INTRODUCTION
Lanternfishes (family Myctophidae) occupy the mesopelagic and bathypelagic realms of the world oceans, and are usually known to undertake considerable diurnal vertical migrations. But some species of myctophids are pseudo-oceanic or epibenthic, restricted to the waters overlaying continental slopes (Hulley, Reference Hulley1981) and form a major constituent of the mesopelagic-boundary communities. Myctophids and other pseudo-oceanic species feature prominently in the diet of a wide variety of demersal fauna, mainly benthopelagic fishes. They usually occur in close association with other pelagic fauna such as ctenophores, sergestids and amphipods, taking advantage of the protection against predation and availability of prey organisms (Auster et al., Reference Auster, Griswold, Youngbluth and Bailey1992). A wide variety of predators including fishes, squids, seabirds and marine mammals depend on myctophids as a food source (Kozlov & Tarverdieva, Reference Kozlov and Tarverdieva1989). A detailed understanding of the feeding habits and vertical migration patterns of myctophids is essential to understand their trophic position in marine ecosystems. Myctophids are characterized by rapid growth rates, early sexual maturity, short lifespans and high mortality rates. Females are oviparous and both sexes are non-guarding pelagic spawners. The females typically produce 100–2000 unfertilized eggs per spawn (Balu & Menon, Reference Balu and Menon2006). But there are species with fecundity up to 25,800 per spawn (Flynn & Paxton, Reference Flynn and Paxton2012).
The Indian Ocean is reported to harbour rich resources of lanternfishes, in terms of diversity and standing stock (Gjosaeter & Kawaguchi, Reference Gjosaeter and Kawaguchi1980). The estimated stock of myctophids in the Arabian Sea (100 million metric tonnes), dominated by a single species (Benthosema pterotum) is believed to be the largest fish stock in the world (Gjosaeter, Reference Gjosaeter1984; GLOBEC, 1993; FAO, 1997; Valinassab et al., Reference Valinassab, Pierce and Johannesson2007; Karuppasamy et al., Reference Karuppasamy, George and Menon2008). The sizeable stock in the Arabian Sea is sustained by the high secondary productivity in the region (Nair et al., Reference Nair, Madhupratap, Gopalkrishnan, Haridas and Gauns1999). Moreover, this group is reported to be well-adapted to survive in the Oxygen Minimum Zones (OMZ) which occur at around 150–1250 m in the Arabian Sea (Kinzer et al., Reference Kinzer, Bottger-Schnack and Sculz1993). In the north-eastern parts of the Arabian Sea, myctophid stocks are reported from certain pockets along Ratnagiri-Mormugao and off Mumbai, while in the south-eastern Arabian Sea (SEAS), high-density pockets occur off Mangalore and Kochi (Raman & James, Reference Raman and James1990).
The most species-rich genus among the Myctophidae is Diaphus, with 75 known species, generally referred to as head-light fishes, owing to the prominent secondary light organs on the head (Sebastine et al., Reference Sebastine, Bineesh, Abdussamad and Pillai2013). Vipin et al. (Reference Vipin, Pradeep, Ravi, Jose Fernandez, Remesan, Madhu and Boopendranath2011) reported 42 species of this genus in the Indian Ocean, and Karuppusamy et al. (Reference Karuppasamy, Muraleedharan, Dineshkumar and Nair2010) reported 13 species of which five are from the eastern Arabian Sea. Balu & Menon (Reference Balu and Menon2006) estimated a biomass of 100,000 tonnes of myctophids within the Indian EEZ of Arabian Sea, dominated by Diaphus spp. (Menon, Reference Menon2002). In SEAS, the myctophids are dominated by Diogenichthys panurgus, Diaphus fragilis, D. aliciae and Bolinichthys longiceps. Diaphus coeruleus is one of the large-sized species (Menon, Reference Menon2002). This species is reported from tropical waters from the western Indian Ocean to the western Pacific and shares its habitat with the closely related D. watasei, through most of its range (Gjosaeter, Reference Gjosaeter1981a). Within the Indian Ocean, D. coeruleus is recorded (Gjosaeter, Reference Gjosaeter1981b) from the Western Arabian Sea and Andaman Sea (Kao & Shao, Reference Kao and Shao1996).
Taxonomic identification of myctophids by conventional approaches can be relatively difficult as the samples are affected by preservation, and the numbers and position of photophores are affected through damage. Such difficulties are compounded when considering closely related species such as D. coeruleus and D. watasei, where differences are subtle and difficult to discern. A novel approach to resolve such ambiguities is the otolith structure as a key taxonomic character (Chen & Yan, Reference Chen and Yan2002). Myctophid otoliths are readily recognized by the presence of a caudal pseudocolliculum, believed to be a synapomorphic character (Schwarzhans, Reference Schwarzhans1978).
During surveys of the Fishery Oceanographic Research Vessel ‘Sagar Sampada’ (FORV-SS), specimens of D. coeruleus were collected in bottom trawls from the shelf edge in SEAS. This is the first detailed study of the systematics, food and feeding behaviour and reproductive biology of the species from the Arabian Sea. Considering that the species is difficult to distinguish from the closely related species D. watasei, which is also widely reported in the same region, a detailed description of the specimens is provided with conventional morphological characters along with the structure of the otoliths as an additional taxonomic character for identifying this species. An attempt has been made to understand the systematics, feeding behaviour, reproductive biology, length–weight relationship and otolith structure (otolith shape, relationship between otolith size and fish size) of D. coeruleus in this paper.
