INTRODUCTION
Copepods constitute a major part of the mesozooplankton community in the coastal as well as oceanic habitat of the Arabian Sea (Madhupratap et al., Reference Madhupratap, Nair, Haridas and Padmavati1990) and in the other parts of the world oceans (Roman & Gauzens, Reference Roman and Gauzens1997; Lo et al., Reference Lo, Hwang and Chen2004). Being a crucial prey, they support secondary consumers of the marine food web (Madhupratap et al., Reference Madhupratap, Nair, Gopalkrishnan, Haridas, Nair, Venugopal and Gauns2001). Also, copepods generate carbon rich faecal pellets as a result of their grazing. Collectively, copepods greatly influence the transfer of energy and carbon compounds to the different trophic levels throughout the marine food web. Being noteworthy contributors to the marine biological pump, it is crucial to understand copepods’ feeding habits. Copepods are known to feed on a wide range of food (Turner, Reference Turner2004) but their tendency to switch diet based on the locale makes it vital to understand their feeding in every habitat (Stern, Reference Stern1986; Peters et al., Reference Peters, Dutz and Hagen2013).
The common method to establish copepod feeding types is based on morphology of mouth parts (Madhupratap Reference Madhupratap1999). However, copepod feeding behaviour might be selective (Go et al., Reference Go, Oh and Terazaki1998) or non-selective (Tseng et al., Reference Tseng, Kumar, Dahms, Chen and Hwang2008) based on assorted dietary type, algal type and toxicity (Atkinson, Reference Atkinson1996; Jansen et al., Reference Jansen, Riser, Wassmann and Bathmann2006). Copepods impact microbial assemblages (Schnetzer & Caron, Reference Schnetzer and Caron2005) by consuming the bacterial biomass (Gowing & Wishner, Reference Gowing and Wishner1998). Copepod feeding studies have been mostly conducted with either direct gut examination (Gowing & Wishner, Reference Gowing and Wishner1998) or faecal pellets study (Turner, Reference Turner2002). However, these techniques may not be practical to determine the diet composition as lots of feed could go unclaimed with swift digestion. Also, bottle incubations have been used to study diets of calanoid copepods (Jansen et al., Reference Jansen, Riser, Wassmann and Bathmann2006), but the experimental pressure on organisms may lead to a feeding habit different from an in situ feeding type.
While in situ studies on natural feeding observation of copepods are difficult, gut fluorescence method is widely used for this purpose by numerous researchers (Mackas & Bohrer, Reference Mackas and Bohrer1976; Kleppel & Pieper, Reference Kleppel and Pieper1984; Rodriguez & Durbin, Reference Rodriguez and Durbin1992; Tsuda & Sugisaki, Reference Tsuda and Sugisaki1994; Saito & Taguchi, Reference Saito and Taguchi1996; Takatsuji et al., Reference Takatsuji, Hamasaki, Toda and Taguchi1997; Tseng et al., Reference Tseng, Dahms, Chen and Hwang2009). Fluorometric analysis gives quantitative estimates of only Chl a and its derivatives and HPLC proves qualitative composition of gut pigments (Kleppel & Pieper, Reference Kleppel and Pieper1984). These gut pigments, chiefly canthaxanthin and astaxanthin, remain stable in copepod gut (Lotocka & Styczynska-Jurewicz, Reference Lotocka and Styczynska-Jurewicz2001), also conveniently are eluted by chromatography (Jeffrey, Reference Jeffrey1974) and act as indicators of feed (Lewin, Reference Lewin and Stewart1974).
Only autotrophs can manufacture carotenoids de novo (Lotocka & Styczynska-Jurewicz, Reference Lotocka and Styczynska-Jurewicz2001; Van Nieuwerburgh et al., Reference Van Nieuwerburgh, Wänstrand, Liu and Snoeijs2005), which are grazed by primary consumers as β-carotene and processed as astaxanthin and canthaxanthin via metabolic pathway (Kleppel et al., Reference Kleppel, Willbanks and Pieper1985; Van Nieuwerburgh et al., Reference Van Nieuwerburgh, Wänstrand, Liu and Snoeijs2005). The exploitation of precursors and successive synthesis of astaxanthin by herbivorous zooplankton thus represents a vital entry point of astaxanthin into marine food webs.
This is the first study on in situ copepod gut pigments from the continental shelf of the eastern Arabian Sea. To understand the copepod feeding types, we studied the gut pigments of copepod orders quantitatively (fluorometric) as well qualitatively (pigment composition) using the gut fluorescence method. This paper presents copepod feeding habits and gut pigment contents over different seasons at the coastal time series station along the central western coast of India.
MATERIALS AND METHODS
Sampling
The sampling site was located at 15°31.17′N 73°44.200′E (G5) off Candolim, Goa, on the continental shelf of the central western coast of India (Figure 1) with depth of ~28 m. The sampling was carried out in daytime during November 2011, March 2012, August 2012, October 2012, November 2012, December 2012, January 2013, February 2013, April 2013, May 2013, July 2013, August 2013, September 2013 and October 2013. It covered monsoon (June–September), post-monsoon (October–January) and pre-monsoon (February–May) seasons. A single mesozooplankton sample, representative of each month, was collected by vertically towing a Heron Tranter net (0.25 m2 mouth area; 200 µm mesh size) from ~26 m to the surface. Sampling of water Chl a was carried out from four depths (0, 9, 18 and 27 m) using a 5 l Niskin sampler coupled with reversible thermometer enabling temperature measurement. A sub-sample of known volume (0.5 l) was collected for each depth in an amber-coloured bottle during each month.
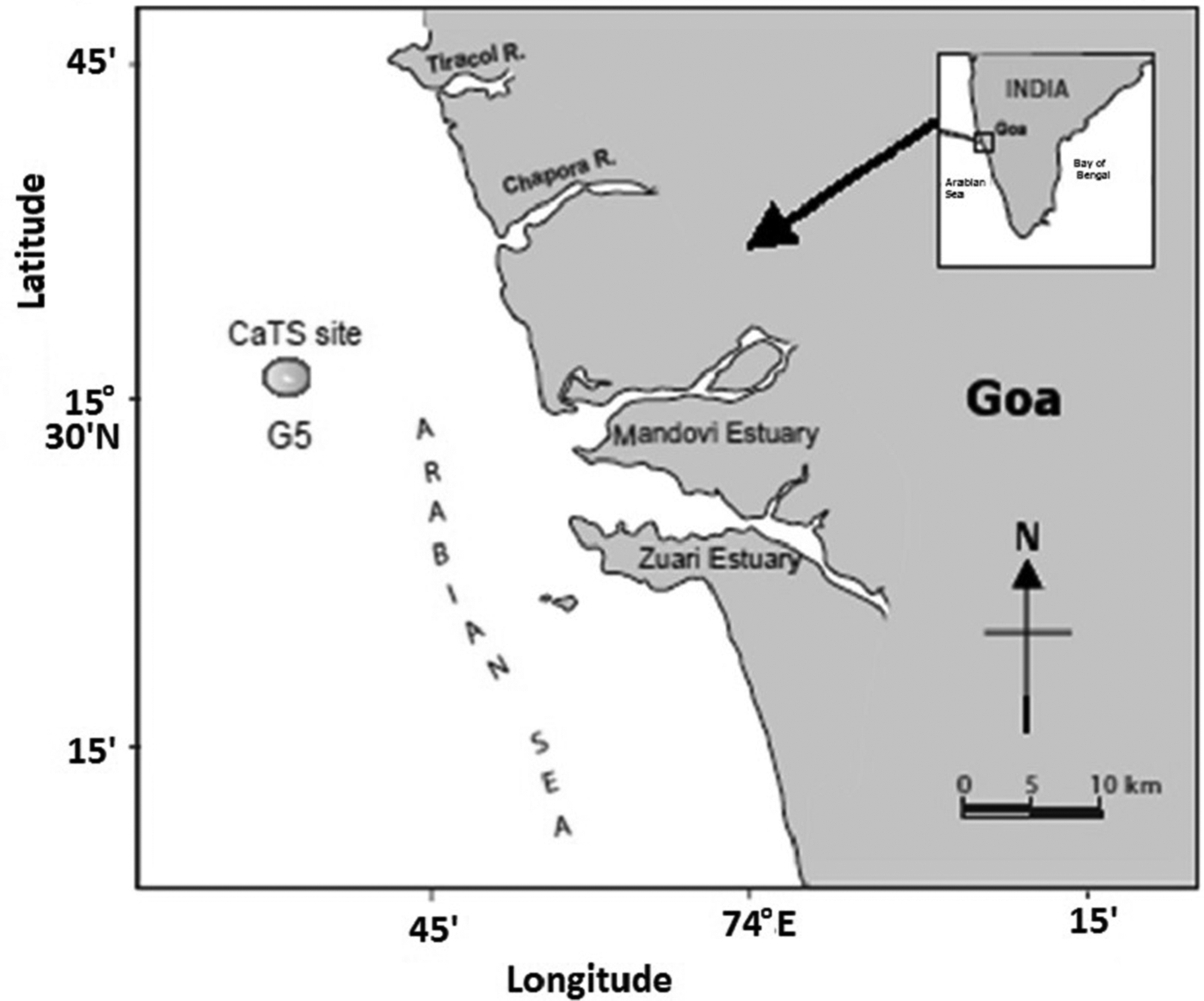
Fig. 1. Location of sampling site (G5) off Goa, in the Arabian Sea.
All the samples were stored in an icebox until transferred to the laboratory for further processing. The data on salinity were obtained using CTD (Conductivity-temperature-depth; Sea-Bird electronics).
