INTRODUCTION
In marine invertebrates with complex life cycles, such as those composed of a benthic juvenile-adult phase and a prolonged pelagic embryonic/ larval phase (Pechenik, Reference Pechenik1999; McEdward, Reference McEdward2000), the environmental conditions experienced by adult individuals can influence reproduction and subsequently the survival and successful development of offspring (Calado & Leal, Reference Calado and Leal2015). For example, in Pleocyemata decapod crustaceans, embryos carried beneath the female's pleon are brooded and cared for maternally until larval hatching occurs (Meusy & Payen, Reference Meusy and Payen1988; Fernandez et al., Reference Fernandez, Calderon, Cifuentes and Pappalardo2006; Hartnoll, Reference Hartnoll2006). Thus, embryos experience similar environmental conditions to their mothers.
In temperate coastal environments, temporal variation in environmental conditions (e.g. productivity and/or food availability, temperature, etc.) is common (Medellin-Mora et al., Reference Medellin-Mora, Escribano and Schneider2016). Because of this, decapod crustaceans in temperate regions must adopt strategies that allow them to invest energy in reproduction to ensure successful survival of their broods (Urzúa et al., Reference Urzúa, Paschke, Gebauer and Anger2012; Guzmán et al., Reference Guzmán, Olavarría and Urzúa2016). Consequently, decapod crustacean mothers provide recently laid embryos with all of the energy reserves necessary for successful development (Wenner & Kuris, Reference Wenner and Kuris1990). Additionally, the subsequent use of these energy reserves during the incubation period can be influenced by environmental factors (e.g. temperature: Fischer et al., Reference Fischer, Thatje, Graeve, Paschke and Kattner2009; García-Guerrero, Reference García-Guerrero2010; oxygen: Taylor & Leelapiyanart, Reference Taylor and Leelapiyanart2001; Alter et al., Reference Alter, Paschke, Gebauer, Cumillaf and Pörtner2015; salinity: Giménez & Anger, Reference Giménez and Anger2001; Taylor & Seneviratna, Reference Taylor and Seneviratna2005), and this, in turn, can affect larval biomass at the time of hatching (Andrés et al., Reference Andrés, Estévez, Simeó and Rotllant2010; Rotllant et al., Reference Rotllant, Simeó, Guerao, Sastre, Cleary, Calado and Estévez2014).
In decapod crustaceans, lipids are known to be the main source of energy reserves used during starvation (Sánchez-Paz et al., Reference Sánchez-Paz, García-Carreño, Muhlia-Almazán, Peregrino-Uriarte, Hernández-López and Yepiz-Plascencia2006). Fatty acids, which are one component of lipids, play a fundamental role in the reproductive cycle of decapods (Glencross, Reference Glencross2009). Fatty acids are important in many life phases, from reproductive maturation to vitellogenesis, both requiring large amounts of polyunsaturated fatty acids (Middleditch et al., Reference Middleditch, Missler, Hines, McVey, Brown and Lawrence1980). Additionally, fatty acids are components of tissues and structures (e.g. cellular membranes, nervous system) of vital importance during ontogeny (Kayama et al., Reference Kayama, Hirata, Kazanawa, Tokiwa and Saito1980; Bell & Dick, Reference Bell and Dick1990; Beltz et al., Reference Beltz, Tlusty, Benton and Sandeman2007). Also, during the initial stages of the life cycle, individuals are often exposed to periods of famine and/or planktonic food shortages, and lipid reserves accumulated internally are crucial for survival and successful growth (Kattner et al., Reference Kattner, Wehrtmann and Merck1994, Reference Kattner, Graeve, Calcagno, Lovrich, Thatje and Anger2003; Rosa et al., Reference Rosa, Calado, Narciso and Nunes2007; Urzúa & Anger, Reference Urzúa and Anger2013).
In crustaceans, the hepatopancreas is the main site of lipid storage and processing (Chang & O'Connor, Reference Chang and O'Connor1983). This organ is responsible for synthesizing enzymes for food digestion, and also hepatopancreas absorbs and stores large quantities of energy, especially fats (Vogt, Reference Vogt1994). During reproduction and growth, this energy is then transferred to gonads and muscles, respectively (Yamaguchi, Reference Yamaguchi2004; Ying et al., Reference Ying, Yang and Zhang2006; Fátima et al., Reference Fátima, Ayub, Ali and Siddiqui2013). The hepatopancreas is also involved in important physiological processes associated with reproduction, for example vitellogenesis, and the synthesis of vitellogenin and sex hormones (Li et al., Reference Li, Chen, Zhou, Li, Zhao and Guo2006).
The model study organism, red squat lobster Pleuroncodes monodon from the South-eastern Pacific, reproduces up to four times per year and has an extended reproductive cycle (from winter, throughout spring and until summer) (Thiel et al., Reference Thiel, Espinoza-Fuenzalida, Acuña and Rivadeneira2012; Guzmán et al., Reference Guzmán, Olavarría and Urzúa2016). This reproductive cycle is characterized by production of multiple broods or clutches (i.e. eggs laid): 3–4 different broods during an annual cycle with ‘larger but few winter eggs’ vs ‘smaller and numerous summer eggs’ (Guzmán et al., Reference Guzmán, Olavarría and Urzúa2016). Consistently, compared with larvae originating from summer eggs, those hatching from winter eggs show reduced nutritional vulnerability and contain high reserves of energy to face planktonic food limitation (Espinoza et al., Reference Espinoza, Guzmán, Bascur and Urzúa2016). Also, a recent study (Bascur et al., Reference Bascur, Guzmán, Mora and Urzúa2017) indicated that seasonal variation in the biochemical composition of the females reflects seasonal differences in reproductive output/investment of this species.
The red squat lobster Pleuroncodes monodon, which in the Humboldt Current Large Marine Ecosystem (HCLME) is distributed from Isla Lobos de Afuera in Peru to Ancud in Chile, is a key species and important fishery resource (Yannicelli et al., Reference Yannicelli, Castro, Parada, Schneider, Colas and Donoso2012; Kiko et al., Reference Kiko, Hauss, Dengler, Sommer and Melzner2015). The HCLME is characterized by spatial-temporal variation in oceanographic phenomena (i.e. upwelling) that is highly seasonal (Thiel et al., Reference Thiel, Macaya, Acuña, Arntz, Bastías, Brokordt, Camus, Castilla, Castro, Cortés, Dumont, Escribano, Fernández, Gajardo, Gaymer, Gomez, González, González, Haye, Illanes, Iriarte, Lancellotti, Luna-Jorquera, Luxoro, Manriquez, Marín, Muñoz, Navarrete, Pérez, Poulin, Sellanes, Sepúlveda, Stotz, Tala, Thomas, Vargas, Vásquez and Vega2007; Escribano & Morales, Reference Escribano and Morales2012). Consequently, there is temporal variation in temperature and quantity/quality of planktonic food (i.e. phyto- and zooplankton); higher temperatures and greater availability of planktonic food have been registered in spring-summer compared with winter (Daneri et al., Reference Daneri, Dellarossa, Quiñones, Jacob, Montero and Ulloa2000; Escribano & Schneider, Reference Escribano and Schneider2007; Escribano et al., Reference Escribano, Hidalgo, Fuentes and Donoso2012). Of course, this, in turn, directly affects quantity and quality of food consumed by the red squat lobster, as well as the quantity of energy reserves (i.e. fats) that can be stored. In this way, environment has a direct impact on the reproduction and growth of this species. Thus, the aim of this study was to determine the extent of seasonal variation in the primary energy reserves (i.e. lipids and fatty acids) of P. monodon females and their embryos. Additionally, the potential implications of female and embryo survival on the HCLME ecosystem are discussed.
MATERIALS AND METHODS
Sampling of squat lobster females from the field
Pleuroncodes monodon females were captured in summer (January–March 2015) and winter (July–September 2015) near Concepción, Chile (35°34′S 72°52′W). The Altaír vessel of Camanchaca Pesca Sur S.A. was used for sampling lobsters from a depth of ~100 m. In both summer and winter, a total of N = 60 ovigerous females per season were selected, measured with a vernier calliper, and transported live to the Hydrobiological Resources laboratory of the Universidad Católica de la Santísima Concepción. Upon arrival, lobsters were immediately frozen at −80 °C until later analyses were performed. Additionally, periodical data of environmental parameters (temperature, chlorophyll and day length) from sites (i.e. fishing grounds) near Concepción, Chile were obtained from the long-term data series of National Marine Fisheries Services of NOAA (www.st.nmfs.noaa.gov).
Collection and preparation of samples for analyses
Embryo masses on the pleopods of adominal area of the ovigerous females were observed using a stereo-microscope (Motic-102 M). Masses were separated according to their initial development characterized by bright orange colouration, using descriptions of Palma & Arana (Reference Palma and Arana1997). Then, embryo masses were washed with distilled water for 5 s, dried with filter paper, and transferred to 1.5 ml Eppendorf tubes. Subsequently, hepatopancreases (the main energy storage organ used in reproduction; Nagaraju, Reference Nagaraju2011) of the same ovigerous females were extracted via dissection using a scalpel; hepatopancreases were stored in 1.5 ml Eppendorf tubes. Embryo and hepatopancreas samples were dried at −80 °C for 48 h in a lyophilizer (Operon, FDU-7012). Then, dry weight was measured using a precision balance (Precisa, model 120A) to the nearest 0.01 mg; the standard method of Anger & Harms (Reference Anger and Harms1990) was followed.
Analyses were performed in triplicate (i.e. analytical replicates): three 20 mg of dry weight (DW) hepatopancreas sub-samples were taken from each of total female hepatopancreas (total N = 120). These three sub-samples were used for measurements of total lipid and fatty acid. Similarly, three 25 mg (DW) embryo samples (summer 25 mg of embryos was equivalent to ~1666 embryos; winter 25 mg of embryos was equivalent to ~1315 embryos) were separated from total embryo masses of each female (total N = 120). These three sub-samples were used for lipid and fatty acid analyses. For statistical analyses the mean composition per female of the lipid content and fatty acid profile of hepatopancreas and embryo clutch were considered.
Lipid and fatty acid analyses
To obtain the best possible results, 5 ml of a solution of dichloromethane: methanol (2:1) was added to the preweighed embryo and hepatopancreas samples. Samples were incubated in this solution in a 3 l ultrasonic bath (MRC, AC-120H) for 10 min at 6 °C.
Total lipid content of the hepatopancreases and embryos was quantified gravimetrically after dichloromethane/methanol (2:1) extraction and solvent evaporation using a digital rotary evaporator (DragonLab, model RE100-Pro) at 80 rpm and 45 °C (Folch et al., Reference Folch, Lees and Stanley1957; Cequier-Sánchez et al., Reference Cequier-Sánchez, Rodríguez, Ravelo and Zárate2008; Urzúa & Anger, Reference Urzúa and Anger2013). The lipid extract was determined to the nearest 0.01 mg, using a balance (Precisa model 120A) and stored at −20 °C in dichloromethane/methanol (2:1) containing 0.01% butylated hydroxytoluene for further fatty acid composition analyses.
The composition of fatty acids was determined using methods presented by Urzúa & Anger (Reference Urzúa and Anger2011). In short, fatty acid methyl esters (FAMEs) were measured after preparation using the total lipid extracts (total lipids). Total lipid extracts were esterified using methanolic sulphuric acid incubations at 70 °C for 1 h in a Thermo-Shaker (MRC model DBS-001). Then, fatty acids were rinsed using 6 ml of n-hexane. Finally, fatty acids were concentrated using a rotary evaporator (DragonLab model RE100-Pro). The measurement of FAMEs was performed using a gas chromatograph (Agilent, model 7890A) at set temperature equipped with a DB-225 column (J&W Scientific, 30 m in length, 0.25 internal diameter, and 0.25 mm film). Using chromatograph software (Agilent ChemStation, USA), individual FAMEs were identified by comparison to known standard fatty acids of marine origin (certificate material, Supelco 37 FAME mix 47885-U; Malzahn et al., Reference Malzahn, Aberle, Clemmesen and Boersma2007; Urzúa & Anger, Reference Urzúa and Anger2013) and quantified by means of the response factor to internal standard (23:0 FA added prior to transmethylation; Malzahn et al., Reference Malzahn, Aberle, Clemmesen and Boersma2007; Urzúa & Anger, Reference Urzúa and Anger2011).
Statistical analysis
The statistical analyses were conducted using the software STATISTICA 8 (StatSoft), PRIMER 6 (Plymouth Routines In Multivariate Ecological Research) and FactoMineR program (R package dedicated to multivariate Exploratory Data Analysis) employing standard methods (Sokal & Rohlf, Reference Sokal and Rohlf1995; Zuur et al., Reference Zuur, Ieno and Graham2007; Le et al., Reference Le, Josse and Husson2008) with 95% confidence levels (P < 0.05). Additionally, graphics were made using the program SigmaPlot 12 (Systat Software Inc., Chicago, USA). As a first step to compare female size (carapace length, CL) among different seasons, a Student's t-test was calculated. Given that no significant differences were found between size of females between summer and winter, comparisons of the energy reserves (lipids and fatty acids) of each season were made. In turn, seasonal variation in environmental parameter were evaluated with a one-way ANOVA. The seasonal variation in the fatty acids composition of female hepatopancreas and embryo were evaluated with a Student's t-test. Normality of the data was evaluated using a Kolmogorov–Smirnov test, and homogeneity of the variance was evaluated using Levene's test. Multivariate analyses (i.e. Principal Component Analysis, PCA; Analysis of Similarity, ANOSIM; Similarity Percentage, SIMPER) were performed to compare fatty acids profile of hepatopancreas and embryos between seasons.
RESULTS
Environmental parameters
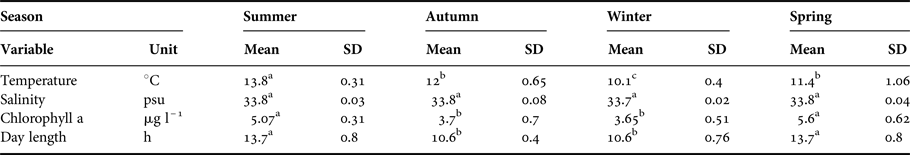
Environmental parameters displayed seasonal variation. Higher temperatures were observed in summer (13.8 ± 0.2 °C; months 1–3 of the year, January–March) than winter (10.1 ± 0.2 °C; months 7–9 of the year, July–September) (ANOVA, F 11,132 = 1209.3; P < 0.001). Meanwhile, chlorophyll also presented a higher average in summer (5.07 ± 0.2 µg l–1) than winter (3.65 ± 0.3 µg l–1) (ANOVA, F 11,132 = 681.7; P < 0.001) (Figure 1B). Finally, day length was significantly longer (ANOVA, F 11,132 = 1044.8; P < 0.001) in summer (13.7 ± 0.8 h) than winter (10.6 ± 0.8 h) (Table 1).
