INTRODUCTION
The kinorhynch biodiversity is still poorly known, and currently only 162 valid species based on descriptions of adult specimens are recognized. The described species are currently distributed in 18 genera (see Sørensen & Pardos, Reference Sørensen and Pardos2008), but the discovery of no less than three new genera within the last three years only (Neuhaus & Blasche, Reference Neuhaus and Blasche2006; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007; Sørensen, Reference Sørensen2008) indicates that also the diversity on generic and higher taxonomic levels is much larger than we know at present.
The kinorhynch interrelationships and character evolution are still far from being understood, and an important stepping stone to reach more clarity is the scrutiny of the taxonomic lineages within the group. Hence, the discovery of, for example, a new genus can provide significant information about the more basal kinorhynch relationships.
In the present contribution, we describe Triodontoderes anulap, a new genus and species from Micronesia in the Central Western Pacific. The new entity is described using light- and scanning electron microscopy, and its morphological characters and putative phylogenetic relationships are discussed.
MATERIALS AND METHODS
Specimens of Triodontoderes anulap gen. et sp. nov. were collected by SCUBA diving at various localities around Chuuk Islands, Micronesia (Figure 1). The locality Sand Dune (7°27′13″N 151°56′05″E) was designated as type locality. The type specimens were collected 24 March 2007, at 17 m depth from subtidal coralline sand with detritus. Additional specimens were found in subtidal coralline sand with detritus at 27 m depth from Benedict Point (7°27′37″N 151°54′15″E), at 6–8 m depth in subtidal coralline sand with detritus and Halimeda debris from Osakura Island (7°28′58″N 151°53′58″E); and at 25 m depth in subtidal coralline sand with detritus from Shark Island (7°29′48″N 151°54′23″E). Specimens of T. anulap gen. et sp. nov. co-occurred with other meiobenthic taxa such as nematodes (Dracograllus sp., Epsilonema sp., Desmoscolex sp., Tricoma sp. and T. (Quadricoma) sp.), isopods, tanaidaceans, ostracods, harpacticoids, halacarids, polychaetes and sipunculans. Various yet undescribed species of Kinorhyncha (Echinoderes spp. nov) were present in other samples from the atoll area of the Chuuk Islands, but they never co-occurred with T. anulap gen. et sp. nov.

Fig. 1. Map showing collecting localities on the Chuuk Islands Atoll. Locality 1, Sand Dune (type locality for Triodontoderes anulap gen. et sp. nov.); locality 2, Benedict Point; locality 3, Osakura Island; locality 4, Shark Island.
The meiofaunal organisms were extracted from the samples through freshwater shocking (see Kristensen & Higgins, Reference Kristensen and Higgins1984; Sørensen & Pardos, Reference Sørensen and Pardos2008), subsequently filtered through a 63 µm mesh sieve and fixed in 4% buffered formalin with seawater. Coarse detritus and tiny shell gravels were removed from the sample by decantation, and the meiobenthos was subsequently roughly extracted by flotation in Ludox® (DuPont) HS 40 (Burgess, Reference Burgess2001). The kinorhynch specimens were picked out from the mixed meiobenthos under a high magnification Leica MZ 8 stereomicroscope with differential interference contrast.
Specimens for scanning electron microscopy (SEM) were dehydrated through a graded series of ethanol, transferred to acetone and critical point dried. The dried specimens were mounted on aluminium stubs, sputter coated and examined with a JEOL JSM-6335F field emission scanning electron microscope. Specimens for light microscopy (LM) were transferred to distilled water, dehydrated through a graded series of glycerin and mounted in Fluoromount G®. The mounted specimens were examined and photographed using Nomarski differential interference contrast with an Olympus BX60 microscope equipped with an Olympus DP20 camera. Measurements were made with Cell ^D software for analysis of light microscopical photographs.
DIAGNOSIS
Mouth cone with nine outer oral styles consisting of a single segment. Introvert with one ring of spinoscalids and four rings of regular scalids. Fourteen trichoscalids present, closely associated with the placids. Fourteen placids present, formed as extensions from segment 1; articulation between placids and segment 1 not visible. Segment 1 consisting of one tergal and one sternal plate; segments 2 to 4 consisting of one tergal and two sternal plates; segments 5 to 11 consisting of one tergal plate with midventral articulation. Segments 1 to 11 with middorsal spines; ventrolateral cuspidate spines and short, flexible acicular spines on segment 2; segments 3 to 4 with lateroventral (lv) acicular spines; segment 5 with lateral accessory (la) acicular spines and lv cuspidate spines; segment 6 with la cuspidate spines and lv acicular spines; segment 7 with lv acicular spines; segment 8 with la cuspidate spines and lv acicular spines; segment 9 with la acicular spines and lv cuspidate spines; segment 10 with lv acicular spines in females and laterodorsal crenulated spines in males; terminal segment with lateral terminal, lateral terminal accessory and midterminal spines. Females with short papillae in ventrolateral positions on segments 7 to 8, and in ventromedial position on segment 9. Cuticle sculptured with broad secondary fringes and scattered, leaf-like cuticular hairs.