MATERIALS AND METHODS
Sample of D. coeruleus were collected during the surveys of FORV ‘Sagar Sampada’ (FORV-SS) from depths between 200–300 m of SEAS (9°00′–10°09′N 75°23′.54″–76°00′E) during August 2013 (FORV-SS, cruise 317 & 318) and January 2014 (FORV-SS cruise 322) (Figure 1). Fifty-five specimens were collected from two stations during the summer monsoon (June to September) and 38 specimens were collected from two stations during the winter monsoon (November to February). Trawling was using High Speed Demersal Trawl II (crustacean version, 2-warp twin-otters bottom trawling net, 58.6 m in total length with a head rope length of 38 m, foot rope of 44.5 m and cod-end with a stretch mesh size of 30 mm, gradually increasing to 130 mm in the front trawl sections; Panicker, Reference Panicker and Mathew1990; Panicker et al., Reference Panicker, Boopendranath and Abbas1993). Trawling speed was between 2.8–3 knots and all the operations were performed during day- and night-time. Simultaneous observations were made on vertical profiles of salinity, temperature and dissolved oxygen using a Sea-Bird electronic CTD profiler (SBE-911 Plus). Sea Surface Temperature (SST) was measured using a bucket thermometer, and salinity values from the CTD were calibrated using the on-board Autosal (GuildLine 8400A).

Fig. 1. Study area indicating bottom trawl stations.
All samples were preserved in 10% formalin and stored for further analysis in the shore laboratory. Data on morphometric and meristic characters standard length (L S), body weight (B W), number (Tables 1 and 2) and arrangement of photophores and photophore status were recorded for each specimen following Nafpaktitis (Reference Nafpaktitis1978) and Nafpaktitis et al. (Reference Nafpaktitis, Robertson and Paxton1995). Length measurements were made to the nearest 0.1 mm and weight was measured to the nearest 0.01 g. Species were identified using identification keys (Wisner, Reference Wisner1974; Nafpaktitis, Reference Nafpaktitis1978; Hulley, Reference Hulley, Smith and Heemstra1986). Since sexual dimorphism was not evident in these specimens, each fish was dissected for determination of sex.
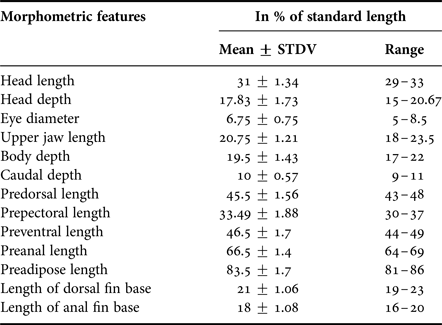
Table 1. Morphometric characteristics of Diaphus coeruleus in relation to % standard length (mm). Standard length ranges from 58.34–132 mm (91 samples).
Abbreviations: as in Figure 2.
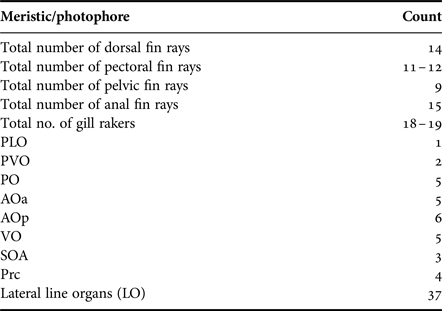
Table 2. Meristic data and photophore count of Diaphus coeruleus (20 samples).
In order to examine gut content, the gut of 93 specimens (58.34–132 mm L S) was removed (from the anterior end of the oesophagus to the pyloric valve). The contents were carefully separated and examined under a binocular microscope, counted and weighed to the nearest 0.01 mg. Organisms in the gut were identified to the lowest possible taxonomic level (Hartel, Reference Hartel1985; FAO, 1998). Only prey occurring in the oesophagus and the gut were analysed in this study, and those found in the mouth were not considered. Relative measures of food items were quantified (Pinkas et al., Reference Pinkas, Oliphant and Iverson1971) using the Index of Relative Importance (IRI):
where N is percentage of a particular food in the gut contents, W is percentage of food weight and O is percentage of frequency of occurrence.
Maturation stages of the gonads were examined and subsequently, juvenile forms of each sex were separated from the adults. The macroscopic stages of gonad development were observed in both sexes during the study and were classified according to the criteria given by Gartner (Reference Gartner1993). Diaphus coeruleus with ripened gonads were used to determine fecundity. The ovaries were slit open to break down the connective tissue, thus releasing oocytes from the ovary. The separated ova were then counted (Prosch, Reference Prosch1991) under a stereomicroscope and fecundity determined. The ratio of males to females (M:F) was taken as the sex ratio (Lessa & Nóbrega, Reference Lessa and Nóbrega2000).
The length–weight relationship was calculated using the formula:
where B W is the total weight, L S the standard length, a is the intercept of the log linear regression and b is the regression coefficient in the log transformed form.
The value of b indicates isometric growth when equal to 3 (Pauly, Reference Pauly1984; Anderson & Neumann, Reference Anderson, Neumann, Murphy and Willis1996). Parameters a and b were estimated by transforming to logarithmic form, as follows,
The Coefficient of Determination (R 2) was noted as the measure of significance of causal effect relationship and robustness of the sample. Analysis of covariance (ANCOVA) was used to identify significant differences in the length–weight among the sexes and among samples (Karuppasamy et al., Reference Karuppasamy, George and Menon2008).
