INTRODUCTION
The Persian Gulf (PG) is a semi-enclosed marginal sea surrounded by landmasses (Figure 1) (Sheppard et al., Reference Sheppard, Al-Husiani, Al-Jamali, Al-Yamani, Baldwin, Bishop, Benzoni, Dutrieux, Dulvy, Durvasula, Jones, Loughland, Medio, Nithyanandan, Pilling, Polikarpov, Price, Purkis, Riegl, Saburova, Samimi-Namin, Taylor, Wilson and Zainal2010). Due to limited water exchange with the Indian Ocean, the PG is characterized by relatively harsh environmental conditions with respect to salinity and temperature to the extent that coral communities surviving therein are inhabiting waters that kill conspecifics elsewhere (Sheppard, Reference Sheppard1993; Coles, Reference Coles2003; Hume et al., Reference Hume, D'Angelo, Burt, Baker, Riegl and Wiedenmann2013). As such, these extraordinary communities are gaining attention for their ability to inform on how coral reefs globally might adapt to increases in water temperatures predicted over the next century (Riegl & Purkis, Reference Riegl and Purkis2012; Hume et al., Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015, Reference Hume, Voolstra, Arif, D'Angelo, Burt, Eyal, Loya and Wiedenmann2016; Mashini et al., Reference Mashini, Parsa and Mostafavi2015; Koupaei et al., Reference Koupaei, Dehghani, Mostafavi and Mashini2016; Ziegler et al., Reference Ziegler, Arif, Burt, Dobretsov, Roder, LaJeunesse and Voolstra2017).
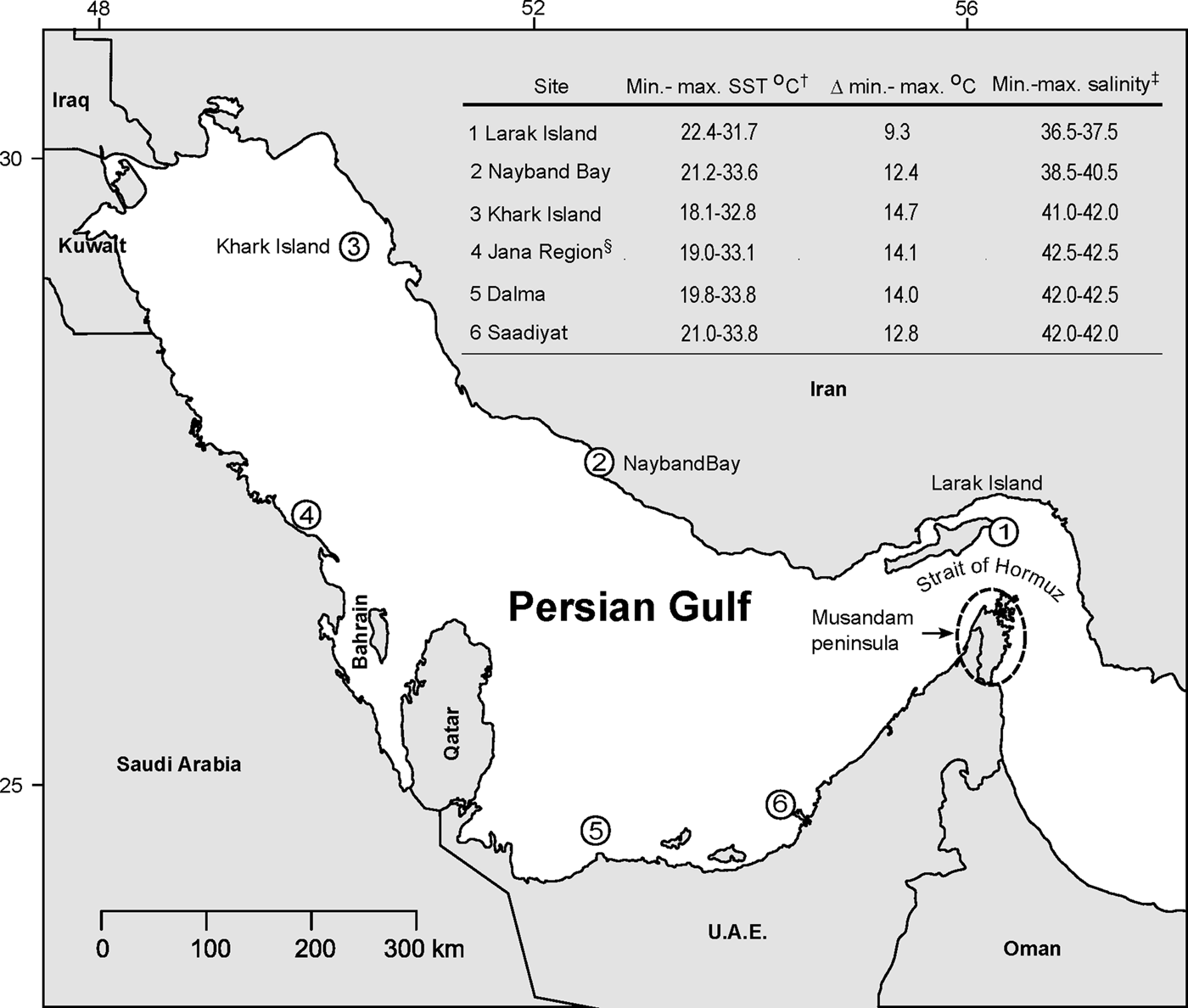
Fig. 1. Study site location, approximate sea surface temperature (SST) and salinity. The base map was created in R using the ‘maps’ package (https://CRAN.R-project.org/package=maps). †Minimum and maximum temperatures are from Aqua MODIS SST (11 µ daytime) 4 km resolution monthly (maximum, August; minimum, February) climatology for 2002–2015 retrieved from OceanColor Web's level 3 browser (http://oceancolor.gsfc.nasa.gov/cms/). The following coordinates were used to represent the site locations: Larak, 26.896′N 56.396′E; Nayband Bay 27.479′N 52.563′E, Khark Island 29.312′N 50.271′E. ‡Salinities are taken from seasonally averaged (maximum, winter; minimum, summer) surface salinity fields of the 10th year model output in the climatological run of Yao & Johns (Reference Yao and Johns2010). §Jana Region is representative of sites from Hume et al. (Reference Hume, Voolstra, Arif, D'Angelo, Burt, Eyal, Loya and Wiedenmann2016) and Baker et al. (Reference Baker, Starger, McClanahan and Glynn2004).