Fluorometric estimation of water Chl a
Chl a levels in ambient water were measured using JGOFS protocol (UNESCO, 1994) with slight modification. Water samples of 0.5 l were filtered using 47 mm GF/F polycarbonate filters and extracted in 90% acetone at −20°C overnight in the dark. The acetone extract was then analysed on a Turner Designs-10 fluorometer before and after acidification with 1.2 M HCl under poorly lit conditions.
All the four depths sampled for water chlorophyll analyses were integrated to get water column Chl a. Further, the depth integrated Chl a was used as ambient water Chl a to the copepods.
Copepod taxonomy and sorting
In the laboratory, the mesozooplankton samples were split into four parts using a Folsom splitter. Two sub-samples were preserved in buffered formalin (4%) for further taxonomic analysis and the remaining two were stored at −20°C until analysed for the gut pigment contents.
Taxonomic identification and enumeration of copepods was carried out from the formalin-preserved sub-samples, placed in Bogorow's chamber under stereoscopic microscope (Olympus SZX 16) using the standard identification keys of Kasturirangan (Reference Kasturirangan1963) and Conway et al. (Reference Conway, White, Hugues-Dit-Ciles, Gallienne and Robins2003). The copepod abundance was expressed as individual 100 m−3. The other two sub-samples were thawed, rinsed with filtered seawater and sorted under microscope with minimum light and then the gut pigments were analysed using a fluorometer and HPLC.
Fluorometric estimation of gut pigments
The gut fluorescence technique described by Mackas & Bohrer (Reference Mackas and Bohrer1976) with modifications proposed by Morales et al. (Reference Morales, Bautista, Hams, Bames and Gibson1990) and followed by Tseng et al. (Reference Tseng, Kumar, Dahms, Chen and Hwang2008) was carried out. For each group, known number of individuals (ranged from 20–40) were picked and kept for extraction in 6 ml of 90% acetone in dark under −20°C (Islam et al., Reference Islam, Ueda and Tanaka2005) for 24 h with no homogenization (Wong et al., Reference Wong, Hwang and Chen1998; Tseng et al., Reference Tseng, Kumar, Dahms, Chen and Hwang2008). Once the pigments were extracted, the upper clear solution was analysed on a Turner Design-10 Fluorometer in low illumination before and after acidification. Acidification was performed using 1.2 M hydrochloric acid. Literature suggested phaeopigment loss when using the gut fluorescence technique (Dagg & Wyman, Reference Dagg and Wyman1983; Tseng et al., Reference Tseng, Kumar, Dahms, Chen and Hwang2008), hence, all the phaeopigment values was multiplied by a factor of 1.51 (Dagg & Wyman, Reference Dagg and Wyman1983). Gut pigment contents were then expressed as ng/copepod for Chl a, phaeopigment and total pigment (obtained from the addition of Chl a and corrected phaeopigment concentrations in the copepod gut; Dam & Peterson, Reference Dam and Peterson1988).
Gut pigment analysis by HPLC
Approximately 300 individuals per copepod order were required for sample analysis using HPLC; the dominant orders such as Calanoida and Poecilostomatoida were analysed for qualitative pigment assessment. The required numbers of copepods were sorted and placed in 2 ml of HPLC grade methanol. The samples were not macerated because previously analysed samples did not show much variation in the pigment extracted with or without sonication. The samples were kept in a refrigerator at −20°C for 24 h in the dark for pigment extraction. The clear extract was then collected in 3 ml amber coloured glass vial and passed directly into the sampler tray for analysis (Gasparini et al., Reference Gasparini, Daro, Antajan, Tackx, Rousseau, Parent and Lancelot2000).
Eclipse XDB C8 HPLC column (4.6 × 150 mm) manufactured by Agilent Technologies was used to carry out the analysis. Methanol and mixture of (70:30) methanol and 1 M ammonium acetate (pH 7.2) were the solvents used for elution. The eluting pigments were detected at 450 and 665 nm (excitation and emission) by the diode array detector. All the chemicals used were of HPLC grade (E. Merck, Germany).
Statistical analysis
Spearman's non-parametric correlation was performed to observe the pattern of variation of gut Chl a and gut phaeopigments in different copepods orders. Further, two-way analysis of variance (ANOVA) was performed to examine the significant seasonal and depth-wise variation in water Chl a and phaeopigment. Similarly, ANOVA was carried out to check significant seasonal variations of total gut pigments in different copepod orders. ANOVA was followed by Tukey's post hoc test to reveal the significant variation within the different seasons. Values were considered significant at 95% level of confidence (Statistica 6.0, Statsoft, OK, USA).
RESULTS
Hydrography
The results on salinity have been adopted from the previously published article by Naqvi et al. (Reference Naqvi, Naik, Jayakumar, Shailaja, Narvekar and Neretin2006) that depicted a decadal variation of this parameter from the study region. This work reports distinct variations during monsoon with lower values in salinity. The minimum salinity value of 34.8 and maximum of 36.0 were examined during monsoon and pre-monsoon.
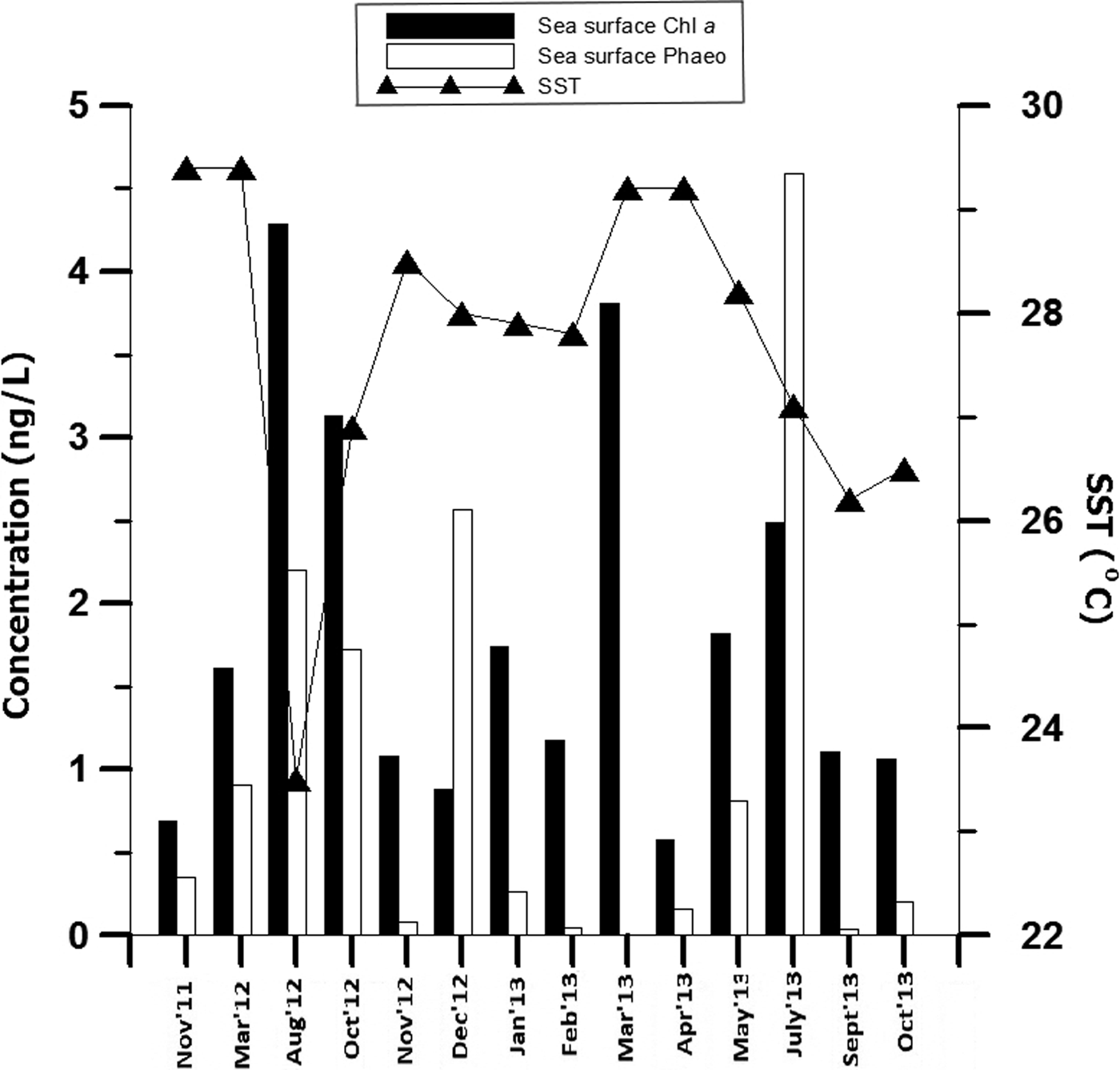
The minimum and maximum temperature recorded during the present study ranged 23.5–29.4°C during monsoon (August 2012) and pre-monsoon season (March 2012; Figure 2). The highest (4.29 ng l−1) and lowest (0.69 ng l−1) concentration of surface water Chl a was observed during monsoon (August 2012) and pre-monsoon (April 2013). The lowest (0.05 ng l−1) and highest (4.59 ng l−1) concentration of water phaeopigment was recorded during pre-monsoon (March 2013) and monsoon (July 2013), respectively.