TYPE SPECIES
Triodontoderes anulap sp. nov.
ETYMOLOGY
The prefix ‘Triodonto-’ is derived from ‘Triodus’ which is the Ancient Greek name for the trident of Poseidon. The name is inspired from the distally tripartite placids. The suffix ‘-deres’ (derived from the Ancient Greek δέρη = neck) refers to the placids' position in the neck region, and is furthermore commonly used in generic names of cyclorhagid kinorhynchs. Gender of genus name: masculine.

Fig. 2. Line art illustrations of Triodontoderes anulap gen. et sp. nov. (A) Female, dorsal view; (B) female, ventral view; (C) male, terminal segments and spines, dorsal view; and (D) male, terminal segments and spines, ventral view. Scales = 100 µm. go, gonopore; ldc, laterodorsal crenulated spine; lva, lateroventral acicular spine; lvc, lateroventral cuspidate spine; ltas, lateral terminal accessory spine; lts, lateral terminal spine; mda, middorsal acicular spine; mts, midterminal spine; pa, papilla; ss1/3, sensory spot type 1/3; tsj, tergosternal junction; vla, ventrolateral acicular spine.

Fig. 3. Diagram of mouth cone, introvert and placids in Triodontoderes anulap gen. et sp. nov. with indication of inner and outer oral style, scalid and placid distribution. Placids are symbolized by the bent bars around the introvert diagram.
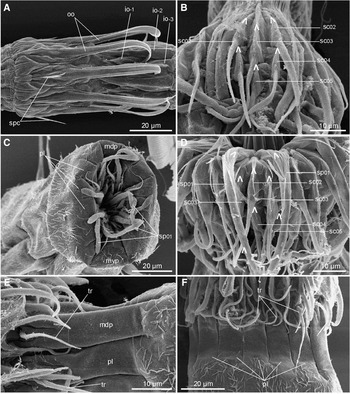
Fig. 4. Scanning electron micrographs showing mouth cone and introvert details in Triodontoderes anulap gen. et sp. nov. (A) Inner and outer oral styles; (B) introvert section 1 (midventral section); (C) head opening in specimen with retracted head; (D) introvert section 10; (E) close-up of middorsal (upper) and left paradorsal placid (lower). Note the distal tripartition; and (F) placids and neck region in lateral view. io, inner oral styles; mdp, middorsal placid; mvp, midventral placid; pl, placid; oo, outer oral styles; sc, scalid; sp, spinoscalid; spc, spicules at bases of outer oral styles; tr, trichoscalid. Digits after labels refer to mouth cone and introvert ring numbers. Lambda symbols Λ mark attachment point of scalids.

Fig. 5. Light micrographs of Triodontoderes anulap gen. et sp. nov. (A) Segments 2 to 3, ventral view; (B) outer oral styles, ventral view; (C) detail of segment 9 in female specimen, showing lateroventral cuspidate spine and ventromedial papilla; (D) segments 10 to 11 in female, dorsal view; (E) segments 10 to 11 in female, ventral view; (F) segments 10 to 11 in male, dorsal view; and (G) segments 10 to 11 in male, ventral view. go, gonopore; ldc, laterodorsal crenulated spine; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lva, lateroventral acicular spine; lvc, lateroventral cuspidate spine; mda, middorsal acicular spine; mdc, middorsal crenulated spine; msj, midsternal junction; mts, midterminal spine; oo, outer oral styles; pa, papilla; ss3, sensory spot type 3 (modified sensory spot); spc, spicules; tsj, tergosternal junction; vla, ventrolateral acicular spine; vlc, ventrolateral cuspidate spine.

Fig. 6. Scanning electron micrographs showing overview and details on anterior segments of Triodontoderes anulap gen. et sp. nov. (A) Ventrolateral overview of female specimen; (B) segment 1, ventrolateral view; (C) segment 2, ventrolateral view; (D) segments 1 to 2, lateral view; and (E) segment 1, dorsal view. mda, middorsal acicular spine; msj, midsternal junction; pl, placid; sp, spinoscalid; ss1, sensory spot type 1; tr, trichoscalid; tsj, tergosternal junction; vla, ventrolateral acicular spine; vlc, ventrolateral cuspidate spine.