The phenotype of the coral holobiont (i.e. the coral host and all associated biological components; algal, bacterial etc.) may be altered according to which algal symbiont(s) the coral host is associated with. Specifically, increases in coral holobiont stress tolerance have received much attention (Warner et al., Reference Warner, LaJeunesse, Robison and Thur2006; Jones & Berkelmans, Reference Jones and Berkelmans2011; Ortiz et al., Reference Ortiz, Gonzalez-Rivero and Mumby2013; Silverstein et al., Reference Silverstein, Cunning and Baker2015). Whilst the exact mechanisms of change and the scope of host-symbiont combinations that may undergo/facilitate such a change are still poorly understood, coral ecosystems have the potential to adapt to changing physical conditions. This change may happen at both the community (change in relative proportion of given coral-algal symbiont associations) and individual (change in predominant symbiont harboured by a given coral) level (Jones et al., Reference Jones, Berkelmans, van Oppen, Mieog and Sinclair2008; LaJeunesse et al., Reference LaJeunesse, Smith, Finney and Oxenford2009; Stat et al., Reference Stat, Loh, LaJeunesse, Hoegh-Guldberg and Carter2009; Ortiz et al., Reference Ortiz, Gonzalez-Rivero and Mumby2013; Pettay et al., Reference Pettay, Wham, Smith, Iglesias-Prieto and LaJeunesse2015). Therefore, characterization of the algal component is a critical factor in determining and understanding stress tolerance of coral communities. Whilst considerable work has been conducted towards characterizing (genotyping) the algal components of associations off the Arabian coastline in the PG there is a paucity of such information available for coral communities off the Iranian coast (Baker et al., Reference Baker, Starger, McClanahan and Glynn2004; Hume et al., Reference Hume, D'Angelo, Burt, Baker, Riegl and Wiedenmann2013, Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015, Reference Hume, Voolstra, Arif, D'Angelo, Burt, Eyal, Loya and Wiedenmann2016; D'Angelo et al., Reference D'Angelo, Hume, Burt, Smith, Achterberg and Wiedenmann2015; Mahmoud & Al-Sarraf, Reference Mahmoud and Al-Sarraf2016).