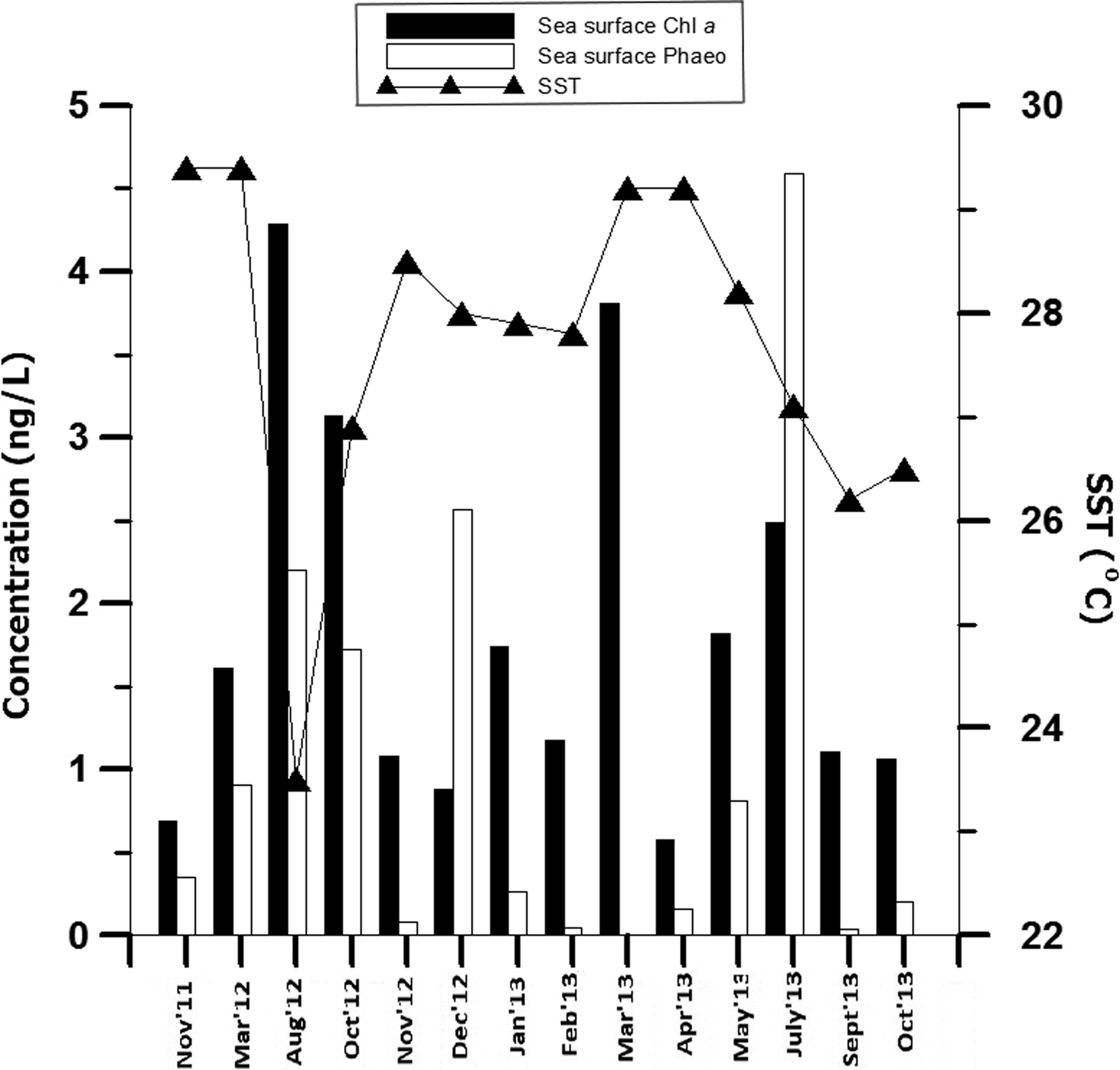
Fig. 2. Temporal variation in the sea surface physical parameters (chlorophyll a, phaeopigment, temperature) recorded at coastal station (G5).
Seasonal variation in Copepoda
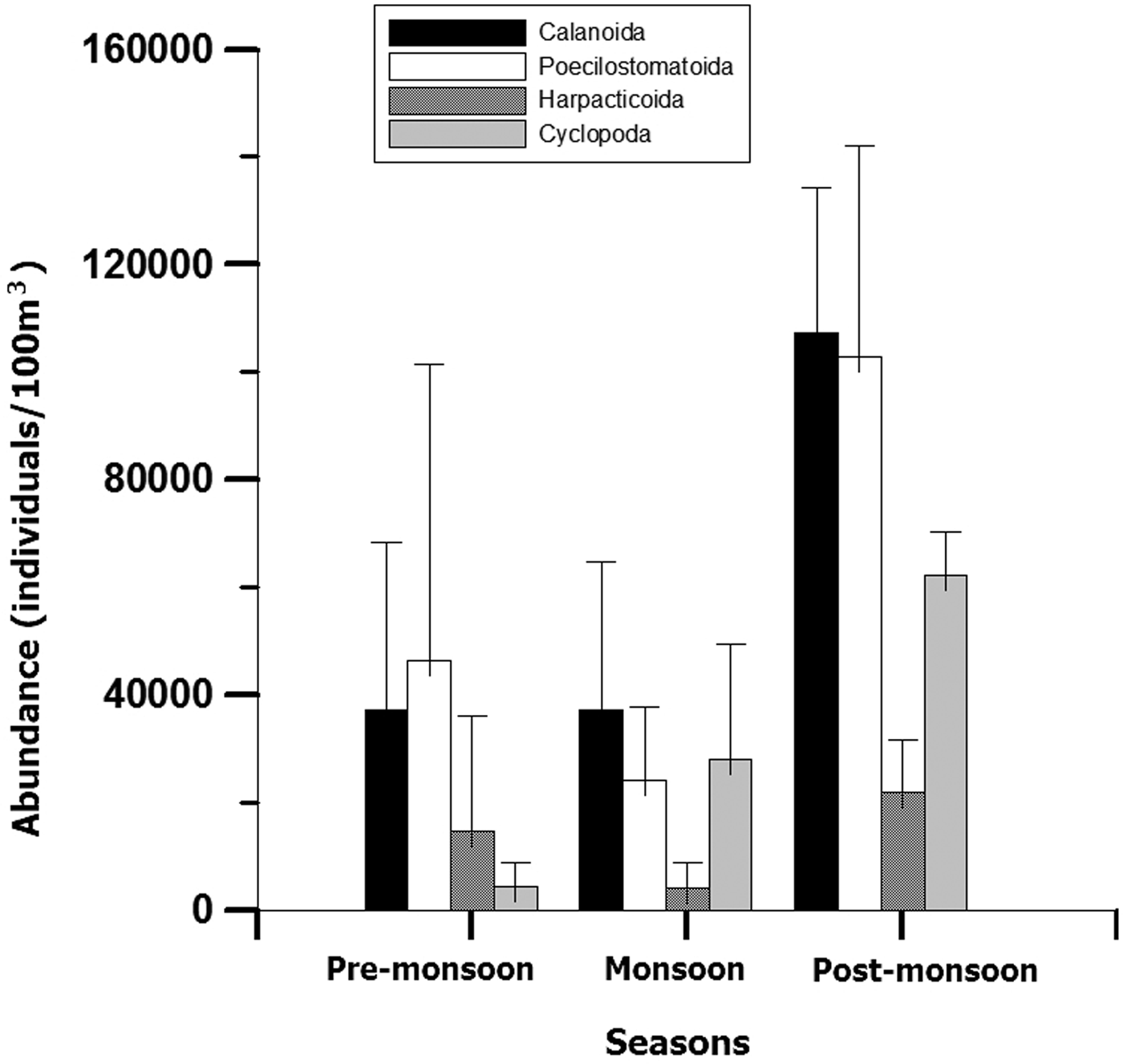
Copepods, the dominant mesozooplankton group, comprised >80% of the abundance. Seasonally, low copepod abundance accounted for 93,600 ± 30,399 ind. 100 m−3 (Figure 3) with dominance of species Oncaea venusta during monsoon. Highest copepod density was observed in post-monsoon (133,062 ± 76,342 ind. 100 m−3), dominated by Acrocalanus spp. Amongst the copepod orders, Calanoida dominated the copepod community throughout the year with an occasional dominance of Poecilostomatoida (46,543 ± 28,178 ind. 100 m−3; during pre-monsoon). The dominant Calanoida and Poecilostomatoida families represented annually were Paracalanidae and Oncaeidae, respectively.

Fig. 3. Seasonal variation of abundance (mean value ± SD) for different copepod orders at coastal station (G5).
The species best represented continuously throughout the year were considered for studying feeding habits. Therefore gut pigment analyses on the following species were undertaken. Calanoida comprised Acrocalanus spp., Paracalanus spp., Subeucalanus spp., Temora spp. and Acartia spp.; Harpacticoida was represented by Euterpina sp.; Poecilostomatoida by Oncaea spp. and Corycaeus spp.; Cyclopoida was represented by Oithona spp. Dominant Calanoida species which were potentially herbivores according to the existing knowledge of their feeding biology through literature review were taken into account.