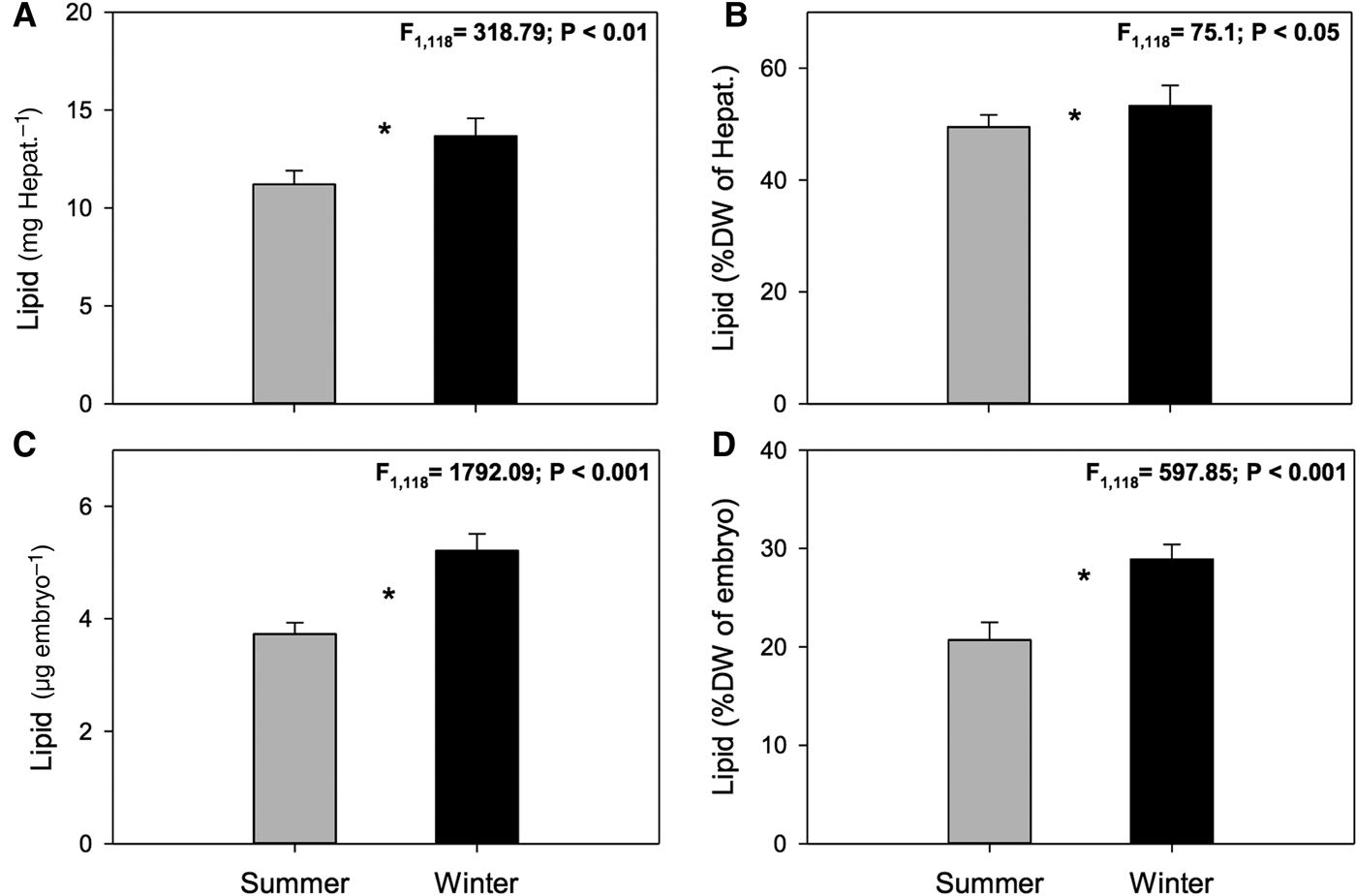
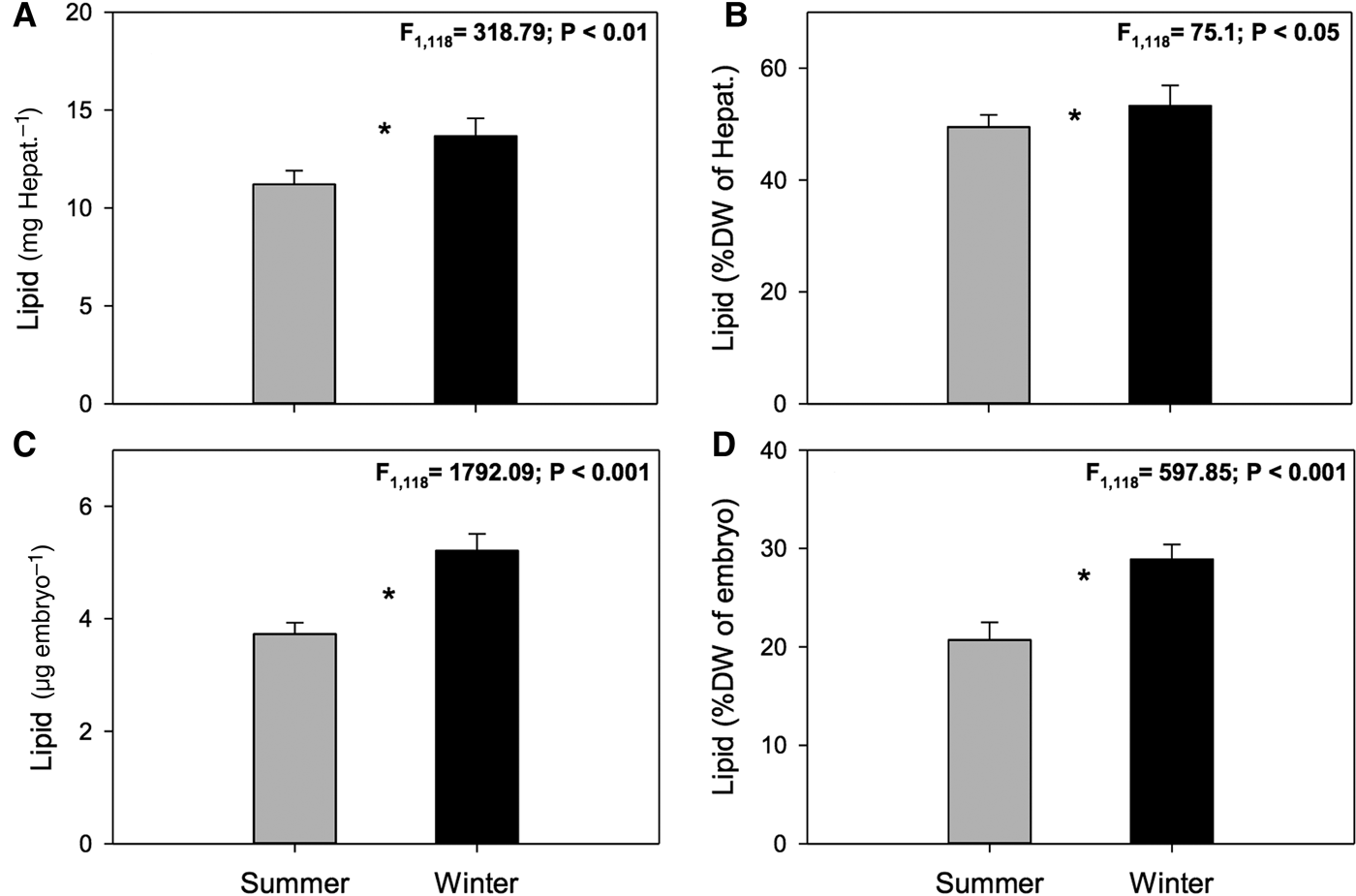
Fig. 1. Pleuroncodes monodon. Seasonal variation in: (A) hepatopancreases total lipids (TL) (mg Hepat.–1), (B) hepatopancreases total lipids (TL) (%DW), (C) total lipids of embryos (μg embryo–1), (D) total lipids of embryos (%DW); mean values ± S.D. Asterisks indicate significant differences between seasons. In all cases N = 120.
Table 1. Environmental variables of capture site near Concepción, Chile during 2015.
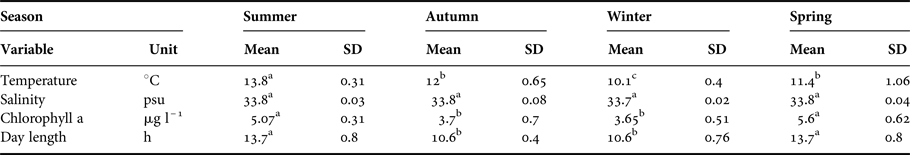
Temperature (°C), salinity (psu), chlorophyll a (μg l–l) and day length (h) (mean values ± SD) from capture site. Summer = January–March; Autumn = April–June; Winter = July–September; Spring = October–December. In all cases N = 144.
Different lower case letters in a row: significant differences between seasons (ANOVA, P<0.05).
Size of ovigerous females
No significant seasonal differences were found in female carapace length (CL) (summer: 43.38 ± 0.21 mm vs winter: 43.84 ± 0.57 mm; (t 1,118 = 0.81; P = 0.42).
Seasonal variation in the amount of lipids in hepatopancreases and embryos
Significant seasonal differences were observed in total lipid content (absolute values, mg Hepat.–1) of ovigerous female hepatopancreas (ANOVA, F 1,118 = 318.79, P < 0.01). Hepatopancreas of females sampled in summer had a lower amount of lipids than hepatopancreas of females sampled in winter (11.21 ± 0.05 mg vs 13.68 ± 0.07 mg; summer and winter, respectively) (Figure 1A). Significant differences were also observed in the percentage of lipids (%DW) in the hepatopancreas (summer: 49.47 ± 2.19% vs winter: 53.27 ± 3.64%; ANOVA, F 1,118 = 75.1; P < 0.05) (Figure 1B).
In turn, highly significant seasonal differences were found in the amount of lipids (absolute values, μg embryo–1) in embryos (ANOVA, F 1,118 = 1792.09, P < 0.001). Embryos laid in summer had fewer lipids than embryos laid in winter (3.73 ± 0.2 µg vs 5.21 ± 0.3 µg; summer and winter, respectively) (Figure 1C). Similarly, significant seasonal differences were also seen in the percentage of lipids as a function of dry embryo weight (%DW) (ANOVA, F 1,118 = 597.85, P < 0.001); embryos laid in summer had a lower percentage of lipids than winter embryos (20.7 ± 1.8% vs 28.9 ± 1.5%; summer and winter, respectively) (Figure 1D).
Multivariate analysis of FAs profiles of hepatopancreas and embryos
HEPATOPANCREAS FAS DATA TO COMPARE DIFFERENCES BETWEEN WINTER AND SUMMER
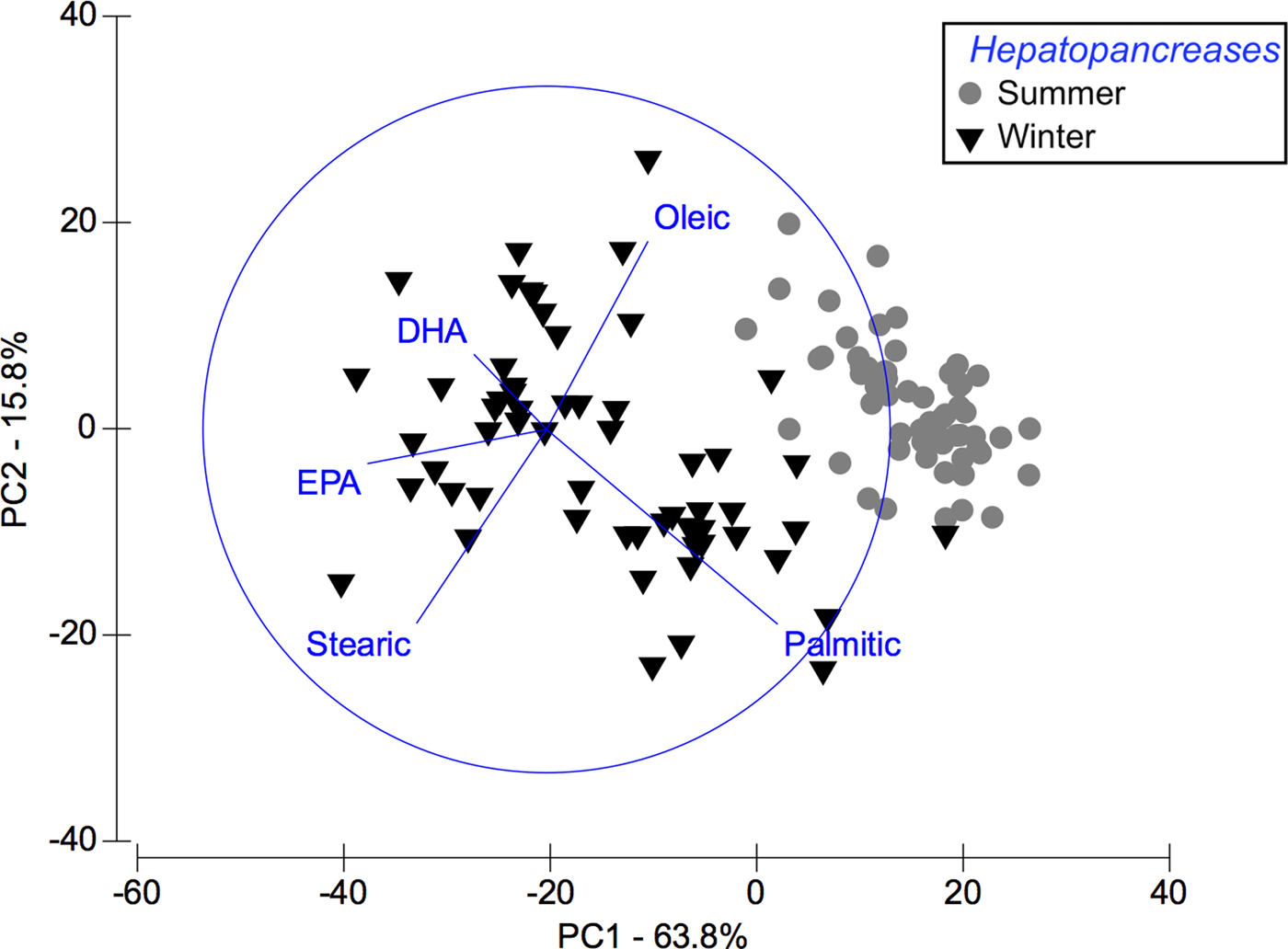
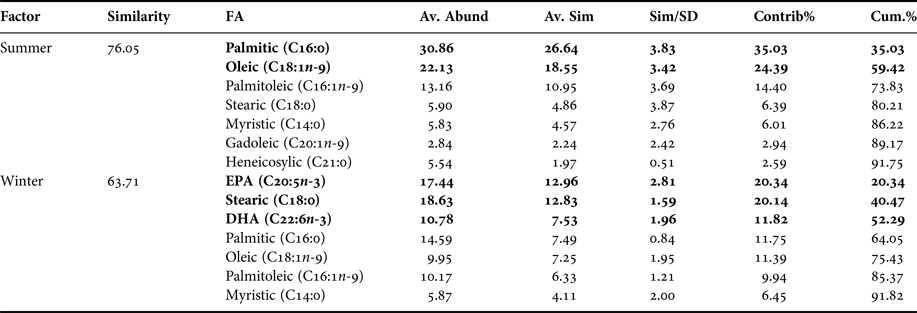
Based on multivariate analysis of PCA, PC1 axis explains 63.8% of seasonal variability and PC2 axis 15.8% (Figure 2). These seasonal tendencies were significant, for example in summer palmitic FA (C16:0) was predominant while in winter stearic FA was (C18:0) (ANOSIM, R ANOSIM = 0.81; P = 0.001; 999 R permutations). According to SIMPER test, in the summer group the FAs palmitic (C16:0), oleic (C18:1n-9) and palmitoleic (C16:1n-7) contributed together with 73.83% of variability. In turn, in the winter group the FAs EPA (20:5n-3) and stearic (C18:0) contributed with 40.47% of variability (Table 2).
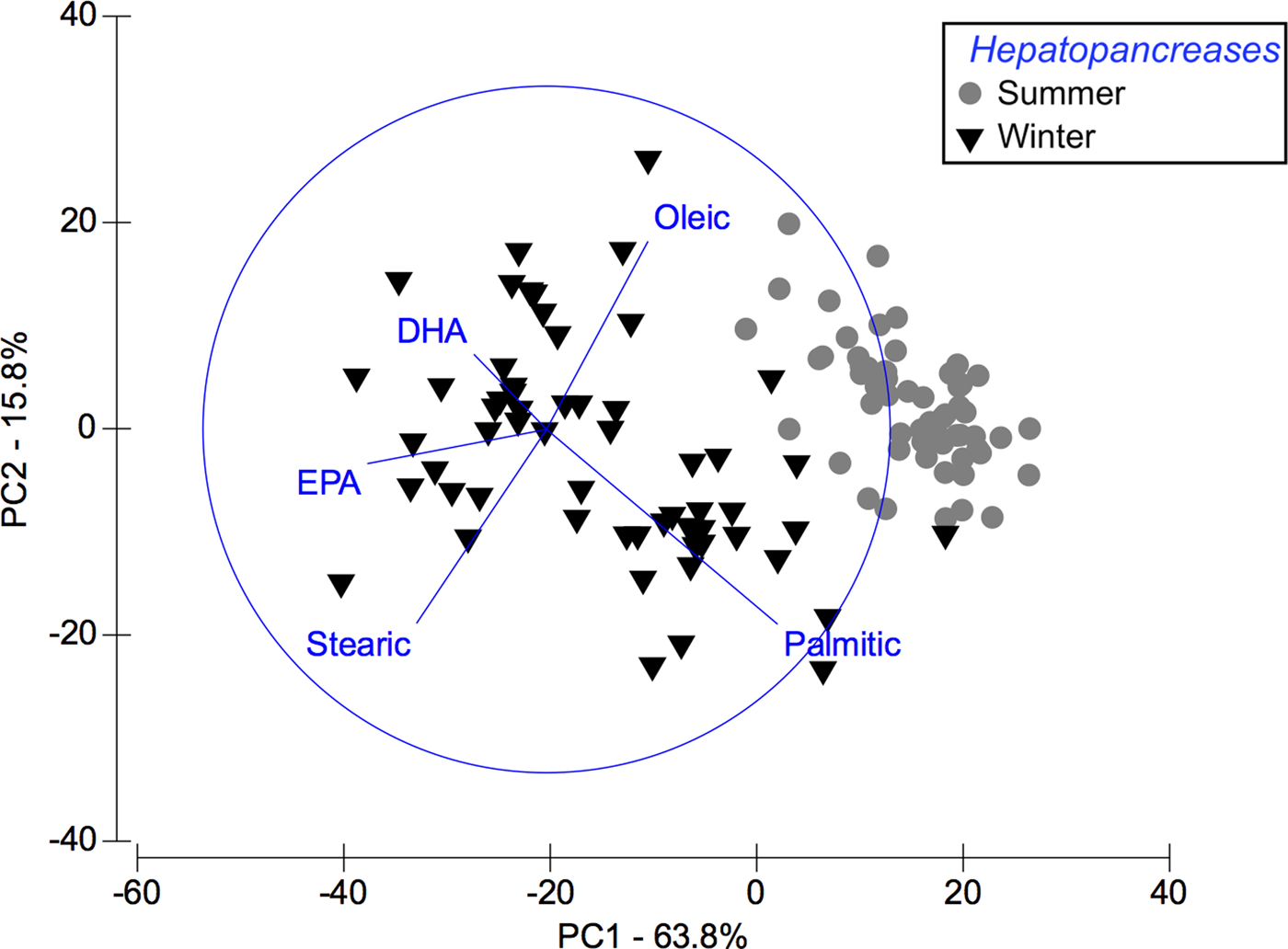
Fig. 2. Pleuroncodes monodon. Principal component analysis (PCA) plot of hepatopancreas fatty acids data to compare differences between winter and summer. N = 120.
