Introduction
The deep-sea seafloor represents the most extensive ecosystem on Earth, maintaining a high diversity of species, including microbial organisms and small-sized metazoans of a scarcely known macroinfauna (Gray, Reference Gray2002; Costello & Chaudhary, Reference Costello and Chaudhary2017). Ecological patterns of the benthic macroinfauna diversity in different deep-sea environments have shown the amphipod crustaceans constitute an important fraction of species composition that play key roles in ecosystem function (Soliman & Rowe, Reference Soliman and Rowe2008; Bernardino et al., Reference Bernardino, Levin, Thurber and Smith2012; De Smet et al., Reference De Smet, Pape, Riehl, Bonifácio, Colson and Vanreusel2017). Recent biological studies on the benthic deep-sea macroinfauna have analysed the evolutionary/ecological implications of morpho-physiological traits of the amphipods expressed on their genotype (Ritchie et al., Reference Ritchie, Jamieson and Piertney2017) and anatomy (Kobayashi et al., Reference Kobayashi, Shimoshige, Nakajima, Arai and Takami2019), which might provide benefits to successfully exploit extreme environmental conditions, such as the hadal zone. This knowledge advances our understanding of the adaptive radiation of amphipods in the colonization of the deep-sea seafloor and will contribute to the known diversity of species observed there.
The Gulf of Mexico (GoM) deep-sea presents a large environmental variability (Escobar-Briones et al., Reference Escobar-Briones, Santillán and Legendre2008; Wei et al., Reference Wei, Rowe, Escobar-Briones, Nunnally, Soliman and Ellis2012) that may offer suitable and heterogeneous benthic habitats for amphipods to develop well-established populations, such as Ampelisca mississippiana Soliman & Wicksten (Reference Soliman and Wicksten2007), with high densities in submarine canyons with abundance up to 26,000 ind. m–2, highly related to the suspended organic matter load from the Mississippi River (Soliman & Rowe, Reference Soliman and Rowe2008). At the southern GoM seafloor environments, benthic amphipod assemblages have been reported to occur in canyon, knoll and ridge habitats from the continental edge to the abyssal plain (Winfield et al., Reference Winfield, Escobar-Briones and Morrone2006; Hernández-Ávila et al., Reference Hernández-Ávila, Pech, Ocaña, Árcega-Cabrera and Enriquez2021), suggesting a taxonomically diverse faunal composition that includes the description of new species recently discovered in the Campeche continental slope (Winfield et al., Reference Winfield, Ortiz and Ardisson2016; Ortiz et al., Reference Ortiz, Winfield and Ardisson2017).
There is potential to describe higher amphipod species diversity in the GoM due to the unexplored habitats (Costello & Chaudhary, Reference Costello and Chaudhary2017). The present study contributes information on the amphipod diversity in the GoM by describing two new genera and six new species sorted from macroinfaunal samples collected on the continental slope and the abyssal zone from the Perdido Fold Belt region. The Perdido Fold Belt is a topographically complex deep-sea environment (Gradmann et al., Reference Gradmann, Beaumont and Albertz2009) located at the western GoM (Figure 1). The sediment is mainly composed of terrigenous clay, calcareous clay and a mixture of pelagic carbonate sediment, dominated by foraminifers and coccoliths (Balsam & Beeson, Reference Balsam and Beeson2003). The surface water mass flows mostly northward coming from a western boundary current along the continental shelf break that weakly invades the deep ocean (Gough et al., Reference Gough, Beron-Vera, Olascoaga, Sheinbaum, Jouanno and Duran2019).

Fig. 1. Perdido Fold Belt, western Gulf of Mexico, with sampling stations where the new benthic amphipods were collected.
Materials and methods
Sampling design and samples processing for sorting amphipods from the Perdido Fold Belt macroinfauna are the same as described by Hernández-Ávila et al. (Reference Hernández-Ávila, Pech, Ocaña, Árcega-Cabrera and Enriquez2021). Prior to dissection, photographs of the habitus were taken for each amphipod species (Figure 2) with a Nikon SMZ800 stereomicroscope equipped with a digital camera. The appendages of the specimens were dissected in glycerine then mounted on slides for drawings using an Olympus CX41 compound microscope equipped with a camera lucida. The following abbreviations are used in the figures: A, antenna; C, coxa; EP, epimeron; G, gnathopod; H, habitus; HD, head; L, left; LL, lower lip; MD, mandible; MP, maxilliped; MX, maxilla; O, oostegite; P, pereopod; PLN, pleonite; R, right; T, telson; U, uropod; UL, upper lip; and UR, urosomite. The type material is deposited in the ‘Colección de Referencia de Bentos Costero (ECOSUR)’, belonging to El Colegio de la Frontera Sur, Unidad Chetumal, Mexico. The systematic classification follows Lowry & Myers (Reference Lowry and Myers2017).

