INTRODUCTION
White Sea bays are gradually separated from the sea due to postglacial isostatic uplift, which presently progresses at an average rate of 32.4 cm per century (Romanenko & Shilova, Reference Romanenko and Shilova2012). As a result lakes at different stages of isolation from the sea can be found. Evolution from marine to the final freshwater state lasts several hundred years (Subetto et al., Reference Subetto, Shevchenko, Lisitsyn, Ludikova, Kuznetsov, Sapelko, Evzerov, Van Beek, Souhaut and Subetto2012), and at intermediate stages a salinity gradient is usually observed (Hakala, Reference Hakala2004; Krasnova et al., Reference Krasnova, Pantyulin, Belevich, Voronov, Demidenko, ZhitinaL, Ilyash, Kokryatskaya, Lunina, Mardashova, Prudkovsky, Savvichev, Filippov and Shevchenko2013a). The condition where layers of lake water do not intermix because of differences in density is known as meromixis. Layers with different hydrological and hydrochemical properties are each inhabited by specific biota. To trace the development of this composite system long-term monitoring of a specific lake or its palaeoecological investigation is needed. However, in this study we employed a third possible method: we studied a set of lakes at different stages of isolation from the sea.
Typical vertical structure of a separating lake (SL) consists of three layers (Krasnova et al., Reference Krasnova, Pantyulin, Belevich, Voronov, Demidenko, ZhitinaL, Ilyash, Kokryatskaya, Lunina, Mardashova, Prudkovsky, Savvichev, Filippov and Shevchenko2013a, Reference Krasnova, Voronov and Voronovab): (i) the surface layer, partially desalinated because of water inflow from the catchment area, snow and rainfall; (ii) the middle layer, oxygenated salty water, well-warmed in summer; (iii) the lower anaerobic salty layer containing hydrogen sulphide because of stagnation; temperature there varies slightly throughout the year, and the salinity may exceed that of the sea (Krasnova et al., Reference Krasnova, Voronov and Voronova2013b). A chemocline with sharp physical and chemical gradients, including redox potential, exists in the border area between the salty aerobic and anaerobic layers. Usually the redox zone is 10–20 cm thick and coloured due to massive development of bright red, green or brown microorganisms. It is bright red when cryptophytic algae Rhodomonas bloom (Krasnova et al., Reference Krasnova, Pantyulin, Matorin, Todorenko, Belevich, Milyutina and Voronov2014), and green or brown when phototrophic bacteria are present (Lunina et al., Reference Lunina, Savvichev, Kuznetsov, Pimenov and Gorlenko2013; Kharcheva et al., Reference Kharcheva, Meschankin, Lyalin, Krasnova, Voronov and Patsaeva2014; Savvichev et al., Reference Savvichev, Lunina, Rusanov, Zakharova, Veslopolova and Ivanov2014).
The presence of coloured layers in the redox zone, indicating dense suspension of microorganisms, is the most interesting feature of SLs. In fact, this zone is the specific biotope for a unique plankton community in SLs. Finding a trend in the composition and quantitative indicators of stages of isolation in the SLs in the study area could serve as a model of ecological succession. With this as our goal, we started with identification of taxa composing the community of redox zones in the White Sea SLs.
MATERIALS AND METHODS
Five SLs with distinct coloured layers in the redox zone were studied in 2012–2014 in the vicinity of the White Sea Biological Station of Moscow State University (Figure 1, Table 1):

Fig. 1. Lake location: 1 – Kislo-Sladkoe, 2 – Lagoon on Zeleny Cape, 3 – Elovoe, 4 – Nizhnee Ershovskoe, 5 – Trekhtzvetnoe. WSBS – White Sea Biological Station of Moscow State University.
Table 1. Characteristics of the studied lakes.

(1) Lake Kislo-Sladkoe (‘Sour-and-Sweet’) – salt lake with a slightly diluted upper layer. There are no regular tidal fluctuations; inflow of seawater occurs several times a year during high syzygy tides; (2) Lagoon on Zeleny (‘Green’) Cape – salt lagoon with tidal oscillations of about 10 cm; in the sea they are about 2 m. Duration of inflow is shorter than that of the ebb tide, so most of the time water flows out of the lake into the sea; (3) Lake Elovoe – lake with vertical salinity gradient from fresh at the surface to marine near the bottom. It is separated from the sea by a rocky barrier with a freshwater stream flowing into the sea. There are no tidal fluctuations; seawater inflow is irregular and happens during floods caused by wind surges; (4) Lake Nizhnee (‘Lower’) Ershovskoe – freshwater lake with brackish water near the bottom; it is separated from the sea by a rocky rise with a freshwater creek flowing into the sea; (5) Lake Trekhtzvetnoe (‘Tricolour’) – freshwater lake with a salty bottom layer. The name meaning ‘tricolour’ was applied to the previously nameless lake because of the impressive differences in the colour of its three layers: surface 2 m layer is brownish due to humus substances released from the surrounding swamp; 10 cm thick redox zone is bright green; and bottom layer is lemon yellow hydrosulphide salty water. The colour is most likely due to polysulphides, turbidity due to suspended sulphur crystals.
Water samples were collected from different depths in the lakes using a Whale Premium Submersible Pump GP1352, with a vertical sampling step of 0.5 m. In coloured layers with thicknesses of 10–15 cm the sampling step was 10 cm. Temperature and salinity were measured using a WTW Cond 315i conductometer. The oxygen content was measured in situ using the ‘MARK 302 E’ oximeter with immersion probe. Acidity (pH) and ORP (E h) were measured with the WaterLiner WMM-73. Illuminance was measured with a regular digital luxmeter AR813A, modified to immerse its measuring unit under water. Water samples were examined using the bright field of a Leica microscope and the epi-fluorescence with filter set N2.1 (excitation/transmittance 515–560/580 nm). Abundance of some species and groups of unicellular eukaryotic organisms was rated using the scale similar to ACFOR in plant ecology or the S.M. Visloukh frequency scale used in calculating the saprobic index (Korde, Reference Korde, Pavlovsky and Zhadin1956). Samples were scored using the following scale: 1 – rare in the sample (one cell in the microscope slide), 2 – negligible (2–5 cells in each slide), 3 – significant amount (up to 50 cells in each slide), 4 – very dense (more than 50 in every slide).