Table 2. Pleuroncodes monodon. Similarity percentage (SIMPER) analysis used to assess the contribution of each fatty acid (FA) recorded in hepatopancreas of both seasons.
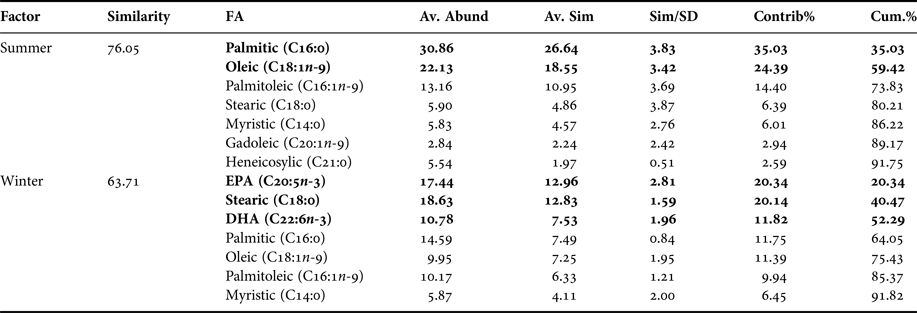
Av. Abund, average abundance of each FA present in each season; Av. Sim, the average similarity contributed by the fatty acid; Sim/SD, the ratio of similarity to standard deviation; Contrib%, the contribution to the fatty acid to the overall similarity; Cum.%, additive overall similarity. The fatty acids with the highest contribution in the Principal Component Analysis (PCA) in Figure 2 are marked in bold.
EMBRYOS FAS DATA TO COMPARE DIFFERENCES BETWEEN SUMMER AND WINTER
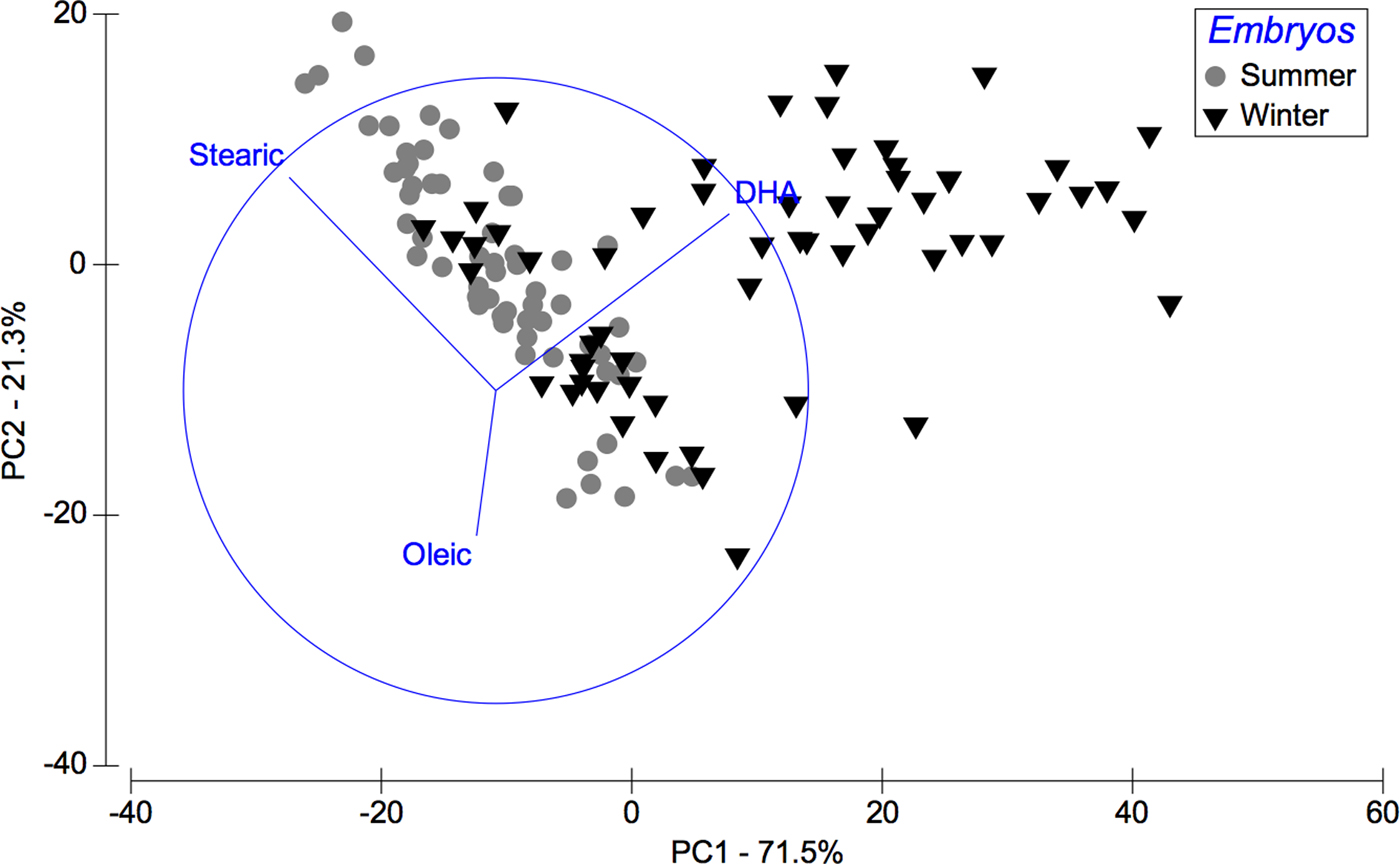
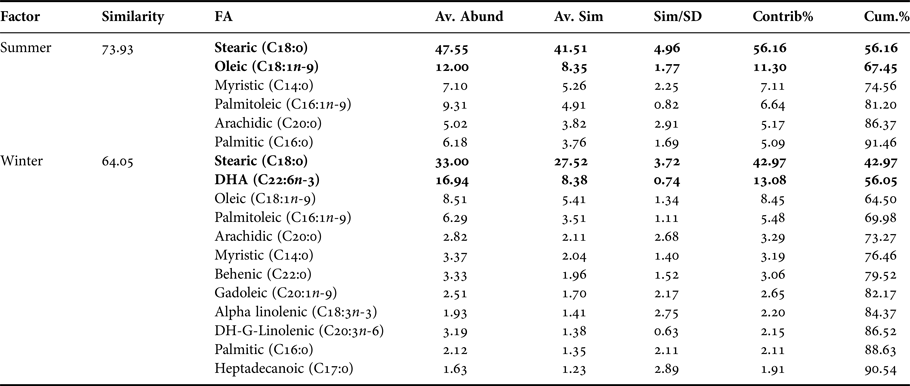
Considering the data set of embryos FAs, in summer stearic FA (C18:0) was predominant whereas in winter DHA FA was (C22:6n-3). 71.5% of this seasonal variability is explained by PC1 axis and 21.3% of the variability by PC2 axis (Figure 3), and according to ANOSIM analysis these trends were significant (R ANOSIM = 0.56; P = 0.001; 999 R permutations). In turn, results of the SIMPER test indicated that for the summer group the FAs stearic (C18:0) and oleic (C18:1n-9) contributed together with 67.45% of cumulative variability, whereas in the winter group the FAs stearic (C18:0) and DHA (C22:6n-3) contributed with 56.05% of variability (Table 3).
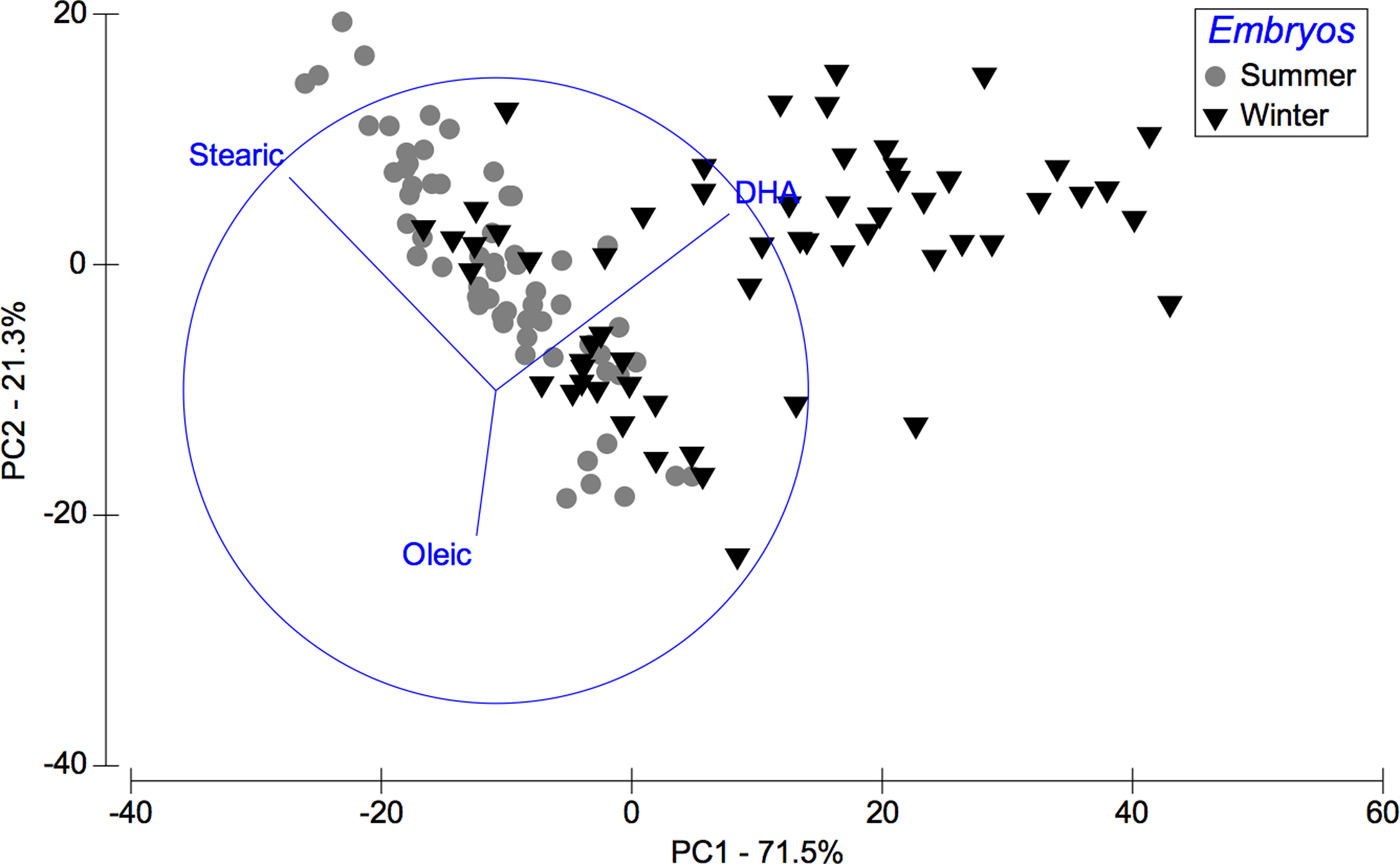
Fig. 3. Pleuroncodes monodon. Principal component analysis (PCA) plot of embryos fatty acids data to compare differences between winter and summer. N = 120.
Table 3. Pleuroncodes monodon. Similarity percentage (SIMPER) analysis used to assess the contribution of each fatty acid (FA) recorded in embryos of both seasons.
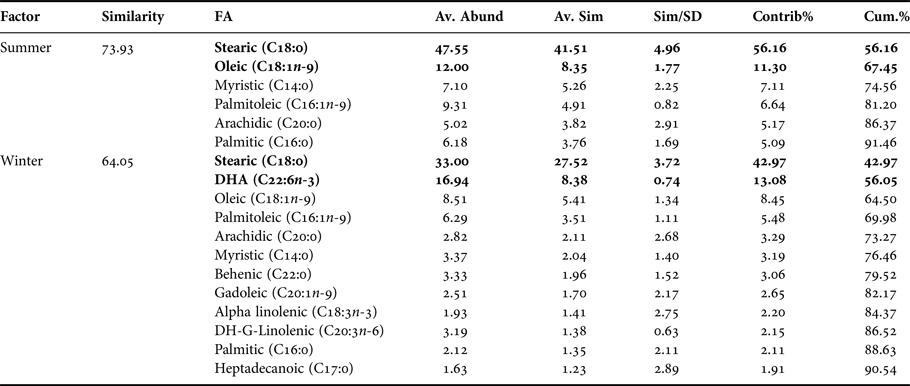
Av. Abund, average abundance of each FA present in each season; Av. Sim, the average similarity contributed by the fatty acid; Sim/SD, the ratio of similarity to standard deviation; Contrib%, the contribution to the fatty acid to the overall similarity; Cum.%, additive overall similarity. The fatty acids with the highest contribution in the Principal Component Analysis (PCA) in Figure 3 are marked in bold.