Fig. 7. Scanning electron micrographs showing details on posterior segments of Triodontoderes anulap gen. et sp. nov. (A) segment 9, ventral view; (B) segment 8, left sternal plate in female; (C) terminal segment in female, lateral view; (D) detail from sternal plate of segment 7 in female showing the leaf-like cuticular hairs, a ventrolateral papilla and a ventromedial sensory spot; (E) terminal segment in male, dorsal view; and (F) terminal segment in female, ventral view. go, gonopores; laa, lateral accessory acicular spine; lac, lateral accessory cuspidate spine; ldc, laterodorsal crenulated spine; ltas, lateral terminal accessory spine; lts, lateral terminal spine; lva, lateroventral acicular spine; lvc, lateroventral cuspidate spine; mda, middorsal acicular spine; mdc, middorsal crenulated spine; mts, midterminal spine; mva, midventral articulation; pa, papilla; po, pores in sensory spot; s, segment (followed by segment number); ss1/3, sensory spot type 1/3.
Table 1. Measurements of adult Triodontoderes anulap gen. et sp. nov. from Chuuk Islands, Micronesia, including number of measured specimens (n) and standard deviation (SD). ac, acicular spine; cu, cuspidate spine; LA, lateral accessory; LD, laterodorsal spine (present in males only); LTAS, lateral terminal accessory spine; LTS, lateral terminal spine; LV, lateroventral (on S10 present in females only); MD, middorsal spine; MTS, midterminal spine; S, segment lengths; TL, trunk length; VL, ventrolateral spine.

Table 2. Summary of nature and location of sensory spots, spines and papillae arranged by series in Triodontoderes anulap gen. et sp. nov. from Chuuk Islands, Micronesia. LA, lateral accessory; LD, laterodorsal; LV, lateroventral; MD, middorsal; ML, midlateral; PD, paradorsal; SD, subdorsal; SL, sublateral; VL, ventrolateral; VM, ventromedial; ac, acicular spine; cu, cuspidate spine; f, female condition of sexually dimorphic character; ltas, lateral terminal accessory spine; lts, lateral terminal spine; m, male condition of sexually dimorphic character; mts, midterminal spine; pa, papilla; ss1/3, sensory spot type 1/3.

TYPE MATERIAL
Holotype: adult female (Sand Dune, Chuuk Islands, Micronesia (Figure 1); 24 March 2007: 7°27′13″N 151°56′05″E, 17 m depth), mounted in Fluoromount G®, deposited at the Zoological Museum, Natural History Museum of Denmark under accession number: ZMUC KIN-232.
Allotype: adult male (same locality as holotype), mounted in Fluoromount G®, deposited at the Zoological Museum, Natural History Museum of Denmark under accession number: ZMUC KIN-233.
Paratypes: two adult females (same locality as holotype), one adult male and two preadults, mounted in Fluoromount G®, deposited at the Zoological Museum, Natural History Museum of Denmark under accession numbers: ZMUC KIN-234 to KIN-238.
Additional material from Sand Dune, Benedict Point, Osakura Island and Shark Island (Figure 1) includes several specimens mounted for either LM or SEM. From the three latter localities, 2 females, 4 males and 5 preadults, mounted in Fluoromount G®, are deposited at the Zoological Museum, Natural History Museum of Denmark under accession numbers: ZMUC KIN-239 to KIN-249. Remaining specimens are stored in the authors' personal collections.
DIAGNOSIS
Same as genus diagnosis.
ETYMOLOGY
The species is named after Anulap; the local Chuuk Islands god of knowledge.
DESCRIPTION
Adult with head, neck and eleven trunk segments (Figure 2A, B). For measurements and dimensions see Table 1; for summary of spine, papilla and sensory spot locations see Table 2.
The head consists of a retractable mouth cone and an introvert (Figures 3, 4A–D & 5B). The inner armature of the oral cone consists of three rings of inner oral styles. The innermost rings, rings –03 and –02, each have five inner oral styles that could not be distinguished morphologically in any of the examined specimens. The inner oral styles of both rings each consists of a single unit with a stout base that tapers into a pointed tip (Figure 4A). Ring –01 has ten smaller inner oral styles consisting of a fringed base and a minute, pointed tip. Nine outer oral styles are located in ring 00 (Figures 3, 4A & 5B). The outer oral styles each consists of a single, flattened unit that distally bends inward and terminates into a pointed tip. Two anteriorly directed spicules are present at the base of each outer oral style (Figures 4A & 5B).