The structure of the sagittal otolith of D. coeruleus and the relation of otolith length and weight of the specimens were studied. The otoliths were removed from 80 specimens, via a cut in the cranium, cleaned and stored; the left and right otoliths were kept separate. To support species identification the identification key by Schwarzhans & Aguilera (Reference Schwarzhans and Aguilera2013), based solely on otolith structure for 28 species of myctophids in the Pacific Ocean was utilized.
Otolith extraction was done from specimens of varying lengths, for more robust data. Each sagittae was systematically placed with the sulcus acusticus oriented towards the observer and its length, defined as the longest dimension along the rostral-postrostral axis (nomenclature following Smale et al., Reference Smale, Watson and Hecht1995), and height, defined as the maximum distance (at right angles to the length) from the dorsal to ventral edge, were determined using a hand-held Vernier calliper (accuracy of 0.02 mm). Individual otolith weight (to nearest 0.01 mg accuracy) was determined using a high precision electronic balance (Mettle Toledo-ML204/A01). The relationships between otolith size and fish sizes were determined using least-squares linear regression using the statistical software R Core Team (2013) for the following pairs of measures: (Otolith length L O) − (standard Length L S), (Otolith weight W O) − (L S), (L O) − (Body weight B W) and (W O) − (B W). These equations were fitted for both left and right otoliths and t-tests were made to check for any possible differences between them.
RESULTS
SYSTEMATICS
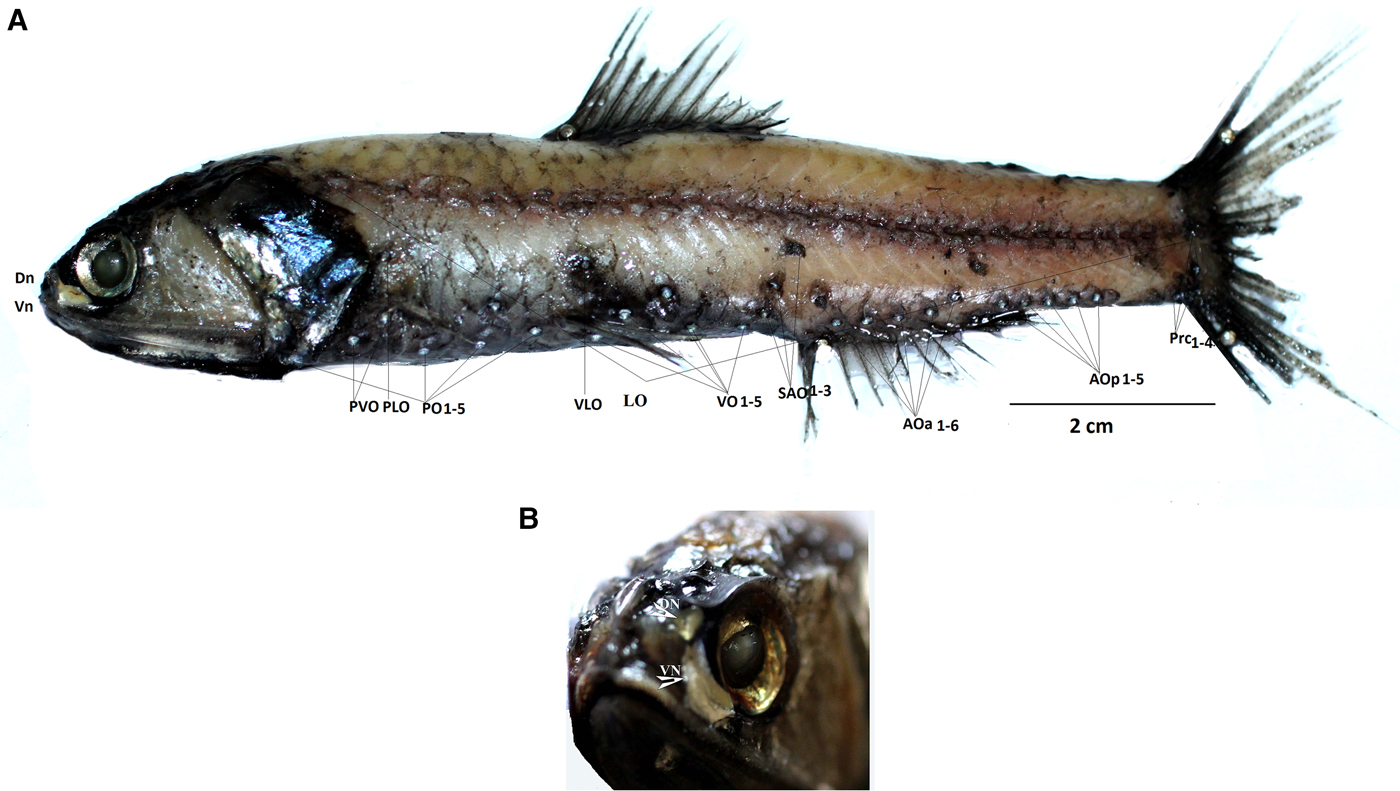
Order Myctophiformes Regan, 1911 Family Myctophidae T N Gill, 1893 Subfamily Lampanyctinae Paxton, 1972 Genus Diaphus Eigenmann and Eigenmann, 1890 Diaphus coeruleus Klunzinger, 1871 (Figure 2A, B)
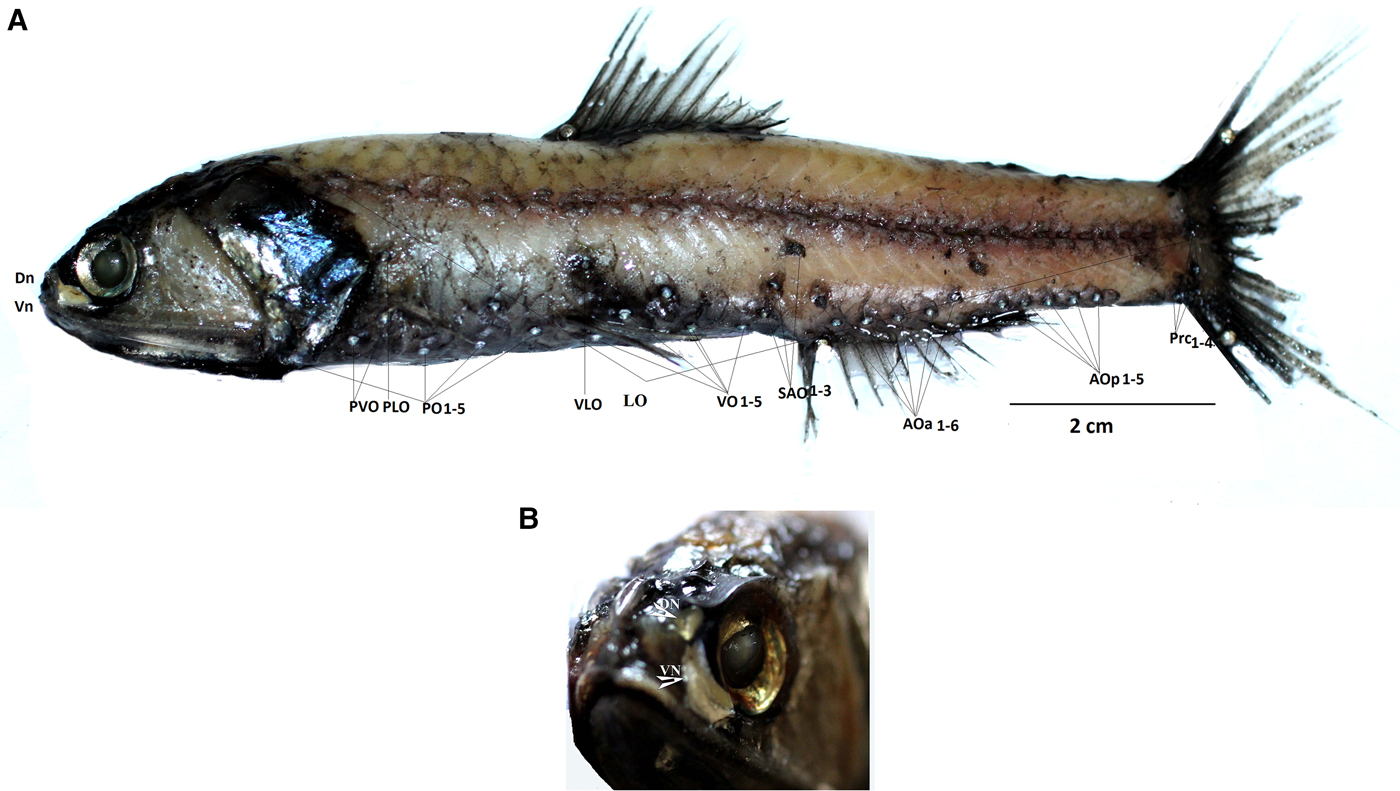