FAS DATA OF HEPATOPANCREAS AND EMBRYOS ONLY FROM SUMMER
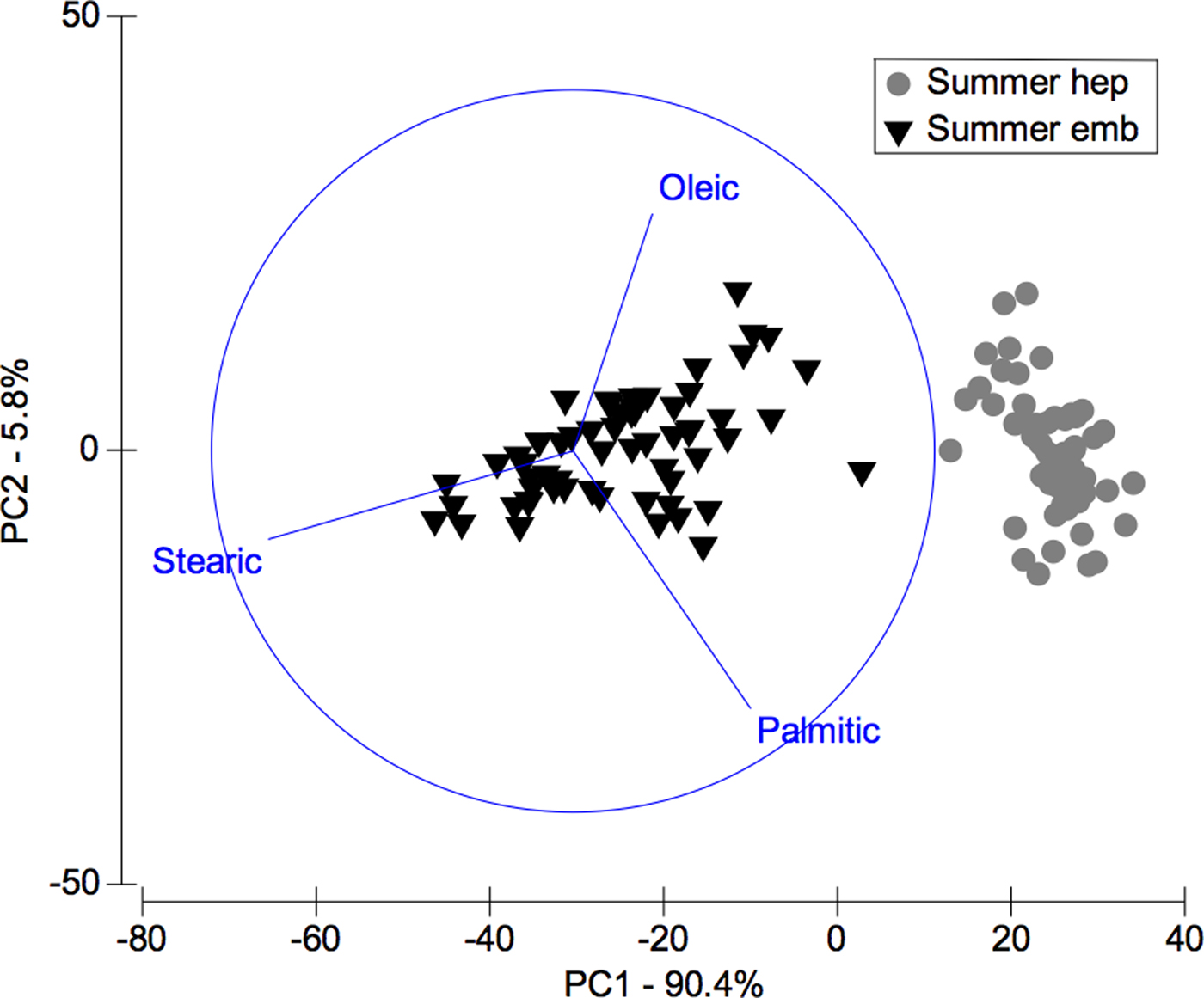
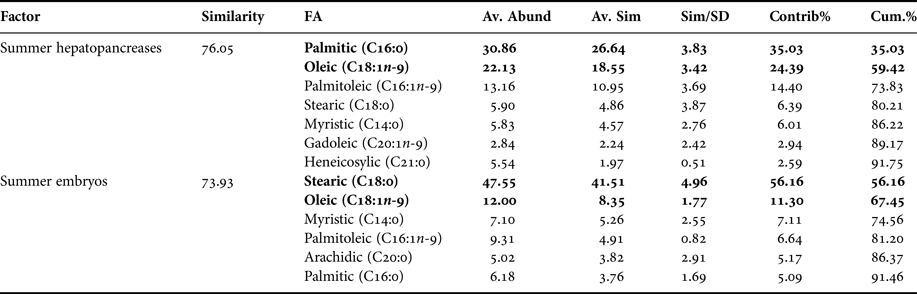
In the comparison between fatty acids of hepatopancreas and embryos only from summer, the PCA plot shows two completely separated groups, with conspicuous variations in fatty acids profile recorded in hepatopancreas and embryos (Figure 4). While PC1 axis explains 90.4% of fatty acid profile variations between ‘structures or organs’ (i.e. summer hepatopancreas vs summer embryos), PC2 axis explains only 5.8% of this variability (Figure 4). Following the ANOSIM test, this indicated significant differences (R ANOSIM = 0.99; P = 0.001; 999 R permutations). In turn, SIMPER test showed that in the hepatopancreas group, oleic (C18:1n-9) and palmitic (C16:0) fatty acids contributed with 59.42% of this cumulative variability (Table 4), whereas for the embryos group the stearic (C18:0) and oleic (C18:1n-9) fatty acids contributed together with 67.45% of this variability (Table 4).
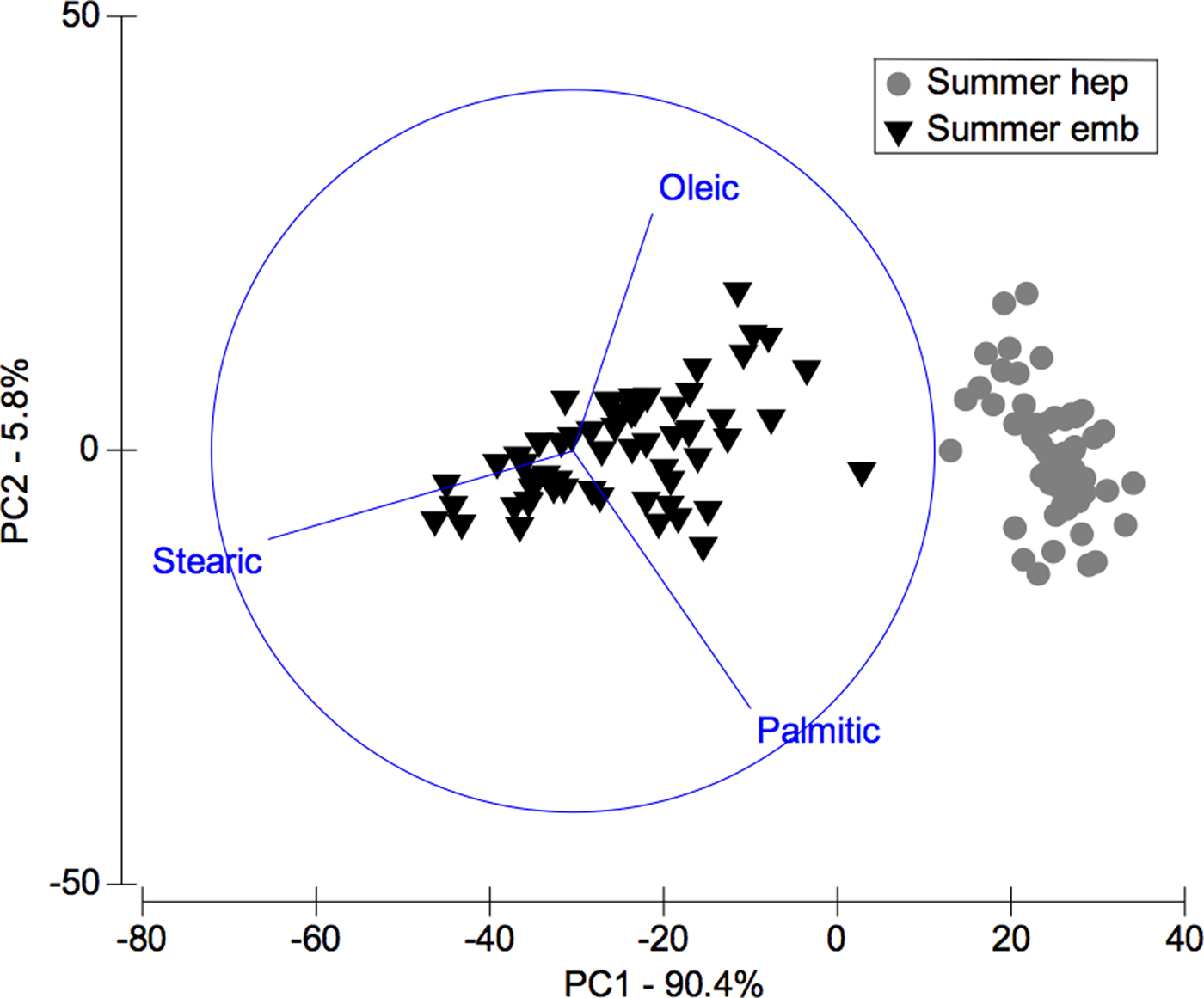
Fig. 4. Pleuroncodes monodon. Principal component analysis (PCA) plot of fatty acids data of hepatopancreas (hep) and embryos (emb) only from summer. N = 120.
Table 4. Pleuroncodes monodon. Similarity percentage (SIMPER) analysis used to assess the contribution of each fatty acid (FA) recorded in hepatopancreases and embryos of summer.
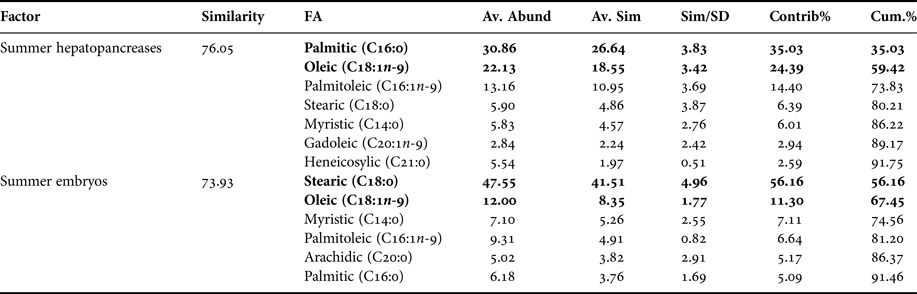
Av. Abund, average abundance of each FA present in each season; Av. Sim, the average similarity contributed by the fatty acid; Sim/SD, the ratio of similarity to standard deviation; Contrib%, the contribution to the fatty acid to the overall similarity; Cum.%, additive overall similarity. The fatty acids with the highest contribution in the Principal Component Analysis (PCA) in Figure 4 are marked in bold.
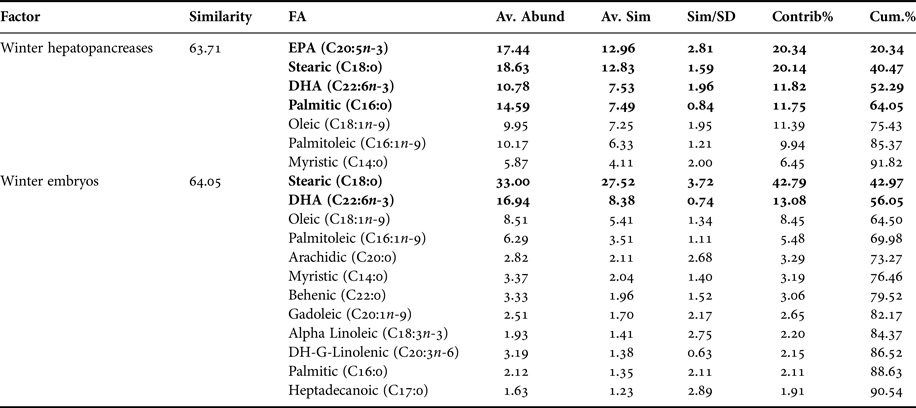
FAS DATA OF HEPATOPANCREAS AND EMBRYOS ONLY FROM WINTER
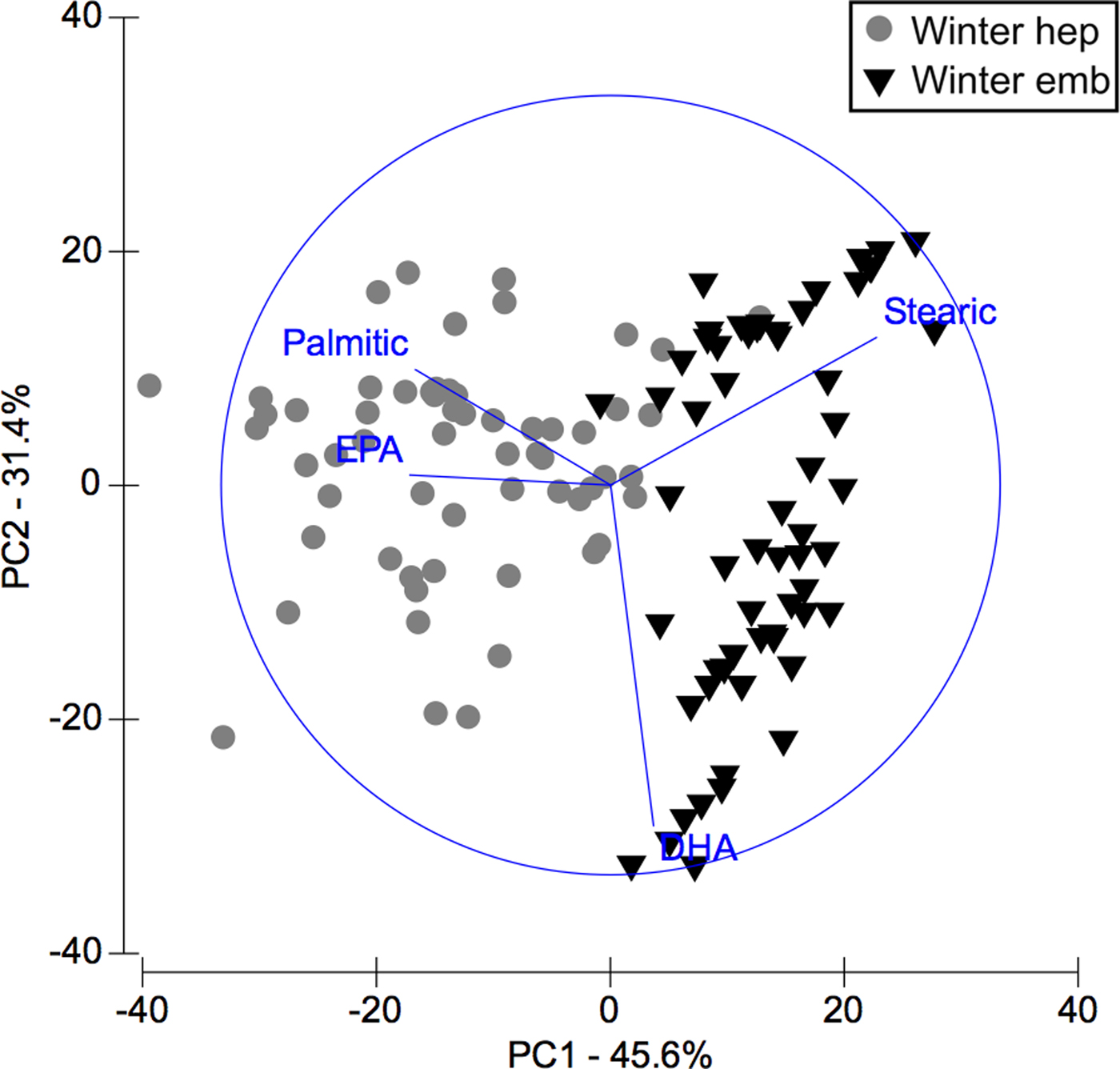
In PCA analysis of fatty acids of hepatopancreas and embryos only from winter, PC1 axis explained 45.6% of variability between them (i.e. winter hepatopancreas vs winter embryos), while PC2 explains 31.4% of fatty acid variability between ‘structures or organs’ (Figure 5). According to the analysis of ANOSIM, this showed significant variations in fatty acid profile ‘structures or organs’ in winter (R ANOSIM = 0.73; P = 0.001; 999 R permutations). In turn, SIMPER test indicated that for hepatopancreas group, the fatty acids EPA (20:5n-3), stearic (C18:0), oleic (C18:1n-9), DHA (C22:6n-3) and palmitic (C16:0) contributed together with 75.43% of variability (Table 5). While for embryo group, stearic acid (C18:0) and DHA (C22:6n-3) contributed 56.05% of cumulative variability (Table 5).