To estimate dominant phototrophs spectrophotometry and spectrofluorometry were used. Height of the band on the absorption and fluorescence spectra indicates abundance of corresponding groups of organisms. Fluorescence spectra were recorded using the Solar CM2203 luminescence spectrometer by excitation of the wavelengths at 270, 410 and 440 nm. Absorption spectra were measured with the use of a Unico 2804 spectrophotometer and quartz cuvettes with an optical path length of 1 cm in the optical range 200–1100 nm. All spectral measurements were performed on unfiltered water samples in laboratory conditions at room temperature.
Identification of non-photosynthetic eukaryotes was done using a molecular genetic approach. Total DNA from water samples was isolated using a Nucleospin Plant Extraction kit according to the protocol of Macherey-Nagel (Germany). PCR was performed using the reagent kit from Dialat (Russia). The 18S rDNA gene was amplified with slightly modified primers recommended for eukaryotic rRNA by Medlin et al. (Reference Medlin, Elwood, Stickel and Sogin1988): Q5 (F) 5′-TCTGGTTGATCCTGCCAGT-3′ and Q39 (R) 5′-GTAGGTGAACCTGCAGAAGGATCA-3′.
PCR conditions were as follows: initial denaturation at 94°C for 3 min; 30 s at 94°C, 20 s at 58°C, and 30 s at 72°C repeated for 27 cycles; and final elongation at 72°C for 5 min. PCR fragments were purified in 1% agarose gel, extracted from agarose using the MinElute Gel Extraction Kit (QIAGEN) and cloned into vector pTZ57R/T (Thermo Scientific). Clones were screened by restriction enzymes of MspI. The DNA sequencing was carried out with the reagent kit, ABI PRISM® BigDye™ Terminator v. 3.1, and followed by an analysis of the reaction products using the Applied Biosystems 3730 DNA Analyzer at the Genome Collective Use Centre. The BLAST program was used to match the closest nucleotide sequences in GenBank (NCBI).
RESULTS
Abiotic conditions in the coloured water layers
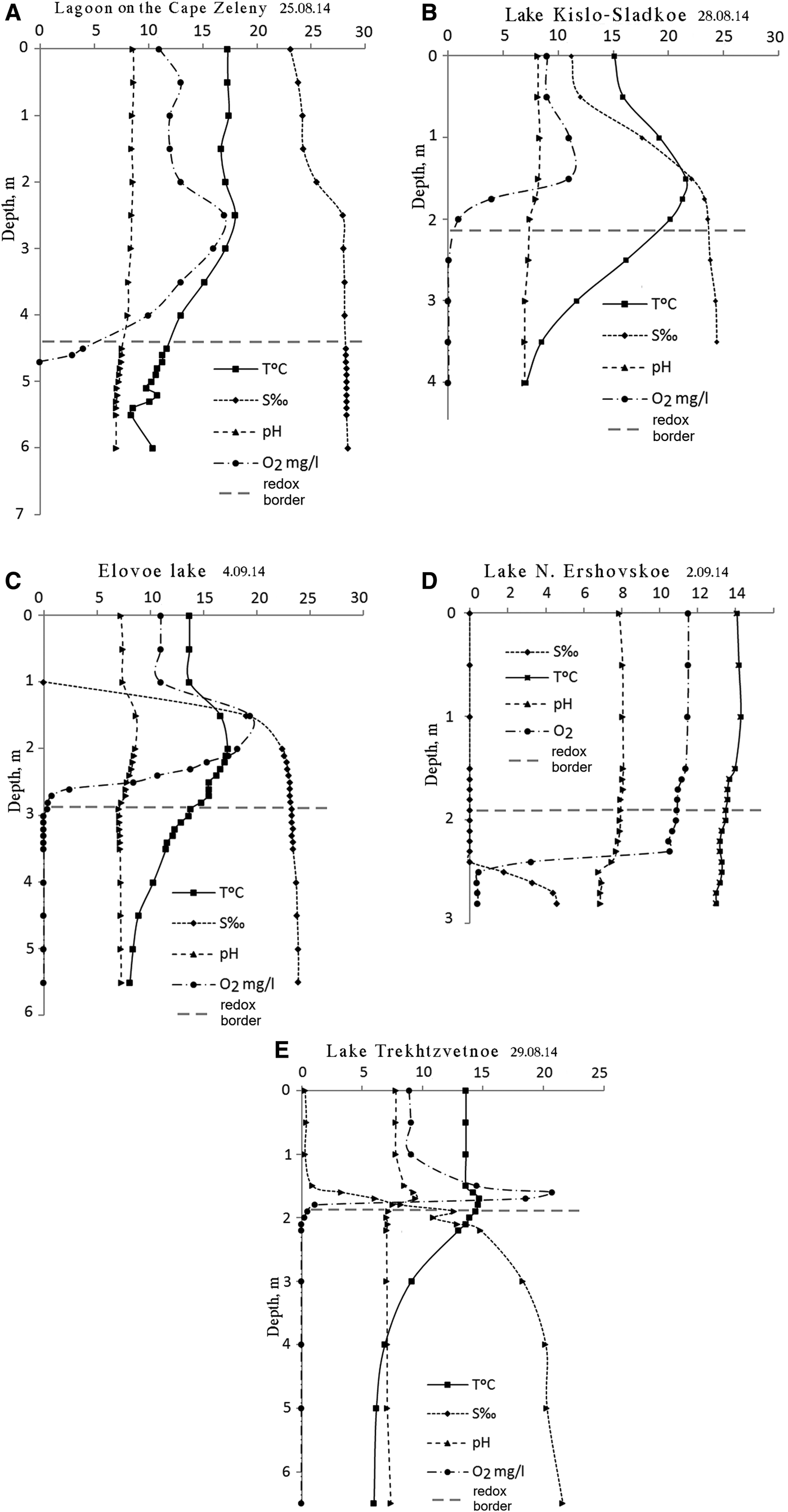
Temperature and salinity profiles in the studied SLs follow the pattern described earlier (Shaporenko et al., Reference Shaporenko, Koreneva, Pantyulin and Pertsova2005; Krasnova et al., Reference Krasnova, Pantyulin, Belevich, Voronov, Demidenko, ZhitinaL, Ilyash, Kokryatskaya, Lunina, Mardashova, Prudkovsky, Savvichev, Filippov and Shevchenko2013a). In the two SLs with the tidal inflow of seawater the upper layer was salty (the lagoon at Cape Zeleny and Kislo-Sladkoe), while in the three others the upper layer was fresh. In all the SLs salinity increased with depth (Table 1, Figure 2). Below 1.5–2 m salinity reached values characteristic for the White Sea in this region (23–27‰) or slightly diluted seawater (16–22‰). In Lake N. Ershoivskoe, which has a maximum depth of less than 2.5 m, the salinity near the bottom was just 4–10‰. The temperature in the upper layer depended on the season, warm in summer and below 0°C in winter. Near the bottom the temperature varied within a narrow range but never dropped below +4°C. In Lake Trekhtzvetnoe temperatures stayed constant throughout the year. During warm periods the temperature in the middle level of the SLs exceeded the temperature in both upper and lower layers. This was caused by the solar pond effect characteristic of lakes with salinity gradients (Zenkevitsch, Reference Zenkevitsch1928; Anderson, Reference Anderson1958; Burton, Reference Burton1981; Ouellet et al., Reference Ouellet, Dickman, Bisson and Pagé1989; Ludlam, Reference Ludlam1996; Williams, Reference Williams1996; Gibson, Reference Gibson1999, and many others).