Fig. 2. (A) Diaphus coeruleus, male (127 mm). PLO (supra pectoral organs), PVO (subpectoral organs), PO (pectoral organs), AOa (anal organs, anterior), AOp (anal organs, posterior), VO (ventral organs), VLO (ventrolateral organs), SAO (supra anal organs), Prc (pre caudal organs), LO (lateral line organs). (B) Head bearing small DN (dorsonasal organ) and enlarged VN (ventronasal organ).
MATERIALS EXAMINED
South-eastern Arabian Sea 93 individuals; FORV ‘Sagar Sampada’ Cruise # 317, Stn.1; 9°57′.898″N 76°00′.022″E, 200–300 m; coll. 1 August 2013, Cruise # 318, Stn 25; 10°030′977N 75°023′542E; coll. 29 August 2013 Cruise # 322, Stn. 1; 10°09′.959′'N 75°38′.96″E, 200–300 m; coll. 7 January 2014, Stn. 7; 9°01′.512″N 75°53′.18″E, 200–300 m; coll. 11 January 2014, High Speed Demersal Trawl.
Diagnosis: Dn lentil shaped, Vn crescent shaped, along the anterior and ventral margin of the eye (Figure 2A, B). From the lateral view it is seen as a massive band of dark tissue obscuring Dn and much of Vn. A luminescence patch near PLO; SAO1 above and slightly posterior to VO5. Pol 2–2.5 times below lateral line, Prc4 almost two photophores below lateral line. Operculum angular, posterio dorsally attenuated to form a sharp point posteriorly.
DESCRIPTION
Ant (anterodorsal organ) absent. Dn (dorsonasal organ) lentil shaped and smaller than a body photophore, located immediately posterio-dorsal to nasal apparatus. Vn (Ventronasal organs) in contact with Dn dorsally; terminating ahead of and ventral to anterior margin of pupil. Origin of dorsal fin in advance of base of ventral fin. PLO 2–2.5 times as close to base of pectoral fin as to lateral line. SAO on a straight line or nearly so; SAO3 on or slightly in front of vertical through origin of anal fin and about 2.5 times its own diameter below lateral view. Pol under or slightly in advance of base of adipose fin, 2–2.5 times its diameter below LL. AOp behind base of anal fin. Prc forming an arc with Prc3-Prc4 interspace often distinctly enlarged; Prc4 about twice its diameter below lateral line. Nafpaktitis (Reference Nafpaktitis1978) provides a full description (Figure 2A) (Tables 1 and 2).
