INTRODUCTION
Seagrass beds are widespread in shallow coastal waters and considered to be highly productive and diverse communities (Heck, Reference Heck, Able, Roman and Fahay1995; Lee et al., Reference Lee, Fong and Wu2001; Jaschinski et al., Reference Jaschinski, Brepohl and Sommer2008; Ouisse et al., Reference Ouisse, Riera, Migné, Leroux and Davoult2012). The Zostera marina is a particularly common species in subtidal habitats from the middle to high latitude regions (Jaschinski et al., Reference Jaschinski, Brepohl and Sommer2008). Seagrass beds support a high diversity of consumers and most likely become potential food sources for a wide range of consumers (Valentine & Duffy, Reference Valentine, Duffy, Larkum, Orth and Duarte2006; Lebreton et al., Reference Lebreton, Richar, Galois, Radenac, Brahmia, Colli, Grouazel, André, Guillou and Blanchard2012).
Meiofauna are often characterized by high densities in seagrass leaves (Bell et al., Reference Bell, Walters and Kern1984; De Troch et al., Reference De Troch, Gurdebeke, Fiers and Vincx2001) and surface sediments (Escavarage et al., Reference Escavarage, Gareía and Castel1989; Danovaro & Gambi, Reference Danovaro and Gambi2002). Meiofaunal communities are thought to rapidly respond to organic matter inputs and may be closely coupled with primary production inputs (Escavarage et al., Reference Escavarage, Gareía and Castel1989; Lebreton et al., Reference Lebreton, Richar, Galois, Radenac, Brahmia, Colli, Grouazel, André, Guillou and Blanchard2012). Detrital organic matter constitutes a substrate for the development of bacteria (Anesio et al., Reference Anesio, Abreu and Biddanda2003; Holmer et al., Reference Holmer, Duarte, Boschker and Barrón2004), which may represent an additional food source for meiofauna (Danovaro, Reference Danovaro1996). In particular, bacteria could contribute significantly to the organic carbon pool, and they may dominate the total pool of organic carbon from living microbes in the Mediterranean seagrass system during certain periods of the year (Danovaro et al., Reference Danovaro, Fabiano and Boyer1994; Danovaro & Faviano, Reference Danovaro and Fabiano1995). Danovaro (Reference Danovaro1996) reported a strong relationship between bacterial and meiofaunal abundance, suggesting that bacteria serve as a link that transfers the carbohydrates in detrital particles to benthic consumers. Similarly, Tenore et al. (Reference Tenore, Cammen, Findlay and Phillips1982) found a highly significant and positive correlation between bacterial abundance and nematode density. Warwick (Reference Warwick, Moriarty and Pullin1987) reported that the structure of the nematode assemblage around the detritus is characterized by selective deposit feeders and suggested that bacteria may be used as suitable food sources for other nematodes.
Stable isotope ratios of carbon and nitrogen have been widely used to identify primary producers at the bases of the food web in several coastal habitats (Fry & Sherr, Reference Fry and Sherr1984; Peterson et al., Reference Peterson and Fry1987; Bustamante & Branch, Reference Bustamante and Branch1996; Riera & Richard, Reference Riera and Richard1996; Fry, Reference Fry2006; Schaal et al., Reference Schaal, Riera and Leroux2008). Moore & Semmens (Reference Moore and Semmens2008) developed MixSIR, an alternative stable isotope mixing model that uses a Bayesian framework to determine the precise proportional contribution ratio of each source to consumers of interest. Bayesian statistics offer a powerful data interpretation method that incorporates information, integrates sources of uncertainty, and explicitly compares the strength of support for competing models or parameter values (Hilborn & Mangel, Reference Hilborn and Mangel1997; Ellison, Reference Ellison2004).
In this study, we aim to understand the energy flow in the benthic ecosystem around seagrass beds by analysing stable carbon and nitrogen isotope ratios of various primary producers and benthic organisms. Additionally, the seagrass-derived detrital organic matter trophic contribution to the benthic communities is determined by the isotope mixing model (MixSIR mixing model). We evaluate the trophic function of meiofauna as a link to transfer the detrital organic matter to higher trophic consumers in the seagrass bed ecosystem.