The introvert has five rings of scalids and one additional ring of trichoscalids that are associated with the placids (Figure 3). Ring 01 has ten spinoscalids consisting of a proximal sheath and an elongate end piece. The proximal sheath has a well-developed median, longitudinally fringed area that extends over the proximal part of the end piece. The end piece has scattered, minute hairs, and consists of an undifferentiated proximal part and a distal part with six to eight pseudosegments that are easily visualized with SEM (Figure 4C), though they appear less prominent in LM. Ring 02 possesses 15 scalids that occur as a pair in uneven numbered introvert sections and single in even numbered sections (Figures 3 & 4B, D). Each scalid consists of a proximal sheath and a distal end piece. The sheath has a basal group of long fringes followed by a median, longitudinal fringe of much shorter hairs, whereas the end piece is densely covered with small hairs. Ring 03 carries 15 scalids, that occur opposite to those in ring 02, hence a single scalid is located in uneven numbered sections, whereas a pair is present in even numbered ones (Figures 3 & 4B, D). The ring 03 scalids resemble those in the previous introvert ring. Ring 04 has 15 scalids that are distributed like those in ring 02 (Figures 3 & 4B, D). The scalids resemble those in the two previous rings, but are shorter and lack the long fringe basally on the sheath. Ring 05 has 15 scalids that are distributed like those in ring 03 and resemble those in the previous ring (Figures 3 & 4B, D).
Fourteen trichoscalids are present in the most posterior part of the introvert, and their occurrence is strictly correlated with placids of the neck region (Figure 3). The trichoscalids are rounded in cross-section, covered with minute hairs and taper gradually to a pointed tip (Figure 4E, F).
The location of scalids in rings 1 to 5 follows a strict pattern around the introvert, and each section carries 6 scalids. A quincunx arrangement pattern is found in all sections, thus even numbered sections display a quincunx posterior to a single scalid in ring 02, whereas odd numbered sections show a quincunx anterior to a single scalid in ring 05 (Figure 3).
The neck consists of fourteen placids (Figures 3 & 4C). The placids are long (around 30 µm) and relatively narrow (7–9 µm), except the broader midventral one (14 µm) (Figure 4C). A marked articulation with segment 1 does not exist hence the placids appear more like extensions of the first segment, rather than individual plates (Figure 4E, F). Anteriorly, each placid has two longitudinal clefts that make the placid distally tripartite (Figures 4E & 6E). A trichoscalid attaches on the median tip of each placid (Figure 4E, F). The neck cuticle in between the placids is densely plicated.
Segment 1 consists of one tergal and one sternal plate (Figures 2B & 6B). The tergosternal junctions are located in a somewhat ventrolateral position. Pachycycli are not developed, and tergosternal junctions on this and the following three segments are extremely indistinct in LM (Figure 5A), though they are easily observed with SEM (Figure 6A–D). This, and the following 10 segments are circular in cross-section. The posterior segment margin is almost smooth, and without fringes or serration. A short middorsal spine, flanked by two paradorsal type 1 sensory spots, is present (Figure 6E). On this and the following nine segments, the middorsal spine attaches in a small notch on the posterior margin of the segment. One additional pair of type 1 sensory spots is present in a dorsolateral position (Figure 2A). The sensory spots in both the paradorsal and dorsolateral positions are rounded, with numerous minute papillae and one or two pores. The surface of the integument is covered with scattered cuticular hairs without any distinct distributional pattern (Figure 6B, D, E). The hairs emerge through rounded perforation sites. They appear soft and are pointed, with a median, longitudinal cleft. Outlets from glandular cells were not found on this or any of the preceding segments.
Segment 2 consists of one tergal and two sternal plates (Figures 2B, 5A & 6C, D). On the anteriormost part of the segment, the tergosternal junctions are located in a ventrolateral position, and extend lateral and slightly caudal until they reach a more lateroventral position. From this point, they extend caudal along the main axis of the animal, until the posterior margin of the segment. The midsternal junction is conspicuous in LM, whereas the tergosternal junctions are much more indistinct (Figure 5A). Both midsternal and tergosternal junctions are easily observed with SEM (Figure 6C, D). The posterior margin of the segment is formed by thin cuticular extensions of the tegumental plates. Fringes or serrations are not present on the margin. A middorsal spine is present in a notch on the posterior margin of the segment. Ventrolaterally, in the same position as the tergosternal junction, a pair of well-developed cuspidate spines and a pair of much smaller, flexible, acicular spines are present (Figures 5A & 6C). The minute acicular spines are located closest to the midsternal junction. A pair of small, rounded paradorsal sensory spots is located close to the base of the middorsal spine. A pair of much larger, oval sensory spots is located in a midlateral position (Figure 6D). The midlateral sensory spots consist of numerous short papillae and have two pores in the anterior end. The anterior third of the segment is covered with curved secondary fringes, composed of tiny scales mixed with slightly longer aciculae. The fringes are in certain positions interrupted by areas with completely smooth cuticle. The posterior two-thirds of the segment are covered with scattered cuticular hairs. The hairs are leaf-like with median longitudinal clefts and truncate tips (Figure 6C, D; for close-up of hair type see also Figure 7D). Only the areas between the ventromedial positions and the midsternal junctions are completely devoid of hairs, whereas the areas in the midlateral position have minute, scale-like hairs only.
