INTRODUCTION
The common cuttlefish, Sepia officinalis (Linnaeus, 1758), is a short lived and semelparous cephalopod distributed from the West African coast (Senegal and Mauritania) to the English Channel and in the Mediterranean Sea (Mangold, Reference Mangold and Boyle1987). It has a life cycle lasting between 1 and 2 years depending on the latitude at which it lives (Domingues et al., Reference Domingues, Bettencourt and Guerra2006; Guerra, Reference Guerra2006). From West African to the Portuguese coasts, the entire population has a 1-year life cycle (Mangold-Wirz, Reference Mangold-Wirz1963; Mangold, Reference Mangold1966; Guerra & Castro, Reference Guerra and Castro1988; Coelho & Martins, Reference Coelho, Martins and Boucaud-Camou1991). The Bay of Biscay is a transition zone where two different groups were identified; Group I Breeding (GIB), can breed at 1 year of age (45% of male specimens and 20% of female specimens), Group II Breeding (GIIB), breeds at 2 years of age (Le Goff & Daguzan, Reference Le Goff and Daguzan1991; Gauvrit et al., Reference Gauvrit, Le Goff and Daguzan1997). In the English Channel, the life cycle of S. officinalis was originally described as lasting exclusively 2 years (Boucaud-Camou & Boismery, Reference Boucaud-Camou, Boismery and Boucaud-Camou1991; Boucaud-Camou et al., Reference Boucaud-Camou, Koueta, Boismery, Medhioub and Boucaud-Camou1991). A decade later, Dunn (Reference Dunn1999a) found that the entire female population and 96% of male specimens had a 2-year life cycle (GIIB). The remaining males (4%) were found to be mature at the age of 1 year old, constituting a GIB.
The English Channel population of S. officinalis was mainly considered as a non-valuable by-catch before the 1980s (Dunn, Reference Dunn1999a). Since then, the increasing price of this species and the depletion of finfish stocks (Royer et al., Reference Royer, Pierce, Foucher and Robin2006; Molfese et al., Reference Molfese, Beare and Hall-Spencer2014) led French and UK fishers to exploit this stock. Landings increased from 4350 tonnes in 1992 to a maximum of 17,400 tonnes in 2004 (Gras et al., Reference Gras, Roel, Coppin, Foucher and Robin2014). During the 2000–2010 decade, an average of 11,000 tonnes was landed annually for an average turnover of €20 M (Pierce et al., Reference Pierce, Allcock, Bruno, Bustamante, Gonzalez, Guerra, Jereb, Lefkaditou, Malham, Moreno, Pereira, Piatkowski, Rasero, Sanchez, Santos, Santurtun, Seixas, Sobrino and Villanueva2010; Portail CHARM III–Interreg IV, ©, Reference Engelhard, Vignot, Leblond, Lesueur and Guitton2012). The English Channel stock of S. officinalis was assessed in trials using a depletion model (Dunn, Reference Dunn1999b), a Virtual Population Analysis (Royer et al., Reference Royer, Pierce, Foucher and Robin2006) and a two-stage biomass model (Gras et al., Reference Gras, Roel, Coppin, Foucher and Robin2014). Whilst Dunn (Reference Dunn1999b) does not give a clear indication of the stock status, Royer et al. (Reference Royer, Pierce, Foucher and Robin2006) and Gras et al. (Reference Gras, Roel, Coppin, Foucher and Robin2014) concluded that Sepia officinalis has been fully exploited since the beginning of the 1990s. Moreover, Gras et al. (Reference Gras, Roel, Coppin, Foucher and Robin2014) showed that S. officinalis abundance decreased during the period 2001–2008 and consequences of the fishing pressure on life history traits should be investigated.
High fishing pressure directly and indirectly affects fish communities and populations. Among the direct effects of high fishing pressure on populations are increased mortality and size selectivity that tend to reduce the proportion of old and large specimens in the population (Smith, Reference Smith1994). After several years of high exploitation, smaller specimens maturing faster are favoured, leading to decrease in mean size and length (or age) at maturity by favouring slow growth and early maturing specimens (Bianchi et al., Reference Bianchi, Gislason, Graham, Hill, Jin, Koranteng, Manickchand-Heileman, Paya, Sainsbury, Sanchez and Zwanenburg2000; Shin et al., Reference Shin, Rochet, Jennings, Field and Gislason2005; Kantoussan et al., Reference Kantoussan, Ecoutin, Fontenelle, Thiaw, Tito de Morais and Laë2009). Observations of such processes have been carried out in various stocks of the N-E Atlantic such as the North Sea herring (Shin & Rochet, Reference Shin and Rochet1998; Engelhard & Heino, Reference Engelhard and Heino2004), the North Sea plaice (Grift et al., Reference Grift, Rijnsdorp, Barot, Heino and Dieckmann2003) and the northern cod (Olsen et al., Reference Olsen, Heino, Lilly, Morgan, Brattey, Ernande and Dieckmann2004). The English Channel has been trawled for over 200 years and from the beginning of the 20th century to date, the fishing pressure has increased considerably (Hawkins et al., Reference Hawkins, Southward and Genner2003). According to Guenette & Gascuel (Reference Guenette and Gascuel2012), the French fishing effort displayed in the Celtic Sea and the Bay of Biscay (including western English Channel, ICES division VIIe where S. officinalis overwinters and is exploited by offshore trawlers) has been multiplied by 10 between 1950 and 2010. In the English Channel, the number of trawling vessels (coming from France and UK) who share the demersal resources has increased from 450 vessels in 2000 to almost 800 ten years later (Portail CHARM III–Interreg IV, ©, Reference Engelhard, Vignot, Leblond, Lesueur and Guitton2012). Finally, a compilation of French and UK data used to derive abundance indices (Gras, Reference Gras2013; Gras et al., Reference Gras, Roel, Coppin, Foucher and Robin2014) shows an increase of the trawling effort from 572,000 h in 1992 to an average of 900,000 h for the period 2003–2007.