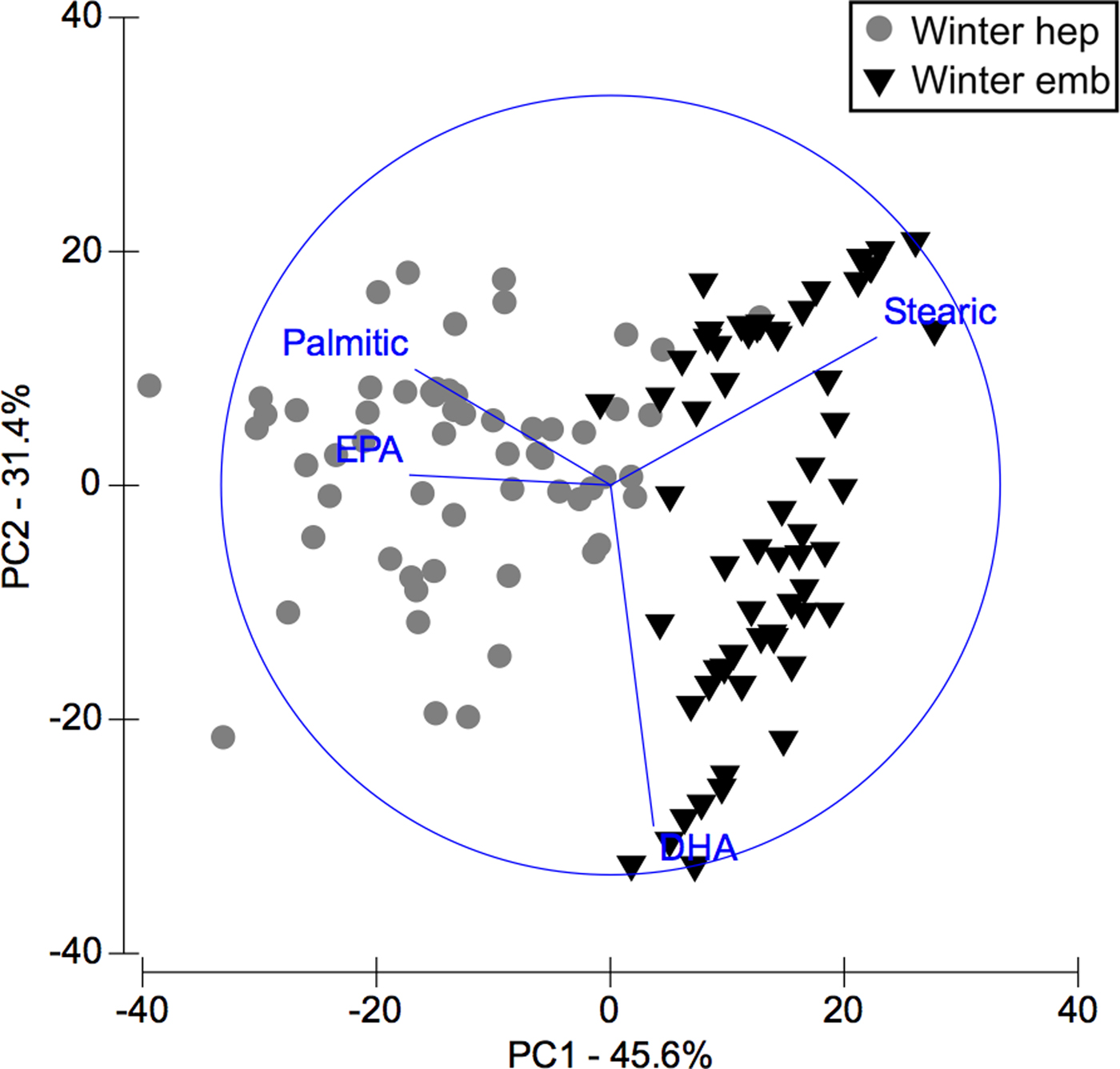
Fig. 5. Pleuroncodes monodon. Principal component analysis (PCA) plot of fatty acids data of hepatopancreas (hep) and embryos (emb) only from winter. N = 120.
Table 5. Pleuroncodes monodon. Similarity percentage (SIMPER) analysis used to assess the contribution of each fatty acid (FA) recorded in hepatopancreases and embryos of winter.
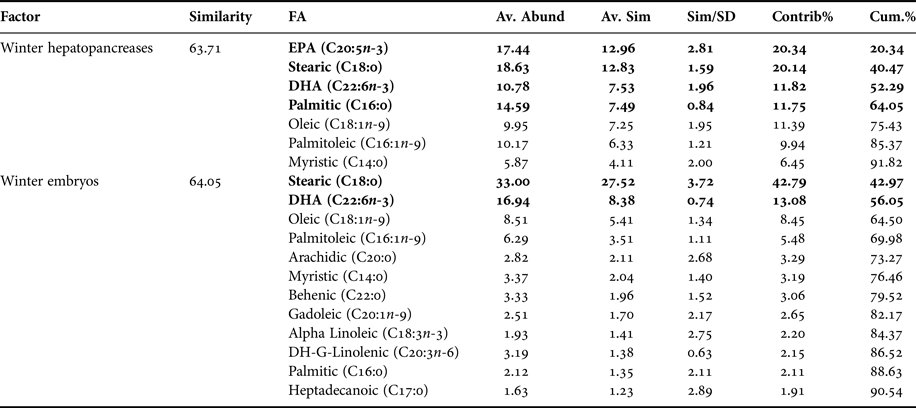
Av. Abund, average abundance of each FA present in each season; Av. Sim, the average similarity contributed by the fatty acid; Sim/SD, the ratio of similarity to standard deviation; Contrib%, the contribution to the fatty acid to the overall similarity; Cum.%, additive overall similarity. The fatty acids with the highest contribution in the Principal Component Analysis (PCA) in Figure 5 are marked in bold.
Seasonal variation in the composition of hepatopancreas fatty acids
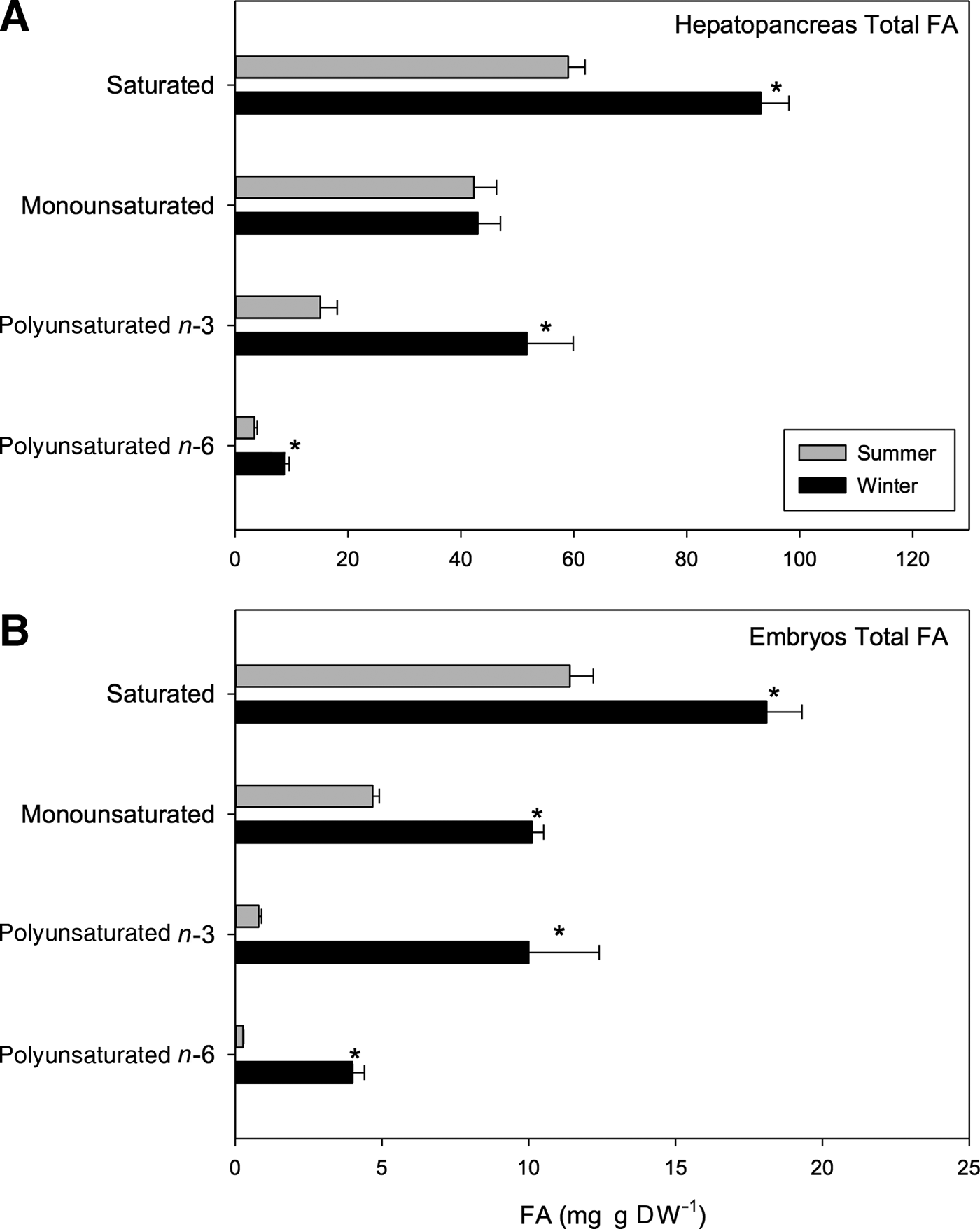
Significant seasonal differences were found in the composition and content of hepatopancreas fatty acids (FA) (t 1,118 = 12.33, P < 0.001; Table 6). For example, a greater amount of saturated (SFA) and polyunsaturated fatty acids (PUFAs) were found in samples from winter than those from summer; the SFA value for summer samples was 57.69 mg g DW−1 while 92.11 mg g DW−1 was recorded for winter samples. Correspondingly, the PUFA value for summer samples was 18.51 mg g DW−1 while 60.4 mg g DW−1 was found for winter samples. Despite this, no significant differences in monounsaturated fatty acids (MUFAs) were found between seasons; values close to 44.6 mg g DW−1 were found for summer samples, and values near 44 mg g DW−1 were found for winter (Figure 6A).
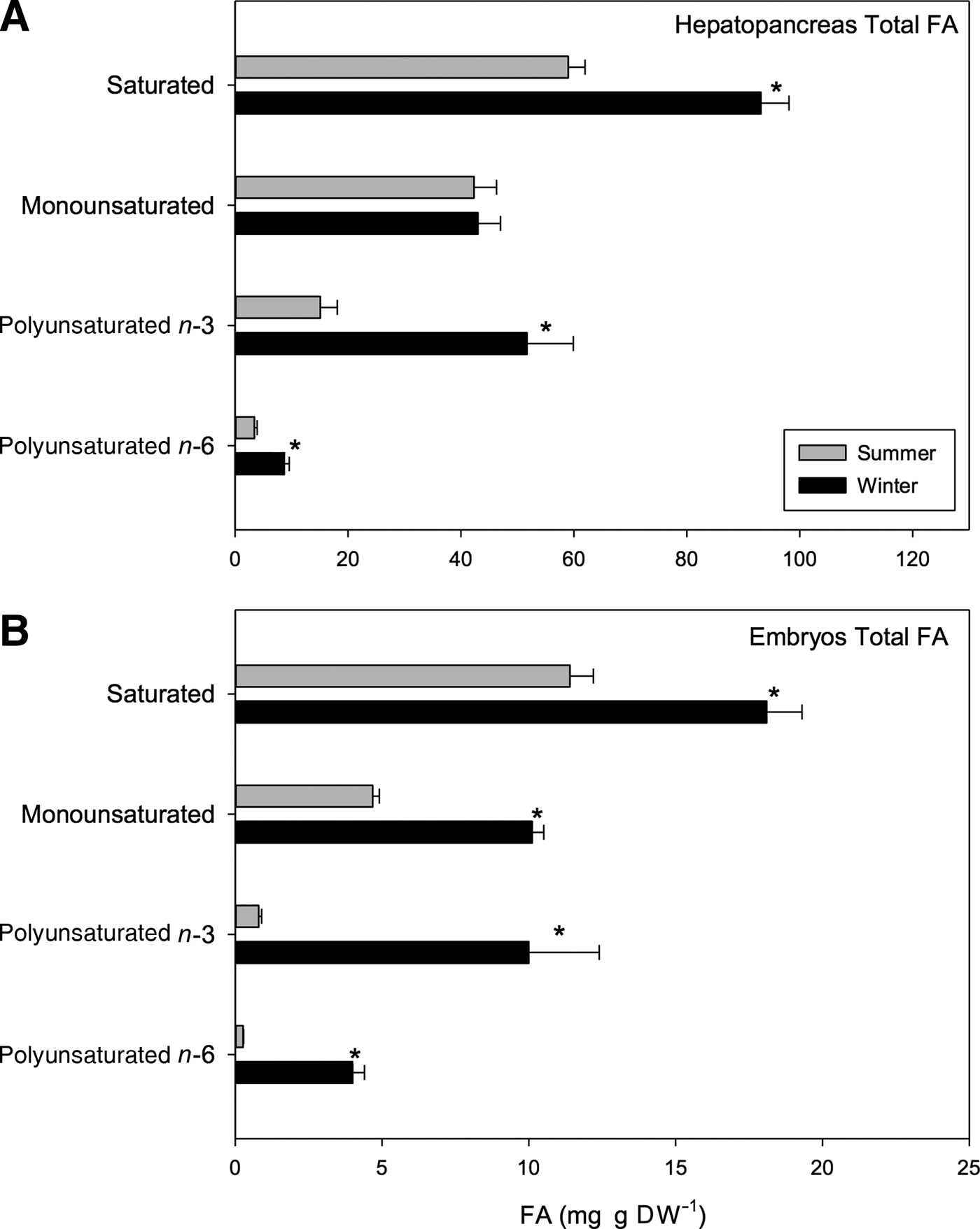
Fig. 6. Pleuroncodes monodon. Seasonal variation in: (A) hepatopancreases total fatty acids (mg g DW–1), (B) total fatty acids of embryos (mg g DW–1); mean values ± S.D. Asterisks indicate significant differences between seasons. In both N = 120.
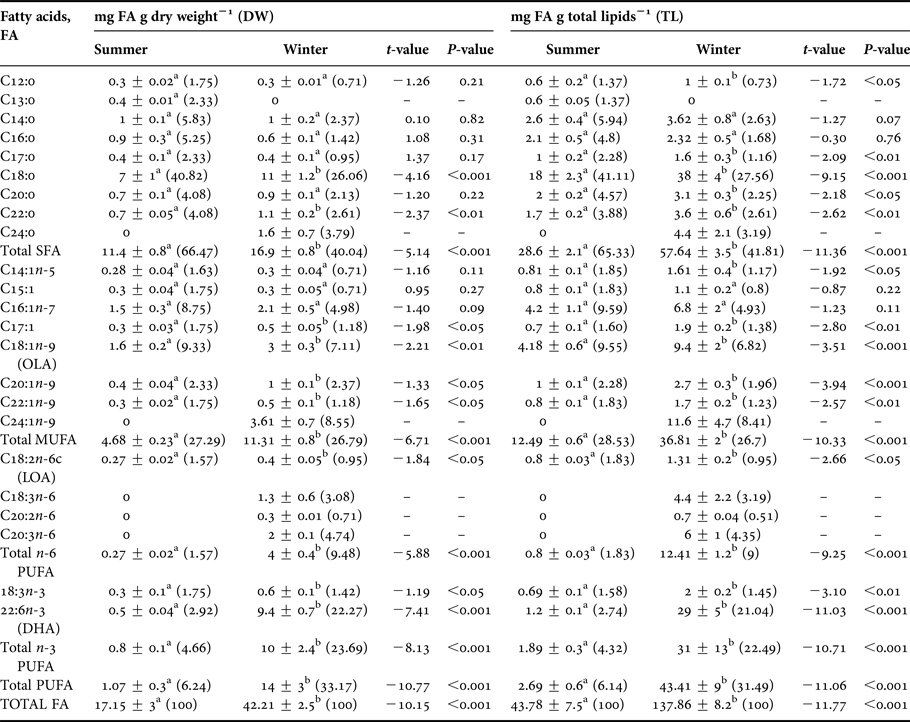
Table 6. Pleuroncodes monodon. Fatty acid (FA) content and profile (given in mg FA g dry weight−1 (DW) and mg FA g total lipids−1 (TL)) of female hepatopancreases between seasons (summer vs winter); mean ± SD.

Fatty acids are expressed in % of total FA pool for both DW and TL (in parentheses, below). Significantly higher values observed in pairwise comparisons are highlighted as bold numbers.
Different lower case letters in a row: significant differences between seasons (Student's t-test, P < 0.05). SFA (Saturated FA): sum of 8:0, 12:0, 13:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 21:0, 22:0 and 24:0; MUFA (Monounsaturated FA): sum of 14:1n-5, 16:1n-7, 18:1n-9, 20:1n-9, 22:1n-9 and 24:1n-9; Total n-6 PUFA (Polyunsaturated n-6 FA): sum of 18:2n-6c, 18:3n-6, 20:2n-6, 20:3n-6; Total n-3 PUFA (Polyunsaturated n-3 FA): sum of 18:3n-3, 22:6n-3, 20:3n-3, 20:5n-3; Total PUFA: sum of n-3 and n-6 PUFA; TOTAL FA: sum of Total SFA, Total MUFA and Total PUFA.
In relation to the percentage of fatty acids found between seasons, summer SFA and summer MUFA values represented 47.76% and 36.92% of the total hepatopancreas FA, respectively. Winter values were slightly lower with SFA and MUFA representing 46.87 and 22.39% of total FAs, respectively. Conversely, an inverse relationship was found for PUFA; PUFA represented 15.32% of total summer FAs while it almost doubled to represent 30.74% of total winter FAs. In this way, the percentage of PUFA in the hepatopancreases of winter females surpassed the percentage of MUFA (~22.39%) (Table 6).