Fig. 2. Habitus of the new deep-sea amphipods from the Perdido Fold Belt, western Gulf of Mexico. (A) Paraeperopeus longirostris gen. nov., sp. nov., (B) Pardaliscella perdido sp. nov., (C) Pardaliscoides ecosur sp. nov., (D) Tosilus cigomensis sp. nov., (E) Dentimelita lecroyae gen. nov., sp. nov., and (F) Neohela winfieldi sp. nov.
Comments on the habitat of the new species include records of environment variables from near-bottom water (temperature, salinity and dissolved oxygen) using a Sea-Bird 9plus CTD© and sediment texture following Bouyoucos (Reference Bouyoucos1962) and organic matter following Gaudette et al. (Reference Gaudette, Flight, Toner and Folger1974). Also, comments on the geographic distribution for congeners of the newly described species were presented for deep-water records only (>200 m), based on information from literature and open access biodiversity databases, such as the Global Biodiversity Information Facility (GBIF), the Ocean Biogeographic Information System (OBIS), and the Smithsonian Invertebrate Zoology Collection of the National Museum of Natural History (NMNH).
Results
SYSTEMATICS
Order amphipoda Latreille, Reference Latreille1816
Suborder Amphilochidea Boeck, Reference Boeck1871
Family Pardaliscidae Boeck, Reference Boeck1871
Genus Paraeperopeus gen. nov.
?gammaridean Amphipoda Wilson, Reference Wilson1987: 9, fig. 1 (lower illustrations)
http://zoobank.org/37A820C6-AEEE-4320-95EF-AEA563C92414
Diagnosis
Head with a long rostrum, slightly curved downward. Antennae short, equal in length. Mandible, incisor toothed; palp article 2 expanded. Maxilla 1, palp article 2 expanded distally. Maxilliped, inner plate small, narrow; palp longer than inner edge of outer plate. Coxae 1–4 shallow, subquadrate; ventral margin with long slender setae. Gnathopods 1 and 2 carpo-subchelate; carpus elongate, broad medially; propodus palm serrate. Urosomite 2 with strong dorsal central tooth. Uropods 1 and 2 rami spinose distally. Uropod 3 long; outer rami two-articulate. Telson emarginate.
TYPE SPECIES
Paraeperopeus longirostris gen. nov., sp. nov. (monotypic).
Etymology
The name of the new genus is composed by the prefix para, which means ‘near’ or ‘closely related’, and the generic name of Eperopeus Mills, Reference Mills1967 due to the resemblance with this genus.
Remarks
Paraeperopeus gen. nov., at first glance, is similar to the genus Eperopeus by the general body form but particularly by the expanded mandible palp article 2 and the large carpus of gnathopods 1 and 2. However, the new genus is clearly differentiated from Eperopeus and all other genera in the family Pardaliscidae based on marked differences with species described in the key of Biswas et al. (Reference Biswas, Coleman and Hendrycks2009) by a set of unique characteristics, such as the presence of a long rostrum, the setose ventral margins of coxae 1–4, the medially broad carpus of gnathopods 1 and 2, the presence of a strong tooth on urosomite 2 (except in females), the distally spinose rami of uropods 1 and 2, an emarginate telson, and the maxilla 1 with a distally extended palp article 2, and the maxilliped with a small inner plate and palp longer than the inner edge of the outer plate. Paraeperopeus gen. nov. shares similar gnathopods 1 and 2 (bearing an elongate and medially broadened carpus) with Eperopeus, Necochea Barnard, Reference Barnard1962, Pardaliscoides Stebbing, Reference Stebbing1888 and Princaxelia Dahl, Reference Dahl1959. The new genus is differentiated from those three genera by a slightly emarginate telson (vs deeply cleft). The other genus (also monotypic) with a slightly emarginate telson is Antronicippe Stock & Iliffe, Reference Stock and Iliffe1990, an anchialine taxon, but it differs markedly from Paraeperopeus gen. nov. by many diagnostic morphological characteristics and a contrasting habitat. A putative similar body form to Paraeperopeus gen. nov. was observed by Wilson (Reference Wilson1987) from material collected in deep-sea environments of the Clipperton-Clarion fracture zone. The partially illustrated organism was identified as ‘gammaridean Amphipoda’ and has a certain resemblance to the new genus by a long rostrum, coxae 1–4 ventral margin setose, a large carpus of gnathopods 1 and 2, and a long uropod 3. It is differentiated by produced anteroventrally cephalic lobes, a long article 2 of outer rami on uropod 3, and an absent tooth on urosomite 2. Due to a lacking description associated with the record, no specific illustrations of mouthparts or appendages, and the high morphological diversity in Pardaliscidae (Lörz & Schnabel, Reference Lörz and Schnabel2015), it is not possible to decide at this point if that taxon belongs to Paraeperopeus gen. nov.
Paraeperopeus longirostris gen. nov., sp. nov.
(Figure 2A & Figures 3–6)
http://zoobank.org/525A879A-61D3-4D88-B926-F72A5AE8302D
Type Material
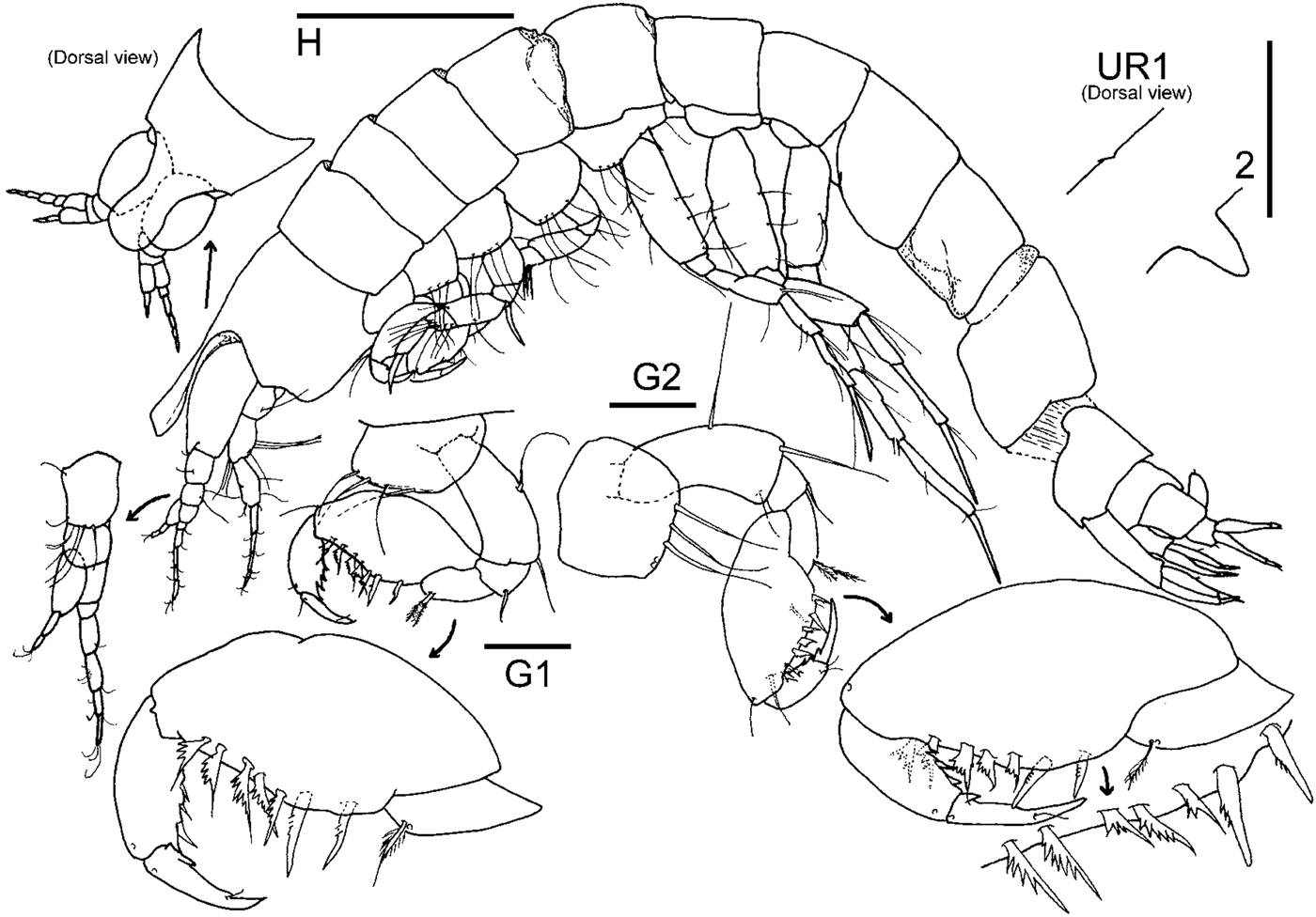
Fig. 3. Paraeperopeus longirostris gen. nov., sp. nov., holotype male (ECOSUR 279). Scale bar for H: 0.5 mm; scale bars for G1–2 and UR1–2: 0.3 mm.

Fig. 4. Paraeperopeus longirostris gen. nov., sp. nov., holotype male (ECOSUR 279). Scale bars: 0.1 mm.

Fig. 5. Paraeperopeus longirostris gen. nov., sp. nov., holotype male (ECOSUR 279). Scale bars: 0.2 mm.