In the southern, hottest and most saline waters of the PG reef building corals form associations with a halo- and a thermo-tolerant member of a recently discovered species of symbionts, the Symbiodinium thermophilum. Members of this group are prevalent in coral associations over almost the entire length of the Arabian coast in waters spanning from north of Al Jubail, Saudi Arabia to waters at the entrance to the PG in the Musandam peninsula (D'Angelo et al., Reference D'Angelo, Hume, Burt, Smith, Achterberg and Wiedenmann2015; Hume et al., Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015, Reference Hume, Voolstra, Arif, D'Angelo, Burt, Eyal, Loya and Wiedenmann2016) (Figure 1). Furthermore, a predominance of clade C Symbiodinium has been found in a range of corals off the Kuwaiti coast (Mahmoud & Al-Sarraf, Reference Mahmoud and Al-Sarraf2016). Lack of genetic information prevents certain identification of these algal symbionts as members of the S. thermophilum group. However, it is highly probable that these symbionts belong to the S. thermophilum group given the scarcity of other clade C symbionts in the PG.
Water enters the PG by means of a surface current from the adjacent Gulf of Oman (Johns et al., Reference Johns, Jacobs, Kindle, Murray and Carron1999; Yao & Johns, Reference Yao and Johns2010). Given its high evaporation rates and minimal freshwater input the PG is a negative estuary with the export of water from the PG being relatively small, hyper-saline and deep compared with the input. The inflow current from the Gulf of Oman acts to buffer the PG, cooling in the summer whilst warming in the winter. After entering the PG water travels some distance up the northern coast before feeding into the more southern waters. As a result, water salinities and annual thermal ranges increase with distance from the PG's entrance (Figure 1).
Waters along the Iranian coast are also characterized by salinities and annual thermal ranges greater than those found in the majority of reef-containing waters globally (Figure 1). With waters ranging in salinity from 37–42 and annual temperature ranges of 8–15°C, these waters represent extraordinary environments in which to find coral communities. However, previous studies have been limited in their spatial coverage of this coastline with previous sampling efforts of scleractinian corals having been conducted at one of five island locations within 300 km of the Musandam peninsula at the entrance of the PG (Mostafavi et al., Reference Mostafavi, Fatemi, Shahhosseiny, Hoegh-Guldberg and Loh2007, Reference Mostafavi, Ashrafi and Dehghani2013; Rahmani et al., Reference Rahmani, Ghavam Mostafavi, Shahhosseiny, Vosoughi and Faraji2011; Shahhosseiny et al., Reference Shahhosseiny, Mostafavi, Fatemi and Karimi2011; Mashini et al., Reference Mashini, Parsa and Mostafavi2015; Koupaei et al., Reference Koupaei, Dehghani, Mostafavi and Mashini2016), waters characterized as being closer to oceanic salinities and experiencing relatively small thermal fluctuations for the region (Figure 1). The results of these sampling efforts have revealed an overall predominance of clade D symbionts (likely Symbiodinium trenchii; LaJeunesse et al., Reference LaJeunesse, Wham, Pettay, Parkinson, Keshavmurthy and Chen2014) with a lesser proportion of clades C and A also reported (Mostafavi et al., Reference Mostafavi, Fatemi, Shahhosseiny, Hoegh-Guldberg and Loh2007, Reference Mostafavi, Ashrafi and Dehghani2013; Rahmani et al., Reference Rahmani, Ghavam Mostafavi, Shahhosseiny, Vosoughi and Faraji2011; Shahhosseiny et al., Reference Shahhosseiny, Mostafavi, Fatemi and Karimi2011; Mashini et al., Reference Mashini, Parsa and Mostafavi2015; Koupaei et al., Reference Koupaei, Dehghani, Mostafavi and Mashini2016).
In this study, we have genotyped the algal symbiont within two species of ecologically dominant scleractinian corals (Porites harrisoni and Cyphastrea microphthalma) from three sites (Larak Island, Khark Island and Nayband Bay) spanning the length of the Iranian coastline. These sites are representative of the full range of temperature and salinity environments found on the Iranian coastline with temperature maximum, minimum and average salinities increasing with increasing distance from the Musandam peninsula (Figure 1). Here, we document a predominance of corals in association with Symbiodinium thermophilum at all three sites sampled, a finding in contrast to previous studies off the Iranian coast and one that may be explained by the physical properties of the waters harbouring the reefs.