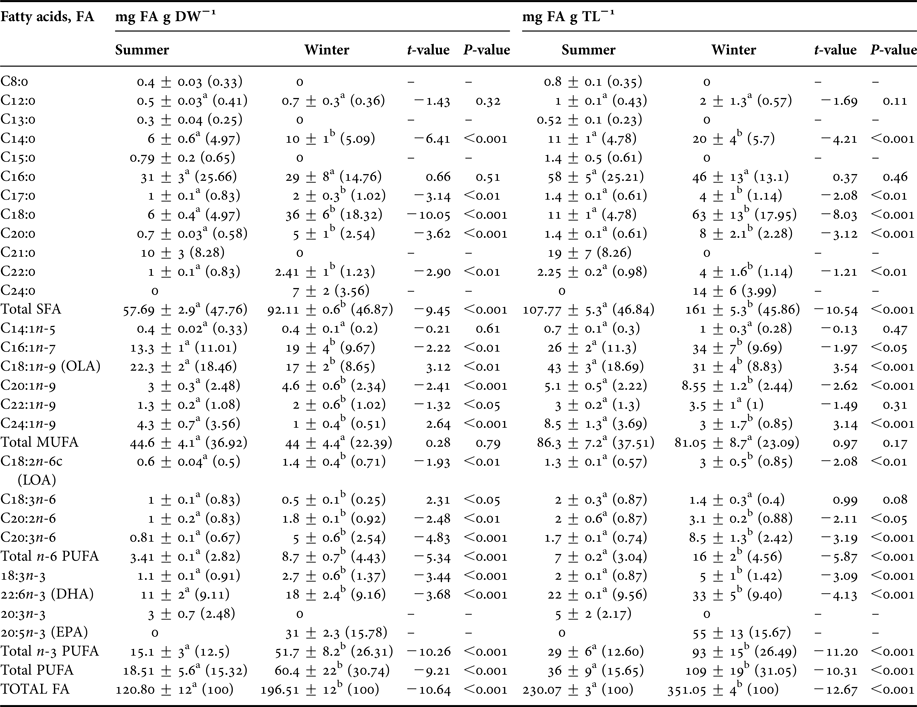
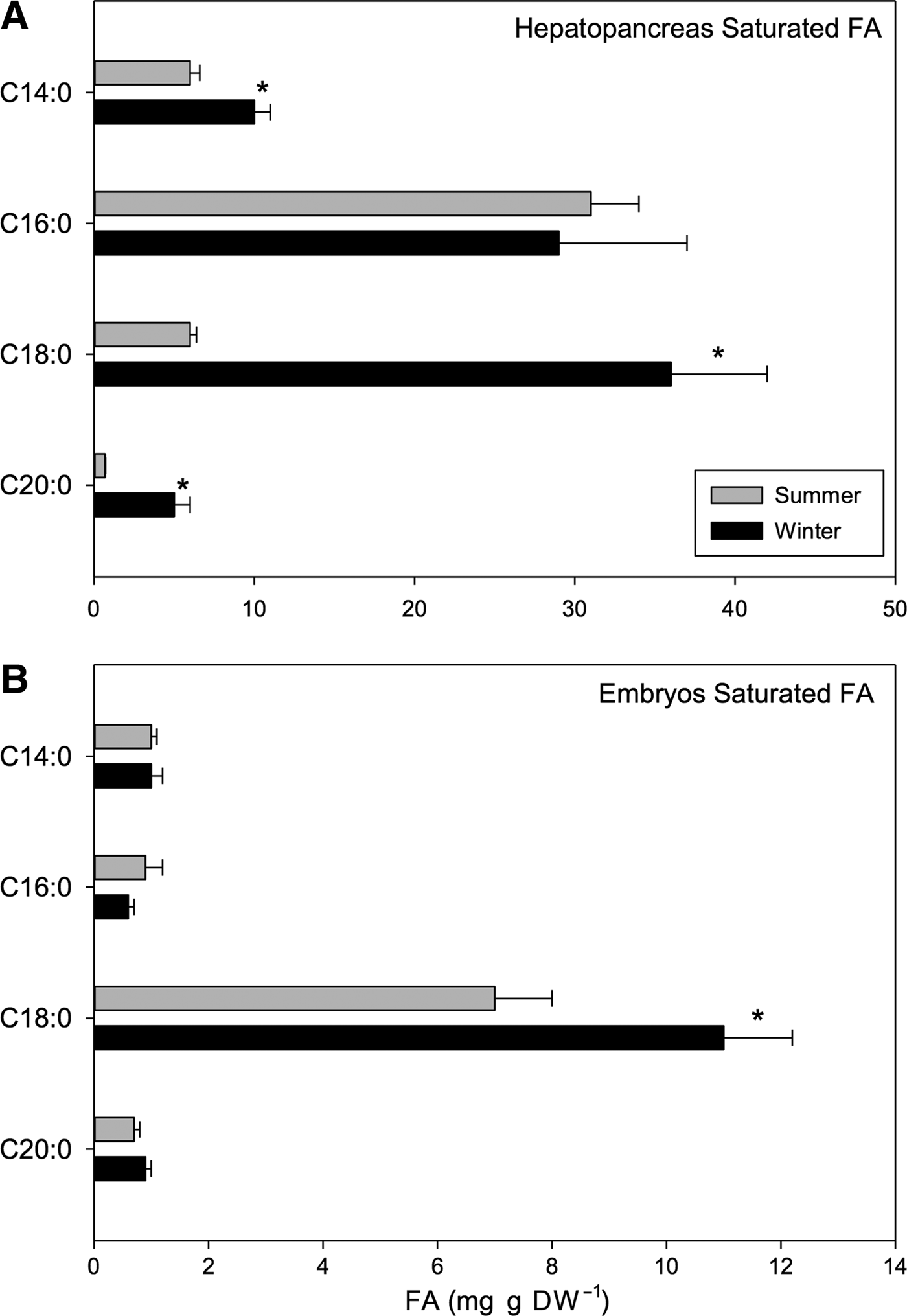
Within the SFAs, palmitic acid (C16:0) and stearic acid (C18:0) were the most abundant components making up 14.76% and 18.32% of total FAs of winter hepatopancreases, respectively. The following fatty acids also varied seasonally: meristic acid (C14:0), stearic acid (C18:0) and arachidic acid (C20:0) (t 1,118 = 11.06, P < 0.001). Greater values of these acids were found in winter rather than summer (meristic: 10 vs 6 mg g DW−1, stearic: 36 vs 6 mg g DW−1, arachidic: 5 vs 0.7 mg g DW−1; winter vs summer, respectively) (Figure 7A). On the other hand, caprylic (C8:0), tridecylic (C13:0), pentadecylic (C15:0) and heneicosylic (C21:0) fatty acids were only present in hepatopancreases of summer females, and they were present only in small quantities representing only 0.33%, 0.25%, 0.65% and 8.28%, respectively of total FA pool (Table 6).
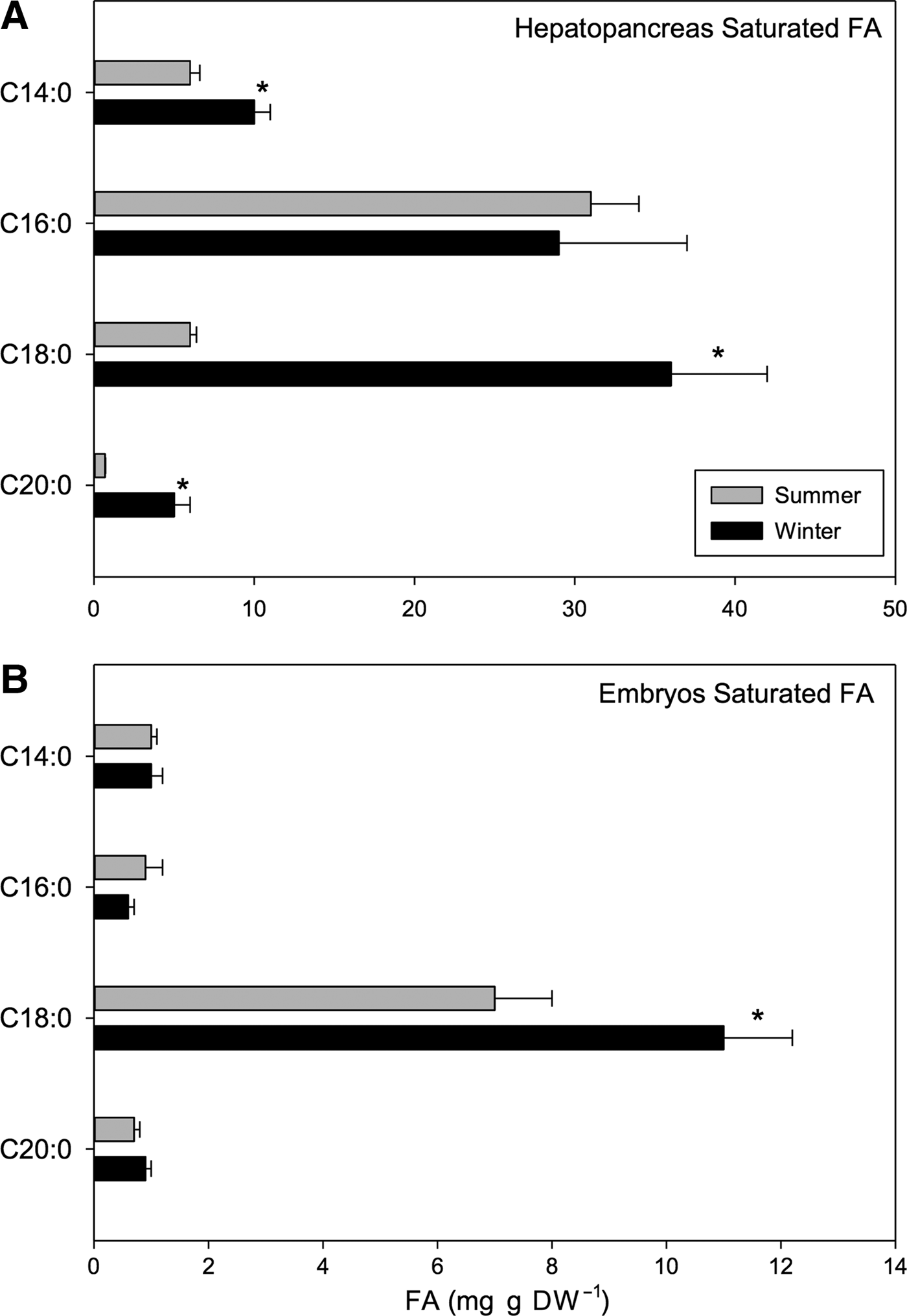
Fig. 7. Pleuroncodes monodon. Seasonal variation in: (A) hepatopancreases saturated fatty acids (mg g DW–1), (B) saturated fatty acids of embryos (mg g DW–1); mean values ± S.D. Asterisks indicate significant differences between seasons. In both N = 120.
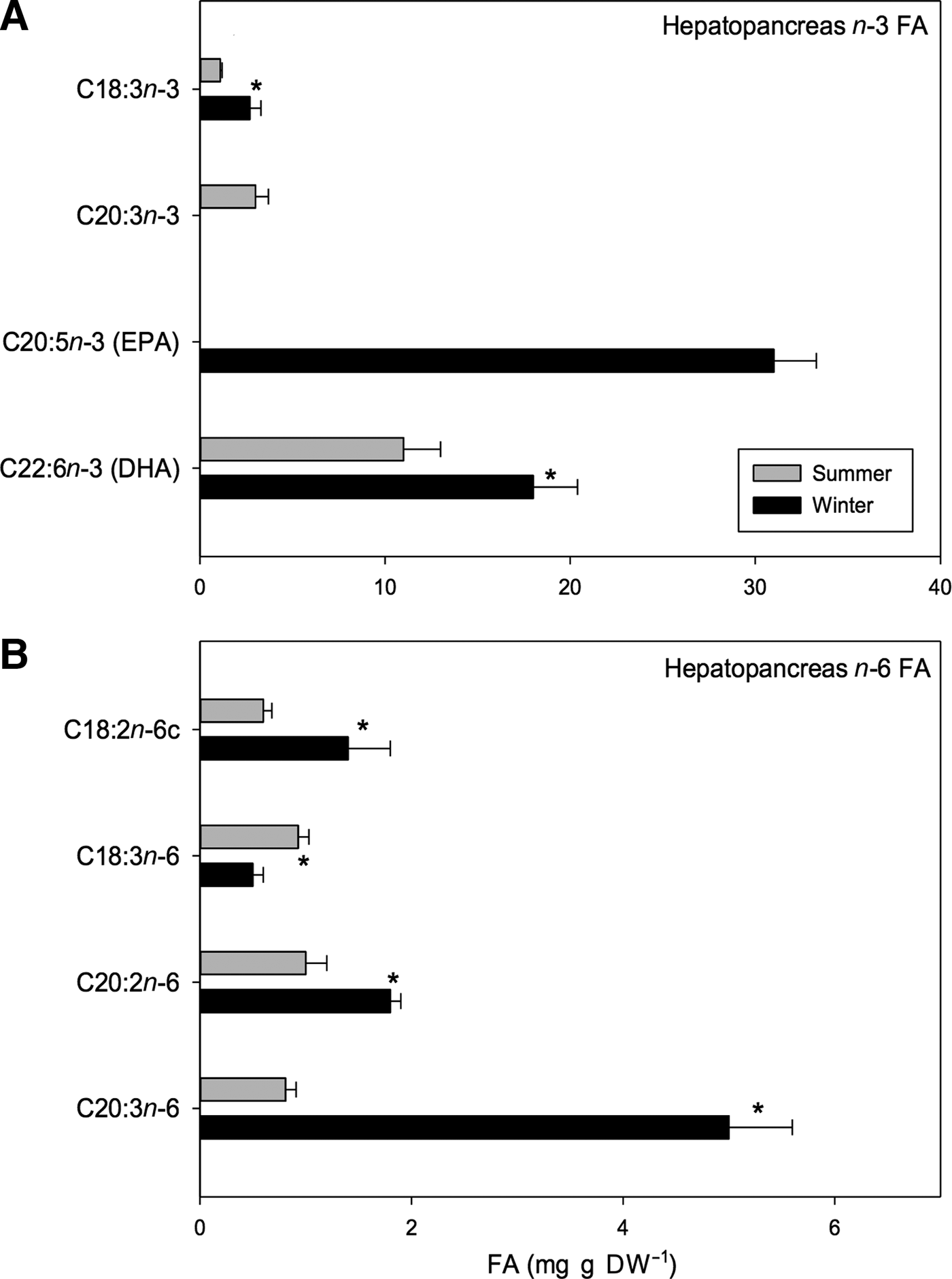
Regarding the n-3 PUFA, significant seasonal differences were only found in certain types of acids such as docosahexaenoic acid (DHA; C22:6n-3) and α-linolenic acid (C18:3n-3); higher values were found for winter samples than for summer samples (DHA: 18 vs 11 mg g DW−1; α-linolenic: 2.7 vs 1.1 mg g DW−1, respectively) (t 1,118 = 9.76, P < 0.001; Table 6). Moreover, some n-3 PUFAs were only found during one season. For example, eicosatrienoic acid (C20:3n-3) was only found in summer and was only found in small amounts (3 mg g DW−1) while eicosapentaenoic acid (EPA; C20:5n-3) was found in large amounts (31 mg g DW−1) but only in winter samples (Figure 8A).