MATERIALS AND METHODS
Study area
All samples were collected around seagrass (Zostera marina) beds in Dong-dae Bay, South Korea (Figure 1). Dong-dae Bay is an inner bay, measuring 5 km long and 1 km wide and covered with the seagrass Z. marina. The water depth at the sampling site was 3–12 m at full tide, and the meadow was exposed to air at low tide. The sediment size distribution was sandy-mud (4.3–5.4 Ф). The organic matter content of the sediment was 6.9–7.7%, and the seawater temperature was 4.6–6°C in winter.

Fig. 1. Map of study area on the southern coast of the Korean peninsula. ●, sampling site at low tide. The shaded portion is the Zostera marina meadow.
Sample collection and isotope analyses
Particulate organic matter (POM), sedimentary organic matter (SOM), benthic microalgae, seagrass, macroalgae (red algae and green), meiofauna (copepods and nematodes), oysters and polychaetes were collected from the intertidal areas every month from December 2004 to March 2005. The POM was collected by filtering 1l of seawater through pre-combusted filter paper (47 mm GF/F) and stored at −80°C. Sediment samples were taken from the top 0.5 cm of cylinder core samples that were 8 cm in diameter. The sediment was dried at 60°C and completely homogenized. Green algae and new leaves from the seagrass Z. marina were collected from the tidal flat, and red algae attached to old leaves of seagrass were isolated using a toothbrush. Macroalgae and seagrass were washed by filtered seawater, and any attached microorganisms and detritus were gently scraped off. Meiofauna (copepods and nematodes) was collected from the surface sediment (above 0.5 cm on top) and separated from the sediment using 20 µm mesh, and approximately 300 individuals were gathered for stable isotope analysis under a microscope. The oyster Crassostrea gigas and polychaetes Lumbrineris spp., Nris spp. and Glycera spp. were additionally hand-collected from this study site. Polychaetes were dissected into the body and internal organs, and only the body was analysed. Benthic shrimps collected from this study area were kept alive overnight in filtered seawater to clear their gut. These consumers and macrophyte samples for stable isotope analysis were dried at 60°C, finely ground with a mortar and pestle, and stored in glass vials at −80°C until the analysis.
Benthic microalgae were separated using a slightly modified method reported by Couch (Reference Couch1989). The details are described by Riera & Richard (Reference Riera and Richard1996). Approximately 0.5–1 cm of the surface sediment was used in this study. A sieve (60 µm pore size) was placed on top of the sediment, and silica was spread on the sieve. Then, these samples were exposed to light for 24 h to allow adsorption of the benthic microalgae onto the silica (pre-burned). The collected benthic microalgae and seagrass were preserved at −80°C until analysis. Each sample was put in a glass vial, and inorganic carbon was removed by 1N HCl treatment before measuring the organic stable carbon isotope ratio. This process was not conducted before measuring the stable nitrogen isotope ratio to prevent any change in the isotope ratio during the HCl treatment (Bunn et al., Reference Bunn, Loneragan and Kempser1995). In this study, the carbon and nitrogen isotope ratios were measured by an elemental analyser coupled to an isotope ratio mass spectrometer (Delta plus, Thermo Fisher Scientific), which was operated by the Alaska Stable Isotope Facility at WERC (Water Environmental Research Center), University of Alaska Fairbanks. V-PDB (Vienna-Pee Dee Belimnite) and AIR (atmospheric nitrogen) were used as reference standards for δ13C (‰) and δ15N (‰), respectively.
Mixing model analyses
We estimated the feasible contribution ratio of each food source with the MixSIR model version 1.0.4 and an associated graphical user interface (GUI) using MATLAB; the number of iterations was 10,000, and we displayed the 0–100% range for each food source (Moore & Semmens, Reference Moore and Semmens2008). The dual isotope ratios of δ13C and δ15N were used after trophic enrichment correction by approximately 0.8‰ for δ13C and 3.4‰ for δ15N at each trophic level shift.