MATERIALS AND METHODS
Sample collection and DNA extraction
Samples were collected at between 3–6 m depth in waters off Larak Island (26.8868′N 56.3838′E; eight colonies of P. harrisoni), Khark Island (29.2686′N 50.3099′E; nine colonies of P. harrisoni, one colony of C. microphthalma) and Nayband Bay (27.4682′N 52.6025′E; eight colonies of P. harrisoni and two colonies of C. microphthalma) in April, July and September 2012, respectively (Figure 1; Table 1). Approximately 4 cm2 specimens were collected by hammer and chisel from the tops of colonies and placed in DMSO buffer (20% DMSO, 25% mM EDTA, saturated with NaCl at pH 8.0). Specimens were kept on ice (~4°C) before a tissue slurry was extracted by airbrush using DNAB buffer (0.4 M NaCl, 50 mM EDTA, pH 8.0). Slurries were stored frozen at −20°C before defrosting to perform DNA extractions using the CTAB/chloroform method of Baker (Reference Baker1999). Extracted DNA was stored at −20°C.
Table 1. Predominant Symbiodinium identity, genotyping marker and sequence accession number of Porites harrisoni and Cyphastrea microphthalma colonies sampled in this study.

a cp23S, chloroplastic ribosomal DNA large subunit; ITS2, nuclear ribosomal DNA second internal transcribed spacer region.
b Symbiodinium species or ITS2 types that returned cp23S sequences that were 100% matches (coverage and identity from the NCBI database) to the sequence in question. S. thermophilum+=exact match for S. thermophilum and ITS2 types C1, C3, C39, C41, C91, C79.
c (P)=putative species designation. Please see the last paragraph of the Methods section for more information on identifying S. thermophilum in this context.
d ITS2 PCR amplicons were characterized as containing two sequences, exact matches for ITS2 sequence C3 (KM487748) and ITS2 sequence S. thermo-indel (KP234524; Hume et al., Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015). Given that all samples genotyped with the ITS2 marker returned the same two sequences, only one representative of each sequence (KX671923 and KX671924, ITS2 sequence C3 and S. thermo.-indel, respectively) was submitted to NCBI's GenBank.
Amplification of the chloroplastic ribosomal 23S and nuclear ribosomal ITS1-5.8S-ITS2
The partial (domain V) chloroplastic ribosomal large subunit (cp23S) RNA gene was amplified by polymerase chain reaction (PCR) using the 23S4F and 23S7R primer pair (Pochon et al., Reference Pochon, Montoya-Burgos, Stadelmann and Pawlowski2006) using an initial denaturation for 4 min at 94°C followed by 35 cycles of 94°C for 30 s, 54.2°C for 30 s and 68°C for 90 s, followed by a final extension at 68°C for 10 min.
The nuclear ribosomal ITS1-5.8S-ITS2 region was PCR amplified using the primer pair ITS1Z1 and ITSZ2 (Forsman et al., Reference Forsman, Barshis, Hunter and Toonen2009) using an initial denaturation for 4 min at 94°C followed by 35 cycles of 94°C for 30 s, 50°C for 30 s and 68°C for 90 s, followed by a final extension at 68°C for 10 min.
All PCRs were conducted in a 25 µl final volume with 50 ng μl−1 of genomic DNA, 2.5 µl of 10× buffer, 2.5 µl of 10 mmol μl−1 dNTPs, 1.2 µl of 10 µmol μl−1 for each primer, 0.3 µl of 5 U μl−1 Taq DNA polymerase, and 10 µl of ddH2O.
Sequencing and phylogenetic analysis
PCR amplicons were directly sequenced by Macrogen (Korea) using the forward primer of each primer pair. Any sequences returning chromatograms containing multiple peaks that could not be explained by the presence of multiple sequences were discarded from the rest of the study. All cp23S and a representative selection of the ITS2 sequences were deposited in GenBank (Table 1).