Fig. 6. Paraeperopeus longirostris gen. nov., sp. nov., holotype male (ECOSUR 279): Urosome and EP1–3, paratype male (ECOSUR 280): UR’1–3 and T’, paratype female (ECOSUR 281). Scale bars: 0.1 mm.
Holotype: Male (dissected and drawn), 3.5 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P2-B6, 25.64°N 95.41°W, 1789 m, 4 October 2016, coll. V. Papiol, ECOSUR 279. Paratypes: Male (dissected and drawn), 3.1 mm, data as for holotype, ECOSUR 280. Female with setose oostegites (dissected and drawn), 3 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P3-F8, 23.58°N 95.13°W, 3462 m, 16 June 2017, coll. S. Balan-Zetina, ECOSUR 281.
Additional Material Examined
Female, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P1-D6, 24.53°N 95.54°W, 2085 m, 12 May 2016, coll. S. Balan-Zetina, ECOSUR-C1186. Female, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P1-B5, 25.63°N 95.39°W, 1610 m, 18 May 2016, coll. S. Balan-Zetina, ECOSUR-C1187. Female, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P2-D6, 24.53°N 95.54°W, 2109 m, 2 October 2016, coll. V. Papiol, ECOSUR-C1188. Female, data as for paratype female, ECOSUR-C1189.
Type Locality
Perdido Fold Belt, offshore Tamaulipas, Mexico, 25.64°N 95.41°W.
Etymology
The name of the new species is composed by the Latin words longus, meaning ‘long’, and rostrum, meaning ‘platform’, due to the presence of an elongated rostrum.
Diagnosis
Head, lateral cephalic lobes rounded medially; eyes absent. Accessory flagellum three-articulate, the first one broad. Mandible, incisor with an acute tooth; palp article 3 setose apically. Maxilla 1, inner plate with one apical plumose seta. Maxilla 2, inner plate shorter than outer. Maxilliped, inner plate small, with one apical slender seta. Coxae 1–5, ventral margin with long slender setae. Gnathopods 1 and 2, carpus with stout serrate setae on the ventral margin; dactylus with one tooth on posterior margin. Urosomite 2 with strong dorsal central tooth. Telson, lobes with one robust seta.
Description
Based on holotype male (ECOSUR 279) and one male paratype (ECOSUR 280). Head with a long rostrum; lateral cephalic lobes rounded medially; eyes absent. Antennae short, equal in length; accessory flagellum three-articulate, the first one broad. Upper lip, slightly concave medially, lobes asymmetrical. Mandible, incisor with an acute tooth; lacinia mobilis wide, multidentate; accessory setal row with two raker spines and fine setulae; palp article 2 expanded with two distal plumose setae; palp article 3 with 10–11 slender setae apically. Lower lip, lost. Maxilla 1, inner plate with one apical plumose seta; outer plate with seven robust setae; palp article 2 expanded distally, with five or six robust setae apically. Maxilla 2, inner plate shorter than outer, with three apical plumose setae on both plates. Maxilliped, inner plate small, narrow, with one apical slender seta; outer plate not reaching end of palp article 1, with a few slender setae on inner margin and apex; palp four-articulate, nearly twice as long as inner edge of outer plate, article 2 scarcely setose on inner margin, article 3 with distal slender setae on inner and outer margin, article 4 falcate and relatively stout.
Gnathopod 1 carpo-subchelate; coxa subquadrate, ventral margin with long slender setae; merus with a single plumose seta on anteroventral margin; carpus large, elongate, broad medially, with seven stout serrate setae on ventral margin; propodus palm serrate; dactylus with one tooth on posterior margin. Gnathopod 2, similar shape as gnathopod 1, slightly larger in size. Pereopods 3 and 4 short, similar shape and size; coxa slightly broader than coxae 1 and 2, with ventral margin, long slender setae, and posteroventral margin oblique; basis, ischium, merus, with a few slender setae on posterior margin; carpus with row of long slender setae on posterior margin; propodus, posterior surface with cuticle denticles, and three slender setae and one long serrate seta on ventral margin; dactylus subequal or longer than propodus in length. Pereopods 5–7 increasing in size. Pereopod 5, coxa broader than long, concave ventrally, with anteroventral lobe produced roundly and long slender setae on ventral margin; basis posterior margin straight; carpus, distal margin with five or six long setae minutely serrated at distal half; propodus with long slender setae on distal margin; dactylus longer than propodus. Pereopod 6, similar shaped as pereopod 5, except by the shallow coxa; basis slightly broad medially. Pereopod 7, coxa small, with anteroventral lobe roundly produced; basis posterior margin straight; merus, carpus and propodus, with long robust setae on anterior margin; dactylus long, about 0.75 times of propodus length.
Pleonites 1–3 smooth. Epimeral plates 1–3 subquadrate, posteroventral corners rounded. Urosomite 2 with strong dorsal central tooth. Uropods 1 and 2, peduncle longer than rami, with robust setae on outer margin; rami subequal in length; inner ramus with one medial robust seta; rami with three apical robust setae, the central one larger. Uropod 3, peduncle shorter than rami, with one distal robust seta; outer ramus longer than inner ramus, two-articulate. Telson, tapering distally, slightly emarginate, with lateral margin with one or two plumose setae, and one apical robust seta on each lobe of emargination.
Paratype female (ECOSUR 281). Accessory flagellum three-articulate, the first one regular. Urosomite 2 with small central tooth. Pereopods 3 and 4 dactylus longer. Pereopod 7, merus, carpus, and propodus, with longer robust setae on anterior margin.
Remarks
Paraeperopeus longirostris gen. nov., sp. nov. presents some intraspecific morphological variations in the central tooth of male urosomite 1, from nearly smooth in the male holotype to produced in the male paratype, and in the apical margin of the telson in male holotype, with a very weak sinuosity that could indicate the emargination on the male and female paratypes. Mills (Reference Mills1967) had already noticed a common variability in the telson structure of pardaliscids. Here, two of seven observed specimens of P. longirostris gen. nov., sp. nov. have an entire telson, a characteristic shared with its closest genus Eperopeus; the other genus with an entire telson is Parpano Barnard, Reference Barnard1964 (Caribbean Sea). The presence of an entire telson only occurs in North-western Atlantic pardaliscids species so far.
Habitat
Paraeperopeus longirostris gen. nov., sp. nov. was collected in the continental slope (1610–2109 m) and abyssal zone (3462 m) at temperatures from 4–13 °C, salinity of 35 PSU, dissolved oxygen of 2.2–4.6 mg l−1, and sediments with high organic matter content (3–11%), very fine sand (11–74%) and low content of medium sand (2–57%).
Distribution
Paraeperopeus longirostris gen. nov., sp. nov., is known so far from the type locality, Perdido Fold Belt, offshore Tamaulipas, western GoM. The geographic distribution of the genus Paraeperopeus gen. nov. might be extended in the future if the North-eastern Pacific material of Wilson (Reference Wilson1987) turns out to correspond to the new genus described here.
Genus Pardaliscella Sars, Reference Sars1895
Pardaliscella perdido sp. nov.
(Figure 2B & Figures 7–10)
http://zoobank.org/244B0006-FBF3-45A1-B000-55B2EC724874
Type Material

Fig. 7. Pardaliscella perdido sp. nov., holotype male (ECOSUR 282). Scale bar for H: 1 mm; scale bars for G1–2: 0.1 mm.

Fig. 8. Pardaliscella perdido sp. nov., holotype male (ECOSUR 282). Scale bars: 0.1 mm.

Fig. 9. Pardaliscella perdido sp. nov., holotype male (ECOSUR 282). Scale bars: 0.1 mm.