Variation in water column Chl a and phaeopigment
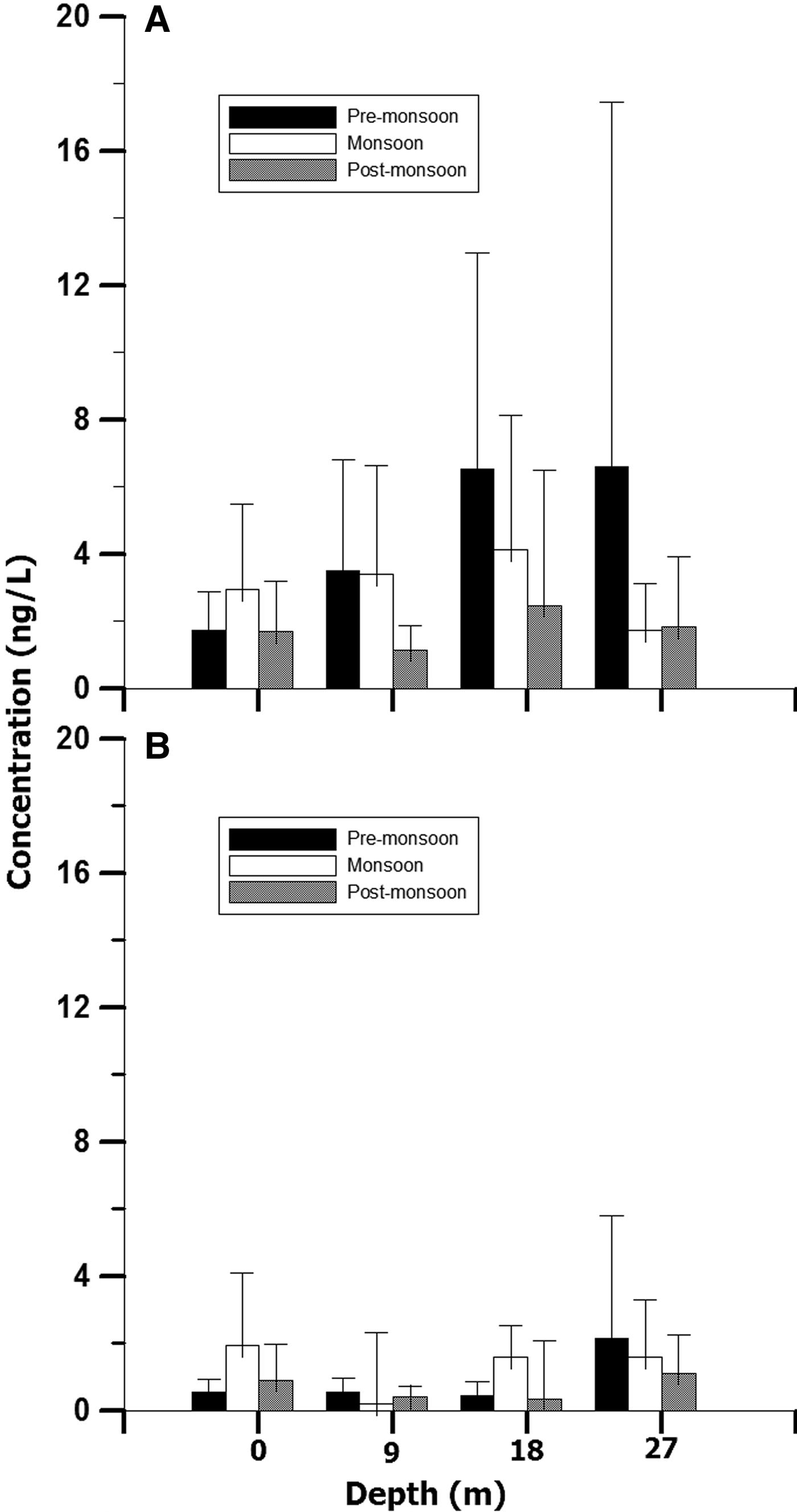
The water Chl a content in the surface water ranged 1.69–2.96 ng l−1; minimum and maximum concentration recorded during post-monsoon and monsoon (Figure 4A). The minimum (0.59 ng l−1) and maximum (1.93 ng l−1) concentration of phaeopigment content in the surface water was recorded during pre-monsoon and monsoon season (Figure 4B). The water Chl a content at 9 m depth ranged 1.14–3.50 ng l−1, at 18 m 2.47–6.52 ng l−1 and at 27 m 1.73–6.60 ng l−1. The phaeopigment concentration ranged 0.20–0.54 ng l−1 at 9 m, 0.35–1.58 ng l−1 at 18 m and 1.11–2.15 ng l−1 at 27 m depth. On the contrary to the surface water Chl a, the other depth zones (9, 18 and 26 m) showed highest water Chl a values during pre-monsoon. Besides, highest phaeopigment concentrations for 9 and 26 m deep waters were observed during post-monsoon. Seasonally, the Chl a variation differed significantly (P < 0.001; Table 1), nevertheless no significant variation was observed within the depths and the interaction between the season–depth. Further, post hoc test revealed significantly high Chl a in monsoon (P < 0.001).

Fig. 4. Seasonal variation of water pigments (A) chlorophyll a and (B) phaeopigments (mean value ± SD) at different depths at coastal station (G5).
Table 1. Results of two-way ANOVA comparing seasonal and depth-wise variation of water chlorophyll a and phaeopigment.
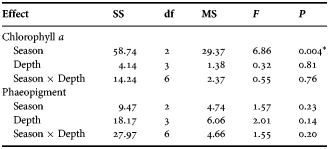
*Indicates variables that were significant.
Temporal variation in gut Chlorophyll a and phaeopigment content in Copepoda
Quantitative analysis of gut pigment content of Calanoida, Poecilostomatoida, Harpacticoida and Cyclopoida was carried out on a monthly basis from November 2011 to October 2013. Lapse in data for a few months is due to either difficulty in sampling during the rough weather or absence of sufficient number of individual species required for analysis. Also, duplicate samples analysis was carried out wherever adequate numbers of organisms of copepod orders were available.
The gut Chl a and phaeopigment in copepods showed distinct variation (Figure 5). Estimates of gut Chl a and phaeopigment content for the Calanoida copepods were highest during monsoon (July 2013; Figure 5A). However, the lowest value for gut Chl a and phaeopigment content was observed during pre-monsoon (April 2013) and post-monsoon (November 2012), respectively. Also, their gut pigments showed the highest variability for gut Chl a (0.02–1.02 ng copepod−1) and gut phaeopigment (0.11–3.26 ng copepod−1; Figure 5A) amongst Poecilostomatoida, Harpacticoida and Cyclopoida. Calanoida exhibited comparatively larger variability in body size (0.93–2.6 mm). In Poecilostomatoida, the gut Chl a content ranged between 0.013–0.548 ng copepod−1 and gut phaeopigment ranged from 0.07–1.23 ng copepod−1 (Figure 5B). The highest copepod gut Chl a was observed in the month of October 2012 and least in August 2013.The highest (1.11 ng copepod−1) and lowest (0.07 ng copepod−1) gut phaeopigment content was observed in August 2012 and December 2012, respectively. Furthermore, its total body size ranged from 1.00–1.27 mm.

Fig. 5. Temporal variation of gut chlorophyll a and gut phaeopigment contents (mean value ± SD) in copepod orders (A) Calanoida, (B) Poecilostomatoida, (C) Harpacticoida and (D) Cyclopoida at coastal station (G5).
Although Harpacticoida comprised minimal body size (0.35–0.66 mm), it yielded the second highest gut Chl a pigment range (0.03–0.693 ng copepod−1; Figure 5C). The highest concentration of harpacticoid gut Chl a was observed in August 2012 and the lowest was recorded in July 2013. The phaeopigment content was in the range of 0.098–1.712 ng copepod−1 (Figure 5C) with least value recorded in November 2012 and highest during March 2012. Cyclopoida had the most slender body and their size ranged from 0.75–1.50 mm. Comparatively, this order yielded the least content of gut Chl a pigment and phaeopigment content that ranged from 0.014–0.42 and 0.06–1.45 ng copepod−1 (Figure 5D), respectively. The highest and lowest values for copepod gut Chl a were observed in October 2012 and March 2012, respectively. The gut phaeopigment concentration was least during December 2012 and highest in July 2013.
Overall, inter-annual and temporal variability were observed in gut Chl a pigment and gut phaeopigment content of copepods. Equally, the pattern of variation of gut Chl a and phaeopigment differed. The Chl a was always less copious than phaeopigment in the gut of copepods; however, no significant statistical relation was observed. An exception was noted in March 2013, when all copepod orders had gut Chl a higher compared with gut phaeopigment.