Segment 3 consists of one tergal and two sternal plates (Figures 2B & 6D). Tergosternal junctions are located between the lateroventral and ventrolateral positions. Acicular spines are present in the middorsal and lateroventral positions (Figure 2A, B). The lateroventral spines are minute and flexible. Sensory spots are only present in the paradorsal position, close to the middorsal spine. Posterior segment margin, secondary fringes and cuticular hairs appear as on the previous segment. From this segment, and on the following seven, all fixed specimens tend to have a deep, midlateral fold. This is most certainly a fixation artefact and not an indication of additional tegumental plate junctions. However, it is noteworthy that the folds always are formed in this position.
Segment 4 consists of one tergal and two sternal plates (Figure 2B). Tergosternal junctions are located in same position as on the previous segment. Acicular spines are present in the middorsal and lateroventral positions (Figure 2A, B). The lateroventral spines are longer and more rigid than those on the previous segment. Paired sensory spots are present in the paradorsal, laterodorsal and ventromedial positions. The ventromedial sensory spots are large and resemble those in the midlateral position on segment 2. The laterodorsal ones look similar but are smaller. Posterior segment margin, secondary fringes and cuticular hairs appear as on the previous segment, with the addition that two paired groups of longer cuticular hairs are located in the ventromedial positions near the posterior segment margin. The hairs form two combs that extend over the posterior segment margin.
Segment 5 and the remaining six segments consist of one tergal plate with a midventral articulation (Figure 2B). Acicular spines are present in the middorsal and lateral accessory positions; cuspidate spines are present in the lateroventral positions (Figure 2A, B). Paired sensory spots are present in the paradorsal and laterodorsal positions. All sensory spots resemble those in the corresponding positions on the previous segment. Posterior segment margin, secondary fringes and cuticular hairs appear as on the previous segment, except that the area between the ventromedial positions and the midventral articulation on this and the following segments are covered with minute, scale-like hairs.
Segment 6 has acicular spines in the middorsal and lateroventral positions; cuspidate spines are present in the lateral accessory positions (Figure 2A, B). Paired sensory spots are present in the paradorsal and ventromedial positions. Sensory spots resemble those in the corresponding positions on segment 4. Posterior segment margin, secondary fringes and cuticular hairs appear as on the previous segment.
Segment 7 has acicular spines in the middorsal and lateroventral positions (Figure 2A, B). Cuspidate spines are not present. Paired sensory spots are present in the paradorsal, sublateral and ventromedial positions. The paradorsal and ventromedial sensory spots resemble those in the corresponding positions on the previous segment. The sublateral sensory spots are oval and resemble those in the ventromedial positions, though they cover a slightly smaller area. In female specimens only, a pair of minute tubular papillae with a basal collar of short, flexible hairs is present in the ventrolateral positions, about medially on the segment (Figure 7D). The posterior segment margin tends to become slightly serrated on this segment. The secondary fringes and cuticular hairs appear as on the previous segment, however, the ventromedial rows of more elongate hairs near the posterior segment margin are broader and cover the area from the lateroventral to the ventromedial positions.
Segment 8 has acicular spines in the middorsal and lateroventral positions; cuspidate spines are present in the lateral accessory positions (Figures 2A, B & 7A). Paired sensory spots are present in the paradorsal and ventromedial positions, and resemble those in the corresponding positions on the previous segment. In female specimens only, a pair of tubular papillae, similar to those on the previous segment, is present in the ventrolateral position (Figure 7B). The serration of the posterior segment margin is stronger than on the previous segment and shows more resemblance with an actual pectinate fringe (Figure 7B). The secondary fringes and cuticular hairs appear as on the previous segment.
Segment 9 has acicular spines in the middorsal and lateral accessory positions; cuspidate spines are present in the lateroventral positions (Figures 2A, B, 5C & 7A). Paired sensory spots are present in the paradorsal and sublateral (Figure 7A) positions, and resemble those in the corresponding positions on the segment 7. In female specimens only, a pair of tubular papillae, similar to those on the previous segment, is present in the ventromedial position (Figure 5C). Protonephridial outlets in form of sieve plates are not present. Posterior segment margin, secondary fringes and cuticular hairs appear as on the previous segment. Two of the examined specimens deviated from all others, in having a cuspidate spine in the left side only, and by having a large ventromedial sensory spot on the right sternal plate.
