots on the entire dorsal surface (Figure 5A). These minute dots are very densely distributed giving a spray-like appearance. Some white dots are too close forming clusters appearing like bigger dots. A black marginal band studded with white microdots runs around the whole body including pseudotentacles.
Description
Live: Body large, oval and margin slightly ruffled. Background body colour chocolate brown with numerous small white to cream dots on entire dorsal surface (Figure 5A). Median area slightly darker in colour and raised. White dots are densely distributed giving a spray-like appearance. Some white dots are very close forming small white clusters delimiting bigger dots. Marginal rim black (Figure 5A, C) with white dots which are smaller than dots on dorsum. Pseudotentacles are simple folding of the anterior margin, black and spotted. Intensity of spots decreases towards margin (Figure 5B). Cerebral eyes cluster horseshoe shaped, tentacular eyes hard to recognize due to black colour of pseudotentacles however some eyes can be seen on ventral side of pseudotentacles. Ventral surface light brownish.
Preserved: Measurements based on holotype. Specimen brown in colour even after fixation but spots turned pale. Mouth and male gonopore are 2.9 mm, male and female gonopore are 2.2 mm while female gonopore and sucker are 2.8 mm apart (Figure 5C). Pharynx ruffled with nine folds (eight in paratype) and male gonopore is situated immediately behind pharynx. Cerebral eyes cluster with about 35–40 eyes (Figure 5B).
Reproductive system: The male copulatory apparatus consists of a seminal vesicle (Figure 6A), a free prostatic vesicle and an armed penis papilla (Figures 6 A, B & 7) housed in male atrium which opens outside via male gonopore. An oval seminal vesicle, 517 × 257 μm, its rounded part oriented towards prostatic vesicle while tapered part oriented towards cement glands (Figure 6A). A free, small and circular prostatic vesicle (75 × 48 μm) present anterior to the seminal vesicle (Figure 6B). Thickness of prostatic vesicle's muscular wall varies from 10–14 μm. The male atrium is wide housing a reduced penis papilla armed with a stylet (197 μm) (Figure 6B). Female copulatory apparatus consists of oviduct, vagina, cement pouch surrounded by dense cement glands and female atrium (Figure 6C, D). The vagina is oriented towards posterior region of body connecting to oviducts. The vagina opens to a very short female atrium. Cement pouch wide which receives secretion from numerous cement glands distributed in parenchyma tissue. The female atrium opens to exterior via female gonopore (Figures 6C & 7).
Taxonomic remarks
The presence of characters such as ruffled pharynx, male copulatory apparatus right behind pharyngeal cavity, free prostatic vesicle, marginal tentacles formed by upfolding of anterior margin and centrally located sucker behind female gonopore (Faubel, Reference Faubel1984) matches with the characters of the family Pseudocerotidae. The presence of characters such as smooth dorsal surface, single male copulatory apparatus with seminal vesicle and armed penis papilla, pseudotentacles as simple folds of anterior margin, female gonopore equidistant from male gonopore and sucker (Faubel, Reference Faubel1984; Newman & Cannon, Reference Newman and Cannon1998) matches the diagnosis of the genus Pseudoceros. The newly described species differs from all other congeners on the basis of spotted pattern and marginal rim. However, species such as Pseudoceros astrorum Bulnes & Torres, Reference Bulnes and Torres2014, Pseudoceros bicolor Verrill, Reference Verrill1901, Pseudoceros canadiensis Hyman, Reference Hyman1953, Pseudoceros josei Newman & Cannon, Reference Newman and Cannon1994, Pseudoceros kylie Newman & Cannon, Reference Newman and Cannon1998 and Pseudoceros stellans Dixit, 2019 exhibit similar background colour, dorsally with blotches or spots of different sizes and marginal band. These species are compared with the newly described species below.
Pseudoceros astrorum, described from Brazil, is characterized by dark brown ground colour, net-like pattern of small black granules, white spots of different sizes uniformly distributed, a thin black submarginal band with white rim and anterior margin and cerebral region devoid of pigment. Pseudoceros galaxea sp. nov. differs from P. astrorum because of absence of the white rim, net-like pattern of black granules and pigment devoid area in cerebral region.
Pseudoceros bicolor, described from Bermuda, its dorsal colouration varies from yellow to dark brown with white spots on dorsum, white marginal band with greyish black transverse stripes. The marginal band in P. galaxea sp. nov. is black with no transverse stripes which differentiates it from the former species.
Pseudoceros canadiensis, described from Canada, is apparently brown or flecked with brown as per its original description. This species differs from P. galaxea sp. nov. which possesses white to cream dots on dorsum and black rim.
Pseudoceros josei, described from Papua New Guinea, is transparent black with numerous small and densely packed yellow spots which turn smaller and white towards the margin while in P. galaxea sp. nov., the spots are white to cream and a black marginal band is present.
Pseudoceros kylie, described from Australia, is dark brown in background with cream microdots but has a bright orange broken band just before the rim while the marginal band is black and continuous in P. galaxea sp. nov.
Pseudoceros stellans, described from India, has a thick black marginal band with white to yellow spots and yellow blotches; median area marbled with irregular white shading while P. galaxea sp. nov. has a thin black marginal rim and dorsum is without any yellow blotches or shading.
As no other species was found comparable in the genus, in light of the above-mentioned characters and comparisons, P. galaxea sp. nov. is described as a new species to science.