In addition to the fishing pressure, climate variations (including global warming which is made of natural and anthropogenic components) are also known to influence the abundance and spatial distribution of marine species (Beaugrand & Reid, Reference Beaugrand and Reid2003; Hawkins et al., Reference Hawkins, Southward and Genner2003; Beaugrand, Reference Beaugrand2009). According to the data collected by the Marine Biological Association of the United Kingdom (MBA), significant warming of the English Channel occurred throughout the 20th century (Southward & Roberts, Reference Southward and Roberts1987). Between 1986 and 2006, Saulquin & Gohin (Reference Saulquin and Gohin2010) estimated this warming to be between 0.5 °C in the southern part of the Channel and 1.5 °C in its northern part. This warming has led to modifications in phytoplankton assemblages, fish larval abundances and pelagic fish abundances (Southward et al., Reference Southward, Boalch and Maddock1988). Moreover, a decline in community level, average length and length-at-maturity in demersal communities has been observed, suggesting that fish assemblages become dominated by species maturing faster and reproducing at a shorter length (Hawkins et al., Reference Hawkins, Southward and Genner2003). Due to their short life cycle and their high metabolic rate, cephalopods are assumed to be very sensitive to environmental warming (Pierce et al., Reference Pierce, Allcock, Bruno, Bustamante, Gonzalez, Guerra, Jereb, Lefkaditou, Malham, Moreno, Pereira, Piatkowski, Rasero, Sanchez, Santos, Santurtun, Seixas, Sobrino and Villanueva2010).
In the context of high fishing pressure and global warming observed between the end of the 1980s and the end of the 2000s in the English Channel, this work aims to establish whether modifications in the life cycle of the S. officinalis exist. By focusing on the stock structure in the reproduction period, we would like to highlight whether the percentage of 1-year-old mature males observed by Dunn (Reference Dunn1999a) has changed and whether a percentage of 1-year-old mature females exists or not. To achieve this goal, a 2-year sampling programme was carried out in spring at French landing sites within the English Channel. Length frequencies associated with measurement of lipofuscin were used to discriminate between the two cohorts and estimate the percentage of mature 1-year-olds. The size-at-maturity and the Gonado Somatic Index estimated are compared with results previously published (Dunn, Reference Dunn1999a). Results obtained are finally discussed in the framework of the environmental pressures exerted on the S. officinalis population inhabiting the English Channel.
MATERIALS AND METHODS
Study area: the English Channel
The English Channel is situated in the N-E Atlantic between the Celtic Sea and the North Sea. Following the International Council for the Exploration of the Sea (ICES), two subdivisions exist, VIId to the East and VIIe to the West. The western English Channel is characterized by a maximum depth of around 170 m and is strongly influenced by Atlantic waters. With a minimum temperature >10 °C in winter, this area has favourable conditions for the overwintering of Sepia officinalis (Wang et al., Reference Wang, Pierce, Boyle, Denis, Robin and Bellido2003). The eastern English Channel (ICES division VIId) is characterized by shallow waters, maximum depth of 50 m and a homogeneous water column. Winter sea temperature in this part of the Channel can reach as low as 5 °C, making it unsuitable for overwintering of S. officinalis (Pingree, Reference Pingree, Banner, Collins and Massie1980; Boletzky, Reference Boletzky and Boyle1983).
Previously described migration cycle of S. officinalis within the English Channel
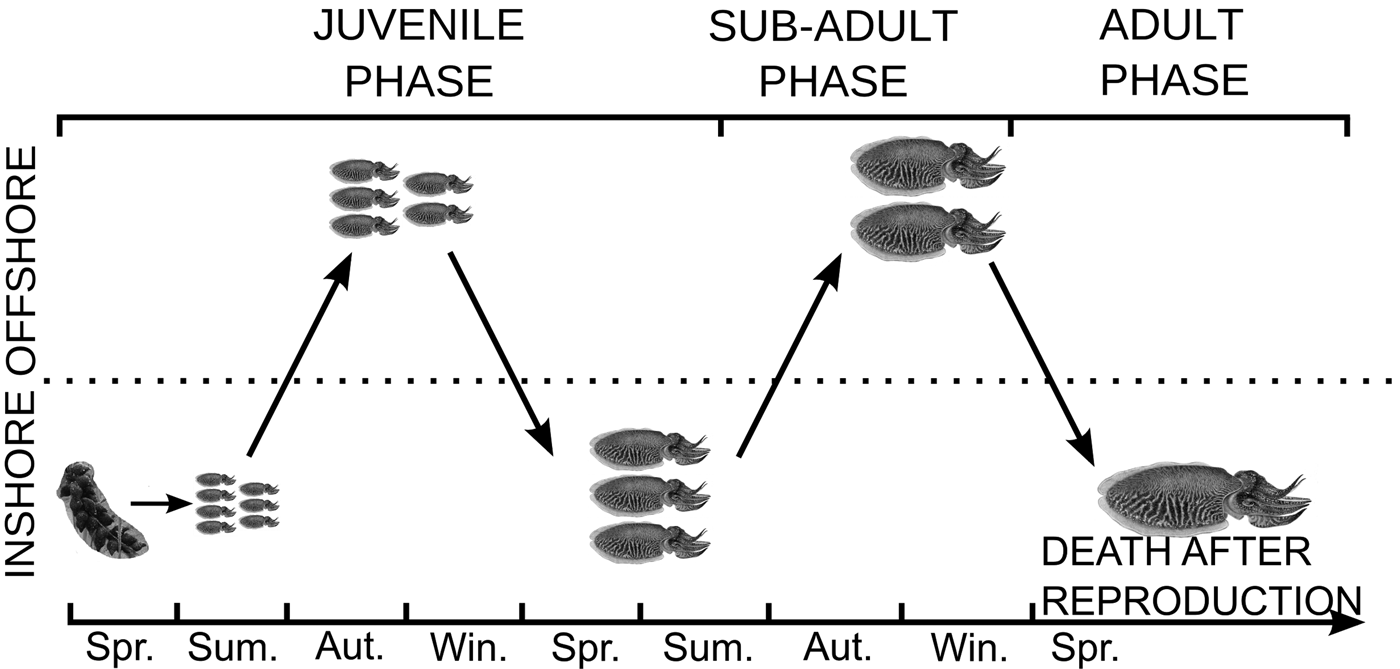
In the English Channel, S. officinalis hatch inshore at the beginning of summer (Figure 1). After spending the entire summer feeding inshore, they migrate offshore to overwinter in the central western English Channel (Boucaud-Camou & Boismery, Reference Boucaud-Camou, Boismery and Boucaud-Camou1991; Dunn, Reference Dunn1999a; Royer et al., Reference Royer, Pierce, Foucher and Robin2006) where sea bottom temperature is favourable (Wang et al., Reference Wang, Pierce, Boyle, Denis, Robin and Bellido2003). The following summer is also spent feeding inshore. At the end of this second summer, male specimens finish their maturation (in approximately September). In October, they perform their second offshore migration to the wintering grounds where female specimens complete their maturation. The final migration is performed at approximately 20 months old to reach the inshore spawning zones. Mass mortality of sexually mature adults occurs after the reproduction period (Boucaud-Camou & Boismery, Reference Boucaud-Camou, Boismery and Boucaud-Camou1991; Dunn, Reference Dunn1999a; Guerra, Reference Guerra2006; Royer et al., Reference Royer, Pierce, Foucher and Robin2006).