Fig. 10. Pardaliscella perdido sp. nov., paratype female (ECOSUR 283). Scale bars: 0.1 mm.
Holotype: Male (dissected and drawn), 3 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P1-B6, 25.64°N 95.41°W, 1847 m, 18 May 2016, coll. V. Papiol, ECOSUR 282. Paratypes: Female with oostegites (dissected and drawn), 3.1 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P1-B4, 25.28°N 96.07°W, 1074 m, 19 May 2016, coll. S. Balan-Zetina, ECOSUR 283.
Type Locality
Perdido Fold Belt, offshore Tamaulipas, Mexico, 25.64°N 95.41°W.
Etymology
The new species is named for the type locality, the Perdido Fold Belt, western GoM.
Diagnosis
Head, short rostrum, curved downward; lateral cephalic lobes slightly rounded; eyes absent. Antennae subequal in length; accessory flagellum three-articulate. Coxa 1 with anteroventral corner produced. Gnathopods 1 and 2, dactylus with one strong tooth on posterior margin. Pereopod 5 propodus, anterodistal margin serrate. Epimeral plates 1–3, posteroventral corner with small acute tooth. Urosomites 1 and 2 with strong dorsal tooth. Uropods 1–2, peduncles with strong and broad distolateral tooth; rami serrate dorsally. Telson, cleft; acute lobes with one apical tiny and slender seta.
Description
Based on holotype male (ECOSUR 282). Head with a short rostrum, curved downward; lateral cephalic lobes broad, slightly rounded; eyes absent. Antenna 1 slightly longer than antenna 2; peduncle of antenna 1 longer by half as peduncle of antenna 2; accessory flagellum three-articulate. Upper lip, concave medially, lobes symmetrical. Mandibles asymmetrical; palp article 3 longest with 3–5 slender setae apically. Right mandible incisor with three strong acute teeth (one of them bifid), accessory setal row with two raker spines and fine setulae. Left mandible with incisor smooth, weakly toothed, lacinia mobilis wide, minutely dentated, accessory setal row with two raker spines and fine setulae. Lower lip, inner lobes coalesced; mandibular process well developed with lobes rounded apically. Maxilla 1, inner plate with one apical plumose seta; outer plate with seven robust setae and one plumose seta; palp dilated distally, with nine robust setae apically. Maxilla 2, plates long and slender; inner plate shorter than outer. Maxilliped, inner plate small; outer plate not reaching end of article 1 on palp; palp four-articulate.
Gnathopod 1 simple, moderately stout; coxa subquadrate, broader than long, with anteroventral corner produced and posteroventral margin oblique; merus with a few slender setae on anteroventral margin; carpus, elongate, broad medially, with serrate setae on ventral margin; propodus palm with serrate setae and fine setulae along, and two small protuberances at the distal half; dactylus with one strong tooth on posterior margin. Gnathopod 2, similar shape and size as gnathopod 1, except by the coxa broader than long and propodus palm smooth. Pereopods 3 and 4 of similar shape and size; coxa broader than long, with posteroventral margin oblique; basis, ischium, merus scarcely setose; merus shorter than carpus; basis posterior margin wide on pereopod 3 and straight on pereopod 4; carpus with row of five long slender setae on posterior margin; propodus with two or three slender setae on posterior margin; dactylus about 0.7 times of propodus length. Pereopods 5–7 increasing in size. Pereopod 5, coxa broader than long, with medioventral lobe roundly produced; basis posterior margin straight; carpus posterior margin with five robust setae; propodus anterodistal margin serrate with a robust seta; dactylus long, about 1.3 times of propodus length. Pereopod 6, coxa broader than long, with anteroventral lobe roundly produced; basis posterior margin straight; dactylus long, about 1.3 times of propodus length. Pereopod 7, coxa broader than long, with anteroventral lobe roundly produced; basis posterior margin wide, tapering distally; dactylus long, subequal to propodus length.
Pleonites 1–3 smooth. Epimeral plates 1–3 posteroventral corner weakly acuminate, with a small acute tooth; posterior margin with one short robust seta. Urosomites 1 and 2 each with strong posterodorsal tooth. Urosomite 3 smooth. Uropod 1, peduncle slightly shorter than rami, with three robust setae along inner and outer edge and a strong and broad distolateral tooth; rami subequal in length, serrate dorsally, with one robust seta. Uropod 2, peduncle slightly longer than rami, with one robust seta on distal inner and outer edge and a strong and broad distolateral tooth; rami subequal in length, serrate dorsally, with one robust seta. Uropod 3, peduncle shorter than rami, with one ventrodistal robust seta; rami slender and scarcely setose, with one apical tiny and slender seta; outer ramus two-articulate. Telson, cleft; acute lobes with one apical tiny and slender seta.
Paratype female (ECOSUR 283). Oostegites present on pereonites 2–5, more setose on pereonite 5; length subequal to basis. Gnathopods 1 and 2 similar in shape and size; propodus elongate with palm smooth. Pereopod 5, dactylus long, about 1.5 times of propodus length. Epimeral plate 3 with posteroventral corner roundly produced. Urosomites 1 and 2 each with an acute dorsal tooth. Urosomite 3 smooth (not illustrated). Uropod 1, outer ramus slightly shorter than inner, weakly serrate dorsally.
Remarks
The genus Pardaliscella is a small taxon formed by seven species, including the new species described here (Barnard & Karaman, Reference Barnard and Karaman1991): Pardaliscella axeli Stebbing, Reference Stebbing1906, Pardaliscella boecki (Malm, Reference Malm1871), Pardaliscella inermis Ledoyer, Reference Ledoyer1986, Pardaliscella lavrovi Gurjanova, Reference Gurjanova1934, Pardaliscella perdido sp. nov., Pardaliscella symmetrica Barnard, Reference Barnard1959 and Pardaliscella yaquina Barnard, Reference Barnard1971. Pardaliscella perdido sp. nov. presents a unique set of characteristics that separate it from its congeners: the dactylus of gnathopods 1–2 with a strong tooth on posterior margin and the uropods 1–2 peduncle with strong and broad distolateral tooth and with rami serrate dorsally. Particularly, the new species differs from P. axeli, P. boecki, P. lavrovi and P. symmetrica by the presence of a strong posterodorsal tooth on urosomites 1 and 2 (vs a small tooth on urosomite 1 of P. symmetrica). Furthermore, it differs from P. axeli and P. boecki by a shorter inner plate on the maxilliped and a small acute tooth on the posteroventral corner of epimeral plate 3, and from P. lavrovi and P. symmetrica by the uropod 3 rami scarcely setose and the lobes of telson with one apical tiny and slender seta. Pardaliscella perdido sp. nov. is similar to P. inermis and P. yaquina by the presence of a posterodorsal tooth on urosomites 1–2 but differs from the first species by a small tooth on the posteroventral corner of epimeral plates 1–3 (vs corner with a slightly upturned large tooth) and from the second species by a smooth inner margin of the maxillipedal palp article 4 (vs inner margin serrated).
According to variability of the characteristics in Pardaliscella, the species P. inermis deviates from the generic description by the following: maxilla 2 plates short and broad, gnathopods 1–2 dactyl with smooth posterior margin, coxae 1–4 longer than broad, pereopods 3–4 merus longer than carpus, urosomal teeth strongly developed, epimeral plates 1–3 with a large slightly upturned tooth on the posteroventral corner, and pereopod 7 basis with posterior margin convexly expanded. Based on these characteristics, P. inermis aligns well with the genus Caleidoscopsis Karaman, Reference Karaman1974, so we propose to relocate it to this genus, such as occurred with Caleidoscopsis simplignathia (Barnard, Reference Barnard1962), formerly removed from Pardaliscella (see Barnard & Karaman, Reference Barnard and Karaman1991).