Segment 10 has paired sensory spots in the paradorsal, subdorsal, sublateral and ventromedial positions. The paradorsal sensory spots resemble those in the corresponding positions on all previous segments, whereas the subdorsal and ventromedial ones are larger and oval, resembling those in the ventromedial positions on some of the previous segments. The sublateral sensory spots are smaller and resemble those in the corresponding position on the previous segment. Tubular papillae are not present. The position and appearance of spines display sexual dimorphism on this segment. In female specimens, regular acicular spines are present in the middorsal and lateroventral positions (Figures 2A, B, 5D, E & 7C). In males, crenulated spines are present in the middorsal and laterodorsal positions, whereas lateroventral spines are lacking (Figures 2C, D, 5F, G & 7E). Posterior segment margin, secondary fringes and cuticular hairs appear as on the previous segment.
Segment 11 has a very long middorsal spine, lateral terminal accessory spines, lateral terminal spines and a midterminal spine (Figure 2A, B). The latter almost reaches same length as the trunk of the animal. Two pairs of modified sensory spots (type 3 sensory spots) are present in the subdorsal positions (Figures 5D, F & 7C, E), whereas one pair is located in the lateroventral positions, close to the bases of the lateral terminal spines (Figures 5E, G & 7F). The anteriormost pair of subdorsal modified sensory spots is located close to the base of the middorsal spine, and emerges through a transverse slit in the cuticle (Figure 7E). The most posterior pair is located on the tips of a pair of elongate, cone-like extensions of the dorsal part of the segment (Figures 5D, F & 7C, E). One additional pair of type 1 sensory spots is located in a ventromedial position, but in female specimens only (Figure 7F). A pair of large and very conspicuous gonopores is furthermore present in a ventrolateral position on the anterior part of the segment in female specimens (Figures 5E & 7F). The dorsal part of the segment has in both sexes a single secondary fringe only, whereas two secondary fringes are present on the ventral side. Cuticular hairs are small and scale-like on all parts of the segment. A well-developed pectinate fringe on the posterior segment margin is present in the laterodorsal positions and along the whole ventral margin.
DISCUSSION
Notes on diagnostic features
The composition of the segments, namely the combination of segment 1 consisting of one tergal and one sternal plate, segments 2 to 4 consisting of one tergal and two sternal plates, and the remaining segments 5 to 11 consisting of one tergal plate with midventral articulation (Figure 2B), is not found in any other known kinorhynch species. This rather fundamental unique trait justifies the erection of a new genus, Triodontoderes gen. nov., to accommodate the species Triodontoderes anulap gen. et sp. nov.
The new genus and species can most easily be confused with species of Cateria or Zelinkaderes, due to its overall appearance of the trunk. In all three genera, the trunk is characteristic in being almost perfectly circular in cross-section, and being quite long relative to its width (Figure 6A) (see also Higgins, Reference Higgins1968, Reference Higgins1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1995; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007; Sørensen & Pardos, Reference Sørensen and Pardos2008). However, besides the abovementioned characters unique to Triodontoderes gen. nov., species of the new genus can be discriminated from both genera by the presence of middorsal spines on all segments (Figure 2A). In the known species of Zelinkaderes, middorsal spines are present on segments 4, 6 and 8 to 11 only (see Higgins, Reference Higgins1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1995; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007), whereas species of Cateria possess middorsal spines on segments 2 to 4, 6, and 8 to 10 only (see Gerlach, Reference Gerlach1956; Higgins, Reference Higgins1968). Cuspidate spines are furthermore lacking in species of Cateria, and spines in the lateral series are restricted to segments 5 to 11. Besides the traits mentioned above, Triodontoderes anulap gen. et sp. nov. differ from species of Zelinkaderes by the deviating trunk sculpturing. In species of Zelinkaderes, the flattened leaf-like cuticular hairs are always arranged in distinct longitudinal rows, whereas the cuticular hairs in T. anulap gen. et sp. nov. are more randomly scattered within well-delimited patches.