ITS1-5.8S-ITS2 sequences were aligned with each other and cropped to the ITS2 region (e.g. KP234524) in MEGA 6 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). Sequences that returned a characteristic majority sequence (the sequence representative of the most abundant intragenomic ITS2 variant sequence) of ITS2 type C3 (e.g. KM487748) but also had a chromatogram characteristic of a secondary sequence of the S. thermo-indel (e.g. KP234524; Hume et al., Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015; identified from the mixed peaks in the chromatogram at the point of differentiation of the C3 and S. thermo-indel sequences) were characterized as belonging to the S. thermophilum group.
Cp23S sequences were aligned with other clade C reference sequences with a clade H sequence used to root the tree (Figure 2) in MEGA 6. All sequences were queried against the NCBI database using the nucleotide BLAST tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Substitutions or indels from the consensus in sequences obtained in this study were reverted to the consensus unless supported (i.e. found in any other Symbiodinium sequence in the NCBI database or found in a second sequence from this study). In this way, the probability of artifacts from sequencing or PCR error was minimized and the estimate of Symbiodinium diversity was conservative. A best-fit evolutionary model was inferred (Tamura 3 parameter with a gamma distribution with five categories) and a maximum likelihood analysis was conducted also using MEGA 6 with nodal support tested by bootstrap analysis (100 times).
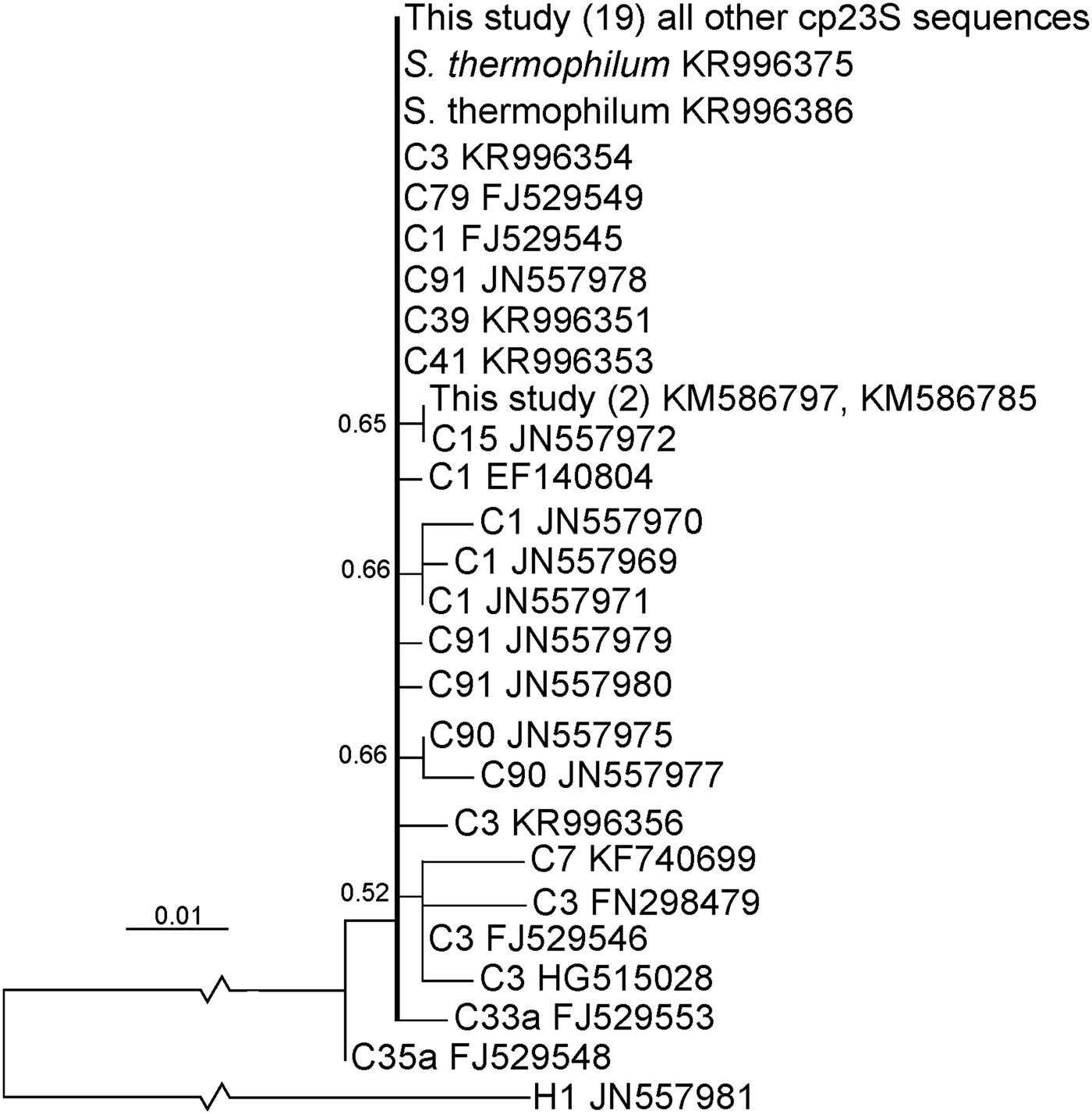
Fig. 2. Maximum likelihood analysis of domain V of the chloroplastic ribosomal 23S. Nomenclature of Symbiodinium are according to their predominant ITS2 sequence unless species classifications have been made. Accession numbers are given after all reference samples. Accession numbers for sequences from this study are given in Table 1. Numbers within parentheses represent multiple samples with identical sequences. Other numbers represent percentage bootstrap support values.