Habitat
Pardaliscella perdido sp. nov. was collected on the continental slope (1074–1847 m) at temperatures between 5–13°C, salinity of 35 PSU, dissolved oxygen of 4–4.7 mg l−1, and sediment with moderate organic matter content (5.6%), dominated by medium sand (57.7%), and a poor content of fine (25.7%) and very fine sand (16.5%).
Distribution
Pardaliscella perdido sp. nov. is known so far from the type locality, Perdido Fold Belt, offshore Tamaulipas, western GoM. The geographic distribution of the genus Pardaliscella in the deep-sea (225–3015 m) includes five species: P. axeli and P. boecki distributed in the North-east Atlantic (Norway Sea and Barents Sea) and P. symmetrica and P. yaquina in the North-east Pacific (California and Oregon offshore). Pardaliscella perdido sp. nov. represents the most southern geographic range for the genus and the first recorded species in the deep-sea from the North-western Atlantic.
Genus Pardaliscoides Stebbing, Reference Stebbing1888
Pardaliscoides ecosur sp. nov.
(Figure 2C & Figures 11–13)
http://zoobank.org/80A9F3E3-2591-4D47-89D5-411C55EE8008
Material Examined

Fig. 11. Pardaliscoides ecosur sp. nov., holotype male (ECOSUR 284). Scale bar for H: 1 mm; scale bars for G1–2: 0.2 mm.

Fig. 12. Pardaliscoides ecosur sp. nov., holotype male (ECOSUR 284). Scale bars: 0.1 mm.

Fig. 13. Pardaliscoides ecosur sp. nov., holotype male (ECOSUR 284). Scale bars: 0.1 mm.
Holotype: Male (dissected and drawn) 3.2 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P2-C6, 25.33°N 95.62°W, 1998 m, 4 October 2016, coll. A. León-Hernández, ECOSUR 284.
Type Locality
Perdido Fold Belt, offshore Tamaulipas, Mexico, 25.33°N 95.62°W.
Etymology
The new species is named for the acronym of the research institute ‘El Colegio de la Frontera Sur, ECOSUR’ in recognition of the sui generis socio-ecological approach to understand the diversity of the south of Mexico and its contribution and commitment to the sustainability development goals.
Diagnosis
Head, moderately developed rostrum, curved downward; lateral cephalic lobes slightly rounded. Antenna 1, peduncular article 2 longer than article 1; callynophore present; accessory flagellum four-articulate, the first one longer. Upper lip, slightly concave medially; lobes asymmetrical. Mandibles with two raker spines; incisor toothed. Gnathopods 1 and 2 carpus broad at the distal half. Pereopods 3 and 4 dactylus subequal to propodus in length. Urosomite 1 with acute dorsal tooth. Urosomite 2 with dorsal carina. Telson poorly cleft.
Description
Based on holotype male (ECOSUR 284). Head, moderately developed rostrum, curved downwards; lateral cephalic lobes slightly rounded; eyes absent. Antenna 1, peduncular article 2 longer than article 1; callynophore present; accessory flagellum four-articulate, the first one longer and scale-like. Antenna 2, ventral margin of peduncular articles setose. Upper lip, slightly concave medially; lobes asymmetrical. Mandibles asymmetrical; palp article 2 longest, with five slender setae medially. Right mandible incisor with two strong acute teeth (one of them bifid); accessory setal row with two raker spines; palp article 3 with four slender and one plumose seta apically. Left mandible incisor weakly toothed, with a basal strong acute tooth; lacinia mobilis wide, minutely dentated, with several blunt teeth; accessory setal row with two long raker spines; palp article 2 slightly expanded. Lower lip, lost. Maxilla 1, inner plate short with one apical slender seta; outer plate with six or seven long robust setae; palp article 2 dilated distally, with seven or eight robust setae and four slender setae apically. Maxilla 2, plates long and slender; inner plate slightly shorter than outer; inner plate with four plumose setae; outer plate with three plumose setae. Maxilliped, lost.
Gnathopod 1 simple, moderately stout; coxa subquadrate, broader than long; basis anterodistal margin with row of four long slender setae; carpus elongate, broad at the distal half, with serrate setae on ventral margin; propodus palm with serrate and slender setae along; dactylus posterior margin smooth, about 0.85 times of propodus length. Gnathopod 2, similar shape and size as gnathopod 1, except by the basis anterior margin with two short slender setae and carpus less broad at the distal half. Pereopods 3 and 4 similar; coxa subquadrate; basis, ischium, merus scarcely setose; basis posterior margin straight; carpus and propodus with a fringe of slender setae on ventral margin; dactylus subequal to propodus in length. Pereopod 5, coxa broader than long, with one small slender seta on posteroventral margin; basis posterior margin slightly broad medially; dactylus broken. Pereopod 6, coxa subquadrate, with one small slender seta on posteroventral corner; articles missing. Pereopod 7, coxa broader than long; margin posteroventral concave, with one small slender seta on posteroventral corner; articles missing.
Pleonites 1–3 smooth. Epimeral plates 1–3 uneven; plate 1 subtriangular, ventral margin slightly produced roundly; plate 2 subquadrate, posteroventral corner notched with a small acute tooth; plate 3 subquadrate, posteroventral corner acute. Urosomite 1 with acute dorsal tooth. Urosomite 2 with dorsal carina. Uropod 1, peduncle longer than uropod 2 peduncle; ramus broken. Uropod 2, ramus broken. Uropod 3, missing. Telson, tapering distally, poorly cleft (about 0.2 times of telson length), with slender setae on distolateral margin and subapical on each lobe of emargination.
Remarks
The genus Pardaliscoides is a small taxon formed by five species, including the new species described here (Barnard & Karaman, Reference Barnard and Karaman1991): Pardaliscoides ecosur sp. nov., Pardaliscoides fictotelson Barnard, Reference Barnard1966, Pardaliscoides longicaudatus Dahl, Reference Dahl1959, Pardaliscoides stebbingi Ledoyer, Reference Ledoyer1970, and Pardaliscoides tenellus Stebbing, Reference Stebbing1888. Pardaliscoides ecosur sp. nov. is in poor condition (the antenna 1, pereopod 5, and uropods 1–2 are incomplete) and the pereopods 6–7 and uropod 3 are missing. However, it can be distinguished from all of its congeners by the dactylus of pereopods 3–4 subequal to propodus (vs shorter than propodus), the article 1 of urosome with one acute tooth on the posterior dorsal margin (vs tooth on articles 1–2), and the angular epimeral plate of pleonite 3 (vs plate rounded or produced with a tooth). In addition, P. ecosur sp. nov. differs from P. fictotelson, P. tenellus, and P. stebbingi by the shape and cleft depth on the telson; we could not consider the telson of P. longicaudatus because it has not been described. Particularly, the new species differs from P. fictotelson and P. tenellus by maxilla 2, which has only slender setae on the apical margin of the inner plate (versus apical and subapical setae). Furthermore, it differs from P. fictotelson and P. stebbingi by the asymmetrical lobes of the upper lip (vs symmetrical lobes).
Habitat
Pardaliscoides ecosur sp. nov. was collected on the continental slope (1998 m) at a temperature of 3.8°C and dissolved oxygen of 4.6 mg l−1.
Distribution
Pardaliscoides ecosur sp. nov. is known so far from the type locality, Perdido Fold Belt, offshore Tamaulipas, western GoM. The genus Pardaliscoides is mostly distributed in the deep-sea (218–9820 m) from the eastern and western Pacific (P. fictotelson, P. longicaudatus and P. tenellus) and the eastern Atlantic in the Mediterranean Sea (P. stebbingi) (Karaman, Reference Karaman1974); P. ecosur sp. nov. is the first described species of the genus in the western Atlantic, with its type locality in the GoM.
Genus Tosilus Barnard, Reference Barnard1966
Tosilus cigomensis sp. nov.
(Figure 2D & Figures 14–16)
http://zoobank.org/4C67D91C-5432-42A4-B85F-0EBED3D0BD89
Type Material