REMARKS
The present specimens match accurately with descriptions of D. coeruleus on the basis of the shapes and positions of the Dn and Vn and lack of an Ant. They are clearly distinct from the larger, deep-bodied species D. watasei, which is characterized by a small Ant, round Dn and oval shaped, well developed Vn extending under to vertical of anterior margin of the pupil. In D. coeruleus the length of the head is greater than the distance between the posterior end of the mouth and the origin of dorsal fin, while it is equal to or smaller than the distance in D. watasei. The caudal peduncle in D. watasei is deeper (least depth is less than 10 times in L S) than in D. coeruleus (least depth more than 10 times in L S) (Nafpaktitis, Reference Nafpaktitis1978). Diaphus watasei has Prc4, SAO3 and Pol about 3 photophore diameters below lateral line (Wang & Shao, Reference Wang and Shao2005), compared with about 2–2.5 photophore diameters below in D. coeruleus. The SEAS specimens obtained from the cruises are consistent with D. coeruleus rather than with D. watasei in all these characters mentioned previously, thus justifying the placement of present specimens in D. coeruleus.
OTOLITH STRUCTURE
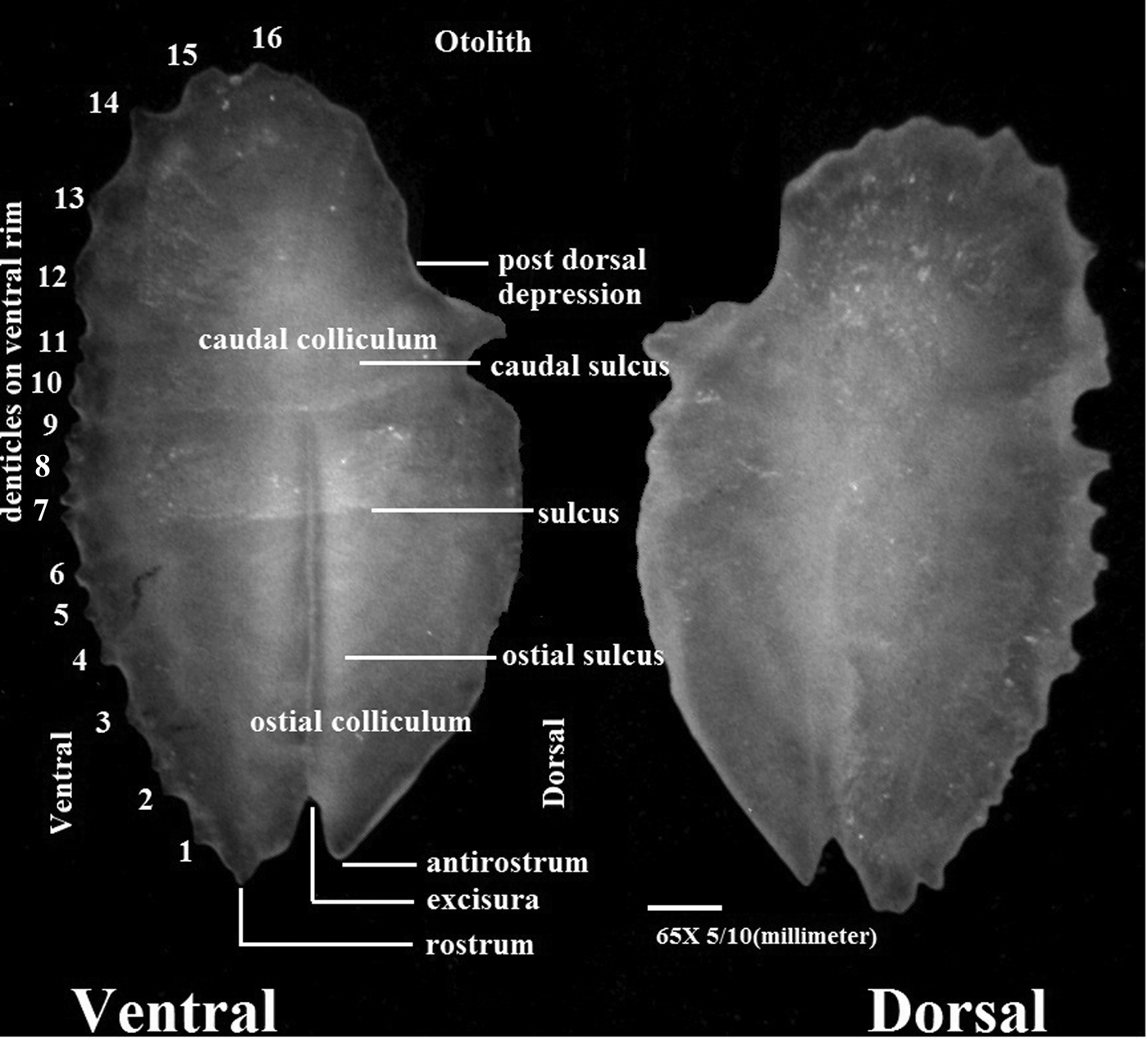
Large, thin, elliptical otoliths, ranging in length from 4.5 to 6.4 mm and weighing 7.1–19.6 mg were obtained from the specimens. Anterior tip bearing a short, pointed rostrum, a small anti-rostrum and excisura. Rostrum 13–15% of otolith length, with a serrate ventral margin and sinuate dorsal one; dorsal rim not expanded anteriorly, regularly curved, and highest at about its middle. Post-dorsal angle moderate, followed by a broad post-dorsal depression of variable depth. Ventral rim moderately deep, very regularly curved, with 16–17 delicate denticles, extending to near posterior tip. The enlarged denticle that protrudes at the post-dorsal angle in both otoliths (Figure 3). Posterior tip projecting almost to the level of upper margin of cauda. Inner face slightly convex, with a long median sulcus. Ostium large, its dorsal rim slightly curved; cauda more or less straight, terminating close to posterior tip of otolith. Ostial colliculum reduced anterio-dorsally, caudal pseudo-colliculum about as long as caudal colliculum. Dorsal depression moderately wide, with indistinct dorsal margin; ventral furrow indistinct. Outer face slightly concave, smooth (Figure 3). Otoliths of D. coeruleus are recognized by their elongate shape and the long depressed predorsal rim. The post-dorsal depression is distinct, but the posterior reduction rather weak. Otoliths of D. watasei are remarkable for their expanded predorsal and the deeply cut and reduced post-dorsal rim and the rostrum not exceeding the antirostrum in length (Schwarzhans & Aguilera, Reference Schwarzhans and Aguilera2013). Otoliths were extracted from the specimens with L s 75 mm to 132 and weight between 4.6 to 6.1 g. Otolith lengths (OL) and weights (OW) ranged from 4.5 mm, 7.1 mg to 6 mm, 13.4 mg with a mean of 5.25 mm and 10.25 mg.