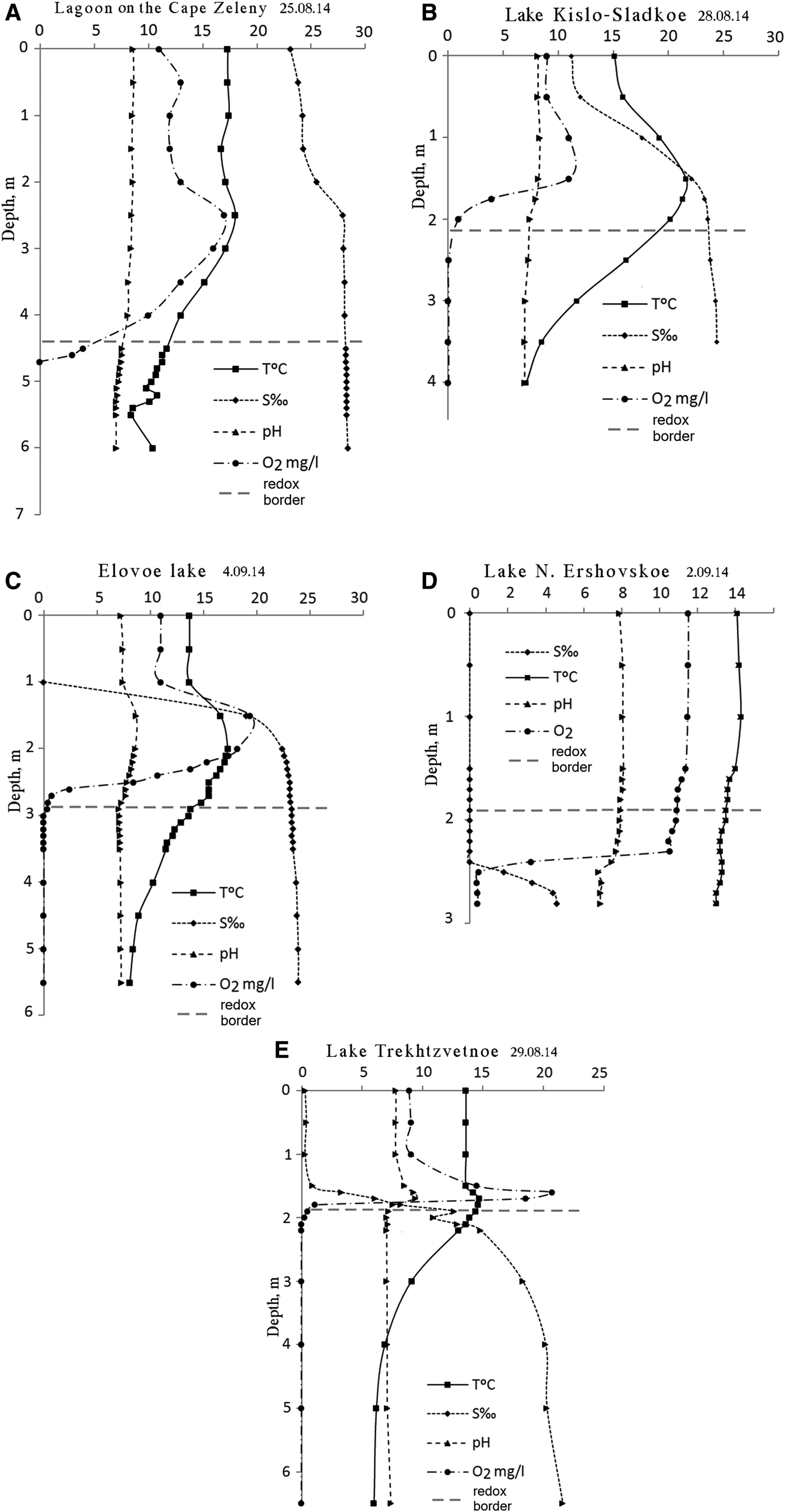
Fig. 2. Abiotic parameters of SLs at the end of summer and early autumn 2014: (A, B) lakes with tidal inflow of seawater; (C, D, E) lakes with a freshwater upper layer. Y-axis – depth in metres, numbers on the X-axis correspond to units indicated in the figure for each parameter.
Oxygen was detected in upper layers only. In the subsurface salty layers local increases of oxygen content would occasionally occur due to phytoplankton. As depth increased concentrations of dissolved О2 rapidly declined and disappeared simultaneously with the change in redox potential from positive to negative. The border between aerobic and anaerobic zones (redox zone) was marked by coloured microbial or algal layers. In the bottom water layer H2S was always present (Table 2). Maximum H2S concentrations in the SLs exceeded the values registered in the aphotic zone of the Black Sea (9.6 mg L−1), which is noted for having one of the highest concentrations of hydrogen sulphide of any body of water in the world (Nekludov et al., Reference Nekludov, Bortz, Polevich, Tkacjenko and Shilyaev2006). In one of the SLs, Trekhtzvetnoe, H2S concentrations was equal or even exceeded those found in the Norwegian Framavaren fjord (6 mM L−1 which corresponds to 204 mg L−1), considered to have the highest H2S concentration in any open water basin (Millero, Reference Millero1991; Behnke et al., Reference Behnke, Bunge, Barger, Breiner, Alla and Stoeck2006).