RESULTS
Nineteen of the 21 cp23S sequences analysed in this study returned one of two sequences: an ambiguous sequence giving an exact match for S. thermophilum and ITS2 types characterized by predominant ITS sequences C1, C3, C39, C41, C91 and C79 (e.g. KR996386), or a second sequence (e.g. KR996375) matching S. thermophilum only (Figure 2; Table 1). The remaining two sequences, both from P. harrisoni colonies collected at Larak Island (at the PG entrance) were matches for a type with predominant ITS2 sequence C15.
All seven of the ITS2 PCR amplicons sequences analysed in this study had intragenomic variant profiles that were exact matches for S. thermophilum (two sequences were present in the ITS2 PCR amplicon, the predominant C3 sequence, KM487748, and the lesser S. thermo-indel sequence, KP234524; Hume et al., Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015).
Where the given cp23S sequence was an exact match for S. thermophilum and ITS2 types C1, C3, C39, C41, C91, C79 cp23S sequences (e.g. sequence KR996386; 10 out of 21 samples) the predominant Symbiodinium complement harboured by the coral sample was putatively identified as an S. thermophilum group member. This is supported by the fact that all ITS2 sequences returned in this study identified S. thermophilum and that ITS2 types characterized by a predominant ITS2 sequence of C3 (other than S. thermophilum), C39, C41, C91 and C79 have not previously been documented in the Persian Gulf. Furthermore, it should be noted that corals of the genus Porites form a close fidelity with Symbiodinium from the ITS2 group C15. Exceptions to this fidelity are uncommon in the PG region except to form associations with S. trenchii and S. thermophilum (D'Angelo et al., Reference D'Angelo, Hume, Burt, Smith, Achterberg and Wiedenmann2015). The fact that the cp23S sequences returned in this study do not match C15 type Symbiodinium further supports the likelihood that the sequences are from S. thermophilum symbionts. Finally, whilst Symbiodinium types characterized by a majority ITS2 sequence of C1 (referred to here as ‘C1 types’) have been documented at very low abundance in the PG (one study; Hume et al., Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015), C1 types have returned multiple different cp23S sequences. This further indicates that the cp23S sequences returned in this study are unlikely to represent the C1 type symbiont.
DISCUSSION
Symbiodinium thermophilum has not previously been documented off the Iranian coastline. Here however, we document the presence of S. thermophilum group members in association with two species of scleractinian coral abundant at the three reef sites sampled in this study, sites that span almost the entire length of the Iranian coast.
Our findings are in contrast to previous studies off the Iranian coast that observed a predominance of Clade D Symbiodinium (likely S. trenchii; T.C. LaJeunesse personal communication) in scleractinian coral associations (Mostafavi et al., Reference Mostafavi, Fatemi, Shahhosseiny, Hoegh-Guldberg and Loh2007, Reference Mostafavi, Ashrafi and Dehghani2013; Rahmani et al., Reference Rahmani, Ghavam Mostafavi, Shahhosseiny, Vosoughi and Faraji2011; Shahhosseiny et al., Reference Shahhosseiny, Mostafavi, Fatemi and Karimi2011; Mashini et al., Reference Mashini, Parsa and Mostafavi2015). No Symbiodinium in Clade D were found in this study. This observed difference in Symbiodinium types could be due to differences in sampled corals between the studies (likely from variation in symbiont-specificities in different coral taxa). However, both C. microphthalma and Porites spp. were sampled as part of previous studies in the region and in all cases returned a predominance of clade D. It should also be noted that whilst some clade C symbionts were identified (e.g. Shahhosseiny et al., Reference Shahhosseiny, Mostafavi, Fatemi and Karimi2011; Mostafavi et al., Reference Mostafavi, Ashrafi and Dehghani2013), genetic resolution was to a cladal level and therefore species associations were not possible.