Fig. 14. Tosilus cigomensis sp. nov., holotype female (ECOSUR 285). Scale bar for H: 1 mm; scale bars for the others: 0.1 mm.

Fig. 15. Tosilus cigomensis sp. nov., holotype female (ECOSUR 285). Scale bars: 0.1 mm.

Fig. 16. Tosilus cigomensis sp. nov., holotype female (ECOSUR 285). Scale bars: 0.3 mm.
Holotype: Female (dissected and drawn), 3 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P4-D5, 24.87°N 96.06°W, 1296 m, 21 September 2017, coll. S. Balan-Zetina, ECOSUR 285.
Type Locality
Perdido Fold Belt, offshore Tamaulipas, Mexico, 24.87°N 96.06°W.
Etymology
The new species is named for the acronym of the research group ‘Consorcio de Investigación del Golfo de México, CIGoM’ in recognition of the extensive sampling effort carried out on deep-sea benthic habitats at southern GoM.
Diagnosis
Head, short rostrum; lateral cephalic lobes rounded. Antenna 1, accessory flagellum four-articulate. Antenna 2, peduncular article 3 short. Upper lip, apically notched, lobes symmetrical. Gnathopods 1 and 2, propodus elongated and tapering distally; dactylus smooth, long, claw-shaped. Coxae 3 and 4, subquadrate, anteroventral margin oblique. Coxa 6, broader than long, ventral margin slightly concave, with posteroventral corner notched. Pereopod 7 basis, posteroproximal margin notched. Epimeral plates 3, produced roundly. Urosomites 1 and 2, with rounded dorsal tooth. Telson, pentagonal, cleft.
Description
Based on holotype female (ECOSUR 285). Head, short rostrum; lateral cephalic lobes roundly produced; eyes absent. Antenna 1, peduncular articles 1–3 decreasing in size; accessory flagellum four-articulate. Antenna 2, peduncular article 3 short. Upper lip, apically notched, lobes symmetrical. Mandibles asymmetrical; palp article 2 with one medial slender seta; palp article 3 longest, with three apical slender setae. Right mandible incisor weakly toothed, with a basal weak blunt tooth; lacinia mobilis wide, minutely dentated; accessory setal row with two raker spines and fine setulae; palp straight, three-articulate. Left mandible incisor with two strong blunt teeth (one of them bifid); accessory setal row with two raker spines and fine setulae; palp straight, three-articulate. Lower lip, lost. Maxilla 1, inner plate short with one apical slender seta; outer plate with seven long robust setae; palp article 2 dilated distally, with six apical robust setae and three marginal slender setae. Maxilla 2, plates long and slender; inner plate slightly shorter than outer; inner plate with three or four plumose setae; outer plate with two plumose setae. Maxilliped, inner plate small, with one apical slender seta; outer plate not reaching end of article 1 on palp, with a few slender setae on inner margin and apex; palp four-articulate, article 2 setose on inner margin, article 3 longest and scarcely setose distally, article 4 falcate and relatively stout.
Gnathopod 1 simple, slender; coxa subquadrate, slightly longer than broad; carpus very short; ischium, merus and carpus with a few ventral slender setae; propodus elongated and tapering distally in dorsal view, palm with slender setae and fine setulae along; dactylus smooth, as long as palm, claw-shaped, with one proximal facial seta. Gnathopod 2, of similar shape and size as gnathopod 1, except by a longer propodus and shorter dactylus (about 0.8 times of propodus palm length) without facial seta. Pereopods 3 and 4 similar; coxae subquadrate, longer than broad, anteroventral margin oblique; merus, carpus and propodus with a few ventral slender setae. Pereopods 5–7 simple, increasing in size. Pereopod 5, coxa broader than long, produced anterodistally with posterior margin oblique; basis straight; merus and carpus with a few robust setae on anterior and distal margins; dactylus subequal in length to propodus. Pereopod 6, coxa broader than long, ventral margin slightly concave, with posteroventral corner notched; basis slightly expanded; merus and carpus with robust setae on anterior and posterior margins; dactylus long, about 0.8 times of propodus length. Pereopod 7, coxa broader than long, ventral margin slightly concave; basis proximally expanded and tapering distally, with robust setae on anterior margin and posteroproximal margin notched; merus and carpus with robust setae on anterior and posterior margins; dactylus broken.
Pleonites 1–3 smooth. Epimeral plates 1–3 differing; plate 1 subtriangular, ventral margin slightly produced acutely with two or three short robust setae; plate 2 subquadrate, anterior margin with two short robust setae, posteroventral corner with a small acute tooth; plate 3 produced roundly with one robust seta on anteroventral margin. Urosomites 1 and 2 each with moderate posterodorsal tooth, roundly produced. Urosomite 3 smooth. Uropod 1, peduncle subequal or longer than rami; outer ramus slightly shorter than inner ramus. Uropod 2, outer ramus shorter than inner ramus. Uropod 3, very small; rami subequal in length. Telson, pentagonal, cleft; lobes with a pair of distomarginal plumose setae.
Remarks
The genus Tosilus was considered monotypic for a long time (54 years), with Tosilus arroyo Barnard, Reference Barnard1966 as the only described species in the genus until the description of T. cigomensis sp. nov. given herein. The description of T. cigomensis sp. nov. is based on a single specimen, but it clearly differs from its congener by the short peduncular article 3 in antenna 2 (vs article 3 long), the article 3 longer than article 4 in maxilliped (vs article 3 shorter than article 4), the oblique anteroventral margin of coxae 3–4 (vs margin right), the coxa 6 with notched posteroventral corner (vs corner rounded), the epimeral plate 3 roundly produced (vs tooth produced acutely upwards), and a posterodorsal tooth on urosomite 1 and 2 (vs dorsally smooth).
The genus Tosilus is highly similar to the genus Parpano Barnard, Reference Barnard1964, which was described from the Caribbean Sea; the main difference is the telson, which is cleft in Tosilus and entire in Parpano. Comparisons between these genera (see Cadien, Reference Cadien2004) suggest that if more material could be found for a revision that encompasses a higher morphological variability, the two assigned species to Parpano (P. cebus Barnard, Reference Barnard1964 and P. composturus Barnard, Reference Barnard1964) will perhaps have to be included in the genus Tosilus. The new species described here shares characteristics with the Parpano species, previously undescribed for Tosilus, increasing similarity between both genera, such as lateral cephalic lobes roundly produced, epimeral plate 3 roundly produced, and urosomites 1–2 each with posterodorsal tooth.
Habitat
Tosilus cigomensis sp. nov. was collected in the continental slope (1296 m) at a temperature of 4.3°C, salinity of 35 PSU, and dissolved oxygen of 3.9 mg l−1.
Distribution
Tosilus cigomensis sp. nov. is known so far from the type locality, Perdido Fold Belt, offshore Tamaulipas, western GoM. The only species previously known in the genus (T. arroyo) has been recorded from southern California to northern Baja California at 976–1095 m depth (Barnard & Karaman, Reference Barnard and Karaman1991). The discovery of T. cigomensis sp. nov. in the GoM represents a remarkable longitudinal geographic extension for the genus Tosilus from the Pacific to the Atlantic.
Suborder senticaudata Lowry & Myers, Reference Lowry and Myers2013
Family melitidae Bousfield, Reference Bousfield1973
Genus Dentimelita gen. nov.
http://zoobank.org/09B7113C-1B86-4DA7-A4EB-83F0338275AB
Diagnosis
Head with subquadrate lateral lobes. Antenna 1 longer than 2; accessory flagellum present. Mandibular palp short and scarcely setose. Maxilla 1, inner lobe triangular-oval in shape. Maxilla 2, inner lobe with extended transversal row of facial setae. Maxilliped, palp article 2 long, slender and columnar; palp article 3 elongated. Coxae 1–4, pointed anteroventral corners and notched posteroventral corners. Gnathopod 1, carpus elongated. Gnathopod 2, propodus elongated; palm dentate. Pereopods 3–4 similar, dactylar ungues without accessory spine. Pereopods 5–7, basis elongated, anterior and posterior margins straight, subparallel. Pleonites 1–3 with acute dorsal projections. Epimera 1–2, posteroventral corner acuminate, epimeron 3 produced acutely. Urosomites 1–2 with acute dorsal projections. Telson deeply cleft with one robust seta on inner margin of each lobe.
Type Species
Dentimelita lecroyae gen. nov., sp. nov. (monotypic).
Etymology
The name of the new genus is composed by the Latin word dentis, which means ‘tooth’, and the generic name of Melita Leach, Reference Leach1814 due to the presence of acute projections on coxae, gnathopod 2 propodus, pleonites, epimera, and urosomites.
Remarks
Dentimelita gen. nov. is differentiated in the family Melitidae by having a unique set of acute projections on the body: pointed ventral corners on coxae 1–4, palm dentate on propodus of gnathopod 2, posterodorsal teeth on pleonal segments 1–3, acuminate posteroventral corners on epimera 1–3, and posterodorsal teeth on urosomal segments 1–2. This new genus shares a conspicuous characteristic with other genera of the family Melitidae (Abludomelita Karaman, Reference Karaman1981, Armatomelita Labay, Reference Labay2013, Desdimelita Jarrett & Bousfield, Reference Jarrett and Bousfield1996, Dulichiella Stout, Reference Stout1912, Ledoyeromelita Labay, 2016, Megamoera Bate, Reference Bate1862, Melitoides Gurjanova, Reference Gurjanova1934, and Verdeia Lowry & Springthorpe, Reference Lowry and Springthorpe2007) by the presence of an oblique submarginal row (reduced or extended) of numerous setae on the inner lobe of maxilla 2. Dentimelita gen. nov. differs from the genera Desdimelita, Megamoera and Melitoides by the absence of long lateral setae on palp article 1 of maxilla 1 (vs setae present) and the presence of truncated lateral cephalic lobes (vs broadly rounded), basis of pereopods 5–7 with margins straight (vs broad), and dorsal teeth on pleonites 1–3 (vs smooth in Desdimelita and Melitoides). It differs from Dulichiella and Verdeia by having the inner plate of maxilla 1 with long and narrow shape (vs triangular-oval shape), pair of gnathopod 2 similar to each other (vs pair unequal), and the absence of accessory spine on dactylar ungues of pereopods 3–4 (vs spine present). Also, it differs from the genera Abludomelita and Armatomelita by having a mandibular palp short and scarcely setose (except by two apical setae on article 3), coxal plates 1–4 with anteroventral margin produced (vs margin rounded), dactylus of gnathopod 2 slender, narrow distally with tip acute (vs dactylus heavy, broad distally with tip obtuse), and basis of pereopods 5–7 unexpanded (vs posterior margin regularly expanded). The new genus resembles the genus Ledoyeromelita by the short mandibular palp, with two or three apical setae on article 3, the inner lobe on maxilla 2 with transversal row of facial setae, and the telson deeply cleft, but it differs by the shape of the inner lobe of maxilla 1 (truncate and rectangular-elongate in Ledoyeromelita, triangular-oval in Dentimelita gen. nov.), the shape of palp article 3 on maxilliped (with rounded protruding on the inner margin in Ledoyeromelita, subparallel in Dentimelita gen. nov.), the shape of the gnathopod 2 dactylus (broad with a blunt tip in Ledoyeromelita, slender with an acute tip in Dentimelita gen. nov.), and the basis of pereopods 5–7 (posteriorly expanded in Ledoyeromelita, posteriorly straight in Dentimelita gen. nov.).
Dentimelita lecroyae gen. nov., sp. nov.
(Figure 2E & Figures 17–19)
http://zoobank.org/ABE70505-EFED-4A15-B917-539EE8CC2601
Type Material