Table 2. H2S concentration (from Kokryatskaya et al., Reference Kokryatskaya, Krasnova, Losyuk, Tzetlin, Reuter, Patsaeva, Krasnova and Dolenko2014; Losyuk et al., Reference Losyuk, Kokryatskaya, Krasnova, Tzetlin, Reuter, Patsaeva, Krasnova and Dolenko2014; and personal data of N. Kokryatskaya). In Lake Elovoe it was not measured.

The pH values showed a declining trend from the surface to the bottom in all five lakes. Local increases were observed above the redox zone in the most oxygenated layer as a result of active photosynthesis. In the redox zone a sharp decrease in pH of 0.5–1.0 or even two pH units occurs. Near the bottom the pH was usually about 7.
Oxidation-reduction potential (E h) effectively divides the water columns of all SLs into three zones: (1) upper oxidized (aerobic) zone with positive values of E h, (2) narrow redox zone just 10–15 cm thick, where E h declines sharply by 100–350 mV/10 cm; (3) reduced (anaerobic) zone, with E h values below −200 or even −300 mV (Figure 3).

Fig. 3. Redox potential (E h, mV) at the end of summer 2014. Horizontal dash line marks position of the coloured layer. The coloured layer is in the zone of extremely sharp vertical decline of Eh, in the 100–350 mV/10 cm range.
Illuminance in the water column declined gradually from the surface to the redox zone where turbid coloured layers completely absorbed residual light.
Our findings consistently show that the redox zones in the SLs, with steep salinity, oxygen concentration, pH and illumination gradients, support abundant microbial communities in their coloured layers.
Microscopy data
Examination of samples from different layers under the microscope showed significant differences between the freshwater, saltwater aerobic and saltwater anaerobic layers.
In the lagoon on Zeleny Cape the upper 1 m of the water column had negligible quantities of unicellular algae (Figure 4). Significant amounts of phytoplankton were registered in the middle salty water layers starting from a depth of 1 m. In July 2013 many mobile colourless cells were present at 1 m and 3.5–4 m, in August 2013 at 2.5–4.3 m, in July 2014 at 4–4.5 m. In July 2013 small immobile green cells were abundant at 4 m, in August 2013 small green flagellates were abundant from the surface to a depth of 4 m, in June 2014 mass development of dinoflagellates Gymnodinium sp. was registered, in August 2014 Gymnodinium sp. together with small immobile algae cells reproduced at 1.5–4.7 m with maximum abundance at 4–4.7 m. Maximum density of organisms was observed in the redox zone. The location of the boundary between aerobic and sulphide layers varied from 4.2 to 5.2 m. Every summer through autumn a Cryptophitic algae, Rhodomonas sp., bloom was detected approximately 20 cm above this boundary (previously described in Krasnova et al., Reference Kokryatskaya, Krasnova, Losyuk, Tzetlin, Reuter, Patsaeva, Krasnova and Dolenko2014).

Fig. 4. Vertical distribution of protists. The width of vertical bars corresponds to abundance of organisms: 1 – rare in the sample, 2 – negligible, 3 – significant, 4 – very dense. Dashed line indicates border between aerobic and sulphide layers.
Heterotrophic dinoflagellates, Oxyrrhis marina Dujardin, 1841, actively fed on these algae and had red inclusions with bright fluorescence. Plenty of infusorians, belonging to several species, appeared close to the sulphide zone, many of them penetrated 0.1–0.5 m below. They also contained numerous red inclusions inside their cells. One of the identified ciliate species shared 99% similarity with Euplotes elegans (GenBank no. DQ309868) from the Mariager Fjord in Denmark (Schwarz et al., Reference Schwarz, Zuendorf and Stoeck2007) and from the Framvaren's fjord (GenBank no. EF527105) in Norway based on comparison of the 18S rRNA gene. Infusorians from the family Plagiopylidae were also found. One more ciliate species had 99% similarity in the 18S rRNA sequence with an uncultivated marine eukaryote of GenBank nos EF527105 and DQ103855 and 98% similarity with the sequence of Prorodon teres (GenBank no. X71140.1). In the deepest part of the sulphide zone eukaryotes were absent or only appeared occasionally.
In the upper 1 m of Lake Kislo-Sladkoe plankton were rare. In July 2013 and 2014 a number of heterotrophic protists occurred there, in August and September they declined. In lower aerobic layers with marine salinity phytoplankton were present. In August 2013–2014 green-coloured cocci 1.5–3 μm in diameter, presumably cyanobacteria, and phytoflagellates were present. Dinoflagellates O. marina were also found in this layer. Maximum number of protists occurred 0.2–0.3 m above the sulphide zone. Cells of cryptophitic algae Rhodomonas sp. dominated during summer and caused red colouration of the water near the chemocline. Infusorians of several species were numerous 0.1–0.2 m above and below the border between the aerobic and sulphide zones. Euglena sp. were observed near the boundary. In lower anaerobic areas of the chemocline numbers of Euglena sp. exceeded those in the adjacent aerobic layer. Single ciliates and Rhodomonas sp. cells were observed down to a depth of 4 m, but usually only bacteria inhabited the lower part of the sulphide zone. In winter protozoan plankton were absent in this lake.
In Lake Elovoe the upper 0.5 m of the water column is fresh water and free of protozoan plankton. Immediately below mass development of heterotrophic cells occasionally appeared. In July 2013 very small colourless flagellates were registered at a depth of 1.5 m, in August small protists were reproducing at 2–2.8 m, and were detected in the sulphide zone down to a depth of 3 m. In July–September 2014 there were no mass occurrences of small protozoans. Phytoplankton mass development was observed in the intermediate salt layer between the freshwater and sulphide layers. Dense cultures of small Chl-containing flagellates and cocci were registered at 2.0–2.8 m in the summer of 2014–2015 as well as the dinoflagellates O. marine preying on them. The redox boundary is located ~2.8 m deep in this lake, and is marked by a green layer with infusorians and green sulphur bacteria blooms. Partial sequence of the18S rRNA gene from a heterotrophic protozoan in the Cercozoa group found in Lake Elovoe had high sequence similarity with uncultivated Cercozoa with GenBank nos FN263034, JQ226494.1 from oxygen-deficient zones in the north-east Pacific, and JN090864.1 from freshwater Lake Karl in Greece (Oikonomou et al., Reference Oikonomou, Katsiapi, Karayanni, Moustaka-Gouni and Kormas2012). Below 3 m eukaryotes were not observed.