Fig. 1. Life cycle of the English Channel population of S. officinalis L. as described by Boucaud-Camou & Boismery (Reference Boucaud-Camou, Boismery and Boucaud-Camou1991), Boucaud-Camou et al. (Reference Boucaud-Camou, Koueta, Boismery, Medhioub and Boucaud-Camou1991) and Dunn (Reference Dunn1999a).
Sample collection
Samples of S. officinalis were collected from three French landing sites along the English Channel coastline in spring 2010 and 2011. Specimens were caught by trawlers or trap fishers in the Normano-Breton Gulf (ICES rectangles 26E8 and 27E8) and in the Bay of Seine (ICES rectangles 27E9 and 28E9). In 2010, from 12 April to 9 July, 734 specimens (395 males, 339 females) were collected in Erquy, Cherbourg and Port-en-Bessin landing sites. In 2011, from 5 April to 30 June, 655 specimens (444 males and 211 females) were collected in Port-en-Bessin and Cherbourg landing sites. The sex ratio was tested using a chi-square test.
Biometry dissection and sex determination
The Dorsal Mantle Length (DML) of each specimen was measured to the lowest cm and length frequencies were represented on four histograms, one per sex and per year. Length classes are defined every cm, immature specimens are represented in white; mature animals are represented in grey. An attempt was made to fit two Gaussian models associated with a mixing effect to the two cohorts. If the model fitted the first cohort well, due to its shape, it was not possible to fit it to the second cohort. A truncation point between the first and second cohort is then defined by the least numerous length class after the first mode. However, due to the high inter-individual variability of the growth rate in S. officinalis (Challier et al., Reference Challier, Royer and Robin2002, Reference Challier, Dunn and Robin2005), this criteria cannot be used alone to determine which cohort a particular specimen belongs to. The measurement of lipofuscin (age pigment accumulated in tissues; Zielinski & Portner, Reference Zielinski and Portner2000; Doubleday & Semmens, Reference Doubleday and Semmens2011) was used in this work to give a second indicator of age estimation. Fresh total Body Weight (BW) and Gonad Weight (GW) were also measured for each specimen.
The mantle of each specimen was opened by a ventral incision to determine the sex and maturity stage using the macroscopic scale developed in the framework of the international Workshop on Sexual Maturity Staging of Cephalopods (WKMSCEPH; ICES, 2010). Six maturity stages are described from undetermined to spent (post-reproduction) for each sex. In female specimens, maturity stages are mainly defined using oocyte size and development of the Nidamental Glands (NG). In male specimens, maturity stages are defined using testis size and spermatophore position in the Spermatophoric Complex (SC). This international scale is based on the same number of stages and the same criteria as the one developed by Dunn (Reference Dunn1999a). Mantle tissue samples were collected from a subsample of specimens to measure lipofuscin concentrations. Tissue samples were kept frozen in liquid nitrogen to avoid tissue degradation and transported to the laboratory to be analysed.
Lipofuscin measurement
Experiments carried out in the past to study English Channel S. officinalis based age estimation only on polymodal decomposition of length frequencies (Boucaud-Camou & Boismery, Reference Boucaud-Camou, Boismery and Boucaud-Camou1991; Boucaud-Camou et al., Reference Boucaud-Camou, Koueta, Boismery, Medhioub and Boucaud-Camou1991; Dunn, Reference Dunn1999a). In cephalopods, growth rate is influenced by environmental conditions and presents a high inter-individual variability making this method inaccurate. Various hard structures have been tested for age determination in S. officinalis (Le Goff et al., Reference Le Goff, Gauvrit, Pinczon Du Sel and Daguzan1998; Neige, Reference Neige2006) but the only consistent results were obtained from statolith daily rings during the juvenile phase (Challier et al., Reference Challier, Royer and Robin2002, Reference Challier, Pierce and Robin2006). However, statolith rings are very difficult to read in S. officinalis and are not usable after 240 days of age (Bettencourt & Guerra, Reference Bettencourt and Guerra2001). Above this threshold, some studies proposed to use the concentration of lipofuscin as a proxy of age: lipofuscin is a pigment that accumulates in tissues through the lifetime of some invertebrates (Sheehy et al., Reference Sheehy, Shelton, Wickins, Belchier and Gaten1996; Ju et al., Reference Ju, Secor and Harvey1999; Bluhm & Brey, Reference Bluhm and Brey2001). This method has been successfully used in cephalopods to provide a rough estimation of S. officinalis age (Zielinski & Portner, Reference Zielinski and Portner2000; Doubleday & Semmens, Reference Doubleday and Semmens2011).