Fig. 17. Dentimelita lecroyae gen. nov., sp. nov., holotype (ECOSUR 286). Scale bar for H: 1 mm; scale bars for G1–2 and P3–4: 0.3 mm.

Fig. 18. Dentimelita lecroyae gen. nov., sp. nov., holotype (ECOSUR 286). Scale bars: 0.1 mm.

Fig. 19. Dentimelita lecroyae gen. nov., sp. nov., holotype (ECOSUR 286). Scale bars: 0.3 mm.
Holotype: Unsexed (dissected and drawn), 5.5 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P3-D3, 24.53°N 96.35°W, 434 m, 10 June 2017, coll. S. Balan-Zetina, ECOSUR 286.
Type Locality
Perdido Fold Belt, offshore Tamaulipas, Mexico, 24.53°N 96.35°W.
Etymology
The new species is named in honour of Sara E. LeCroy (University of Southern Mississippi) in recognition for her contribution to the taxonomic knowledge of amphipods.
Diagnosis
Subquadrate lateral cephalic lobes. Coxae 1–4 with anteroventral corners acutely produced; coxae 1–3 with posteroventral corners notched; coxa 4 with posteroventral margin serrate. Gnathopod 2 propodus palm oblique with two protuberances at the proximal half and one at the distal half, and palmar corner defined by an acute tooth. Pleonites 1–3 with central tooth; pleonites 2 and 3 with one lateral tooth subequal on each side. Urosomite 1 with central tooth and one lateral tooth subequal on each side. Urosomite 2 with two pairs of dorsolateral teeth with one robust seta between each pair.
Description
Based on holotype (ECOSUR 286). Head, slightly shorter than pereonites 1 and 2 combined; truncated lateral cephalic lobes; eyes small, subovate; anteroventral corner notched. Antennae 1–2, peduncle and flagellum weakly setose. Antenna 1, about 0.75 times of body length; peduncular article 1 shorter than article 2, with distolateral tooth produced acutely on outer surface; accessory flagellum three-articulate. Antenna 2, peduncular article 2 gland cone reaching the half of peduncular article 3 length; article 4 slightly longer than article 5. Upper lip, with apical notch, pubescent apically. Mandible, incisor five-dentate; lacinia mobilis four-dentate; raker setae row with five accessory blades; molar triturative, with a short plumose seta; palp reduced, article 1 short, produced distally and subequal to article 2, article 3 slightly longer than articles 1–2 with 2 apical setae only. Lower lip, inner and outer lobe rounded, pubescent medially; mandibular process well developed with lobes subacute apically. Maxilla 1, inner plate subtriangular, with five plumose setae at the proximal half; outer plate with nine bifurcated robust setae; palp two-articulated with six apical robust setae and seven slender setae. Maxilla 2, inner plate smaller than outer, with marginal inner row of slender setae and facial slender setae, located submarginal-dorsally (omitted in the drawing); outer plate with apical slender setae. Maxilliped, inner plate with five apical robust setae and an oblique row of 11 plumose setae on surface reaching the apex; outer plate not reaching end of palp article 2, with seven apical robust setae and a marginal inner row of five robust setae; palp four-articulate, article 2 longest and scarcely setose, article 3 with apical slender setae on inner and outer margin, article 4 with nail and inner margin with a row of setae.
Gnathopod 1 subchelate; coxa expanded distally, with anteroventral corner acutely produced and posteroventral corner notched; basis with short and long slender setae on anterior and posterior margin, respectively; merus, carpus and propodus setulose facially; carpus longer than propodus, with four transversal rows of slender setae and tufts of serrate setae on ventral margin; propodus expanded distally, subtriangular in shape; palm oblique, longer than posterior margin, with three transversal rows of slender setae and tufts of serrate setae at the distal half of ventral margin, and posterodistal corner distinctive, defined by one robust seta; dactylus shorter than palm, not reaching the angle, with one long slender seta on anteroproximal margin. Gnathopod 2 subchelate; coxa slightly longer than coxa 1 with anteroventral corner produced acutely and posteroventral corner notched; basis scarcely setose on anterior and posterior margin; merus with posterodistal tooth; carpus long, subtriangular; propodus elongate, with tufts of slender setae on dorsal and ventral margin, palm oblique with two protuberances at the proximal half and one at the distal half, and palmar corner defined by an acute tooth; dactylus slightly longer than palm, with two short slender seta on anteroproximal margin. Pereopod 3, coxa similar size as coxa 2, slightly narrowed distally, with anteroventral corner produced acutely and posteroventral corner notched; basis long with few long slender setae on posterior margin; merus long; dactylus long, about 0.75 times of propodus length. Pereopod 4, similar shape and size as pereopod 3, except by the coxa expanded distally, with posterior margin concave, anteroventral corner produced acutely and posteroventral corner oblique and serrate; basis with row of long slender setae in the medial part of posterior margin. Pereopod 5, coxa broader than long, excavate ventrally, with anteroventral lobe produced roundly and posteroventral lobe with a small acuminate notch; basis posterior margin straight, weakly serrate; propodus with few robust and slender setae on posterior margin. Pereopod 6, coxa similar shape as coxa 5; basis posterior margin straight, longer than basis of pereopods 5 and 7, weakly serrate. Pereopod 7, coxa small, with ventral lobe roundly produced; basis tapering distally, weakly serrated, with posterodistal corner acute.
Pleonite 1 with dorsal central tooth; epimeral plate acuminate posterodistally with posterior margin slightly convex. Pleonite 2 with dorsal central tooth and one longer lateral tooth on each side; epimeral plate acuminate posterodistally, with posterior margin slightly convex. Pleonite 3 with dorsal central tooth and one longer lateral tooth on each side; epimeral plate, posteroventral corner acutely produced, with posterior margin concave, notched, and ventral margin serrate weakly. Urosomite 1 with dorsal central tooth and one longer lateral tooth on each side. Urosomite 2 with two dorsal subequal pairs of teeth and one robust seta between each lateral pair. Urosomite 3 smooth dorsolaterally. Uropod 1 peduncle subequal in length to rami with small robust setae along outer edge, basofacial seta, and inter-ramal spur developed; rami subequal in length, linear, and slender with robust setae on apex. Uropod 2 shorter than uropod 1; peduncle shorter than rami; outer ramus shorter than inner, linear and slender, with robust setae on apex. Uropod 3, missing. Telson, deeply cleft, with one dorsal robust seta on inner marginal notch of each lobe.
Remarks
The species Quasimelita serraticoxae Labay, Reference Labay2014 from the North-western Pacific is similar to Dentimelita lecroyae gen. nov., sp. nov. by the acutely produced anteroventral corners of coxal plates 1–4 and the serrate posterior margin of coxal plate 4. However, characteristics at the genus category differentiate them (see Labay, Reference Labay2014 for Quasimelita). In Dentimelita gen. nov., pleonites 1–3 have strong dorsal teeth (vs weakly toothed to smooth dorsally), the mandibular palp is short (vs palp long) with scarce setae on the distal article of mandible (vs article setose), the palp segment 1 of maxilla 1 lacks lateral setae (vs bearing lateral setae), the maxilla 2 presents a distinctive transversal row of facial setae (vs few facial setae submarginally positioned), the propodus of gnathopod 2 has teeth/protuberances on palm (vs teeth/protuberances missing), the dactylus of gnathopod 2 is slender with an acute tip (vs broad with a blunt tip), and without outer marginal setae (vs numerous outer marginal setae).
Habitat
Dentimelita lecroyae gen. nov., sp. nov. was collected on the continental slope (434 m) at a temperature of 9.5°C, salinity of 35 PSU, dissolved oxygen of 2.42 mg l−1, and sediments with high organic matter content (11%) and fine-medium sand (32–49%) and low content of very fine sand (19%).
Distribution
Dentimelita lecroyae gen. nov., sp. nov. is known so far only from the type locality, Perdido Fold Belt, offshore Tamaulipas, western GoM.
Family unciolidae Myers & Lowry, Reference Myers and Lowry2003
Genus Neohela Smith, Reference Smith1881
Neohela winfieldi sp. nov.
(Figure 2F & Figures 20–23)
http://zoobank.org/E872F6FA-633D-4055-8A82-BD6A93D1D72B
Type Material

Fig. 20. Neohela winfieldi sp. nov., holotype male (ECOSUR 287). Scale bar for H: 1 mm; scale bars for G1–2 and HD: 0.2 mm.

Fig. 21. Neohela winfieldi sp. nov., holotype male (ECOSUR 287). Scale bars: 0.1 mm.

Fig. 22. Neohela winfieldi sp. nov., holotype male (ECOSUR 287). Scale bars for P4–7: 0.5 mm; scale bars for U1–2: 0.2 mm; scale bar for T: 0.1 mm.