The freshwater layer in Lake N. Ershovskoe is poor in plankton, similar to other lakes with surface fresh water. Green flagellates were documented only once at a depth of 0.5–1 m. Phytoplankton blooms were observed in summer close to the chemocline located at 2.0–2.2 m. In summer 2014 in Lake N. Ershovskoe the green layer consisted of two sub-levels with slightly different colour and definitively different taxonomic compositions. Above the border of the aerobic and sulphide zones, at a depth of 1.9 m, enormous quantities of several species of green-coloured cocci and phytoflagellates were present, whereas just 10 cm below at a depth of 2.0 m there were no algae but dense cultures of green-coloured bacteria were present. Mass development of ciliates was registered in summer 2014 in the redox zone, both in oxidized and reduced parts. A bloom of unidentified cryptomonads was observed; in August they were present above the border of the sulphide zone, in September beneath it. In winter no eukaryotic cells were found in this lake and the green layer contained bacteria only.
In Lake Trekhtzvetnoe's upper 1 m of fresh water plankton were rare with one exception: in February 2014 Euglena sp. were found there in significant numbers. Maximum numbers occurred above the sulphide water at 1.5 m, and a second layer with abundant numbers was located deep in the sulphide zone at 6 m where they floated motionless. Immobile Euglena sp. cells were also observed at the same 6 m depth at the end of August 2014. In March 2014 no Euglena cells were found in this lake. This was similar to the other lakes studied where phytoplankton development was usually located in the intermediate salty aerobic layer. In this lake it lays at 1.5–1.8 m. In July 2014 a bloom of unidentified cryptophyte algae was observed at 1.7–1.8 m, close to the border of the aerobic and sulphide zones. Ciliates are not abundant in this lake, nevertheless they were observed in small amounts above the chemocline in August 2013 and July 2014, and in great numbers 0.4 m below the chemocline at a depth of 2.1 m at the end of August 2014. One of the ciliate species was the same as was found in the lagoon on Zeleny Cape, having 98% similarity with the sequence of P. teres. The chemocline in this lake is marked by a green layer containing green sulphur bacteria.
Light absorption measurements
This method allows identification of photosynthetic pigments composition corresponding to different taxa of phototrophs. Light absorption maxima at 450–460 and 720 nm correspond to absorption of light by bacteriochlorophylls (Bchls) d and e, 740–755 to BChl c. The peak in the 670–680 nm range corresponds to chlorophyll (Chl) a, so we can estimate the dominance of phototrophic bacteria or algae in the sample by the peak position.
The absorption spectra of water from the red layer (Figure 5A) peaked at 675 nm, this is attributed to the absorption of light by Chl a in phytoplankton. In Lake Elovoe, which has a brown layer in the redox zone, BChls d and/or e, characteristic of green sulphur bacteria, peaked at 722–726 nm (Figure 5B). They were also found below the redox zone where they peaked at 717–724 nm. The samples from the bright green layers (Figure 5C, D) are characterized by light absorption maxima near 720 nm corresponding to BChls d and e. In the sulphide zone the concentration of cells corresponding to this absorption value declined.

Fig. 5. Absorbance spectra of water samples from different depths. A – in the lagoon on Zeleny Cape with a red layer in the redox zone, B – in Lake Elovoe with a brown layer, C – in Lake N. Ershovskoe and D – in Trekhtzvetnoe; the last two have green layers. ‘O2’ means high oxygen concentration at this depth; ‘red layer’, ‘brown layer’ and ‘green layer’ indicate samples from red, brown or green coloured layers; ‘fresh’ means the layer at this depth is fresh water, ‘H2S’ indicates sulphide layer. Surface layers as well as middle highly oxygenated layers are almost clear. In the red layer algae containing Chl a are present. Brown colour in the redox zone is caused mostly by BChl d and/or e, the same pigments occur in the sulphide zone in small amounts. The green colour in the redox zone of Lakes N. Ershovskoe and Trekhtzvetnoe is caused mostly by BChl d and/or e. The lower sulphide layers contain BChls and sunken cells with Chl a.
Fluorescence measurements
In water samples from the coloured water layers, under UV excitation with wavelength λex = 270 nm and shorter, two overlapping emission bands were observed. The first, with a maximum of 330–350 nm, is characteristic of protein-type fluorescence as a result of a high concentration of microorganisms. A broad peak with a maximum at 420–460 nm (so-called humic-type fluorescence) corresponds to dissolved organic matter (humus substances) in natural water (Patsayeva & Reuter, Reference Patsayeva and Reuter1995; Gorshkova et al., Reference Gorshkova, Milukov, Patsayeva and Yuzhakov2006; Shubina et al., Reference Shubina, Patsaeva, Yuzhakov, Gorshkova and Fedoseeva2009).
The position of the fluorescence emission peak excited at 440 nm varied slightly in samples of coloured water from the different SLs. Its maximum wavelength was 755 nm for the lagoon at Zeleny Cape (Figure 6A), 752 nm for Kislo-Sladkoe (Figure 6B), 750 nm for Elovoe (Figure 6C), 769 nm for Nizhnee Ershovskoe (Figure 6D) and 763 nm for Trekhtzvetnoe (Figure 6E). This was close to fluorescence emission of BChls in green sulphur bacteria usually located near 770 and 815 nm.

Fig. 6. Fluorescence emission spectra excited at wavelength of 440 nm. In the red layers of the lagoon at Cape Zeleny (A) and Lake Kislo-Sladkoe (B) the peaks at 686 nm indicate presence of the algae Rhodomonas sp. containing Chl a. The extrema in the region of 748–759 nm in the bottom layers match emission of bacterial photosynthetic pigments. Maxima in the region of 745–750 nm in the water samples from brown layer (Lake Elovoe, C) and 765–780 nm in the green layers of the lakes N. Ershovskoe (D) and Trekhtzvetnoe (E) correspond to different taxa of green sulphur bacteria.