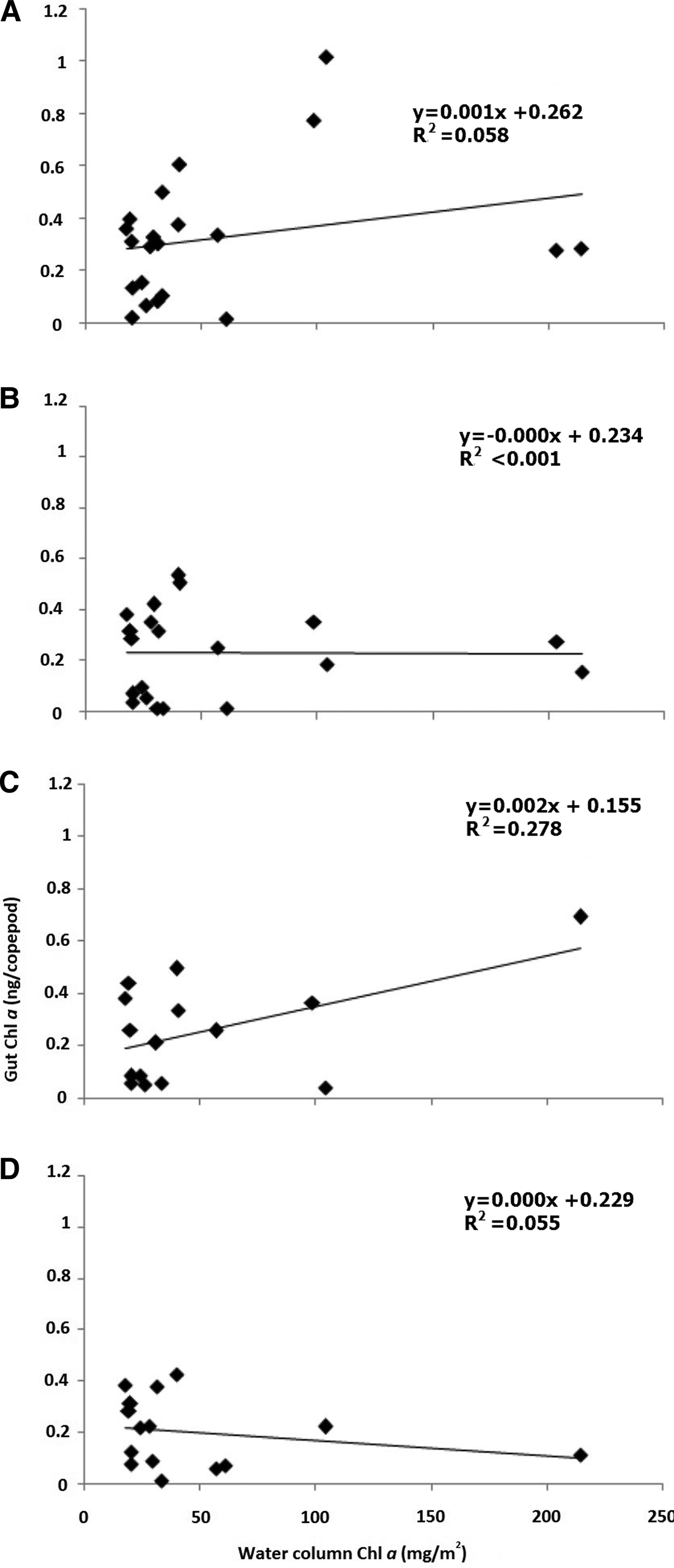
The integrated water column Chl a recorded highest during monsoon (86.08 mg m−2) followed by post-monsoon (46.26 mg m−2) and the least during pre-monsoon (42.80 mg m−2). When integrated water column Chl a was correlated with gut Chl a of all the four copepod orders, no significant correlation was observed. The lack of correlation apprehended for Calanoida (N = 21, r 2 = 0.06; Figure 6A), Poecilostomatoida (N = 20, r 2 ≤ 0.01; Figure 6B), Harpacticoida (N = 15, r 2 = 0.28; Figure 6C) and Cyclopoida (N = 15, r 2 = 0.06; Figure 6D).
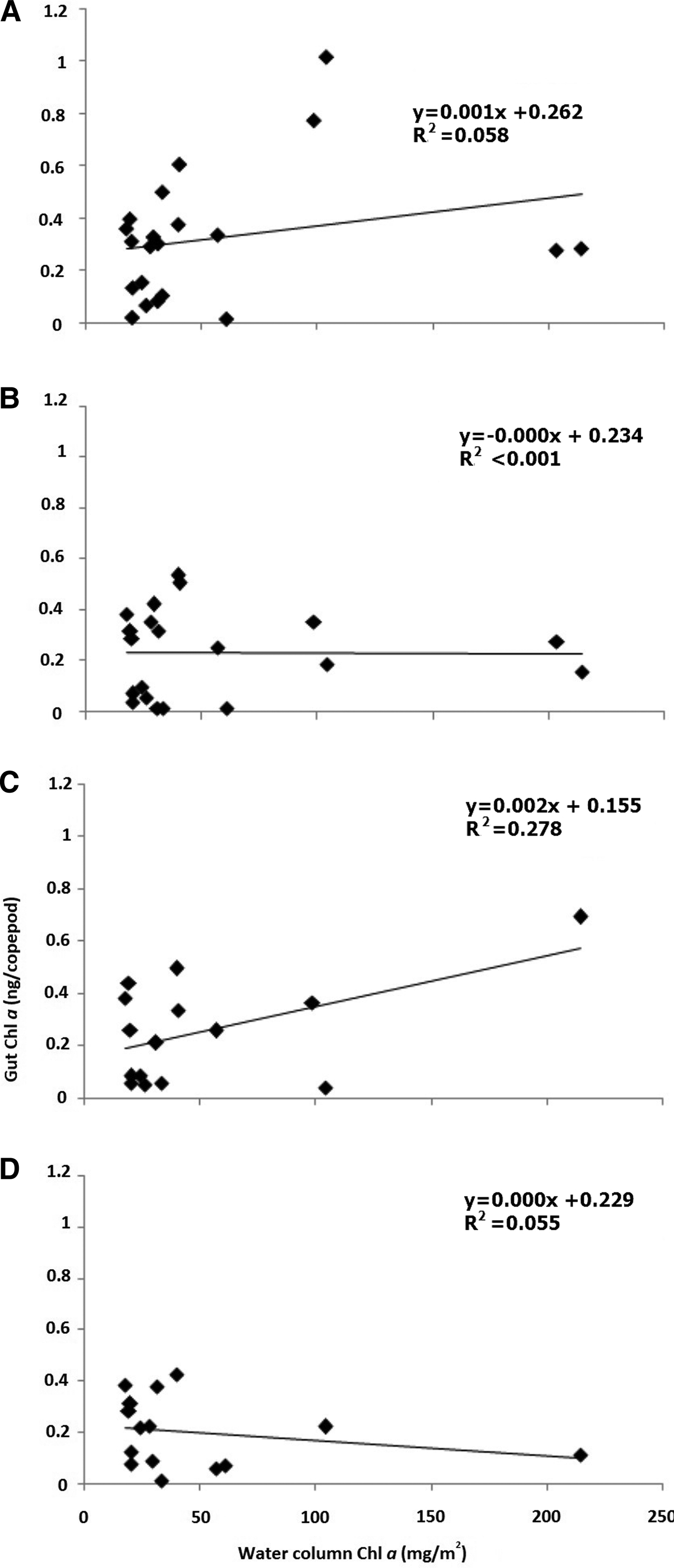
Fig. 6. Correlation between integrated water column chlorophyll a and copepod gut chlorophyll a content for copepod orders (A) Calanoida, (B) Poecilostomatoida, (C) Harpacticoida and (D) Cyclopoida from November 2011 to October 2013. The water column Chl a has been integrated for four depths viz., 0, 9, 18 and 27 m.
Seasonal variation in total gut pigment in Copepoda
The data were presented on a seasonal scale; specifically, monsoon (June–September), post-monsoon (October–January) and pre-monsoon (February–May). Further, qualitative analysis of gut pigment content was carried out following HPLC technique on a seasonal basis for the copepod orders Calanoida and Poecilostomatoida. Due to unavailability of the required number of individual species of Harpacticoida and Cyclopoida, those orders were not taken into consideration for analysis.
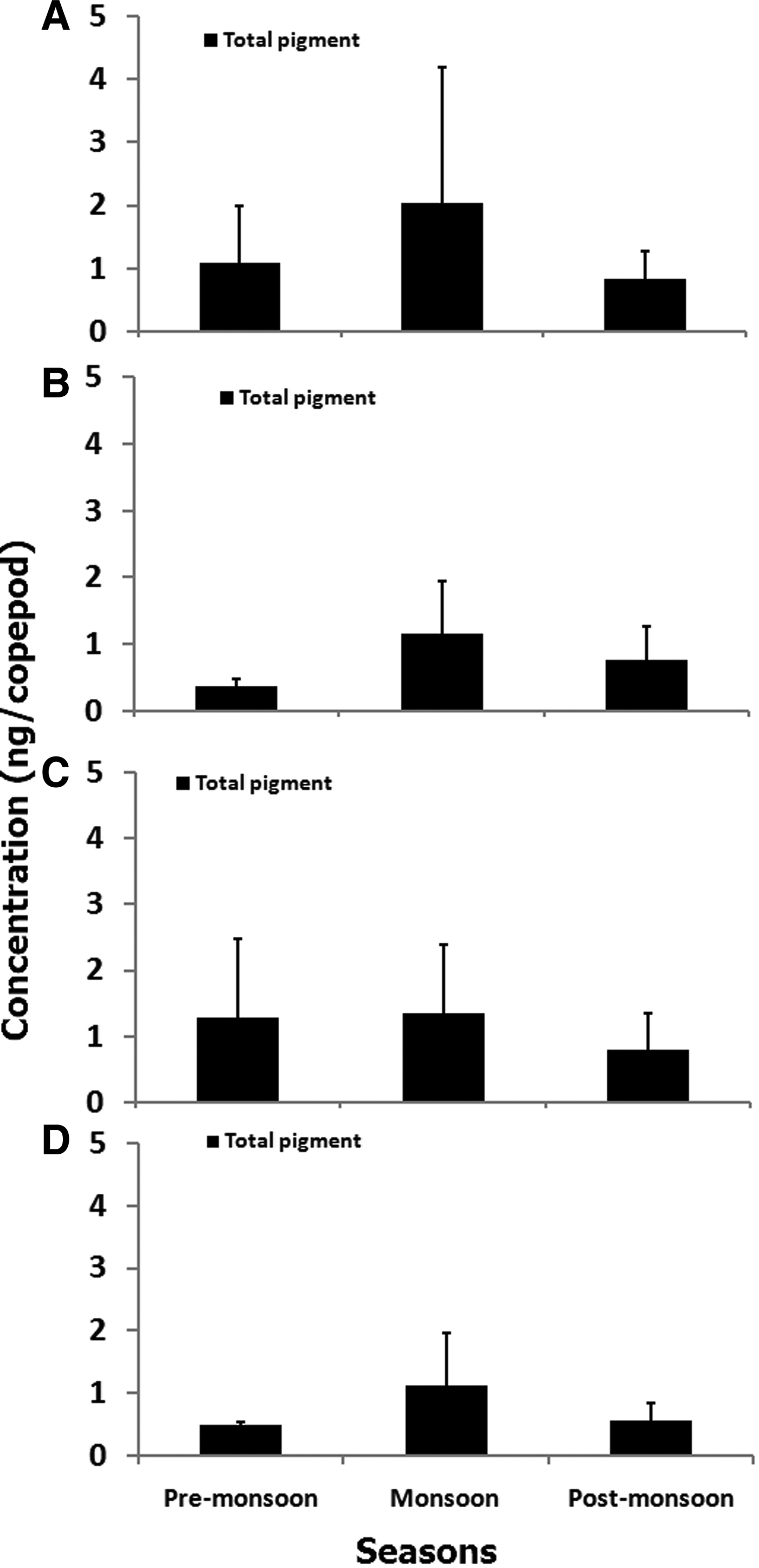
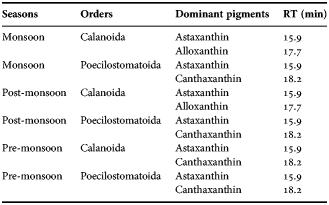
Estimates of total gut pigment contents for the copepod orders showed higher concentration during monsoon than during pre-monsoon and post-monsoon. The total gut pigment (Chl a and corrected phaeopigments) in Calanoida varied from 0.27–5.93 ng copepod−1 (Figure 7A) more than that of Poecilostomatoida, Cyclopoida and Harpacticoida. Seasonally, monsoon depicted the highest gut pigment content (2.02 ± 2.15 ng copepod−1). Further, qualitative analysis revealed predominantly astaxanthin, canthaxanthin and alloxanthin pigments (Table 2). However, alloxanthin, the marker pigment of Cryptophyta, was conspicuous by its absence in pre-monsoon suggesting seasonality in gut pigment composition of Calanoida. The total gut pigments ranged between 0.12 and 2.01 ng copepod−1 (Figure 7B) for Poecilostomatoida. Again, the highest gut pigment content, 1.15 ± 0.79 ng copepod−1 was noticed in monsoon. The qualitative gut pigment composition revealed predominantly canthaxanthin and astaxanthin (Table 2). In addition, an HPLC absorbance chromatogram depicted a few tiny peaks that were eluted at lower limits of detection hence confirmation of their identity was critical.