It should be noted that at sites ~200 km from the Khark Island site, off the Kuwaiti coast, clade C Symbiodinium was predominant in all corals sampled (Mahmoud & Al-Sarraf, Reference Mahmoud and Al-Sarraf2016). These Clade C symbionts are likely S. thermophilum, due to the fact that few other clade C species occur in the PG. However, the lack of material available for genetic analyses prevents an exact species assignment. Given the strong inflow to the PG from the neighbouring Gulf of Oman and the movement of water in a north-westerly direction along the Iranian coast from the Musandam peninsula (Johns et al., Reference Johns, Jacobs, Kindle, Murray and Carron1999; Yao & Johns, Reference Yao and Johns2010), limited physical connectivity (i.e. impairment of symbiont distribution due to contra-flowing currents) is likely not responsible for the lack of clade D found at the two most westerly sites in this study. Furthermore, the findings of clade D in previous studies at north-western PG sites (e.g. Jana Region; Figure 1) (Baker et al., Reference Baker, Starger, McClanahan and Glynn2004; Ziegler et al., Reference Ziegler, Arif, Burt, Dobretsov, Roder, LaJeunesse and Voolstra2017) evidences the ability of clade D to physically distribute throughout the PG. As such, the predominance of S. thermophilum group members in all three of this study's sites, and especially in the two more northerly sites, is likely due to these symbionts being better suited to local conditions rather than a lack of competition from other symbiont types.
Reefs off the Arabian coast of the PG, especially in the southernmost waters (for example reefs off Saadiyat and Dalma island, e.g. sites 5 & 6, see Figure 1) (D'Angelo et al., Reference D'Angelo, Hume, Burt, Smith, Achterberg and Wiedenmann2015), have gained attention due to the prevalence of associations hosting S. thermophilum; symbionts that are likely in part responsible for the exceptional resilience of these corals (although other factors such as host physiology and the exceptionally high salinity of the region probably contribute to this resilience too; D'Angelo et al., Reference D'Angelo, Hume, Burt, Smith, Achterberg and Wiedenmann2015). In contrast, coral associations surviving in reefs off the Iranian coast are typified as having narrower annual temperature ranges and closer to oceanic salinities, probably owing to the majority of symbiont characterization studies being conducted in the region within 300 km of the Strait of Hormuz (Mostafavi et al., Reference Mostafavi, Fatemi, Shahhosseiny, Hoegh-Guldberg and Loh2007, Reference Mostafavi, Ashrafi and Dehghani2013; Rahmani et al., Reference Rahmani, Ghavam Mostafavi, Shahhosseiny, Vosoughi and Faraji2011; Shahhosseiny et al., Reference Shahhosseiny, Mostafavi, Fatemi and Karimi2011; Mashini et al., Reference Mashini, Parsa and Mostafavi2015; Koupaei et al., Reference Koupaei, Dehghani, Mostafavi and Mashini2016). Coral associations at these sites are characterized as containing a mix of symbiont types, a large proportion of which are clade D, likely the stress-tolerant host-generalist S. trenchii (the only clade D Symbiodinium species identified in the PG to date; D'Angelo et al., Reference D'Angelo, Hume, Burt, Smith, Achterberg and Wiedenmann2015). However, reefs along the majority of the Iranian coastline remain uncharacterized. As represented by this study's Khark Island and Nayband Bay sites, a large proportion of this understudied coastline experiences temperature extremes and ranges significantly higher than those waters closer to the Strait of Hormuz (Figure 1). These Iranian coastal waters experience more variation in salinity across the year than waters in the southern Gulf, with salinities experienced closer to those of the southern waters than those in the Strait of Hormuz. Thus, it is unsurprising to find a predominance of halo- and thermophilic S. thermophilum group members. However, it should be noted that Cyphastrea and Porites spp. corals demonstrate a relative propensity to form an association with S. thermophilum group members compared with other corals. This was demonstrated in Hume et al. (Reference Hume, Voolstra, Arif, D'Angelo, Burt, Eyal, Loya and Wiedenmann2016) where at the southernmost PG sites, 90 out of 91 corals sampled across eight genera associated with S. thermophilum members. In comparison, at the more north-westerly sites where only 21 out of 68 of the corals associated with S. thermophilum group members, only four of the 10 most commonly sampled genera harboured group symbionts at an abundance over 50% (Porites, Favia, Cyphastrea and Favites). The other six genera harboured less than 20% in three cases (Acropora, Pavona and Platygyra) and none at all in the remaining three (Pocillopora, Stylophora and Montipora). This heterogeneity in affinity to S. thermophilum group members, specifically the higher likelihood of Porites and Cyphastrea spp. corals to form group member associations, has implications for the northern sites of Khark Island and Nayband Bay, as well as the southern Larak Island site.