Lipofuscin was measured according to Zielinski & Portner (Reference Zielinski and Portner2000). Mantles of S. officinalis were ground under liquid nitrogen and homogenized in a chloroform-methanol mixture (1:2, v/v). After centrifugation for 10 min at 2000 g, lipofuscin was found in the chloroform phase. In this phase an emission spectrum between 350 and 550 nm was obtained at an excitation wavelength of 350 nm using a Mithras LB940 fluorimeter (Berthold, Thoiry, France). The luminescence of the sample was determined at the maximum emission at 420 nm. Lipofuscin concentrations were expressed as relative fluorescence intensity according to Hill & Womersley (Reference Hill and Womersley1991), using 0.1 μg quinine per mL 1N H2SO4 as a standard.
In a first step, we tested the applicability of lipofuscin concentrations to fish market samples of S. officinalis by measuring post-mortem variability over the 3 days following the death. A mantle was sampled on one living animal and lipofuscin was measured on the fresh tissue. The tissue was then kept on ice during the following 3 days. A large piece of mantle was taken every 24 h randomly on the mantle and used to perform replicated measures of lipofuscin. Differences were tested using an ANOVA. In a second step, lipofuscin concentrations were measured on two subsamples. A subsample of 11 immature specimens was taken from the first cohort (DML ranging 10–13 cm and assumed to be 1 year olds as they are short and immature) and a subsample of 17 mature specimens was taken from the group which length is highly over the boundary between the two cohorts (DML ranging 20–31 cm and therefore assumed to be 2-year-olds). This experiment describes inter-cohort differences in lipofuscin concentrations. Finally, results of lipofuscin concentration measured from the smallest mature specimens (two animals of 11 cm DML and one of 13 cm DML) were used in a Student t-test to estimate the probability of each small mature specimen to belong to each cohort.
Size-at-maturity estimation
The maturity stage of each specimen, observed in each length class, was used to fit a binomial error Generalized Linear Model (GLM) to estimate size-at-maturity. Specimens were considered as mature when they reached the ‘Maturing stage’ (2b), assuming that if they are maturing during the spring, they will become mature and able to breed and spawn before the end of the reproduction season in late summer.
Gonado-Somatic Index
An index of sexual development was computed with the Gonado-Somatic Index (GSI):