Fig. 3. Otolith of Diaphus coeruleus.
DISTRIBUTION
Diaphus coeruleus has been reported from the Red Sea and the Andaman Sea off the coast of Burma (Kao & Shao, Reference Kao and Shao1996), Gulf of Elat (Gunther, Reference Gunther1887), Papua New Guinea (Kailola, Reference Kailola1987), Indonesia (Gloerfelt-Tarp & Kailola, Reference Gloerfelt-Tarp and Kailola1984), Taiwan (Wang & Chen, Reference Wang and Chen2001), Chesterfield Islands (Kulbicki et al., Reference Kulbicki, Randall and Rivaton1994), Australia (Paxton et al., Reference Paxton, Hoese, Allen and Hanley1989) and South China Sea (Yang et al., Reference Yang, Huang, Chen and Li1996). This is the first detailed study of the species from SEAS.
FOOD AND FEEDING
The gut contents of the 93 specimens of Diaphus coeruleus were examined, and amongst these, empty guts were not observed. The gut of all specimens contained digestive fluids, with 13% being fully filled, 7% three-quarters filled, 45% half-filled and 35% one-quarter filled. Prey items mainly included cephalopods and crustaceans. Among the cephalopods, the deep-sea squid Onychoteuthis banskii was the predominant species (occurrence 55%), whereas among crustaceans Plesionika martia (occurrence 30.8%) was dominant. The percentages of index of relative importance (% IRI) were Plesionika martia (shrimp) 20.73%, Onychoteuthis banskii (squid) 75.97%, semi-digested food particles 3.32% and fish as <0.01%. A single semi-digested myctophid of the genus Benthosema was found in the gut of a single individual.
REPRODUCTIVE BIOLOGY
Sex ratio and fecundity: A total of 90 specimens of Diaphus coeruleus were dissected and examined for reproductive studies. Three specimens were not included in subsequent analysis due to difficulty in identifying their reproductive stage. The sex ratio for the specimens as a whole was 1 male: 0.2 female (χ 2 = 9.09), with no seasonal variation being observed in the ratio. The calculated chi-square value indicates that the sex ratio deviates significantly from the hypothetical 1 : 1 ratio. In males, the six maturation stages observed were I –immature (29.58%), II –developing (quiescent) (25.35%), III –mature (18.31%), IV – ripe (11.27%), V – spawning (7.04%) and VI – spent (8.45%); while in females the stages observed were I – immature (11.76%), II – developing (35.39%), III – mature (17.65%), IV – ripe (17.65%) and V – spawning (17.65%). No spent stages were observed in females (Figure 4). The estimated fecundity of ripe and spawning females ranged between 1900 eggs (gonad weight 0.31 g in a fish with length 96 mm and weight 8.5 g) and 2400 eggs (gonad weight of 0.78 g in a fish with length 115 mm and weight 18.7 g) with a mean of 2150 ± 353 eggs.

Fig. 4. Percentage of fish maturity stages I–VI during the sampling period for both male and female.
LENGTH–WEIGHT RELATIONSHIP
The L S of D. coeruleus ranged from 58.32 to 132 mm (100 ± 1.52 mm) and B w ranged from 2.22 to 23.81 g (13.73 ± 0.53 g Table 3 and Figure 5). The length-weight relationship differed significantly between males and females. The exponential value (b) for males (N = 76) did not differ significantly from 3, with a coefficient of determination (R 2) of 0.944, which indicates an isometric growth pattern. In females (N = 17), the value was 1.71 and significantly different from the isometric benchmark (P < 0.05), implying a strong negative allometry. This may perhaps be due to the small sample size and the poor representation of large females. When considering both sexes together, the coefficient of exponentiation value (b) was close to 3, and coefficient of determination was 0.929.