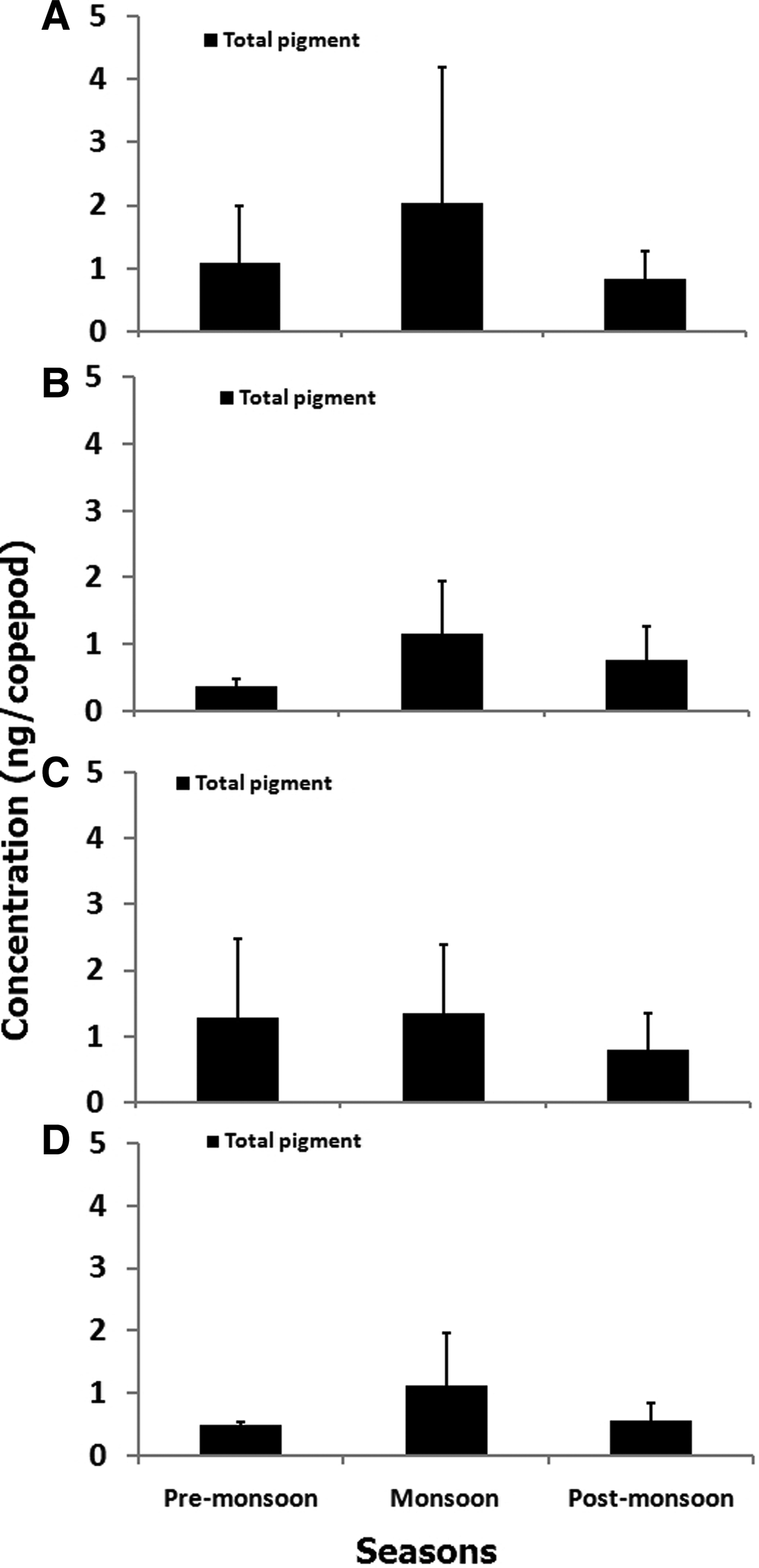
Fig. 7. Seasonal variation of total pigment (±SD) in orders of copepods: (A) Calanoida, (B) Poecilostomatoida, (C) Harpacticoida and (D) Cyclopoida at coastal station (G5). Total gut pigment is summation of gut chlorophyll a and corrected gut phaeopigment (gut phaeopigment × 1.51) content.
Table 2. Seasonal variation of gut pigment composition in copepods.
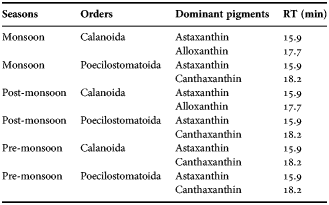
RT is the retention time.
Harpacticoids attained the second highest total gut pigment values that ranged from 0.20–2.75 ng copepod−1 (Figure 7C). However, a decline in total gut pigment was prominent in post-monsoon although gradual elevation was observed during the pre-monsoon season. Furthermore, the seasonal variation showed a similar trend to that observed in Calanoida copepods. Similarly, total gut pigment content in Cyclopoida varied from 0.17–2.41 ng copepod−1 (Figure 7D) with highest values noticed in monsoon (1.12 ± 0.82 ng copepod−1). Further, the variation of gut pigment concentration was similar to that of Poecilostomatoida, with descending concentration from monsoon to post-monsoon and pre-monsoon.
Total gut pigment content of copepods revealed a greater contribution of phaeopigment than chlorophyll. Also, copepods were found to contain photosynthetic pigments in their gut throughout the year. However, gut pigment concentrations were at a maximum during monsoon indicating significant variability (P < 0.01; Table 3). Further post hoc tests revealed high value during post-monsoon (P < 0.01).
Table 3. Two-way ANOVA comparing seasonal variation of total gut pigments for copepod orders.

*Indicates variables that were significant. The season showing significantly highest mean values from the Tukey's post hoc test is shown.
DISCUSSION
The IWCS is governed by the monsoon regime and experiences diverse biochemical phenomena that appear to modulate copepod distribution as well as their feeding habits. The cusp of biochemical phenomena occurring in the region is the reciprocal action of upwelling (from July/August to October/November) and downwelling (during the rest of the year) that hold in check the oxygen conditions in benthic waters (Maya et al., Reference Maya, Karapurkar, Naik, Roy, Shenoy and Naqvi2011). Moreover, IWCS is a productive system due to nutrient enrichment owing to coastal upwelling and riverine run-off due to monsoonal flushing (Pratihary et al., Reference Pratihary, Naqvi, Narvenkar, Kurian, Naik, Naik and Manjunatha2014). In addition, freshening of coastal surface waters is known to occur during the monsoon due to freshwater supply from rivers along the Indian west coast (Jayakumar et al., Reference Jayakumar, Naqvi, Narvekar and George2001; Suprit & Shankar, Reference Suprit and Shankar2008). A distinctive feature of the IWCS is seasonal anoxia (Naqvi et al., Reference Naqvi, Naik, Jayakumar, Shailaja, Narvekar and Neretin2006), although this is confined to the near-shore region of the shelf (Pratihary et al., Reference Pratihary, Naqvi, Narvenkar, Kurian, Naik, Naik and Manjunatha2014). Eventually, the monsoonal effects result in intense anoxia during the early post-monsoon period (October–November). Another crucial event of seasonal fluctuation in phytoplankton composition occurs in the study region as described previously by Parab et al. (Reference Parab, Prabhu Matondkar, Gomes and Goes2006). A pigment study from this region reported a plethora of tiny phytoplankton groups dominated by prymnesiophytes and green algae during monsoon (Roy et al., Reference Roy, Pratihary, Gauns and Naqvi2006). Likewise, blooms of nitrogen fixers, Trichodesmium have long been known to occur in Indian coastal waters during pre-monsoon (Devassy et al., Reference Devassy, Bhattathiri and Qasim1978; Parab et al., Reference Parab, Prabhu Matondkar, Gomes and Goes2006; Roy et al., Reference Roy, Pratihary, Gauns and Naqvi2006).