Firstly, further sampling of ecologically significant scleractinian corals at the two more northern sites would enable a better understanding of whether associations with group members are limited to only some species of corals in these waters or whether their integration is complete, similar to the reefs of the southern PG (e.g. do species other than C. microphthalma and P. harrisoni contain S. thermophilum?). In this way we may better assess to what degree the group symbionts are responsible for the success of reefs in these waters. Secondly, the finding of sequences matching Symbiodinium types with a majority C15 ITS2 sequence (hereafter referred to as C15 type) in two out of the eight corals sampled at Larak Island (all P. harrisoni) is likely indicative of a lower proportion of S. thermophilum group members associating with corals in these waters, especially considering the affinity of Porites spp. corals for associations with S. thermophilum group members. This lower proportion is in agreement with previous findings in the area that demonstrated a mix of symbiont types.
When characterizing symbionts within Porites species, D'Angelo et al. (Reference D'Angelo, Hume, Burt, Smith, Achterberg and Wiedenmann2015) found a transition from C15 type symbionts in the Gulf of Oman, to the stress tolerant S. trenchii in the Strait of Hormuz, and finally to S. thermophilum group symbionts in the PG. D'Angelo et al. (Reference D'Angelo, Hume, Burt, Smith, Achterberg and Wiedenmann2015) hypothesized that the replacement of C15 by S. trenchii was resultant from its out-competition by the more stress tolerant symbiont as thermal ranges and salinities increased. This same reasoning explains the replacement of S. trenchii by S. thermophilum with increasing extremes in environment. As such, the findings of P. harrisoni in association with both C15 type and S. thermophilum member symbionts at Larak Island, as well as the previously identified clade D symbionts, would suggest that these waters represent the terminal end of a transition gradient from the more oceanic waters of the Gulf of Oman to the more saline and thermally stressful conditions of the southern and northern PG.
A considerable diversity of Symbiodinium types have been sampled that contain predominant ITS2 sequences closely related (often 1 bp different) to ITS2 sequence C15 (Krueger & Gates, Reference Krueger and Gates2012). As such it is likely that there is a diversity of Symbiodinium types with different phenotypes all of which contain the C15 sequences as their predominant ITS2 sequence. Given the diversity found in both of these groups of symbionts, an important aspect of further work in the Iranian coastal waters will be to re-characterize coral-symbiont associations using more resolute phylogenetic strategies, either by sequencing to a greater depth with the ITS2 marker so that fine scale intragenomic variations may be taken into account, or by using a marker such as the psbAncr that has the capacity to resolve between types with similar patterns of intragenomic ITS2 sequences. Such studies may demonstrate that the S. thermophilum group members and C15 types found at Larak Island may be phenotypic extremes of their respective groups and that group members at the more northern sites are genetically distinct from each other and other PG sites, potentially an example of local adaptation to these extreme conditions.
ACKNOWLEDGEMENTS
We offer our thanks to Iranian Pars Oil and Gas Company for providing financial support towards field sampling and laboratory resources. We would also like to thank Maryam Mehrbod for her assistance in sample collection and processing and Dr Carmen C. Denman (LSHTM, London, UK), for copyediting the manuscript.