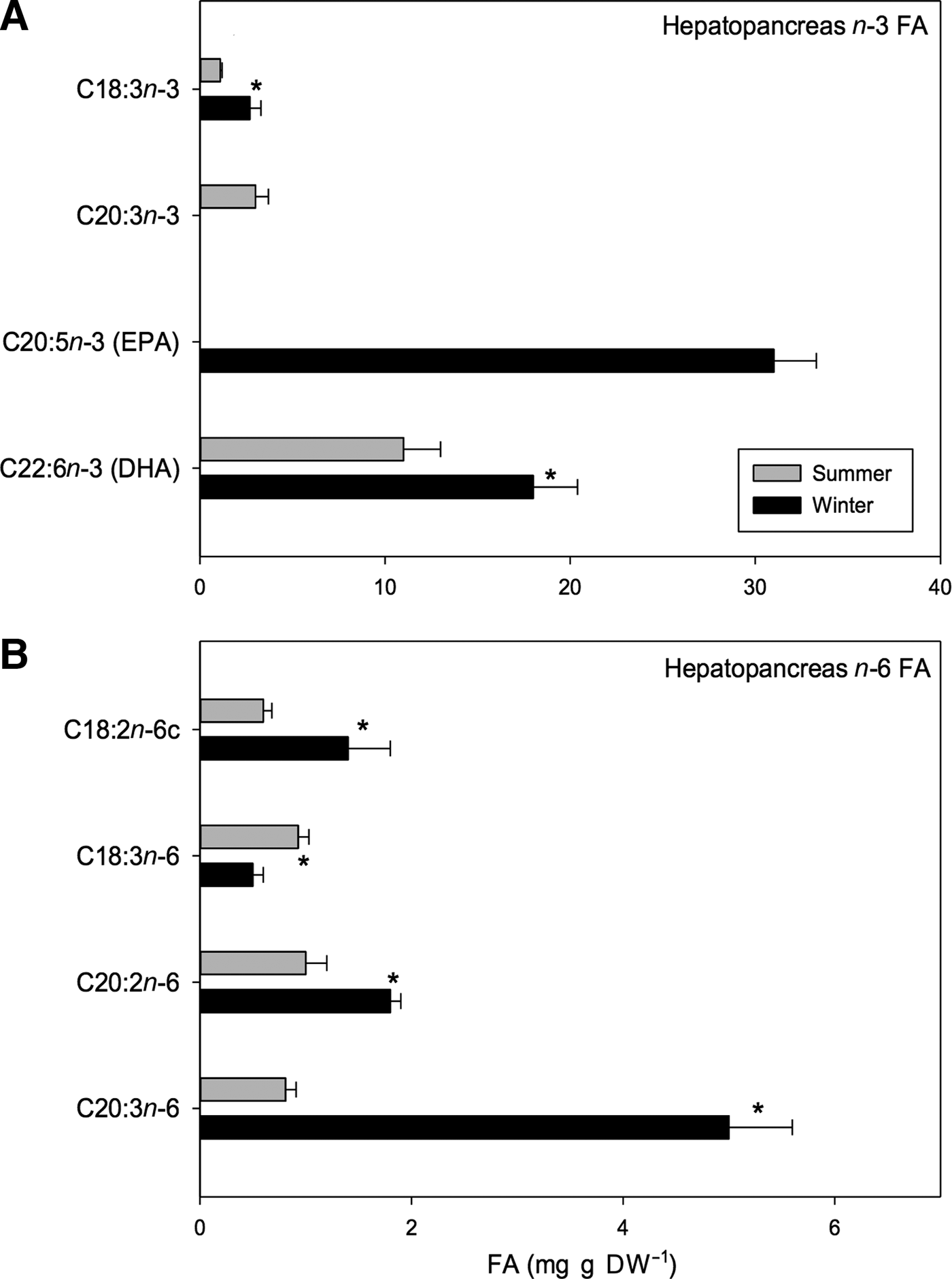
Fig. 8. Pleuroncodes monodon. Seasonal variation in: (A) hepatopancreases n-3 polyunsaturated fatty acid (mg g DW–1), (B) hepatopancreases n-6 polyunsaturated fatty acid (mg g DW–1); mean values ± S.D. Asterisks indicate significant differences between seasons. In both N = 120.
In turn, the n-6 PUFAs such as linoleic acid (C18:2n-6c), eicosadienoic acid (C20:2n-6) and dihomo-gamma-linolenic (DGLA; C20:3n-6) varied seasonally with greater values being registered for winter rather than for summer samples (linoleic acid: 1.4 vs 0.6 mg g DW−1, eicosadienoic acid: 1.8 vs 1 mg g DW−1, DGLA: 5 vs 0.81 mg g DW−1, winter vs summer respectively). Significant differences in γ-linolenic acid (C18:3n-6) were also found between seasons, but the relationship was opposite that for the previously mentioned n-6 PUFAs; a greater amount was found in the hepatopancreases of summer females than in hepatopancreases of winter females (1 vs 0.5 mg g DW−1) (Figure 8B). Within the n-6 PUFAs, DGLA acid was most abundant and represented 2.54% of total FA pool of winter samples. As a whole, EPA and DHA acids represented 15.78% and 9.16% of total FA pool, respectively (Table 6).
Seasonal variation in the amount of fatty acids in embryos
Highly significant differences were seen in fatty acid profiles of embryos between seasons (t 1,118 = 10.27, P < 0.001; Table 7). The content (mg g DW−1) of saturated acids (SFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids was consistently higher in winter embryos than in summer embryos (Figure 6B). In turn, the respective values of these types of FAs also varied between seasons. For example, in summer there was a tendency of SFA > MUFA > PUFA with these types of fatty acids representing close to 66.47%, 27.29% and 6.24% of total fatty acids, respectively. On the other hand, in winter the tendency was SFA > PUFA > MUFA with these fatty acids representing close to 40.04%, 33.17% and 26.79% of total FA, respectively (Table 7).
Table 7. Pleuroncodes monodon. Fatty acid (FA) content and profile (given in mg FA g dry weight−1 (DW) and mg FA g total lipids−1 (TL)) of initial stage eggs between seasons (summer vs winter); mean ± SD.

Fatty acids are expressed in % of total FA pool for both DW and TL (in parentheses, below). Significantly higher values observed in pairwise comparisons are highlighted as bold numbers.
Different lower case letters in a row: significant differences between seasons (Student's t-test, P < 0.05). SFA (Saturated FA): sum of 12:0, 13:0, 14:0, 16:0, 17:0, 18:0, 20:0, 22:0 and 24:0; MUFA (Monounsaturated FA): sum of 14:1n-5, 15:1, 16:1n-7, 17:1, 18:1n-9, 20:1n-9, 22:1n-9 and 24:1n-9; Total n-6 PUFA (Polyunsaturated n-6 FA): sum of 18:2n-6c, 18:3n-6, 20:2n-6, 20:3n-6; Total n-3 PUFA (Polyunsaturated n-3 FA): sum of 18:3n-3 and 22:6n-3; Total PUFA: sum of n-3 and n-6 PUFA; Total FA: sum of Total SFA, Total MUFA and total PUFA.
Within the SFAs, stearic acid (C18:0) was the most abundant; 7 mg g DW−1 was found in summer embryos while 11 mg g DW−1 was found in winter embryos (t-test, t 1,118 = −7.55, P < 0.001; Table 7) (Figure 7B). However, no significant seasonal differences were detected in myristic (C14:0), palmitic (C16:0) or arachidic (C20:0) SFAs; values for these fatty acids were 1, 0.9 and 0.7 mg g DW−1 for summer samples and 1, 0.6 and 0.9 mg g DW−1 for winter samples respectively. Lignoceric (C24:0) SFA was only found in embryos laid in winter; 1.6 mg g DW−1 of this fatty acid was found (Table 7).
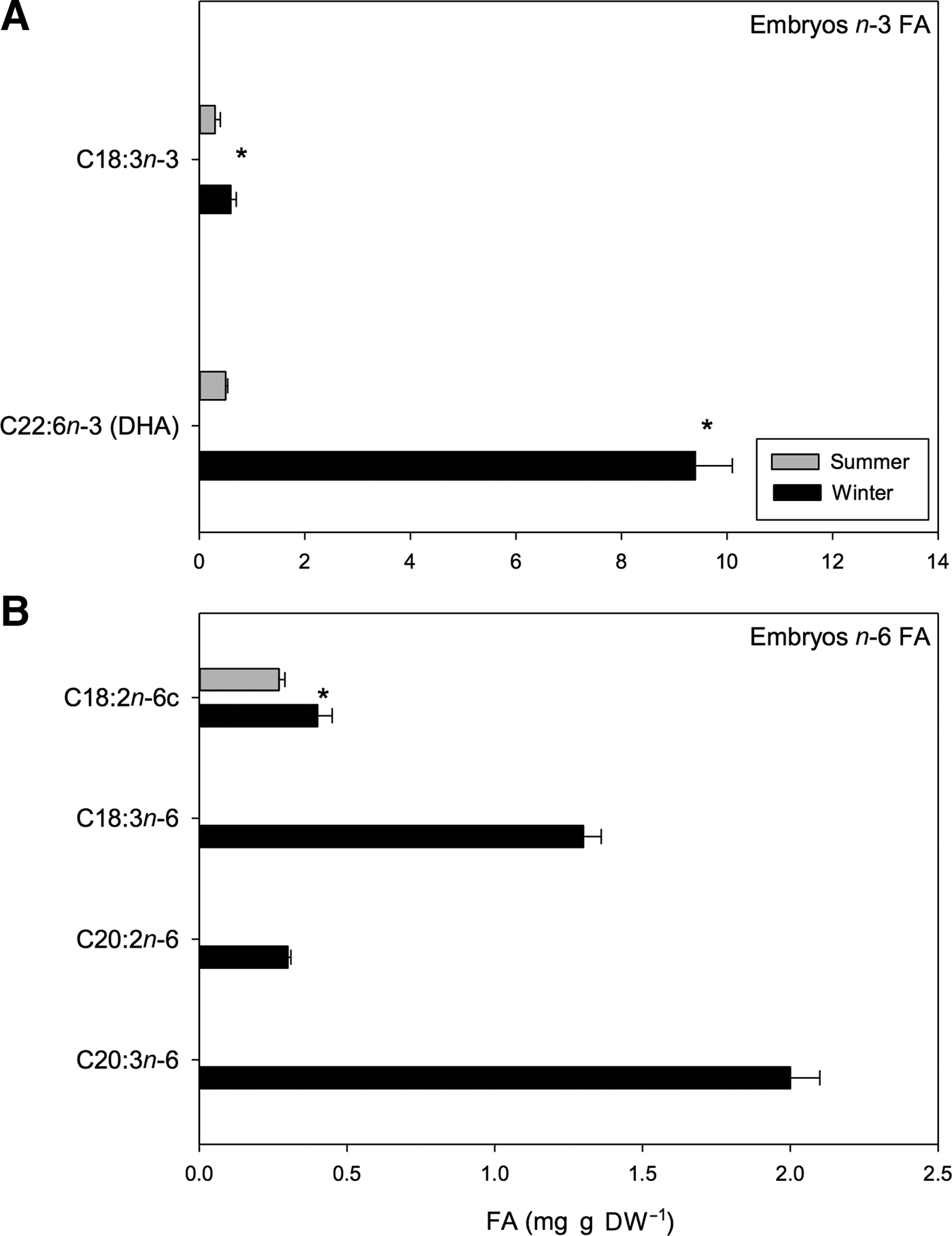
Seasonal variation was also found in n-3 and n-6 PUFAs. For example, in the case of the n-3 PUFAs, the proportion of DHA (C22:6n-3) and α-linolenic acid (C18:3n-3) was greater in winter embryos than in summer embryos (t 1,118 = 3.47, P < 0.01) (Figure 9A). Within the n-3 PUFAs, DHA was consistently the most abundant, representing 22.27% of the total FA pool of winter embryos. Regarding n-6 PUFAs, linoleic acid (C18:2n-6c) varied seasonally with greater amounts of this acid found in winter. Conversely, other n-6 PUFAs, including γ-linolenic (C18:3n-6), eicosadienoic (C20:2n-6) and DGLA (C20:3n-6) PUFAs, were not found in summer embryos (Figure 9B). Overall, combination of these types of n-6 FAs represented 3.08%, 0.71% and 4.74% of the total FA pool found in winter embryos, respectively (Table 7).
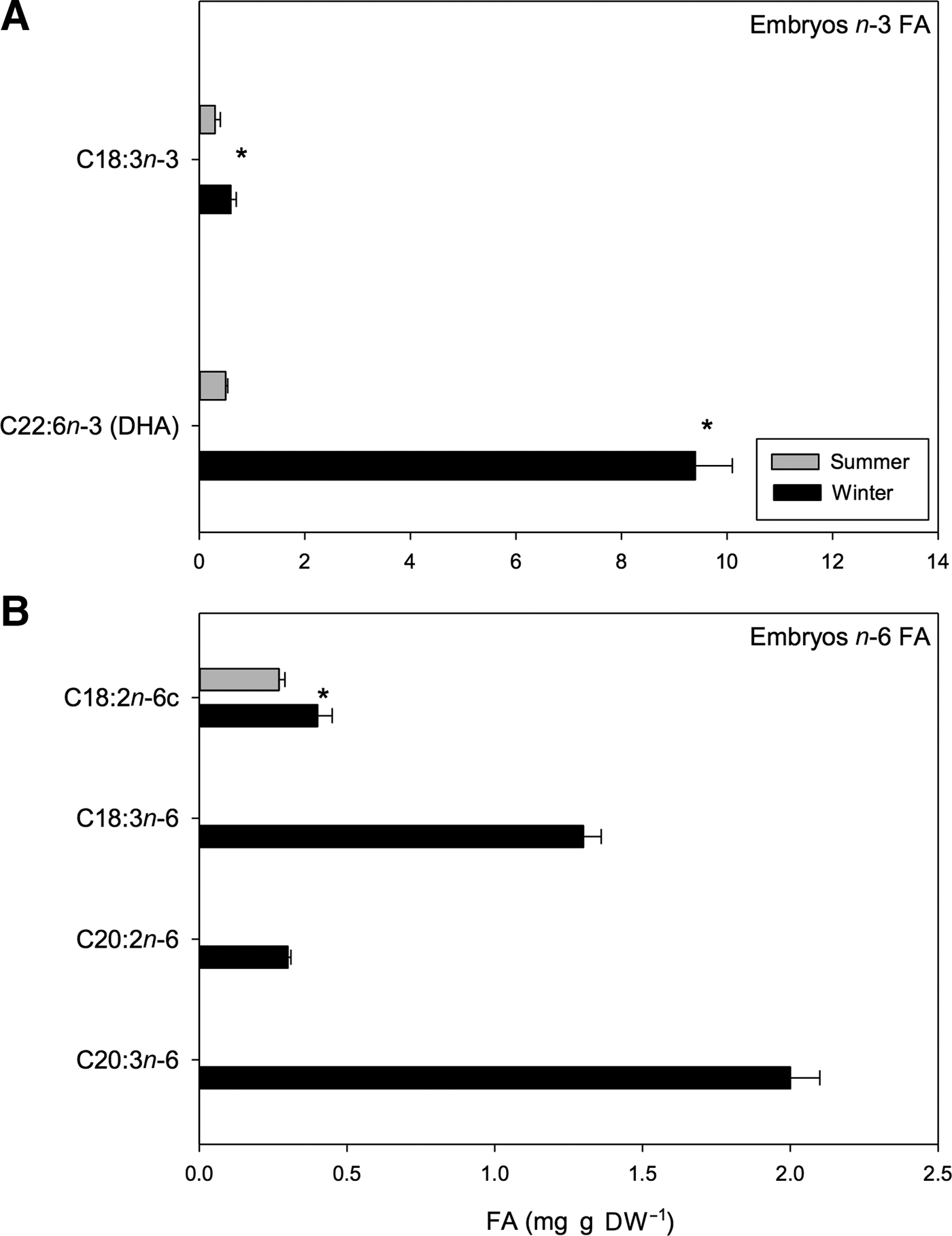
Fig. 9. Pleuroncodes monodon. Seasonal variation in: (A) n-3 polyunsaturated fatty acids of embryos (mg g DW–1), (B) n-6 polyunsaturated fatty acids of embryos (mg g DW–1); mean values ± S.D. Asterisks indicate significant differences between seasons. In both N = 120.