Although mesozooplankton are considered to be an important component of the marine food chain, information on copepod feeding types was based on morphology of mouth parts (Madhupratap, Reference Madhupratap1999). In the present study, copepod abundance and taxonomy are in close agreement with prior data reported from the Arabian Sea by Madhupratap et al. (Reference Madhupratap, Gopalkrishnan, Haridas, Nair, Aravindakshan and Padmavati1996), Padmavati et al. (Reference Padmavati, Haridas, Nair, Gopalakrishnan, Shiney and Madhupratap1998) and Smith & Madhupratap (Reference Smith and Madhupratap2005). Four dominant copepod orders, namely Calanoida, Poecilostomatoida, Harpacticoida and Cyclopoida, were consistently recorded from this study. Interestingly, the low copepod abundance during monsoon may be attributed to factors such as freshening of the system and coastal upwelling. The highest copepod abundance during post-monsoon corresponds to the breeding season of organisms. Also, ambient Chl a concentration was >1 µg l−1 throughout the water column (Figure 3). It is an indicator of productive waters and therefore, feeding preference for herbivorous/omnivorous copepod would be autotrophic prey. All the copepod orders (Calanoida, Poecilostomatoida, Harpacticoida and Cyclopoida) showed presence of undegraded chlorophyll. This indicated that copepods were non-diapausing and were actively grazing on the abundantly available autotrophic biomass throughout the year.
The standard 200 µm mesh used to collect mesozooplankton would obtain samples that are comparable to previous studies (Madhupratap et al., Reference Madhupratap, Gopalkrishnan, Haridas, Nair, Aravindakshan and Padmavati1996; Padmavati et al., Reference Padmavati, Haridas, Nair, Gopalakrishnan, Shiney and Madhupratap1998; Smith & Madhupratap, Reference Smith and Madhupratap2005) albeit absolute values of small copepods may be biased. Therefore, present data on copepod abundance might inefficiently capture small-sized poecilostomatoids, cyclopoids, copepodites and their nauplii. In addition, the seasonal patterns of copepod assemblage require adjustments in fine and coarse mesh size according to the temporal change in diversity. The breeding season for most of the copepods in the Indian waters was during the post-monsoon period, although a few species breed continuously throughout the year (Ummerkutty, Reference Ummerkutty1965). Consequently, in monsoon, copepod nauplii would seldom be encountered and the underestimation would be preferably negligible. From the experience of the present study, for future research it will be best to use a smaller mesh size along with 200 µm.
The findings of the present study showed higher gut pigment contents in Calanoida that belong to the comparatively larger body size (0.93–2.6 mm). First, it may be the result of consideration of only herbivores belonging to Calanoida from the studied region. Second, the larger forms are known to accumulate more gut pigments (Morales et al., Reference Morales, Bautista, Hams, Bames and Gibson1990; Tseng et al., Reference Tseng, Kumar, Dahms, Chen and Hwang2008, Reference Tseng, Dahms, Chen and Hwang2009), as they have larger gut volume and metabolic expenditures. The value of Chl a thus seems to increase in copepod gut with escalating body sizes. Additionally, studies on ingestion of phytoplankton by copepods revealed that the minimal size limit of feed was 2 µm (Roman & Gauzens, Reference Roman and Gauzens1997). In this view, Lie et al. (Reference Lie, Wong and Wong2013) suggested that the large phytoplankton was inadequately grazed by the small copepods in Tolo Harbour as the transfer efficiency was low (1.4%) among phytoplankton (primary production) and copepods (secondary production). Thus, it appears that size range of copepod feed is another important component governing the gut content estimates.
The dissimilar pattern of variation for Chl a and phaeopigment in copepod gut is indicative of governance of diverse processes for their distribution. Among these, photo-degradation of Chl a to phaeopigment could be one of the probable reasons as the organisms are exposed to light in the natural habitat and during sorting of samples under the microscope (Islam et al., Reference Islam, Ueda and Tanaka2005). Furthermore, the growth phase and size of the phytoplankton cell consumed by the copepods also portray the variation in Chl a concentration (Uye, Reference Uye1986; Bautista & Harris, Reference Bautista and Harris1992; Tan et al., Reference Tan, Huang, Chen and Huang2004). On the other hand, the degree of degradation and pigment loss in the copepod guts could fluctuate under diverse circumstances such as the concentration of feed in ambience, digestion of the chlorophyll-bearing material and history of feed of the organism (Dagg & Walser, Reference Dagg and Walser1987; Penry & Frost, Reference Penry and Frost1991; Head, Reference Head1992; Head & Harris, Reference Head and Harris1994). It might have also been affected by ingestion of detritus and coprophagy resulting in varied distribution (Goes et al., Reference Goes, Caeiro and Gomes1999).
In our study, phaeopigment concentration in copepod guts was mostly found to be higher than Chl a values. This might be because of rapid degradation of ingested chlorophyll to phaeopigments that eventually remains unaffected (Shuman & Lorenzen, Reference Shuman and Lorenzen1975). Also, variable phaeopigment:chlorophyll ratio in gut signifies the amount of recently ingested chlorophyll but phaeopigments (phaeophorbide and phaeophytin) generally make up the major part of the total pigments assessed (Shuman & Lorenzen, Reference Shuman and Lorenzen1975; Hallegraeff, Reference Hallegraeff1981; Dagg & Wyman, Reference Dagg and Wyman1983; Islam et al., Reference Islam, Ueda and Tanaka2005; Tseng et al., Reference Tseng, Kumar, Dahms, Chen and Hwang2008). Goes et al. (Reference Goes, Caeiro and Gomes1999) speculate that the higher per cent of phaeopigment is due to the reingestion of the already evacuated particulate organic matter. Conspicuously, in March 2013, gut Chl a was higher than gut phaeopigment in all copepod orders and water phaeopigments were below the detectable limit. Such inter-annual variability between the water pigments and the copepods gut pigments needs to be monitored for longer duration to understand the pigment dynamics.
However, bioconversion of Chl a to detectable phaeopigment is controversial; Shuman & Lorenzen (Reference Shuman and Lorenzen1975) supported complete degradation of chlorophyll into phaeophorbide, while Hallegraeff (Reference Hallegraeff1981) suggested up to 20–50% conversion of Chl a into phaeophorbide and the rest to phaeophytin takes place. Further, Bustillos-Guzman et al. (Reference Bustillos-Guzman, Lopez-Cortes, Mathus and Hernandez2002) suggested that individual phaeopigments could be produced at different rates in accordance with the chemical/enzymatic reaction acting differently on the chlorophylls in the copepod gut. Besides, lack of information on Chl a degradation and turnover rates is the major shortcoming of the present method. Furthermore, we did not evaluate the pigment loss during this study and opted to correct for pigment damage using an average estimate value of 33% (Dam & Peterson, Reference Dam and Peterson1988).
The total gut pigments content of copepods were 0.16–4.13 ng copepod−1 (Figure 7); the values were lower than those documented in Dabob Bay, Washington (0.23–8.35 ng copepod−1; Dagg et al., Reference Dagg, Frost and Walser1989). Sampling in daytime might have recorded the comparably low copepod gut content estimates. Prior studies (Mackas & Bohrer, Reference Mackas and Bohrer1976; Saito & Taguchi, Reference Saito and Taguchi1996; Islam et al., Reference Islam, Ueda and Tanaka2005; Tseng et al., Reference Tseng, Kumar, Dahms, Chen and Hwang2008; Wu et al., Reference Wu, Gao, Wang and Liu2013) suggest that gut pigment contents in copepods were usually higher during the nocturnal hours and minimal at daylight hours. Besides this, faster gut evacuation rate and occurrence of gut pigment destruction also possibly led to underestimation of the observed values (Morales et al., Reference Morales, Bedo, Harris and Tranter1991; Bollens & Landry, Reference Bollens and Landry2000). However, the values were comparable to those estimated by Tseng et al. (Reference Tseng, Kumar, Dahms, Chen and Hwang2008, Reference Tseng, Dahms, Chen and Hwang2009) and higher compared with those reported by Islam et al. (Reference Islam, Ueda and Tanaka2005).
Although copious autotrophic biomass was available in the studied region, particular copepod species may prefer different size fractions to prey upon. This may be why no significant correlation was observed between total water Chl a and gut Chl a of Calanoida, Poecilostomatoida, Cyclopoida and Harpacticoida (Figure 6). The present observations are consistent with the views of Dagg & Wyman (Reference Dagg and Wyman1983), Dam & Peterson (Reference Dam and Peterson1988) and Li et al. (Reference Li, Sun, Wang and Wang2004). Such surveillances are also suggestive of diel feeding rhythms, individual variance and/or feeding synchronization (Uye & Yamamoto, Reference Uye and Yamamoto1995; Li et al., Reference Li, Sun, Wang and Wang2004). On the other hand, significant positive correlation of copepod gut Chl a and ambient water Chl a has been reported from less productive waters (Tseng et al., Reference Tseng, Dahms, Chen and Hwang2009).