Vertical distribution of phytoplankton and green sulphur bacteria
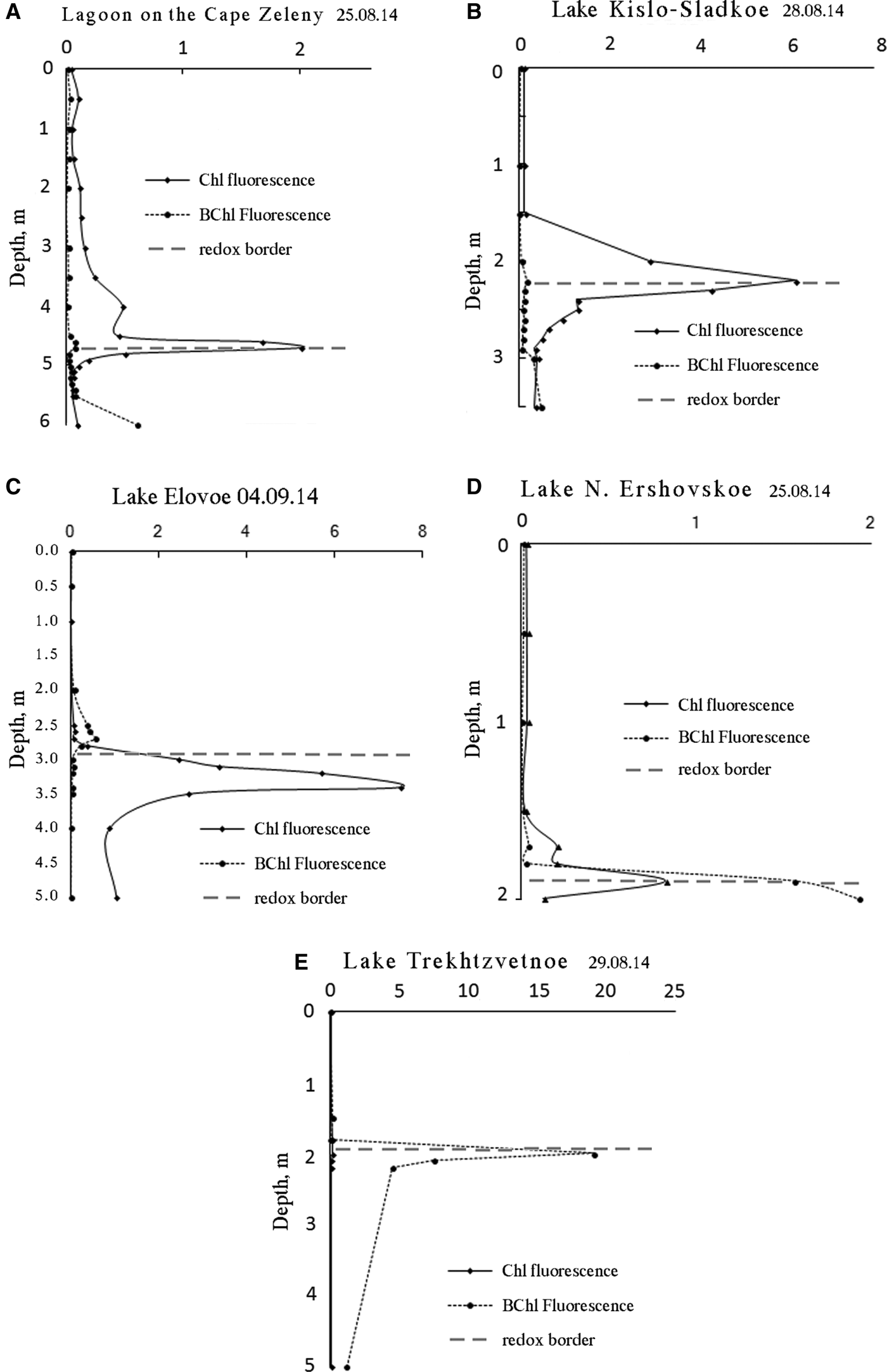
Vertical distribution profiles of main photosynthetic pigments (Chl a and BChls) were developed for fluorescence excited at 440 nm (Figure 7). Intensity at an emission wavelength of 685 nm was recorded to estimate the concentration of Chl a in relative units. To quantify BChl we measured fluorescence amplitude at the wavelength of BChl emission maximum within the 750–770 nm range.