GSI is a proxy of the energy allocated by the specimen to reproduction to the detriment of somatic growth. This index is a quantitative measure of sexual maturity and the relationship between GSI and DML complements the estimation of size-at-maturity. Student t-tests were performed to look for differences in GSI between the two sampled years, between mature and immature animals and between males and females. An F-test was used to highlight differences between GSI variances of mature males and females. Finally, a linear model was fitted to explore the correlation between mature male DML and GSI.
RESULTS
Length frequency of samples
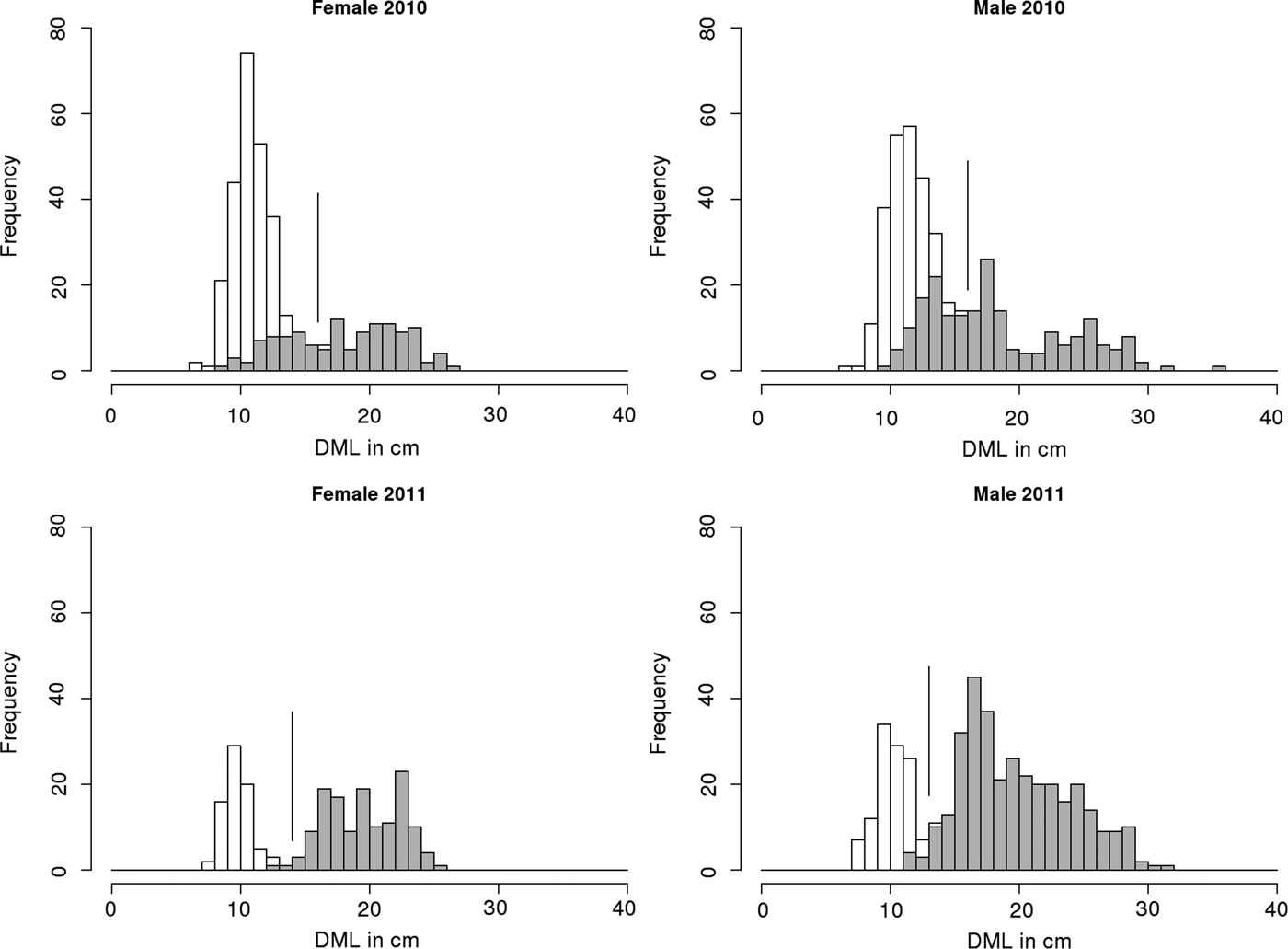
Female specimens ranged from 6 to 27 cm DML in 2010 and from 7 to 26 cm DML in 2011. Male specimens ranged from 6 to 36 cm DML in 2010 and from 7 to 32 cm DML in 2011 (Figure 2). Length frequencies highlighted that a first cohort was identifiable and the maximum size of this first cohort could be determined at 16 cm for both sexes in 2010 and at 14 and 13 cm for females and males respectively in 2011. In 2010, the smallest mature females were 7 cm long while the smallest mature males were 8 cm long. In 2011, the smallest mature females were 11 cm long and the smallest mature males were 10 cm. In 2010, 17% of females and 30% of males assigned to the first cohort were found to be mature and in 2011, 3% of females and 13% of males of the first cohort were found to be mature. For both years, sex ratios were significantly different from 1:1 with males more numerous than females, particularly in 2011.

Fig. 2. Length frequencies of the female and male specimens collected during spring 2010 and 2011 in French English Channel landing sites. Immature specimens are displayed in white and mature specimens are displayed in grey. The vertical line drawn indicates the boundary between the first and the second cohorts.
Size-at-maturity determined using a binomial error GLM
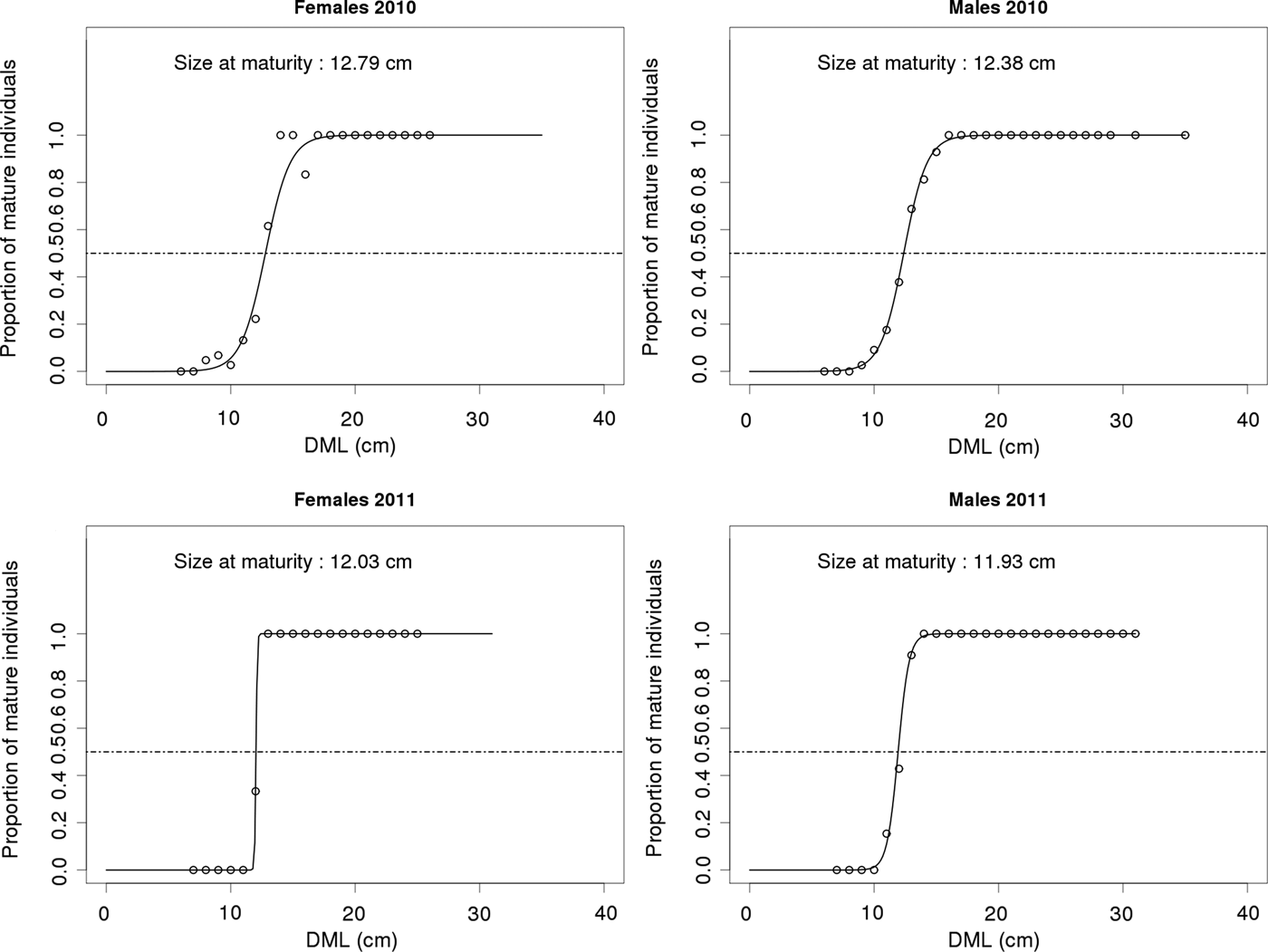
For both sexes, the size-at-maturity (Figure 3) was higher in 2010 than in 2011 and for both years, size-at-maturity in females was larger than in males. For male specimens, the smallest length class within which all individuals were found to be mature was 16 cm DML in 2010 and 14 cm DML in 2011; above these length classes all male specimens were found to be mature. In contrast, for female specimens, the smallest length within which all individuals were found to be mature was smaller at 14 cm in 2010 and 13 cm in 2011; however, above these length classes, immature individuals were still observed in larger length classes.