Discussion
All the studies on the polyclads living in Indian waters have been conducted in the last nine years, except one study conducted a century ago by Laidlaw (Reference Laidlaw and Gardiner1902). A total of nine species were reported from Laidlaw's work which marked the beginning of polyclad research in India. A few species such as Acanthozoon plenhi, Latocestus maldivensis, Planocera langi, Pseudoceros buskii and Pseudoceros tigrinus described by Laidlaw from Lakshadweep have never been recorded since. These species are in need of redescription based on fresh samples and coloured photographs of internal and external details. From the year 1902 until 2011, not even a single study was conducted on these worms from Indian waters, including in the Lakshadweep Islands which are one of the major coral reef areas of India and nurture rich reef-associated biodiversity. However, many works were published on polyclads during this period from nearby geographic areas in Arabian Sea such as Sri Lanka (Kelaart, Reference Kelaart1858; Laidlaw, Reference Laidlaw1904), Maldives (Laidlaw, Reference Laidlaw and Gardiner1902, Reference Laidlaw1903), Djibouti (Mexiner, Reference Meixner1907a, Reference Meixner1907b) and the Red Sea (Meyer, Reference Meyer1922; Prudhoe, Reference Prudhoe1952). Since 2011, efforts from a handful of researchers raised the number of species from nine to 68 from Indian waters (Apte & Pitale, Reference Apte and Pitale2011; Sreeraj & Raghunathan, Reference Sreeraj and Raghunathan2011, Reference Sreeraj and Raghunathan2013, Reference Sreeraj and Raghunathan2015; Dixit & Raghunathan, Reference Dixit and Raghunathan2013; Pitale et al., Reference Pitale, Bhave and Apte2014; Sreeraj et al., Reference Sreeraj, Raghunathan, Raghuraman, Dixit and Venkataraman2015; Dixit et al., Reference Dixit, Sivaperuman and Raghunathan2015, Reference Dixit, Raghunathan and Chandra2017a, Reference Dixit, Raghunathan and Chandra2017b, Reference Dixit, Sivaperuman and Raghunathan2018a, Reference Dixit, Sivaperuman and Raghunathan2018b, Reference Dixit, Bulnes and Raghunathan2018c, Reference Dixit, Bayyana, Manjebrayakat, Saravanane and Sudhakar2019, Reference Dixit, Manjebrayakat and Saravanane2021; Pitale & Apte, Reference Pitale and Apte2017, Reference Pitale and Apte2019; Dixit, Reference Dixit2018; present study). All species of polyclads reported from Indian waters are compiled in a checklist (Table 1) based on published reports. The maximum number of species is reported from Andaman and Nicobar Islands (44 species) followed by Lakshadweep Islands (29 species). The genus Pseuodoceros is dominating with 32 species in Indian waters out of which 14 species are from Lakshadweep waters. The genus Pseudobiceros follows next with 11 species from India while seven species are reported from Lakshadweep Islands alone. Other genera such as Euplanoida Faubel, Reference Faubel1983; Eurylepta Ehrenberg, Reference Ehrenberg1831; Latocestus Plehn, Reference Plehn1896; Maritigrella Newman & Cannon, Reference Newman and Cannon2000; Pericelis Laidlaw, Reference Laidlaw and Gardiner1902; Prostheceraeus Schmarda, Reference Schmarda1859; Prosthiostomum De Quaterfage, Reference De Quatrefages1845; and Stylostomum Lang, Reference Lang1884 are represented by only one species so far from Indian waters. Apte & Pitale (Reference Apte and Pitale2011) reported Pseudoceros cf. susanae Newman & Anderson, Reference Newman and Anderson1997 from Lakshadweep Islands. This species though doubtful in its identification is retained in the checklist until new specimens of this species are collected for confirmation of its identification.
The discovery of five new species (two species from Dixit et al., Reference Dixit, Bayyana, Manjebrayakat, Saravanane and Sudhakar2019; one species from Dixit et al., Reference Dixit, Manjebrayakat and Saravanane2021 and two from the present study) from this archipelago in recent times is a clear indication that more efforts are required for inventorization of polyclad fauna from these islands. Their extreme fragility has made these animals difficult to study (Newman & Cannon, Reference Newman and Cannon2003). During our field surveys, many polyclad specimens of different genera were collected, but some of them tended to curl, distort or dissolve during the course of fixation (personal observation). However, members of the genus Pseudoceros were less difficult to fix in comparison with species from other genera such as Acanthozoon, Bulaceros, Thysanozoon and Pericelis (personal observation). Thus, based on our observations, we speculate this as one of the reasons for a higher number of species being described under the genus Pseudoceros in comparison with other genera under suborder Cotylea. The flamboyant colouration of species in the genus Pseudoceros may also be the reason for more numerous descriptions but more studies are required to test these speculations. We hope that recent publications in polyclad taxonomy from Indian waters will attract the interest of young researchers towards these extraordinary worms. The main food source of polyclads are oysters, bivalves and ascidians (Newman & Cannon, Reference Newman and Cannon2003), which are present in both intertidal and subtidal zones making these islands an ideal habitats for polyclads. Only three islands of the Lakshadweep archipelago have been surveyed for polyclad fauna to date (Agatti, Kavaratti and Minicoy), leaving 33 islands unexplored.
Acknowledgements
The authors would like to thank the Ministry of Earth Sciences (MoES), Government of India for proving the opportunity and facilities to visit Agatti Island. This work would not have been completed without support of the local staff of CMLRE field station at Agatti Island. The first author would like to thank Mr B. Kishore Kumar (CMLRE) for his support in logistics and collection of specimens. The first author would also like to thank his fellow colleagues from CMLRE for their help during fieldwork and comments during manuscript preparation. The support of three reviewers with their critical comments and in improving the manuscript is also acknowledged. This is CMLRE contribution number 132.