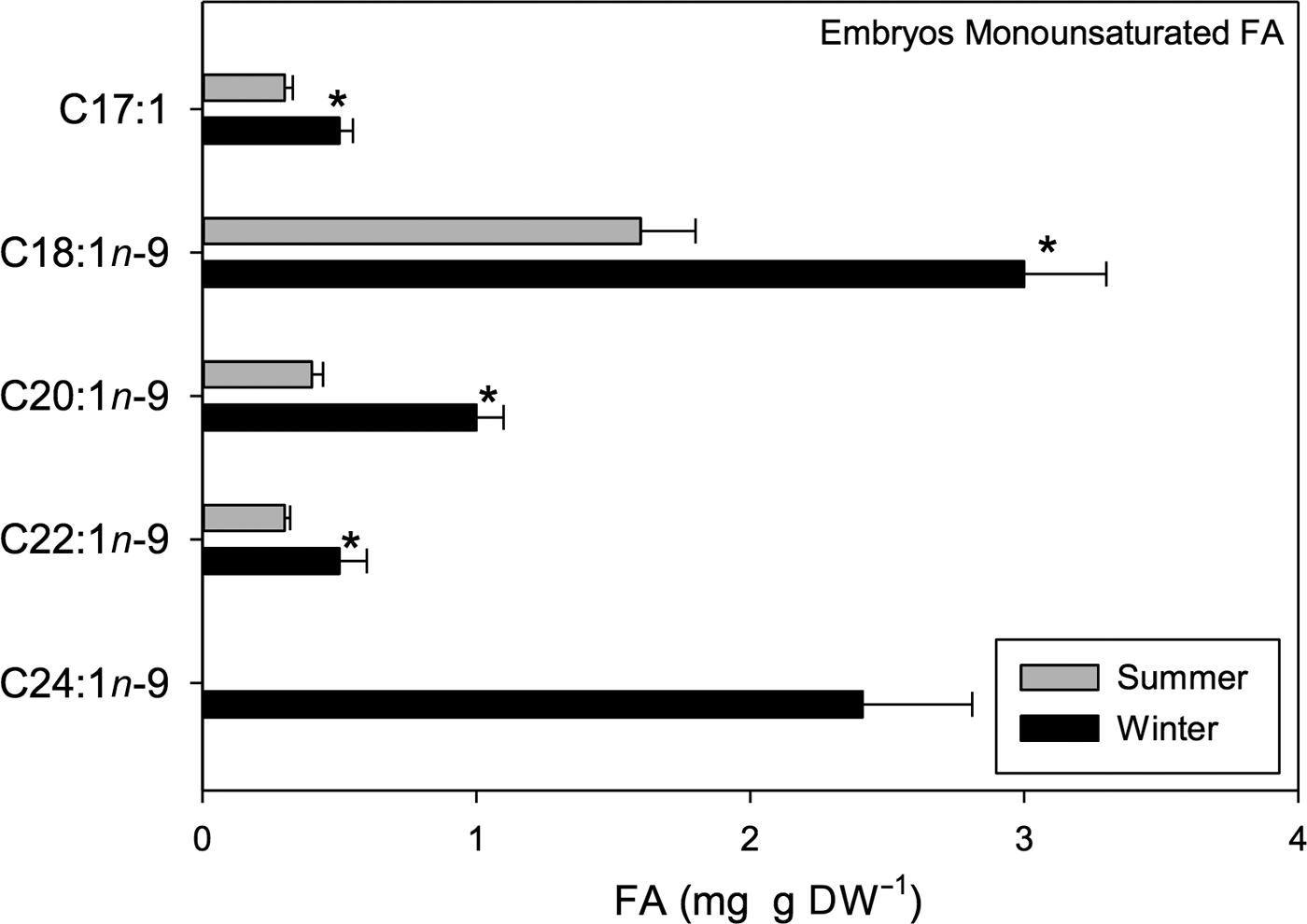
Significant seasonal variation in MUFAs such as heptadecanoic (C17:1), oleic (C18:1n-9), gaddoleic (C20:1n-9), erucic (C22:1n-9) and nervonic (C24:1n-9) acids was detected (t 1,118 = 9.84, P < 0.001). Overall, a greater amount of acids was found in winter samples than in summer samples (Figure 10). Within the MUFAs, oleic FA was the most abundant; 3 mg g DW−1 was found in winter samples while 1.6 mg g DW−1 was found in summer samples (Figure 10).
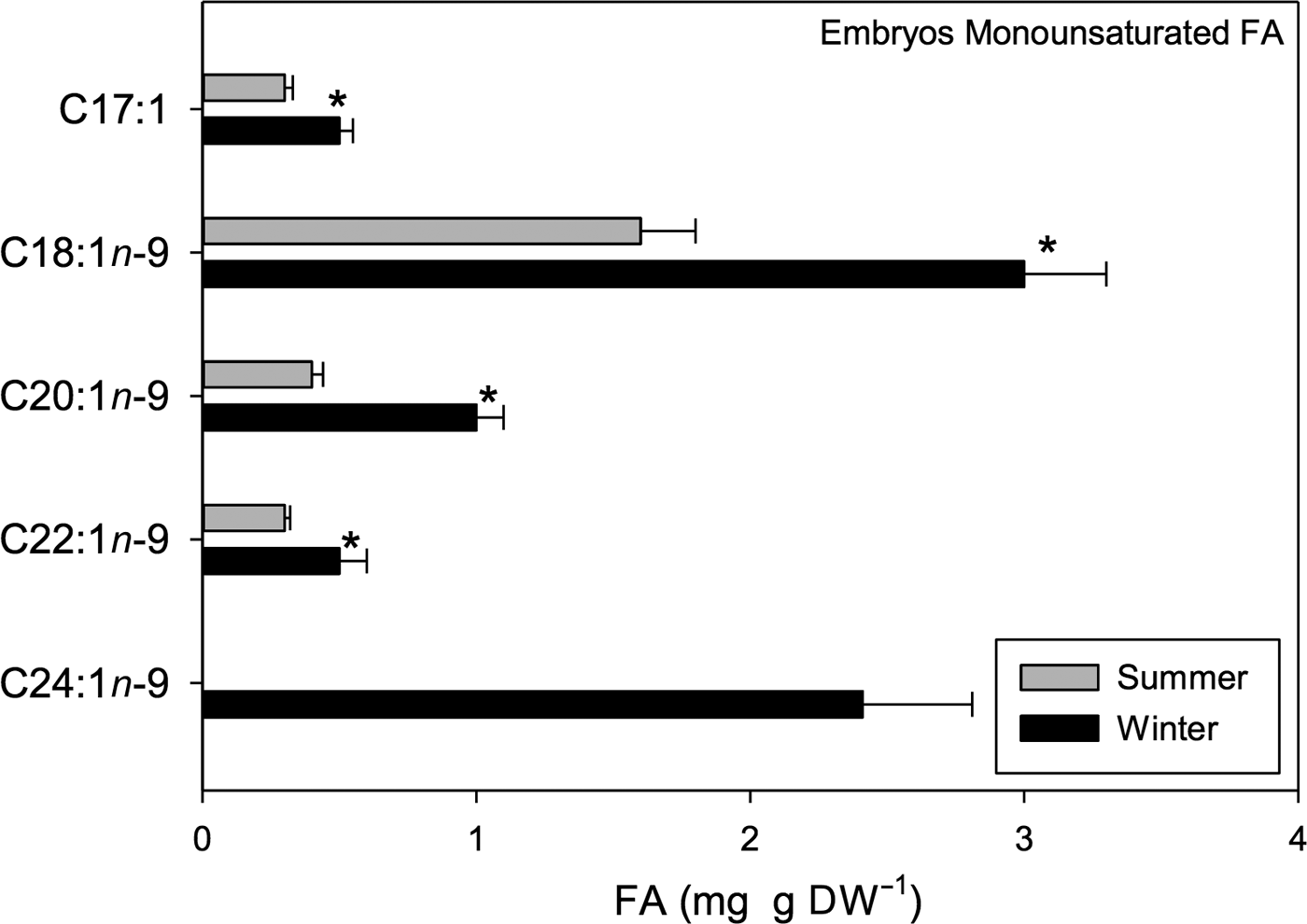
Fig. 10. Pleuroncodes monodon. Seasonal variation in monounsaturated fatty acids of embryos (mg g DW–1); mean values ± S.D. Asterisks indicate significant differences between seasons. N = 120.
DISCUSSION
Seasonal variation in the lipid and fatty acid content of females studied here could represent an adaptive response of female squat lobsters to marked seasonal variation in the environmental conditions of their surrounding habitat. During unfavourable and unpredictable periods (i.e. winter: low primary productivity, cold temperature), the composition of stored energy of P. monodon females is related to production of a single embryo laying; in summer (high primary productivity, warm temperature), energy is likely used for physiological processes of growth (e.g. moulting) and also for the numerous different embryo production events (2–3 embryo laying) during the main reproductive period. In this context, according to PCA, analysis of fatty acid in summer clearly distinguished two isolated groups and with conspicuous variations in fatty acids profile recorded in hepatopancreas and embryos. In winter, the opposite pattern occurs in the fatty acid profile of embryo and hepatopancreas. These seasonal variations in fatty acids profile of P. monodon may be related to relevant physiological processes of adult individuals (e.g. for ‘winter hepatopancreas’ (stearic, palmitic, EPA): reproduction and growth) and also for their ontogeny (e.g. for ‘winter embryos’ (DHA): successful development and survival of offspring) (Glencross, Reference Glencross2009).
This study reveals that there is a greater content of lipids and saturated fatty acids in female hepatopancreases in winter, which could be closely linked to an extension of reproduction. This is reflected in the production of embryos (1–2 broods) (Thiel et al., Reference Thiel, Espinoza-Fuenzalida, Acuña and Rivadeneira2012; Guzmán et al., Reference Guzmán, Olavarría and Urzúa2016) during winter with large amounts of lipids and essential fatty acids (EPA and DHA) (present study). In this context, winter embryos containing high lipid content would be useful for both embryonic development at low temperatures and for larvae hatching (during late winter) when planktonic food availability is poor or unpredictable (low primary productivity).
In turn, lipid content of females in summer is probably related to physiological processes (i.e. moulting and reproduction) and with the production of numerous embryos with high amounts of structural fatty acids that would confer rapid growth to the embryo. Some types of structural fatty acids may help crustacean larvae to present a greater degree of flexibility of their jaw that would allow them to capture a wide spectrum of prey in marine environments with strong seasonality of temperature and food availability (see Anger, Reference Anger2001; Liddy et al., Reference Liddy, Kolkovski, Nelson, Nichols, Phillips and Maguire2005; Pandian, Reference Pandian2016). Similar responses have been described for crustacean species inhabiting coastal areas with marked seasonality of environmental conditions (Kattner et al., Reference Kattner, Wehrtmann and Merck1994, Reference Kattner, Hagen, Lee, Campbell, Deibel, Falk-Petersen, Graeve, Hansen, Hirche, Jonasdottir, Madsen, Mayzaud, Muller-Navarra, Nichols, Paffenhofer, Pond, Saito, Stubing and Virtue2007; Calado et al., Reference Calado, Guerao, Gras, Cleary and Rotllant2013).
Crustaceans, in general, require a large amount of long-chain polyunsaturated fatty acids (lcPUFA; FA with more than 20 carbons) for various important physiological processes (e.g. synthesis of eicosanoid hormones, neural and immune development). These lcPUFA can be directly supplied to the individual through food consumption (i.e. prey rich in lcPUFA: phyto-zooplankton, Arts et al., Reference Arts, Brett and Kainz2009) or by enzymatic degradation and/or bio-conversion of short-chain polyunsaturated fatty acids (scPUFA; FA with less than 20 carbons, e.g. linoleic acid (C18:2n6cis) and alpha linolenic acid (C18:3n6)) to lcPUFA (e.g. docosahexaenoic (DHA; 22:3n6) and eicosapentaenoic (EPA; 20:5n3)) (Glencross, Reference Glencross2009). Several studies have demonstrated that marine crustaceans (e.g. Crangon crangon: Mika et al., Reference Mika, Skorkowski and Stepnowski2014) and freshwater crustaceans (e.g. Macrobrachium rosenbergii: Reigh & Stickney, Reference Reigh and Stickney1989) are capable of enzymatically transforming scPUFA into lcPUFA. It is yet unknown whether Pleuroncodes monodon is capable of enzymatically bio-converting scPUFA to lcPUFA. Furthermore, more studies are needed to determine the adaptive role of reproducing during periods when food availability is of low quality and quantity.
In agreement with the fatty acid profiles in both seasons for female Pleuroncodes monodon hepatopancreas, evaluating fatty acid content and composition can be used to determine the type of food that red squat lobster is eating, and it can also be used to elucidate interspecific interactions within the marine food web (e.g. Connelly et al., Reference Connelly, Deibel and Parrish2014; Kiyashko et al., Reference Kiyashko, Kharlamenko, Sanamyan, Alalykina and Würzberg2014; Legezżynńska et al., Reference Legezżynńska, Kędra and Walkusz2014). In this context, presence of SFA, such as C15:0 and C17:0, indicates that females feed off detritus present in sediments (Volkman et al., Reference Volkman, Barrett, Blackburn, Mansour, Sikes and Gelin1998). The consistent and significant amount of oleic acid (C18:1n-9) in hepatopancreases of winter females suggests that P. monodon is also a carnivore (Dalsgaard et al., Reference Dalsgaard, St John, Kattner, Muller-Navarra and Hagen2003), probably consuming other crustaceans (larvae or pieces of dead adults), polychaetes and molluscs. In turn, large amounts of EPA and DHA (~25% of total FA) present in hepatopancreases of winter females reflect the consumption of phytoplankton and zooplankton that consume primary producers (i.e. micro and macroalgae) (Rosa et al., Reference Rosa, Calado, Narciso and Nunes2007). We therefore propose that female red squat lobsters are omnivores (generalist trophic niche, for concept see Lovrich & Thiel, Reference Lovrich, Thiel, Poore, Ahyong and Taylor2011); this feeding regime has been described for other squat lobster species (e.g. Munida quadrispina: Burd & Brinkhurst, Reference Burd and Brinkhurst1984, Munida gregaria: Romero et al., Reference Romero, Lovrich, Tapella and Thatje2004, Munidopsis subsquamosa: Phleger et al., Reference Phleger, Nelson, Groce, Cary, Coyne and Nichols2005).
In relation to the seasonal variation observed for fatty acid profiles of red squat lobster embryos, the variation detected can be explained by the differential energy input by mothers to their offspring depending on season (i.e. winter vs summer) (Torres et al., Reference Torres, Penha-Lopes, Narciso, Macia and Paula2008). For example, high levels of saturated fatty acids (e.g. stearic acid and myristic acid) and MUFA (e.g. palmitoleic acid and oleic acid) observed in embryos laid in winter would be used during embryogenesis as energy reserves. These would also be the precursors of structural components of cells, membranes and other types of organelles (for examples of decapods see Calado et al., Reference Calado, Dionisio and Dinis2007; Figuereido & Narciso, Reference Figuereido and Narciso2008). Overall, this could indicate that embryos laid in winter will later probably hatch into larvae of larger size and with more biomass than embryos that hatch in summer.
Conversely, high levels of essential fatty acids (e.g. docosahexaenoic acid (DHA), alpha linoleic (C18: 3n6) and linolenic acid (C18: 2n6cis)) in P. monodon embryos are highly related with the development of the nervous and immune systems during embryogenesis (Beltz et al., Reference Beltz, Tlusty, Benton and Sandeman2007). Additionally, these types of fatty acids, with a high amount of DHA and only recorded in the winter season (above the EPA), are highly relevant sources of reserve energy during periods of food scarcity (Anger, Reference Anger2001; Glencross, Reference Glencross2009). In this context, more DHA content in embryos laid in winter would decrease red squat lobster larval nutritional vulnerability at the time of hatching when planktonic food availability is scarce (Espinoza et al., Reference Espinoza, Guzmán, Bascur and Urzúa2016). The newly hatched larvae (without feeding) of P. monodon could use this AG type (DHA) as an energy reserve, transferred directly from the mother. However, it is not known if the DHA and other essential fatty acids are self-catabolized by the larvae through alternative metabolic routes (e.g. enzymatic activities).
Pleuroncodes monodon is a key species in the Humboldt Current Large Marine Ecosystem (HCLME) because it plays an important role in the marine food web, and because it is a main resource for demersal crustacean fisheries. In the HCLME, P. monodon is a predator of a large variety of species spanning various trophic groups (for example, bacteria, phyto-zooplankton, detritus, polychaetes and amphipods; Gallardo et al., Reference Gallardo, Cañete, Roa, Enríquez-Briones and Baltazar1994; Roa et al., Reference Roa, Gallardo, Ernst, Baltazar, Cañete and Enríquez-Briones1995). Pleuroncodes monodon is also prey to a number of fish species including Genypterus maculatus (Henríquez & Bahamonde, Reference Henríquez and Bahamonde1964; Andrade, Reference Andrade and Arana1986), Genypterus chilensis (Chong et al., Reference Chong, Sepúlveda and Ibáñez2006), Genypterus blacodes (Bahamonde & Zavala, Reference Bahamonde and Zavala1981), Merluccius spp. (Arancibia & Meléndez, Reference Arancibia and Meléndez1987) and Hippiglossina macrops (Villarroel & Acuña, Reference Villarroel and Acuña2000). As a general conclusion, given the important role of this species in the trophic web and in fisheries, seasonal variation in lipid content and fatty acid composition of P. monodon females and embryos found in this study probably has a direct impact on reproduction, survival and recruitment during later stages of the life cycle (larvae and juveniles). Overall, this could have global consequences on the trophic marine chain and its commercial exploitation.
ACKNOWLEDGEMENTS
We thank Camanchaca Pesca Sur S.A. and the Instituto de Fomento Pesquero for help capturing and transporting live squat lobster to the Lenga Region in Biobío. We thank PhD Emily Giles (UACh-Chile) for correcting the English and improving this manuscript; PhD Cristina Rodríguez (ULL-Spain) helped in statistical analyses; Esthefany Reyes helped in fatty acid analyses. We also thank two anonymous reviewers for constructive criticism and helpful suggestions. The experiments comply with animal ethics laws in Chile.
FINANCIAL SUPPORT
This work was funded by the Comisión Nacional de Ciencia y Tecnología (CONICYT: grants FONDECYT N° 11140213 and PAI N° 79130025 to AU; FA DI-UCSC).