Notes on other morphological features
Triodontoderes anulap gen. et sp. nov. show several traits that can be found among species of other genera. The arrangement of scalids on the introvert shows much resemblance with the patterns found in species of Zelinkaderes. Among all other species for which the detailed introvert morphology is known, the introvert armature in adult specimens consists of one ring with spinoscalids followed by five rings with regular scalids (see e.g. Brown, Reference Brown1989; Nebelsick, Reference Nebelsick1993; Neuhaus, Reference Neuhaus1995; Bauer-Nebelsick, Reference Bauer-Nebelsick1996; Sørensen, Reference Sørensen2007, Reference Sørensen2008; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007, Reference Sørensen, Heiner and Hansen2009). In T. anulap gen. et sp. nov., however, only four rings with regular scalids are present (Figure 3), whereas this number is reduced further to only three rings in species of Zelinkaderes (Higgins, Reference Higgins1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1995; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007). This could imply that the scalid pattern in T. anulap gen. et sp. nov. represent an intermediate stage between the condition found in species of Zelinkaderes and in all other kinorhynch species. In Zelinkaderes klepali and Z. brightae the scalids in the odd numbered sections attach in a diamond pattern, whereas scalids in even numbered sections form a quincunx. If the scalids of ring 02 in T. anulap gen. et sp. nov. were reduced, the remaining scalid distribution pattern would be identical with the one among these species of Zelinkaderes. Also the flattened outer oral styles appear to be characteristic for species of Zelinkaderes and Triodontoderes gen. nov (Figure 4A). Distal pseudosegmentation of the spinoscalids is present in species of both genera as well, but this trait is also found among species of other genera, such as Antygomonas incomitata (see Sørensen et al., Reference Sørensen, Heiner and Hansen2009); Paracentrophyes praedictus (see Neuhaus, Reference Neuhaus1995); Semnoderes armiger (see Sørensen et al., Reference Sørensen, Heiner and Hansen2009) and Tubulideres seminoli (see Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007).
Placids appearing as extensions from segment 1, rather than isolated articulating plates have been reported from species of Zelinkaderes and Cateria also (Higgins, Reference Higgins1968, Reference Higgins1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1995; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007). The unusual distal tripartition of the placids in T. anulap gen. et sp. nov. (Figures 4E, F & 6E) has not previously been reported from any other species, and was initially considered being a unique trait for the new species. However, reexaminations of Z. brightae mounted for SEM revealed that a similar tripartition of the placids is present in this species. Although it is not mentioned in the description, this particular trait is furthermore documented in the description of Z. klepali (see figure 34 in Bauer-Nebelsick, Reference Bauer-Nebelsick1995), and indications, more indistinct however, are shown for Z. floridensis (see figure 19 in Higgins, Reference Higgins1990).
As stressed above, the overall segmental composition is not present in any other known kinorhynch species. Compared segment by segment only, some similarities should be noted, however. The composition of segment 1 (one tergal and one sternal plate) is present in species of Cateria (Gerlach, Reference Gerlach1956; Higgins, Reference Higgins1968) and in species of the two homalorhagid genera Paracentrophyes (Higgins, Reference Higgins1983) and Neocentrophyes (Higgins, Reference Higgins1969). However, the condition in T. anulap gen. et sp. nov. show most resemblance with segment 1 in species of Cateria, as the tergosternal junctions here are located in a ventrolateral position, opposed to a much more lateral position as found in the two homalorhagid genera. Segments 2 to 4 being composed of one tergal and two sternal plates are found among species of several groups, including Antygomonas, Tubulideres, Centroderidae, Dracoderidae, Semnoderidae, the echinoderid genera Fissuroderes and Polacanthoderes and all Homalorhagida. In other words, only species of Zelinkaderes, Cateria, Echinoderes and Cephalorhyncha deviate from this pattern and the two latter only deviate regarding segments 2 and 3. Segments 5 to 10 consisting of one tergal plate with a midsternal articulation is present in T. anulap gen. et sp. nov., and species of Zelinkaderes only. Species of Cateria have this segment composition also, but only from segment 8. Regarding the terminal segment, T. anulap gen. et sp. nov. differ from species of Zelinkaderes, as the segment consists of a tergal plate only, opposed to one tergal and two sternal plate in Zelinkaderes.
Leaf-like cuticular hairs, viz. non-acicular hairs with a median, longitudinal cleft, have previously been reported from species of Antygomonas and Zelinkaderes (Higgins, Reference Higgins1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1995; Sørensen, Reference Sørensen2007; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007, Reference Sørensen, Heiner and Hansen2009). In species of Zelinkaderes the hairs are miniaturized and arranged in conspicuous longitudinal rows, whereas they are larger and more randomly scattered over the tergal plate in species of Antygomonas. The appearance (Figure 7D) and distribution of hairs on the dorsal and lateral sides (Figures 2A, B, 6 & 7) in T. anulap gen. et sp. nov. show clear resemblance with the condition in species of Antygomonas. Well-developed, scattered leaf-like cuticular hairs on the sternal plates, or eventually, ventral parts of the sternal plates, are present in only T. anulap gen. et sp. nov.