Fig. 3. Size-at-maturity determined using a binomial error GLM for female and male samples collected during spring 2010 and 2011 in French English Channel landing sites. Number of specimens measured per DML class is shown in Figure 2.
Variability in lipofuscin measurement and age estimation
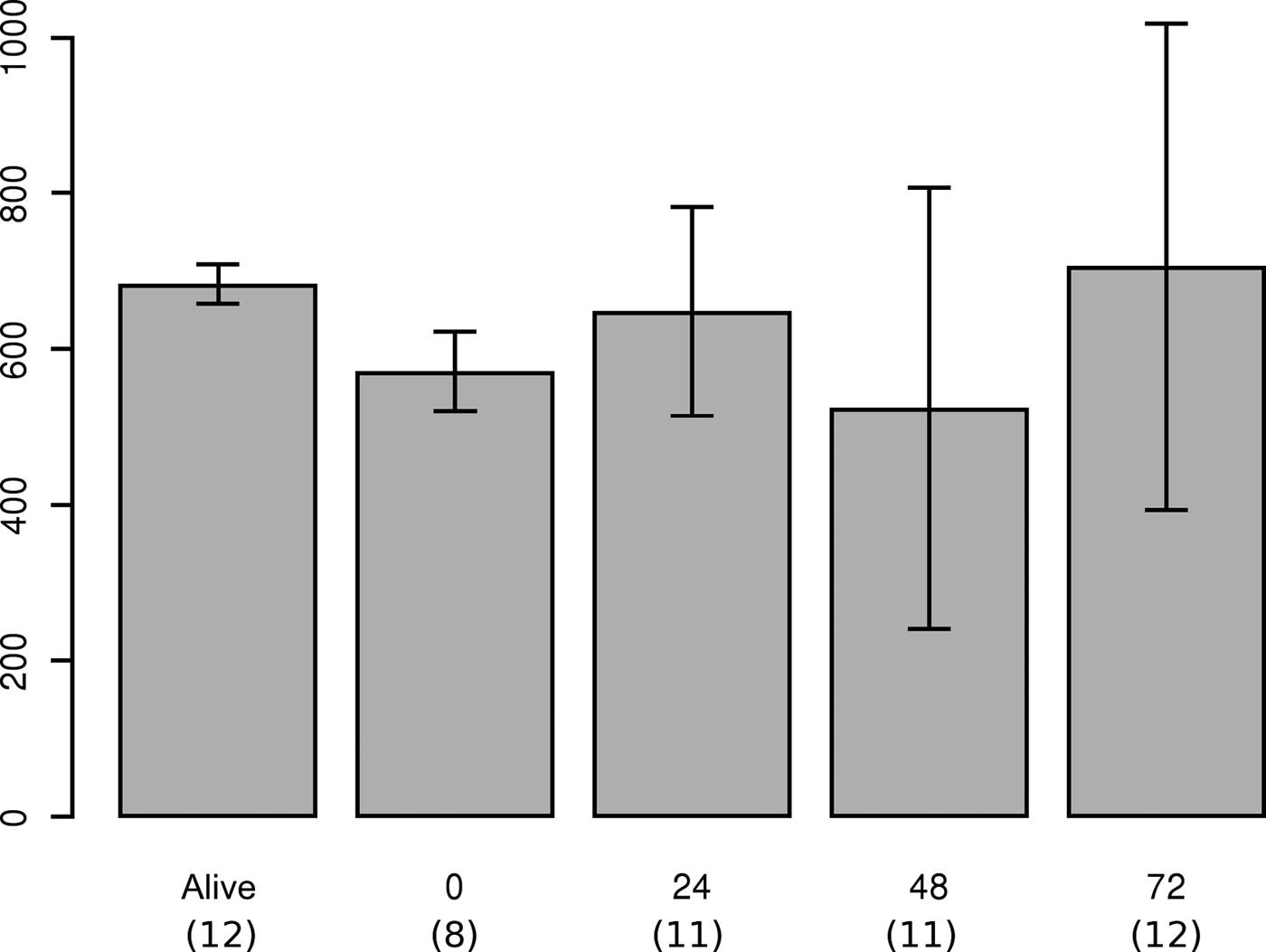
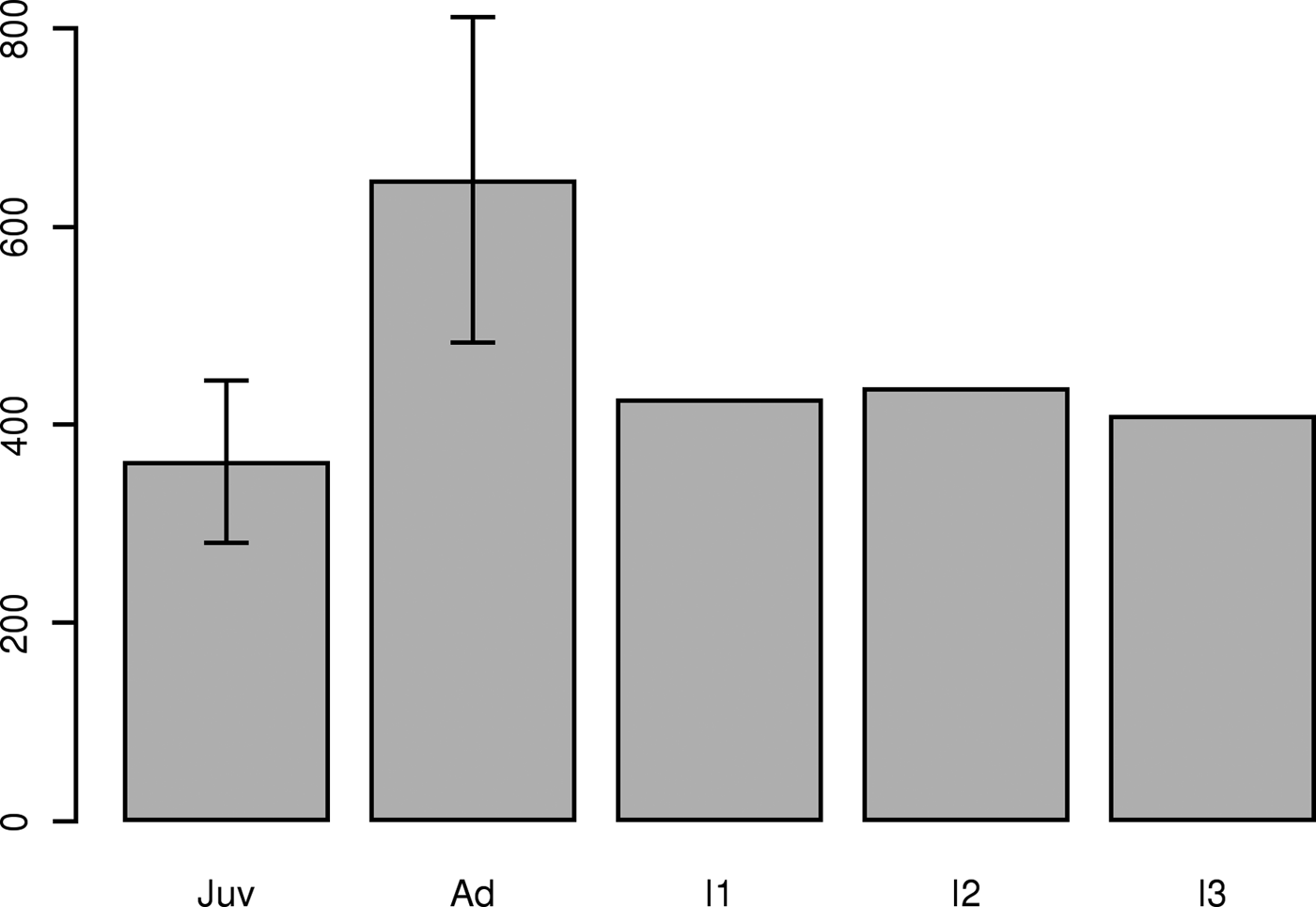
Lipofuscin measurement from the first experiment (Figure 4) showed that, over a period of 3 days, no significant trend was observed in lipofuscin concentration; however, the standard deviation of the measure did increase with time. In the second experiment, lipofuscin accumulation measured in specimens belonging to the first and second cohorts were significantly different (Figure 5). Results of the lipofuscin measurement performed on the three small mature males (Figure 5, Table 1) showed that lipofuscin accumulation of these three specimens was not significantly different from that of the first cohort, but was significantly different from lipofuscin accumulation measured in GIIB. These three specimens could thus be considered as part of the GIB group.

Fig. 4. Trend in lipofuscin concentration measured in the mantle sampled on one living S. officinalis from the death and every 24 h until 72 h after the death. Error bars are the standard deviations of each measure.

Fig. 5. Lipofuscin concentration measured in S. officinalis mantles for juveniles (Juv, 11 specimens, ranging 10–13 cm DML), adults (Ad, 17 specimens, ranging 20–31 cm DML) with the inter-individual standard deviation and lipofuscin concentration measured in three small mature specimens (I1, 11 cm DML; I2 11 cm DML; I3, 13 cm DML).
Table 1. Probabilities of three small male mature specimens to be different from the GIB and GIIB cohorts.