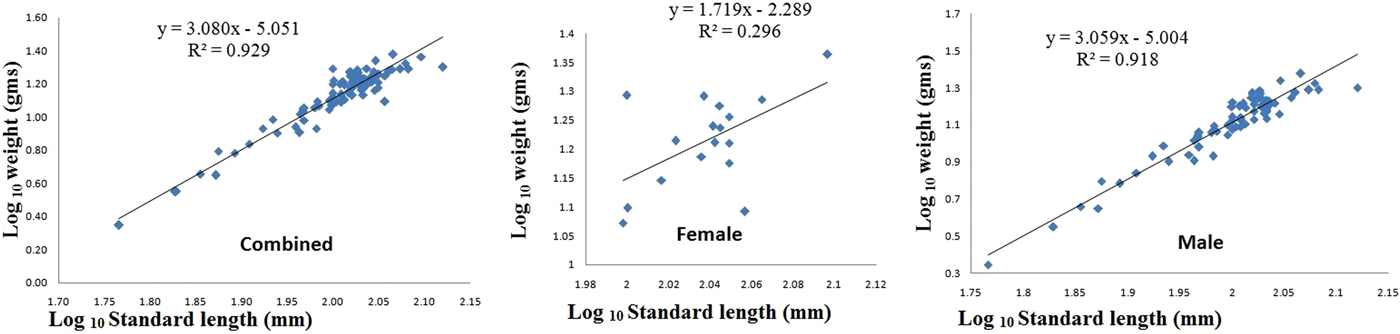
Fig. 5. Length–weight relationship of Diaphus coeruleus.
Table 3. Length-weight relationship in Diaphus coeruleus. Standard length (millimetres), weight (grams) and length-weight regression.

The relationship of otolith length (L O) and weight (W O) with that of the fish standard length (L S) and weight (B W) were tested through linear regression. Statistical analysis (Student's t-test) revealed no significant differences in length (P) and weight between right and left otoliths, so, mean values of right and left L O and W O of each specimen were taken for analysis. Data fitted well to the regression model for both L O and W O, as demonstrated by the high values of the coefficient of determination (R 2) as 90%. The slope of regression lines of otolith length and weight on standard length and weight for the entire set of samples revealed no significant deviation from zero (P > 0.1).
However, when the otolith length and weight to fish length and weight of both male and female fishes were combined, and also for males alone, the R 2 was found to be between 0.47–0.51 (P < 0.01) (Table 4). For females the R 2 of L O and W O on fish L S and B W was 0.06–0.12 and their P value was found to be insignificant. These results reveal that otolith length is correlated with growth parameters of fish during maturing stages and also after attaining maturity.
Table 4. Relationships of fish length and weight to otolith size (length and weight) for D. coeruleus (male, female and combined data – standard length in millimetres and weight in g to otolith length (OL) in millimetres and otolith weight (OW) in milligrams. The number of specimens (N) and the coefficient of determination (R 2) and their significance P are given).

L O, otolith length; W O, otolith weight; L S, fish standard length; B W, fish weight.
DISCUSSION
Diaphus coeruleus is one of the pseudo-oceanic representatives of the Myctophidae (Hulley, Reference Hulley, Fischer and Hureau1985; Kornilova & Tsarin, Reference Kornilova and Tsarin1993) closely related to and difficult to distinguish from the coexisting species, D. watasei. The two species have overlapping geographic distribution in the northern Indian Ocean (Andaman Sea, western Arabian Sea etc.), where they are known to occupy the same habitat. While the occurrence of D. watasei in the eastern Arabian Sea is well documented (Karuppasamy et al., Reference Karuppasamy, Muraleedharan, Dineshkumar and Nair2010; Vipin et al., Reference Vipin, Pradeep, Ravi, Jose Fernandez, Remesan, Madhu and Boopendranath2011), this is the first report of D. coeruleus from this part of the Indian Ocean. Nafpaktitis (Reference Nafpaktitis1978) states that the species are often mistaken for each other or misidentified, as they are widely co-habiting, and have numerous morphological similarities, which reflects their close phylogenetic relationship. In the SEAS, where both species occur at ~200–300 m, D. coeruleus can be readily distinguished from D. watasei based on luminous organs on the head, opercular shape and a few morphometric differences (Nafpakitis, Reference Nafpaktitis1978; Gjosaeter, Reference Gjosaeter1981b), and the present detailed description of D. coeruleus, is expected to help in this regard. It is generally believed that larger species of myctophids, such as D. coeruleus and D. watasei, do not undertake diurnal vertical migrations (Gjosaeter & Kawaguchi, Reference Gjosaeter and Kawaguchi1980) which is corroborated by the present study where D. coeruleus was collected in bottom trawls both during night and day from almost the same depth (200–300 m). This species appears to be restricted to the waters immediately overlaying the seafloor. The Arabian Sea Oxygen Minimum Zone (OMZ) is reported to impinge on the seafloor at these depths (Karuppasamy et al., Reference Karuppasamy, Lalu Raj, Muraleedharan and Nair2011; Abdul Jaleel et al., Reference Abdul Jaleel, Anil Kumar, Nousher Khan, Correya, Jacob, Philip, Sanjeevan and Damodaran2014). At the locations of collection, the depth of the thermocline bottom varied between 160–180 m. The affinity of this species to concentrate on the oxygen minimum zone rather than at the thermocline depth (Karuppasamy et al., Reference Karuppasamy, Lalu Raj, Muraleedharan and Nair2011) is confirmed in the present study.