Gut pigment contents in copepods were observed to be higher in the monsoon (Figure 7) that coincided with low copepod abundance (Figure 2). It implies less inter-species competitive stress on copepods for favoured feed. At the same time, copepods may be facing predation pressure for efficient energy transfer to the higher consumers. The shift in community structure of phytoplankton seems to be a crucial factor leading to seasonality in feeding habits. Existence of cyanobacterial dominance, especially Trichodesmium sp. in pre-monsoon followed by a diatom-rich community in monsoon and dinoflagellates in post-monsoon have been reported from the Arabian Sea (Parab et al., Reference Parab, Prabhu Matondkar, Gomes and Goes2006; Pratihary et al., Reference Pratihary, Naqvi, Narvenkar, Kurian, Naik, Naik and Manjunatha2014). Additionally, in pre-monsoon, Noctiluca scintillans bloom that is considered to be an undesired food by copepods was recorded (Gomes et al., Reference Gomes, Goes, Matondkar, Buskey, Basu, Parab and Thoppil2014). Gaonkar & Anil (Reference Gaonkar and Anil2012) revealed gut pigment content in barnacle larvae from neighbouring waters of the study region. Although our data cannot be directly compared with barnacle larvae, it is interesting to note that the observed seasonality in the gut pigment content were higher in post-monsoon as compared with pre-monsoon season. A report on fishery of the Arabian Sea by Madhupratap et al. (Reference Madhupratap, Nair, Gopalkrishnan, Haridas, Nair, Venugopal and Gauns2001) pointed out abundances of planktonivorous fishes in the region with highest catches observed between October and March. Interestingly, in this study high abundance of copepods was noted during post-monsoon. Combining this information in view of trophic levels suggests that the copepods play a vital role in the sustenance of fishery in this realm.
Predominance of astaxanthin in copepod gut is eminent. Most researchers considered astaxanthin to be derivative of canthaxanthin, which is produced from β- carotene via echinenone in herbivores (Goodwin, Reference Goodwin, Florkin and Scheer1971; Lotocka & Styczynska-Jurewicz, Reference Lotocka and Styczynska-Jurewicz2001; Caramujo et al., Reference Caramujo, de Carvalho, Silva and Carman2012). Such a mode of bioconversion of dietary carotenoid is generally considered the pathway in aquatic organisms (Goodwin, Reference Goodwin, Florkin and Scheer1971; Caramujo et al., Reference Caramujo, de Carvalho, Silva and Carman2012), but a few researchers have considered astaxanthin as an animal pigment (Gasparini et al., Reference Gasparini, Daro, Antajan, Tackx, Rousseau, Parent and Lancelot2000) due to its presence even in starved copepods. However, with the available literature, it is reasonable to consider astaxanthin as a marker pigment for omnivory (see Juhl et al., Reference Juhl, Ohman and Goericke1996). Hence, presence of canthaxanthin in Calanoida and Poecilostomatoida gut (Table 2) is suggestive of herbivorous and astaxanthin of omnivorous feeding habits. The current study portrays presence of astaxanthin and canthaxanthin in Calanoida that is in accordance with Lotocka & Styczynska-Jurewicz (Reference Lotocka and Styczynska-Jurewicz2001) and Holeton et al. (Reference Holeton, Lindell, Holmborn, Hogfors and Gorokhova2009). The pigment, astaxanthin, plays an important role in copepods by being a potent antioxidant for protecting lipids, photo-protection against photosynthetically active radiation and UV light (Hairston, Reference Hairston1980; Terao, Reference Terao1989; Holeton et al., Reference Holeton, Lindell, Holmborn, Hogfors and Gorokhova2009; Hansson, Reference Hansson2004). Additionally, astaxanthin is suggested to act as a precursor of vitamin A and retinoid compounds (Schiedt et al., Reference Schiedt, Leuenberger and Vecchi1985; Holeton et al., Reference Holeton, Lindell, Holmborn, Hogfors and Gorokhova2009). Also, fishes such as salmon require astaxanthin for their characteristic red colour (Olsen et al., Reference Olsen, Kiessling, Milley, Ross and Lall2005) and for certain aspects of immunity (Thompson et al., Reference Thompson, Choubert, Houlihan and Secombes1995) but cannot produce it de novo. Hence, copepods with astaxanthin pigment can be a source of astaxanthin for the fish stock.
A notable observation is the presence of alloxanthin in calanoids that varied at seasonal scale (Table 2). The gut alloxanthin recorded during monsoon and post-monsoon could be due to the feeding on Cryptophyceae from ambient water. This theory is based on previous work of Maya et al. (Reference Maya, Karapurkar, Naik, Roy, Shenoy and Naqvi2011) that reported seasonal variations in water alloxanthin concentration, with trace quantities during pre-monsoon. Also, dominance of Trichodesmium sp. (devoid of alloxanthin) was reported during pre-monsoon (Parab et al., Reference Parab, Prabhu Matondkar, Gomes and Goes2006). Thus, our finding roughly conforms to the universal assumption of selective grazing of calanoids. At the same time, consideration of alloxanthin as marker pigment of cryptophytes is a sensitive statement as sometimes it is considered as an alloxanthin-like animal pigment (Pandolfini et al., Reference Pandolfini, Thys, Leporcq and Descy2000). Nevertheless, our observation of alloxanthin as a cryptophytes marker pigment is favoured by other studies (Breton et al., Reference Breton, Sautour and Brylinski1999; Cotonnec et al., Reference Cotonnec, Brunet, Sautour and Thoumelin2001), and there are reports on presence of astaxanthin and alloxanthin in copepods (Juhl et al., Reference Juhl, Ohman and Goericke1996) in particular Temora longicornis (Calanoida) (Antajan & Gasparini, Reference Antajan and Gasparini2004). These facts imply caution while considering alloxanthin to be a marker pigment in copepod gut.
In this study, the presence of fucoxanthin, a marker pigment for diatoms (Jeffrey, Reference Jeffrey1974) was not detected, probably due to pigment degradation in the gut passage (Head & Harris, Reference Head and Harris1994). It has been reported that fucoxanthin degrades faster than chlorophyll derivatives into undetectable compounds (Antajan & Gasparini, Reference Antajan and Gasparini2004). In addition, it could be due to the low concentration of fucoxanthin eluted on chromatogram as low intensity peaks went unidentified. It is noteworthy that chlorophyll pigments went undetected by HPLC for no known reason. One possibility could be that pigment decomposition occurred during gut passage in copepods. Similarly, Kleppel & Pieper (Reference Kleppel and Pieper1984) and Kleppel et al. (Reference Kleppel, Willbanks and Pieper1985) considered carotenoids to be more conserved compared with chlorophylls in copepod guts. Further investigation on pigment dynamics is required on this aspect on copepod feeding behaviour for better understanding.
Previous documentation on microscopic gut examination of Poecilostomatoida species revealed presence of phyto- as well as zooplankter (Ohtsuka et al., Reference Ohtsuka, Shimozu, Tanimura, Fukuchi, Hattori, Sasaki and Matsuda1996; Metz, Reference Metz1998). Ohtsuka et al. (Reference Ohtsuka, Shimozu, Tanimura, Fukuchi, Hattori, Sasaki and Matsuda1996) explained the presence of diatom chains and appendicularian houses in Oncaea sp. (Poecilostomatoida) gut in detail. Also, an experimental study by Metz (Reference Metz1998) found diatoms to be a preferred food in Oncaea curvata. Conversely, it has been reported that Poecilostomatoida and Cyclopoida undertake carnivorous feeding behaviour in the Arabian Sea (Timonin, Reference Timonin1971; Smith & Madhupratap, Reference Smith and Madhupratap2005). It is interesting that in the current study, both Poecilostomatoida and Cyclopoida reflected presence of Chl a. Poecilostomatoida and Cyclopoida may compensate for their nutritional need mostly from an animal-based diet. This suggests that these tiny organisms exhibit omnivory in the natural habitat of the Arabian Sea. Also, as their sizes are low (<1 mm), based on the observation by Atkinson (Reference Atkinson1996) and Roman & Gauzens (Reference Roman and Gauzens1997), these copepods might exert more grazing pressure on the nominal-sized primary producers. On the other hand, Harpacticoida had a considerable amount of gut Chl a with no significant relation with ambient water Chl a, which suggested a blend of water and benthic Chl a source for its dietary quota.
It is apparent that the copepods from coastal waters of Arabian Sea essentially graze upon the chlorophyll-bearing material and probably can act as an astaxanthin source to the fish stock. This study highlights the temporal variability in copepod gut pigment contents with highest values recorded during monsoon, corresponding to the breeding season of fishes.
ACKNOWLEDGEMENTS
We are grateful to the Director of the National Institute of Oceanography for his support and facilities to pursue the research. Special thanks to Dr Siby Kurian (Scientist), for her helpful suggestions on HPLC results and Mr Chandrasekhar Rao for providing technical help to carry out HPLC analysis. All the members of the aquatic biogeochemistry group are acknowledged for their assistance in sample collections. We would like to thank the anonymous reviewers for their comments and suggestions. This article bears NIO contribution no. 5927.
FINANCIAL SUPPORT
Analiza Maria D'souza acknowledges the Council of Scientific and Industrial Research (India) for the fellowship. Also, we acknowledge SIBER project (GAP 2425) for financial support towards the field trips in coastal Arabian Sea.