RESULTS
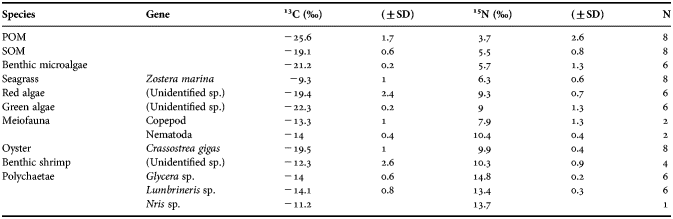
The average δ13C value of POM (−25.6 ± 1.7‰) in the seagrass beds of Dong-dae Bay was significantly lower than the values of other primary producers in Figure 2. The average δ13C value of the SOM was −19.1 ± 0.6‰, and temporal variation was hardly observed. The average δ13C value of the benthic microalgae was −21.2 ± 0.2‰, which was slightly lower compared with SOM. The δ13C values of macroalgae, such as green algae (−22.3 ± 0.2‰) and attached red algae (−19.4 ± 2.4‰), were similar to benthic microalgae and SOM, but slightly higher than POM. In contrast, the seagrass Zostera marina had a more positive δ13C value (−9.3 ± 1.0‰) compared with the other primary producers. The benthic microalgae, green algae, attached red algae, and SOM primary food sources in the food web had more negative δ13C values compared with the seagrass. The POM had the lowest value. The Crassostrea gigas oyster, a filter feeder, had an average δ13C value of −19.5 ± 1.0‰, which was slightly higher than benthic microalgae and similar to SOM. In contrast, the meiofauna had δ13C values (−13.3 ± 1.0‰ for nematodes and −14.0 ± 0.4‰ for copepods) between the benthic microalgae and seagrass. The δ13C values of polychaetes such as Glycera spp., Lumbrineris spp. and Nris spp., which are considered to be the highest trophic level organisms in the study area, were close to the meiofauna.

Fig. 2. A cross plot of stable carbon and nitrogen isotope ratios for macrobenthos and their food sources.
The average δ15N value of POM was 3.7 ± 2.6‰, and the values of SOM and benthic microalgae were very similar to each other (5.5 ± 0.8‰ and 5.7 ± 1.3‰, respectively). However, the δ15N value of the seagrass (6.3 ± 0.6‰) was slightly higher than the values of SOM and benthic microalgae. The primary producers green algae and attached red algae had relatively higher δ15N values of 9.0 ± 1.3‰ and 9.3 ± 0.7‰, respectively. The average δ15N values of the meiofauna (nematodes and copepods were 10.4 ± 0.4‰ and 7.9 ± 1.3‰, respectively) were higher than the primary producers, except for green algae and attached red algae. The δ15N values of the oyster (9.9 ± 0.4‰) were similar to the nematodes (Table 1; Figure 2). The δ15N values of the three polychaete species were 3–4‰ higher than the nematodes, reflecting their higher trophic levels (Glycera spp., Lumbrineris spp. and Nris spp. had average values of 14.8 ± 0.2‰, 13.4 ± 0.3‰ and 13.7‰, respectively). The study period (winter) displayed little temporal variation in the stable isotope ratios for each organism and species.
Table 1. Stable carbon and nitrogen isotope ratios of primary producers and consumers near the seagrass Zostera marina beds in Dong-dae Bay.

Based on the δ15N values, we assumed the two consumer study groups to be herbivores (trophic level = 2.0, δ15N = ~10‰, for oyster and two meiofauna) and carnivores (trophic level = 3.0, δ15N = ~14‰ for polychates). In the MixSIR model input, the trophic enrichment in δ13C and δ15N (0.8‰ and 3.4‰ at each shift of the trophic level, Aberle & Molzahn, 2007; Doi et al., 2011) were corrected for the δ13C and δ15N values of these consumers. The MixSIR model shows that the oysters use multiple food sources, including benthic microalgae (26.4 (2.5–62.9%)), POM (19.4 (2.4–43.2%)), red algae (15.8 (1.4–42%)), and green algae (16.5 (1.5–46%)) and seagrass (62.6 (54.2–71%) and 53.7 (41.1–63.3%), respectively) are the dominant food sources for the copepods and nematodes. The model shows that seagrass significantly contributes to the food sources for benthic shrimp (65.9 (54.7–75.7%)) and three polychaete species, Glcera spp. (41.1 (18.7–53.4%)), Lumbrineris spp. (48.4 (37.4–57.9%)) and Nris spp. (68 (56.8–77.8%)) (Table 2).
Table 2. Feasible contribution ratio (%) of each food source to the consumer, determined by the MixSIR model using two stable isotopes of carbon and nitrogen (after correcting for trophic enrichment by 0.8‰ for δ13C and 3.4‰ for δ15N) (Moore & Semmens, Reference Moore and Semmens2008).