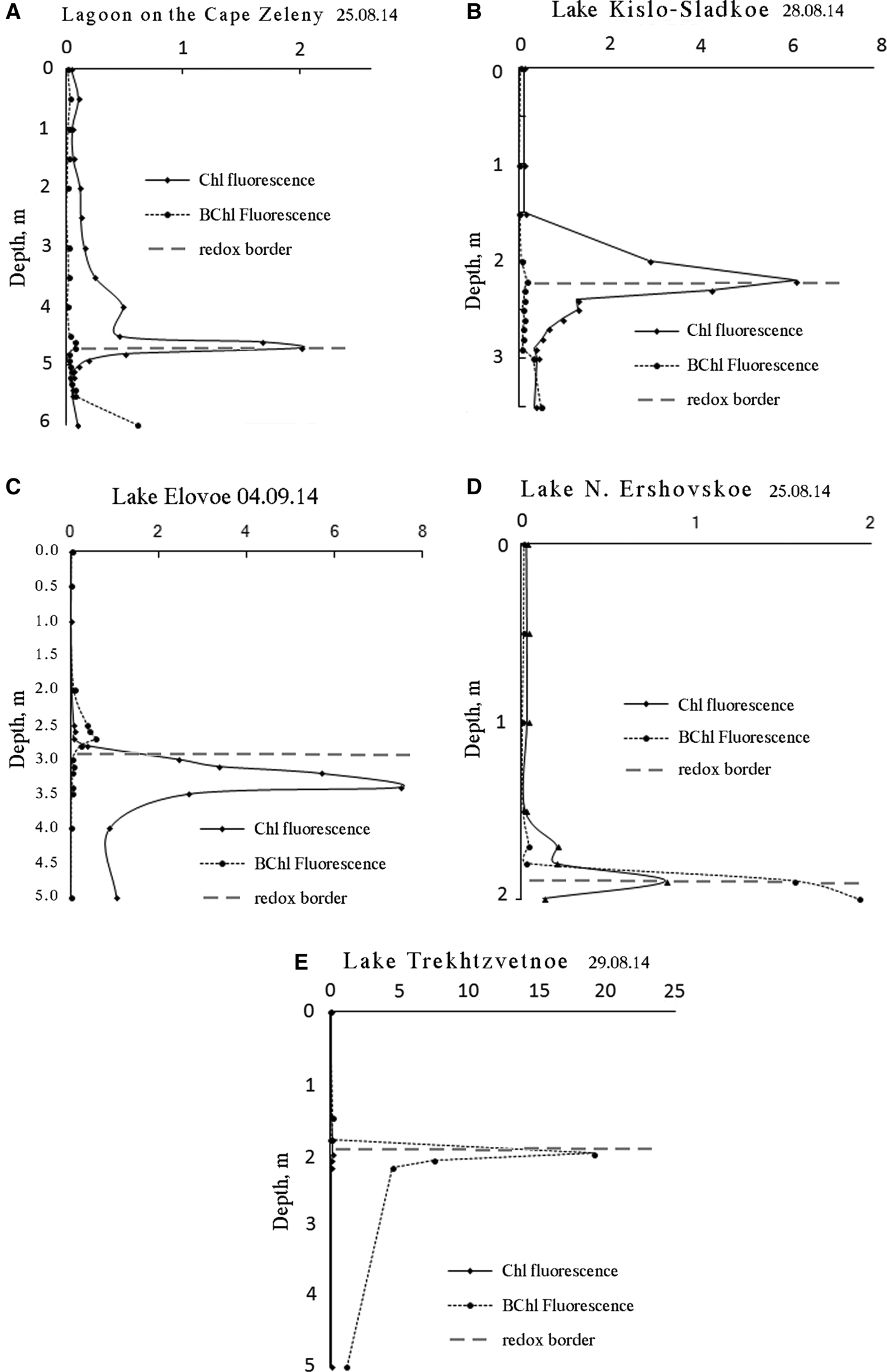
Fig. 7. Vertical distribution of main photosynthetic pigments of phytoplankton (Chl a) and green sulphur bacteria (BChls c, d, e) at the end of summer 2014. Dashed line separates reduced and oxidized zones.
DISCUSSION
Meromixis is a natural stage of ecosystem development for relict lakes along sea shores (Hakala, Reference Hakala2004; Dugan & Lamoureux, Reference Dugan and Lamoureux2011). Different layers separated by density barriers serve as biotopes for different communities of microorganisms. In well-studied meromictic Lake Mogilnoe on the Kildin Island in the Barents Sea, three communities were found to coexist: (1) freshwater species in the surface freshwater layer, (2) marine organisms in the middle saltwater layer, and (3) a bacterial community in the bottom oxygen-depleted layer (Gorlenko et al., Reference Gorlenko, Vainstein and Kachalkin1978; Strelkov et al., Reference Strelkov, Shunatova, Fokin, Usov, Fedyuk, Malavenda, Lubina, Poloskin and Korsun2014). Between the aerobic and anoxic water layers purple sulphur bacteria form a red layer 0.5 m thick (Gorlenko et al., Reference Gorlenko, Vainstein and Kachalkin1978; Strelkov et al., Reference Strelkov, Shunatova, Fokin, Usov, Fedyuk, Malavenda, Lubina, Poloskin and Korsun2014). This is similar to red layers in the White Sea SLs. However, the cause of colouration is different. In White Sea SLs colour is caused by the presence of the cryptophytic algae, Rhodomonas sp.
Microbial coloured layers exist in many meromictic lakes all over the world (Overmann et al., Reference Overmann, Beatty, Hall, Pfennig and Northcote1991; Savvichev et al., Reference Savvichev, Rusanov, Zakharova, Lunina, Bryantseva, Yusupov, Pimenov, Ivanov, Rogozin and Degermendzhi2005; Rogozin et al., Reference Rogozin, Trusova, Khromechek and Degermendzhy2010; Mori et al., Reference Mori, Kataoka, Okamura and Kondo2013; Storelli et al., Reference Storelli, Peduzzi, Saad, Frigaard, Perret and Tonolla2013). These layers can be green, red or brown. Cases of seasonal change of colour because of succession in the microbial community have been documented (Tonolla et al., Reference Tonolla, Peduzzi, Demarta, Peduzzi and Hahn2004). Due to the presence of many SLs at different stages of isolation along the coast of the White Sea, there is an opportunity for chronological reconstruction of their hydrological and ecological evolution including long-term succession in the plankton community in the coloured layers.
The redox zone in the White Sea SLs is a specific biotope characterized by sharp gradients in physical and chemical parameters. This narrow and highly anisotropic zone is much more favorable for plankton in comparison with other zones of the studied SLs. Conditions in the coloured layers in different SLs have some similarities and differences. In all studied SLs the coloured layers were always situated below the pycnocline within the saltwater layer.
The SLs are influenced by varying degrees of freshwater run-off, therefore total salinity varies among the lakes as well as among the coloured layers. Throughout 4 years of observations the salinity in the red layer of the lagoon at Cape Zeleny was about 28‰, in Lake Kislo-Sladkoe 24‰, in the brown layer of Lake Elovoe 23‰, in Lake Trekhtzvetnoe 14‰ and in the green layer of Lake N. Ershovskoe 2‰. Red layers are characteristic of lakes with higher salinity in their redox zones, green layers of lakes with diluted seawater, and brown layers are observed in lakes with intermediate salinity. The temperature in the coloured layers depends on the season; it never drops below 4°C and does not rise above 20°C. The most impressive feature of the coloured layers is the sharpness of E h decline by 350 mV/10 cm, and the steep drop of illuminance to zero because of complete light absorption by microorganisms suspended in the coloured layers. The dark zone below 2–4 m in the SLs with transparent overlying water is definitely a result of development of microorganisms in the coloured layer. The abrupt decrease of E h indicates the transition between the upper aerobic and lower anaerobic zones. The aerobic zone contains oxygen due to its transfer from the surface by convection and diffusion, and due to its production in situ by photosynthesis; in the anaerobic zone the oxygen is consumed for oxidation of excess organic matter sinking from the upper aerobic zone.