Fig. 23. Neohela winfieldi sp. nov., paratype female (ECOSUR 288). Scale bar for A1: 1 mm; scale bars for G1–2 and HD: 0.3 mm; scale bar for T: 0.1 mm.
Holotype: Male (dissected and drawn), 5.7 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P3-C3, 25.16°N 96.21°W, 508 m, 11 June 2017, coll. A. Leon-Hernandez, ECOSUR 287. Paratypes: Female (dissected and drawn), 5.8 mm, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P2-F3, 24.03°N 97.05°W, 490 m, 30 September 2016, coll. V. Papiol, ECOSUR 288.
Additional Material Examined
Female, data as for holotype, ECOSUR-C1190. Female, Perdido Fold Belt, offshore Tamaulipas, Mexico, sampling station P3-B3, 25.45°N 96.14°W, 514 m, 11 June 2017, coll. A. Leon-Hernandez, ECOSUR-C1191.
Type Locality
Perdido Fold Belt, offshore Tamaulipas, Mexico, 25.16°N 96.21°W.
Etymology
The new species is named in honour of Ignacio C. Winfield Aguilar (Universidad Nacional Autonoma de Mexico) in recognition for his contribution to the taxonomic knowledge of amphipods.
Diagnosis
Head, rostrum with a tiny medial tooth; lateral cephalic lobes acutely produced. Upper lip with notch slightly asymmetrical. Mandible palp article 3 shorter than article 2. Coxae 1–2 with a small tooth on anteroventral corner; coxae 3–4 with anteroventral corner acutely produced. Gnathopods 1–2, basis scarcely setose on inner and outer margins; dactylus with a single long slender seta on anterior margin. Gnathopod 1, carpus elongate; palm with two teeth. Pereopods 5–6, carpus distal margin with row of robust setae. Pereopod 5 dactylus about 0.4 times of propodus length. Uropods 1 and 2, peduncle with a few short slender setae on dorsolateral edges; outer ramus straight, shorter than inner ramus. Telson, broader than long, emarginate.
Description
Based on holotype male (ECOSUR 287). Head, short rostrum, with a tiny medial tooth; lateral cephalic lobes acutely produced; eyes absent; anteroventral corner acutely produced. Antennae 1–2 missing; peduncular article 1 of antenna 1 with one distal robust seta on inner margin. Upper lip with a slightly asymmetrical notch, pubescent apically. Mandibles, symmetrical; right incisor five-dentate, left four-dentate; lacinia mobilis four-dentate; raker setae row with two or three accessory blades; molar triturative with a plumose seta; palp article 1 short; palp article 2 longer than article 3, scarcely setose; palp article 3 with two long medial and four apical slender setae. Lower lip, inner and outer lobe rounded, pubescent apically; mandibular process well developed with lobes subacute apically. Maxilla 1, inner plate linguiform, with three medial (one long and two short) and four apical (two long and two short) slender setae; outer plate with nine dentate robust setae; palp two-articulated with three apical robust setae and two slender setae. Maxilla 2, inner plate smaller than outer, with apical and subapical plumose setae; outer plate with apical slender setae and lateral margin with pubescence. Maxilliped, inner plate with six apical robust setae and an oblique row of six plumose setae on surface reaching the apex; outer plate not reaching end of article 2 on palp, with four apical robust setae and a marginal inner row of three robust setae; palp four-articulate, article 2 longest and scarcely setose on inner margin, article 3 with apical and subapical slender setae, article 4 with nail.
Gnathopod 1 subchelate; coxa expanded ventrally with a small tooth on the anteroventral corner; basis scarcely setose on anterior and posterior margins; merus with tuft of long serrate setae on ventral margin; carpus elongated (about 2 times of propodus length) with tufts of long dorsal slender setae and long ventral serrate setae along margins; propodus with tufts of long dorsal slender setae and long ventral serrate setae along margins, palm with two teeth (one medial and one ventral); dactylus elongate (about 3 times of palm length) with a single long slender seta on anteroproximal margin. Gnathopod 2 subchelate; coxa expanded ventrally with a small tooth on the anteroventral corner; basis scarcely setose on anterior and posterior margins; merus with tuft of long serrate setae on the anteroventral margin; carpus slightly longer than propodus with scattered long dorsal slender setae and long ventral serrate setae along margins; propodus with tufts of long dorsal slender setae and long ventral serrate setae along margins, palm oblique and minutely serrated with transversal rows of slender setae; dactylus slightly longer than palm with a single long slender seta on anteroproximal margin. Pereopod 3 of similar shape and size as pereopod 4. Pereopod 4 simple; coxa with ventral margin convex and anteroventral corner produced acutely; basis straight, anterior and posterior margins devoid of setae; merus, carpus and propodus with posterior margins nearly setose; propodus about 0.8 times of merus length; dactylus about 0.5 times of propodus length. Pereopods 5–7 increasing in size. Pereopod 5 simple, coxa subrectangular, broader than long, with anterior margin oblique; basis straight, anterior and posterior margins devoid of setae; merus and propodus with posterior margins sparsely setose; carpus anterodistal margin with transversal row of eight robust setae; dactylus about 0.4 times of propodus length. Pereopod 6 simple, coxa subrectangular, broader than long, with anterior margin oblique; basis straight, anterior and posterior margins sparsely setose; merus and propodus with posterior margins sparsely setose; carpus anterodistal margin with transversal row of five robust setae; dactylus about 0.6 times of propodus length. Pereopod 7 simple, coxa subrectangular, broader than long, with anterior margin oblique; basis straight, anterior and posterior margins sparsely setose; merus, carpus and propodus with anterior and posterior margins sparsely setose; dactylus about 0.8 times of propodus length.
Uropod 1 peduncle longer than rami with a few short slender setae on dorsolateral edges and one distal robust seta on each edge; outer ramus straight, shorter than inner ramus; rami apex with four robust setae. Uropod 2 peduncle slightly longer than rami with one distal robust seta on each edge; outer ramus straight, shorter than inner ramus; rami apex with two or three robust setae. Uropod 3 missing. Telson, subtriangular, broader than long, slightly emarginate, with lateral margins with three plumose setae.
Paratype female (ECOSUR 288). Head, short rostrum, smooth; lateral cephalic lobes unproduced. Antenna 1 peduncle longer than flagellum; accessory flagellum three-articulate. Oostegites present on pereonites 2–5, shorter than basis. Gnathopods 1 and 2 of similar shape, but slender. Gnathopod 1 palm minutely serrated with one small ventral tooth.
Remarks
The genus Neohela is a small taxon formed by six species, including the new species described here (Barnard & Karaman, Reference Barnard and Karaman1991): Neohela intermedia Coyle & Mueller, Reference Coyle and Mueller1981, Neohela lamia d'Udekem d'Acoz, Reference d'Udekem d'Acoz2007, Neohela maxima Stephensen, Reference Stephensen1933, Neohela monstrosa (Boeck, Reference Boeck1861), Neohela pacifica Gurjanova, Reference Gurjanova1953 and N. winfieldi sp. nov. Neohela winfieldi sp. nov. presents a unique set of characteristics that separate it from its congeners: a rostrum with a tiny medial tooth (absent in female), coxal plates 1–4 with acute anteroventral corner, dactylus of gnathopods 1–2 with a single long slender seta on anterior margin, the uropod 1 with outer ramus straight, and the telson emarginate. Particularly, the new species differs from N. intermedia, N. maxima, N. monstrosa and N. pacifica by the gnathopod 1 with the palm bearing two teeth (vs three teeth) and scarce robust setae transversely arranged (vs many robust setae longitudinally arranged) and from N. lamia by the gnathopod 2 palm without tooth (vs one tooth) and the peduncle of uropods 1–2 with a few short slender setae on dorsolateral edges (vs slender setae absent and many robust setae present).
Habitat
Neohela winfieldi sp. nov. was collected on the continental slope (490–514 m) at temperatures from 3.4–9.3°C, salinity of 35 PSU, and dissolved oxygen of 2.4–4.1 mg l−1.
Distribution
Neohela winfieldi sp. nov. is known so far from the type locality, Perdido Fold Belt, offshore Tamaulipas, western GoM. Species of the genus Neohela are distributed in the Arctic and subarctic regions, North Atlantic and North Pacific (Barnard & Karaman, Reference Barnard and Karaman1991; d'Udekem d'Acoz, Reference d'Udekem d'Acoz2007). Three species, including the new one here described have been reported at >200 m depth in the North Atlantic: N. lamia, N. monstrosa and N. winfieldi sp. nov. The geographic record of N. winfieldi sp. nov. in the GoM represents the largest southward extension for any recognized member of the genus into tropical latitudes.
Discussion
In the present study, two new genera (Dentimelita gen. nov. and Paraeperopeus gen. nov.) and six new species (Dentimelita lecroyae gen. nov., sp. nov., Neohela winfieldi sp. nov., Paraeperopeus longirostris gen. nov., sp. nov., Pardaliscella perdido sp. nov., Pardaliscoides ecosur sp. nov. and Tosilus cigomensis sp. nov.) of benthic amphipods from the continental slope and abyssal zone have been described. Also, the transfer of Pardaliscella inermis to the genus Caleidoscopsis is proposed.
The new amphipods described here belong to rare genera, poorly reported in the literature. Each one of those genera has fewer than nine recognized species worldwide, and the genus Tosilus represented a monotypic genus for a long time (54 years) until the description of T. cigomensis sp. nov. in this study. The current distribution of the new species in the Perdido Fold Belt region represents a remarkable geographic extension for the genera, including new records for Neohela to the GoM, Pardaliscella and Pardaliscoides to the western Atlantic and Tosilus to the Atlantic. The discovery of new species in the Perdido Fold Belt region updates the amphipod diversity at the GoM deep-sea (>200 m depth) to 34 spp. according to previous studies (Winfield et al., Reference Winfield, Escobar-Briones and Morrone2006, Reference Winfield, Ortiz and Ardisson2016; Soliman & Wicksten, Reference Soliman and Wicksten2007; Escobar-Briones et al., Reference Escobar-Briones, Santillán and Legendre2008; Ortiz et al., Reference Ortiz, Winfield and Ardisson2017). Their description provides information that enhances the knowledge of amphipod taxonomy in benthic deep-sea habitats.
The new proposed genus Paraeperopeus gen. nov. and four new species (P. longirostris gen. nov., sp. nov., P. perdido sp. nov., P. ecosur sp. nov. and T. cigomensis sp. nov.) belong to the family Pardaliscidae, whose organisms are frequently found in deep-sea benthic samples from the continental slope to the hadal zone (Karaman, Reference Karaman1974; Fujii et al., Reference Fujii, Kilgallen, Rowden and Jamieson2013). Until the present study, no pardaliscid amphipod had been described for any marine zone in the GoM, thus this study increases the morphological diversity of the family and extends its biogeographic range into the deep-sea.
Acknowledgements
We thank the support of the ‘Laboratorio de Biodiversidad Marina y Cambio Climatico, BIOMARCCA’ staff, specially to Anabel Leon Hernandez and Sara Balan Zetina who collected and prepared the amphipod samples. Thanks to Nuno Simões (Universidad Nacional Autonoma de Mexico) by providing access to the microscopy equipment for the photographs. We also thank Charles O. Coleman (Museum für Naturkunde) for his careful review of the first version of the manuscript, and specially to Vjacheslav S. Labay (Sakhalin State University) by his recommendation and advice for establishing the new melitid genus. We thank the reviewer who helped to improve the manuscript. This is a contribution of the Gulf of Mexico Research Consortium (CIGoM).
Financial Support
The research and a postdoctoral fellowship to CEPR was funded by the National Council of Science and Technology of Mexico (CONACYT) – Mexican Ministry of Energy – Hydrocarbon Trust, project 201441.

