DISCUSSION
The role of benthic microalgae as primary food sources
The 13C values of POM were lower than the marine primary producers and sediments, which may reflect large contributions of terrestrial organic matter input from the nearby Sacheon City to the POM in Dong-dae Bay. However, several previous studies reported that carbon sources of terrestrial origin are generally of little importance to estuarine food webs and argued that the influence of terrestrial organic matter input is limited to the riverine or upper estuarine area, potentially due to their poor nutritional quality (Incze et al., Reference Incze, Mayer, Sherr and Makco1982; Sinenstad & Wissmar, Reference Sinenstad and Wissmar1985; Bunn et al., Reference Bunn, Barton, Hynes, Power and Pope1989; Deegan & Garritt, Reference Deegan and Garritt1997; Page, Reference Page1997). This small contribution of terrestrial organic matter to estuarine food webs is consistent with the results of the MixSIR model (i.e. relatively small contribution ratio to consumers) in this study (Table 2). It has been reported that re-suspended benthic microalgal biomass in shallow estuaries is largely responsible for the spatial distribution of chlorophyll-a and either contributes as much as the total phytoplankton biomass or accounts for most of the chlorophyll-a in the water column (Shaffer & Sullivan, Reference Shaffer and Sullivan1988; de Jonge & van Beusekom, Reference De Jonge and van Beusekom1992; Zurburg et al., Reference Zurburg, Smaal, Héral and Danker1994). Flume experiments and field observations in previous studies have shown variation in re-suspended benthic microalgae in response to stepwise increases in current velocity or wind speed and have identified local deposition of the re-suspended microalgal biomass and rapid settling of fine sediment and microalgae at a low current or wind speed (de Jonge & van Beusekom, Reference De Jonge and van Beusekom1992; Blanchard et al., Reference Blanchard, Sauriau, Cariou-Le, Gouleau, Garet and Olivier1997; Widdows et al., Reference Widdows, Brinsley, Bowley and Barrett1998; Herman et al., Reference Herman, Middelburg, Widdows, Lucas and Heip2000). In fact, the results in the MixSIR model show that benthic microalgae significantly contribute as a primary food source for oysters (26.4% in diet) and Lumbrineris polychaete (14.1% in diet). Additionally, the δ15N value of oyster is approximately 4‰ higher than benthic microalgae (Figure 2), which may demonstrate a clear trophic relationship. Most previous studies used approximately 0.8‰ and 3.4‰ as the mean trophic enrichment for carbon (△δ13C) and nitrogen (△δ15N), respectively, from diets to consumers (DeNiro & Epstein, Reference DeNiro and Epstein1978, Reference DeNiro and Epstein1981; Minagawa & Wada, Reference Minagawa and Wada1984; Aberle & Malzahn, Reference Aberle and Malzahn2007; Doi et al., Reference Doi, Chang and Nakano2011). However, Dubois et al. (Reference Dubois, Jean-Louis, Bertrand and Lefebvre2007) argue that trophic enrichment for Crassostrea gigas should be variable and averaged to 1.85‰ for δ13C and 3.78‰ for δ15N values because trophic enrichment reflects numerous parameters, including species, diet, environmental conditions, and nitrogenous wasting (Vander Zanden & Rasmussen, Reference Vander Zanden and Rasmussen2001; McCutchan et al., Reference McCutchan, Lewis, Kendall and McGrath2003; Vanderklift & Ponsard, Reference Vanderklift and Ponsard2003). Thus, the differences in 15N values between benthic algae and oysters can be simply explained by the contribution of benthic algae as a major food source to oyster in Dong-dae Bay.
Contribution of seagrass detritus as an energy source to the benthic ecosystem
The results of the MixSIR model revealed a significant contribution of seagrass to the diet sources of benthic organisms (except for oyster), particularly meiofauna, which is the likely link between seagrass and marcrobenthos (Table 2).