Vertical distribution of pigments indicates that the coloured layers are the most productive parts in all SLs. As the biomass of photosynthetic microorganisms is proportional to pigment content (Antal et al., Reference Antal, Venediktov, Matorin, Ostrowska, Woźniak and Rubin2001) we can compare the quantities of cells in the different layers by height of the corresponding spectra peaks. In the coloured layers the amount of phototrophic cells is 2–15 times more than in the aerobic zone and 2–10 times more than in the bottom sulphide zone.
Light absorption and fluorescence spectra data indicate there are two main phototrophic components in the communities found in the coloured layers: algae containing Chl a, and green sulphur bacteria with the BChls d and e. In SLs with a red water layer Rhodomonas sp. algae make up most of the biomass; green sulphur bacteria predominate below in the sulphide zone. In SLs with green water layers algae can be abundant in the upper oxygenated part of the coloured layer as was found in Lake N. Ershovskoe, or just above it like the Euglena sp. found in Lake Trekhtzvetnoe. Nonetheless in the dense microbial culture of the redox zone green sulphur bacteria play the leading role. In the brown water layer where bacteria are also the main contributor to colouration, they differ from those in the green layers by spectral properties of their photosynthetic pigment as they emit fluorescent light of a shorter wavelength. There is little variety of eukaryotic species found in the coloured layers. The number of different species is limited and the same species are found in different SLs. Dominance of one or two species is characteristic of these communities. Salinity and temperature differ in the 5 SLs, indicating that the species have wide tolerance to these conditions.
Data on eukaryotic species composition found in the coloured layer communities allow for the identification of some common features. Every SL has some mixotrophic dominant (Rhodomonas sp. and other Cryptophyta, Euglena sp.) capable of photosynthesis as well as of consuming organic matter. Mixotrophy is an important adaptation in an environment with hydrogen sulphide present and an abundance of bacteria as a potential food source (Roberts & Laybourn-Parry, Reference Roberts and Laybourn-Parry1999; Jones, Reference Jones2000; Hammer & Pitchford, Reference Hammer and Pitchford2005). Identified infusorians and dinoflagellate O. marine are predators able to phagocytize bacteria as well as large algal cells. In all SLs studied, mass growth of ciliates was occasionally observed, mostly in the lower sulphide level of the redox zone. Some species feed on unicellular algae and can swallow up to several dozen cells. All infusorians have wide tolerance ranges for salinity. Euplotes elegans is tolerant to salinity from 2.5 to 44.5‰ (Schwarz et al., Reference Schwarz, Zuendorf and Stoeck2007) and can grow slowly at +4°C. Optimal salinity and temperature for the population of this species in Mariager Fjord was 12–32‰ and about +20°C. Prorodon teres inhabits a wide variety of environments: freshwater ponds and puddles, brackish marshes, or salt water.
An important adaptation of redox zone species is their resistance to oxygen deficiency. Euplotes elegans can reproduce under low oxygen content and survive anaerobically up to 24 h. The enzyme system of O. marina allows it to stay active in the absence of oxygen (Altenbach et al., Reference Altenbach, Bernhard and Seckbach2012). Plagiopylidae ciliates can occupy different anoxic habitats including invertebrate internal environments (Fenchel & Finaly, Reference Fenchel and Finaly1990; Lynn & Strüder-Kypke, Reference Lynn and Strüder-Kypke2002). Additionally, many anaerobic ciliates including Plagiopylidae harbour a flora of ecto- and endosymbiotic bacteria and metanogenic archaea facilitating their adaptation to anoxic environments (Fenchel et al., Reference Fenchel, Perry and Thane1977).
Thus, the dominant species found in redox zone communities are adapted to the anaerobic and microaerobic conditions prevalent in this zone and are capable of using organic matter produced by phytoplankton from the layers above as well as by anoxic bacterial photosynthesis in the lower water levels. Unicellular organisms and algae could benefit from adjacent phototrophic bacteria in different ways. First, they can consume bacterial cells. Second, bacterial metabolites can serve as a source of organic compounds. They use nutrients produced by green sulphur bacteria presented in the coloured layers of SLs. As these bacteria provide anoxygenic photosynthesis, this source of primary production distinguishes the chemocline community from regular marine and freshwater plankton ecosystems where the main producers are algae.
In relation to degree of isolation from the sea we found that at the initial stages the redox zones are characterized by red cryptophytic layers and at the advanced stages by green or brown layers with green sulphur bacteria. Along this sequence the role of eukaryotic phototrophs declines, and they are replaced by prokaryotic organisms. Thus the colour of the layer in the redox zone and spectral characteristics of the water can be considered indicators of the stage of isolation from the sea.
CONCLUSIONS
In the White Sea SLs most microplankton numbers are concentrated in a narrow layer associated with the redox zone. The amount of phototrophic organisms there is more than 2–10 times the amount found in the aerobic zone and 2–5 times that of the sulphide zone. The redox zone is characterized by sharp physical and chemical gradients; most prominent are Eh gradient up to 350 mV/10 cm, and illuminance declining to zero because of light absorption by high densities of phototrophic organisms. The coloured conglomeration of plankton is about 10 cm thick. According to light absorption and fluorescence spectra algae containing Chl a predominate in the red layer of SLs connected to the sea. The red colour is caused by blooms of cryptophyte algae, Rhodomonas sp. In SLs in more advanced stages of isolation from the sea the redox zone is green because of green sulphur bacteria. A brown colour is caused by green sulphur bacteria the BChl of which absorbs light of shorter wavelength. Eukaryotic biota of the redox zone in SLs is characterized by an abundance of mixotrophic organisms and by species resistant to anoxia. The colour and spectral characteristics of water in redox zones as well as spectral characteristics (i.e. light absorption and fluorescence) of the water can be considered indicators of the stage of lake isolation from the sea.
FINANCIAL SUPPORT
This work was supported by the Russian Foundation for Basic Research [grant numbers 12-04-00534-а, 12-04-01621-а, 13-04-10068, 14-35-10094], and the non-profit, Dynasty Foundation [grant number SS14-63].