The sensory spots on segments 2 to 10 are basically similar to the regular type 1 sensory spots (sensu Nebelsick, Reference Nebelsick1992) that are found in most kinorhynch species. However, the sensory spots in the lateral and ventral series of T. anulap gen. et sp. nov. are characteristic in their distinct oval shape and their remarkable size (Figure 7A, B). Very similar large and oval sensory spots are present in species of Zelinkaderes (Higgins, Reference Higgins1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1995; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007).
The presence of cuspidate spines in the lateral series on some segments is shared with species of Condyloderes (Adrianov et al., Reference Adrianov, Murakami and Shirayama2002), Antygomonas (Nebelsick, Reference Nebelsick1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1996; Sørensen, Reference Sørensen2007), Semnoderes (Sørensen et al., Reference Sørensen, Heiner and Hansen2009), and Zelinkaderes (Higgins, Reference Higgins1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1995; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007). The presence of a cuspidate spine located very close to a short, flexible acicular spine on segment 2 draws special attention as this combination is present in the three latter genera only as well as in T. anulap gen. et sp. nov (Figures 2B, 5A & 6C).
Also the minute papillae located on segments 7 to 9 in females of T. anulap gen. et sp. nov. are noteworthy (Figures 5C & 7B, D). Similar looking structures were recently reported from females of Semnoderes armiger in the same positions on segments 8 and 9 (Sørensen et al., Reference Sørensen, Heiner and Hansen2009), and they have furthermore been observed on segments 5 to 7 in females of Campyloderes cf. macquariae (Sørensen, personal observation). The function of these papillae remains uncertain, but their presence is apparently tied to the female sex.
Another sexually dimorphic character is displayed in the spines of segment 10. Besides the papillae and the conspicuous gonopores, females of T. anulap gen. et sp. nov. can be distinguished by their acicular lateroventral spines on segment 10. Contrarily, males have laterodorsal crenulated spines on this segment (Figure 2). Dorsolateral crenulated spines as a sexually dimorphic character on segment 10 are also known from species of Centroderes (Sørensen & Pardos, Reference Sørensen and Pardos2008), Tubulideres (Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007) and Zelinkaderes (Higgins, Reference Higgins1990; Bauer-Nebelsick, Reference Bauer-Nebelsick1995; Sørensen et al., Reference Sørensen, Heiner, Ziemer and Neuhaus2007).
Taxonomic and phylogenetic considerations
As outlined in the previous sections, T. anulap gen. et sp. nov. share several features with species from various other kinorhynch genera. Since our knowledge of the pathways of kinorhynch character evolution is still scarce, it is impossible to determine which similarities represent synapomorphies, symplesiomorphies or, eventually, convergences. However, we find it noteworthy that T. anulap gen. et sp. nov., and species of Zelinkaderes share a considerable amount of rather specialized features, in particular concerning introvert morphology, the distally tripartite placids and the general circular appearance of the trunk. Hence, based on these potential synapomorphies we preliminarily assign Triodontoderes gen. nov. to Zelinkaderidae, which makes it a putative sister group to Zelinkaderes. This proposed relationship will in the near future be tested in a formal cladistic analysis based on combined morphological and molecular data. The general appearance of the trunk and the composition of segments 8 to 11 make it tempting to propose species of Cateria as closely related with Triodontoderes gen. nov. and Zelinkaderes as well, but we do not wish to address this question before more detailed data, including information revealed through SEM examinations, are present from at least one species of Cateria.
The assignment of Triodontoderes gen. nov. to Zelinkaderidae changes the family's status as monotypic, and has prompted the following emending of the family diagnosis.
Diagnosis for Zelinkaderidae (emended from Higgins, Reference Higgins1990)
Introvert with one ring of spinoscalids followed by three or four scalid rings. Fourteen or sixteen distally tripartite placids, without or with very indistinct articulation with segment 1. Trunk conspicuously circular in cross-section. Segment composition of segments 1 to 4 and 11 varying, segments 5 to 10 composed of single tergal plate with midventral articulation. Acicular spines present in dorsal and lateral positions on all or some segments; cuspidate spines present on some segments; terminal segment with lateral terminal, lateral terminal accessory, and midterminal spines. At least some sensory spots large and oval with two pores in the anterior part. Cuticular hairs leaf-like, present on dorsal, lateral and ventral side of segments. Males with crenulated spines on segment 10; females with regular acicular spines on segment 10.
ACKNOWLEDGEMENTS
We wish to thank Stine Elle for providing the line art illustrations. This work has been conducted with support through the research programme of KORDI with contract No. PE972001 to H.S.R. The work has been conducted with support through the Danish Natural Research Council grant No. 272-08-0576 to M.V.S., and the research programme of KORDI.