Many previous studies have frequently focused on benthic microalgae as major food sources for various benthic invertebrates located on the tidal flats (Page, Reference Page1997; Kang et al., Reference Kang, Kim, Lee, Kim, Lee and Hong2003, Reference Kang, Choy, Paik, Park, Lee and An2007; Kanaya et al., Reference Kanaya, Takagi and Kikuchi2008). However, Doi et al. (Reference Doi, Matsumasa, Fujikawa, Kanou, Suzuki and Kikuchi2009) reported that seagrass greatly contributes to the food sources of the snail and argued that both macroalgae and seagrass play important roles as food sources at their growing sites and tidal flats. Olsen et al. (Reference Olsen, Fox, Teichberg, Otter and Valiela2011) emphasized that benthic invertebrates consume a large amount of seagrass detritus as food sources at the lower N load estuaries.
It has previously been hypothesized that benthic bacteria are a major carbon source for meiofauna (Montagna, Reference Montagna1984). It has been previously reported that other species of the polychaete Nephtys incise could use energy sources derived from aged seagrass detritus (Tenore et al., Reference Tenore, Tietjen and Lee1977; Leduc & Probert, Reference Leduc and Probert2009). If so, benthic bacteria are likely to be important links that transfer the organic carbon and nitrogen pools from seagrass to meiofauna in the seagrass meadow ecosystem rather than the unvegetated sediment ecosystem. Moreover, δ15N values of meiofauna should primarily reflect seagrass and trophic enrichment through the food web. In terms of the energy flow and isotope changes through the benthic food chain, the carbon isotope ratio of bacteria should be close to meiofauna (~−14 to −13‰) if the meiofauna feed on benthic bacteria (Figure 2). Polychaetes (Glycera spp., Lumbrineris spp. and Nris spp.) and their food source meiofauna have similar carbon stable isotope ratios.
Previous investigations of seagrass meadows found that changes in bacterial abundance are closely related to seasonal changes in temperature, food resources (labile organic matter), and primary production (Danovaro et al., Reference Danovaro, Fabiano and Boyer1994; Danovaro & Fabiano, Reference Danovaro and Fabiano1995). In the seagrass sediment of Prelo Bay, bacterial abundance was significantly enhanced by organic matter inputs. The highest bacterial biomass was observed during the winter accumulation of vegetal debris because fluctuations of bacterial abundance and biomass reflect changes in the sediment's carbohydrate content (Danovaro, Reference Danovaro1996). In particular, bacterial abundance that shows a significantly positive correlation with carbohydrates may represent the link between detrital particles and benthic consumers (Danovaro, Reference Danovaro1996). Kenworthy & Thayer (Reference Kenworthy and Thayer1984) reported that structural carbohydrates, which are cell wall constituents in leaves, account for 31.8% of the total carbohydrates in Zostera marina. Williams et al. (Reference Williams, Jaffé, Anderson and Jochem2009) used the isoSource mixing model and reported that seagrass-derived (Thalassia testudinum) organic matter explain 13–67% of bacterial-specific δ13C signatures. Thus, seagrass detritus may promote bacterial abundance, which is usually transformed by benthic bacteria into useful food sources for deposit feeders. The aboveground biomass of Z. marina in this study area is at a minimum in December. The number of spathes per shoot increases from February to the summer, and most leaves and sheathes from Z. marina fall in winter (Lee et al., Reference Lee, Lee and Choi2005). Thus, seagrass detritus could sufficiently supply organic matter to the bacterial community, and benthic bacteria and meiofauna can survive and grow by consuming the organic matter from fallen Z. marina detritus, even in winter.
These stable isotope results demonstrate that filter feeders such as oyster use benthic microalgae as a food source but that polychaetes feed on meiofauna. These meifauna may feed on benthic bacteria and serve as links that transfer organic carbon and nitrogen from seagrass detritus to the food web. When most of the seagrass leaves had fallen in the study area in winter, the benthic microalgae contribution to the food web may have been limited in the seagrass meadow ecosystem. During this time period, however, seagrass detritus could become an important food source, especially for large deposit feeders (i.e. meiofauna and polychaetes) in this benthic food web. Previous research generally emphasized that the ecological role of seagrass beds is a shelter in a coastal ecosystem. However, the present investigation suggests that seagrass detritus are significant energy source for many consumers, including bacteria and meiofauna that serve as important links in the benthic ecosystem of coastal seagrass beds.
FINANCIAL SUPPORT
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012012617), and also by Fishery Commercialization Support Programme (112093-03-1-CG000), Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.