Food availability is a key factor which regulates and influences the growth, reproduction, migration and abundance of fish stocks. Abundance of preferred food organisms may be responsible for the horizontal and vertical migration (both seasonal and diurnal) of the fishes (Philip, Reference Philip1998). Like other members of the myctophidae, D. coeruleus are carnivores. During the present study, this species fed predominantly (55%) on deep-sea squids (Onychoteuthis banskii) followed by crustaceans (31%). Studies have revealed that D. watasei is closely associatied with crustaceans, cephalopods, and other fishes (Sebastine et al., Reference Sebastine, Bineesh, Abdussamad and Pillai2013). This, along with the present observations suggests that the presence of preferred prey may be an important factor in the distribution of myctophids in the region. The current observations also present an example of resource partitioning among myctophids occupying the same region in the eastern Arabian Sea, with D. coeruleus preferentially feeding on deep-sea squids, and D. watasei showing an affinity to deep-sea shrimps.
In the SEAS, the ratio of males to females among D. coeruleus was found to be 1 : 0.2. In the deep-sea realm the chances of finding a mate are uncertain and male dominant population and hermaphroditism are believed to be a great advantage (Randall & Farrell, Reference Randall and Farrell1997). Therefore, the reproductive strategy of deep-sea fishes is manifested by a sex ratio in favour of males (Allsop & West, Reference Allsop and West2004). The present study reveals that the populations of D. coeruleus were highly skewed towards males. In the same region, the sex ratio of D. watasei is reported to be 2 : 1 (Vipin et al., Reference Vipin, Pradeep, Ravi, Jose Fernandez, Remesan, Madhu and Boopendranath2011). In general, sex ratios in fishes deviate from the expected ratio of 1 : 1, and vary among species, and also within populations of the same species, being influenced by several factors such as adaptation of the population, reproductive behaviour, food availability and environmental conditions (Nikolsky, Reference Nikolsky1963; Emlen & Oring, Reference Emlen and Oring1977; Baroiller & D'Cotta, Reference Baroiller and D'Cotta2001; Brykov et al., Reference Brykov, Kukhlevsky, Shevlyakov, Kinas and Zavarina2008; Oliveira et al., Reference Oliveira, Costa, Araújo, Pessoa, Carvalho, Cavalcante and Chellappa2012; Vandeputte et al., Reference Vandeputte, Quillet and Chatain2012).
Length–weight relationships in D. coeruleus differed considerably between the sexes; with isometric growth in males and negative allometric growth in females. In some other deep-sea fishes, females are reported to attain maturity only after reaching adult size, when somatic growth has slowed or ceased (Gordon et al., Reference Gordon, Merrett, Haedrich and Hopper1995). This may be the case with D. coeruleus.
Otoliths are an important feature in fish taxonomy, because of their high inter-specific variability in shape. This is particularly true for myctophids. Schwarzhans & Aguilera (Reference Schwarzhans and Aguilera2013) provides descriptions and images of 63 of the currently recognized 76 recent species of Diaphus based solely on otolith structure. In the present study, the morphology and size of otoliths in D. coeruleus in the SEAS are provided, in order to aid taxonomic identification and to reduce misidentification. Diaphus coeruleus is readily distinguished by their elongate shape and long depressed predorsal rim and their post-dorsal depression is distinct but posterior reduction is weak. Diaphus watasei are remarkable with their expanded predorsal, deeply cut and reduced post-dorsal rim. This is the first detailed description of otolith of Diaphus coeruleus although much research has been carried out on the otoliths of other Diaphus sp. which proves their appearance in the Oliogocene and miocene periods (Nolf & Cappetta, Reference Nolf and Cappetta1988; Brzobohaty & Nolf, Reference Brzobohaty and Nolf1995).
Statistical analyses indicated that the growth of the otoliths in this species is linked with the growth of the fish. Myctophids are important sources of food for a wide variety of mesopelagic fishes as well as larger cephalopods and cetaceans (FAO, 1997), and often otoliths are the only identifiable parts recoverable from gut content or scat samples. Availability of detailed descriptions of otoliths can enable the identification of myctophid species, consumed by these predators. Preferences of species preying on myctophids can be inferred in this manner, which can help to understand the trophic roles of the myctophids. Additionally, an understanding of the exact relationship of otolith size with fish size may also prove useful in estimating myctophid biomass based on sizes of otoliths collected from the guts of the predator species (Yonezaki et al., Reference Yonezaki, Kiyota, Baba, Koido and Takemura2011).
Diaphus coeruleus has been commercially exploited in the south-western Indian Ocean, Taiwan and Atlantic Ocean during the 1970s (Nafpaktitis, Reference Nafpaktitis, Fischer and Bianchi1982; Hulley, Reference Hulley, Fischer and Hureau1985; FAO, 1997; Wang & Chen, Reference Wang and Chen2001; Balu & Menon, Reference Balu and Menon2006). Unlike most other myctophids, this species is reported to have no wax ester content, making it suitable for human consumption (Gjosaeter & Kawaguchi, Reference Gjosaeter and Kawaguchi1980). Detailed studies on the stock of this species, with high spatio-temporal resolution are warranted, as it may prove a potential source of food or other value-added products. To complete our understanding about the size and extent of the stock of D. coeruleus in the Arabian Sea future surveys are required about this species, their identification, and determination of the stock's population dynamics. Although myctophids have no commercial value in India, a sizeable stock of the species if present, can be a potential source of food or may have considerable export value.
ACKNOWLEDGEMENTS
We are thankful to our colleagues of Benthos project and Deep Sea and Distant water fishery (DSDWF) project of Centre for Marine living resources and Ecology (CMLRE) Kochi and staff of FORV Sagar Sampada for their support and kind cooperation during sampling. This is CMLRE contribution number 86.
FINANCIAL SUPPORT
We are grateful to the Ministry of Earth Sciences, New Delhi for funding the project and providing facilities onboard FORV ‘Sagar Sampada’ for collecting the specimens.