Gonado-Somatic Index
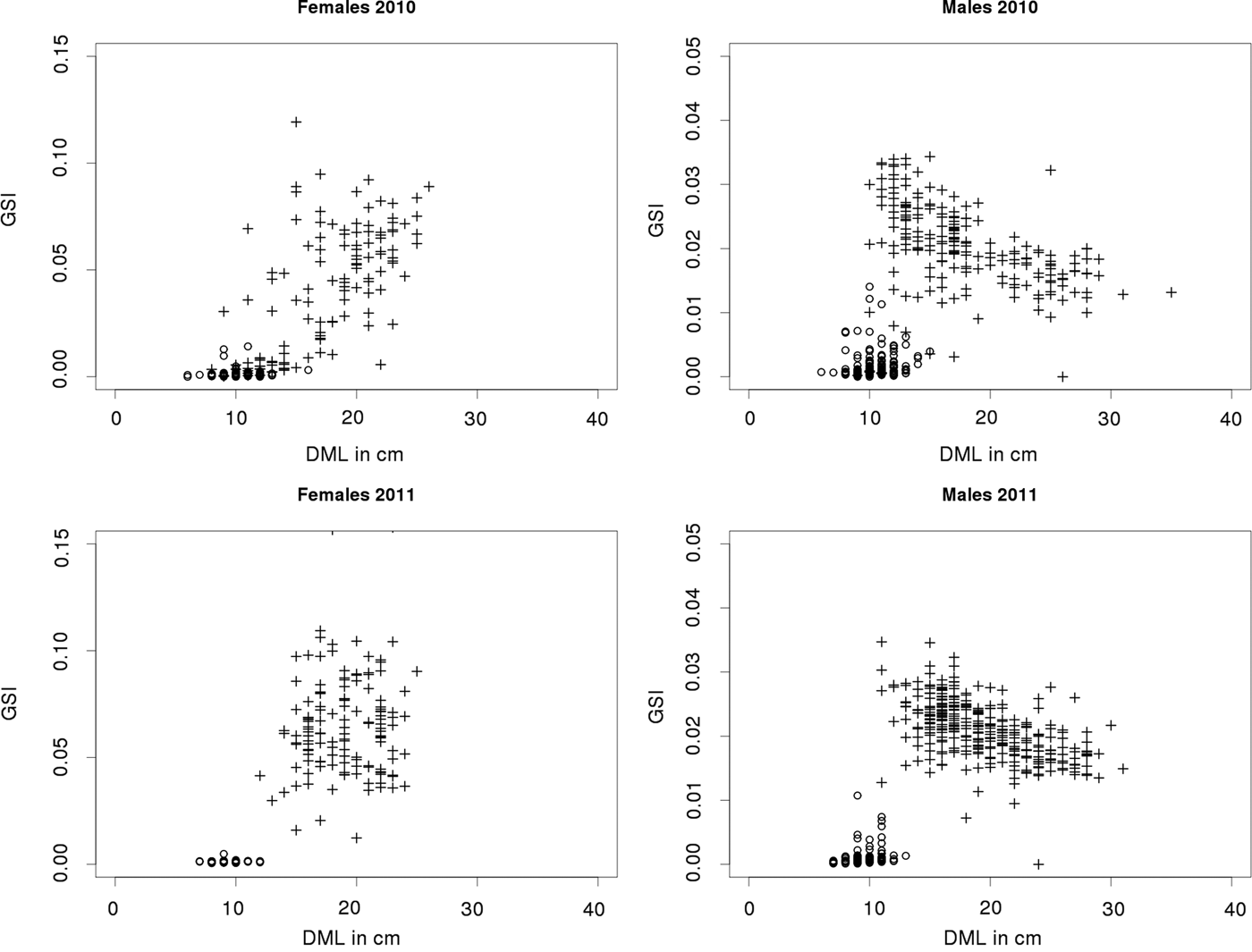
The GSI (Figure 6) for each sex and each year, does not reveal any significant inter-annual differences. In both sexes, the GSI during the reproduction period was significantly higher for mature specimens than for immatures. Mature female specimens were characterized by a significantly higher GSI and a higher inter-individual variability than mature males. Finally, the GSI of mature male specimens was found to be significantly correlated with DML.

Fig. 6. Gonado Somatic Index (GSI) vs Dorsal Mantle Length (DML) for female and male samples collected during spring 2010 and 2011 in French English Channel landing sites. Immature specimens are displayed with circles while mature specimens are displayed with crosses.
DISCUSSION
The life cycle of the Sepia officinalis population in the English Channel was described in the past as lasting 2 years for females and for the large majority of males (Boucaud-Camou et al., Reference Boucaud-Camou, Koueta, Boismery, Medhioub and Boucaud-Camou1991; Dunn, Reference Dunn1999a). The present study highlights that, during the 2010 and 2011 reproduction seasons, a variable percentage of mature male and female S. officinalis belongs to the first cohort and could thus be considered as a GIB. Age determination was based on polymodal decomposition and adaption of lipofuscin measurement protocol (Zielinski & Portner, Reference Zielinski and Portner2000; Doubleday & Semmens, Reference Doubleday and Semmens2011). The association of both methods is more accurate than the polymodal decomposition used alone and enabled to disentangle the two sampled cohorts. It has corroborated that the smallest mature animals observed are most likely 1-year-old S. officinalis and not small 2-year-old specimens. Finally, the GSI illustrates the amount of energy dedicated by each sex to the development of the gonad to the detriment of the somatic growth.
Lipofuscin measurement has helped in determining the cohort to which each specimen belongs, but this method is not accurate enough for age estimation (Zielinski & Portner, Reference Zielinski and Portner2000; Doubleday & Semmens, Reference Doubleday and Semmens2011). Moreover, accuracy decreases with increasing time between death and sampling; as the sampling process was based on commercial fishery landings, this parameter was not under control. Finally, lipofuscin measurement is expensive and time consuming and for this reason experiments cannot be carried out on a large number of specimens. An alternative method for rough age estimation could be set up using statoliths. Although statolith daily rings are not accurately readable in 1-year-old S. officinalis (Bettencourt & Guerra, Reference Bettencourt and Guerra2001; Challier et al., Reference Challier, Royer and Robin2002, Reference Challier, Pierce and Robin2006), studies of several fish species have found that otolith length, thickness and weight continued to grow with age even when body growth rate was null (Reznick et al., Reference Reznick, Lindbeck and Bryga1989; Campana, Reference Campana1990; Newman, Reference Newman2002). Age frequency distribution derived from otolith weights were found to be consistent with those derived from increment counts (Pilling et al., Reference Pilling, Grandcourt and Kirkwood2003; Mc Dougall, Reference Mc Dougall2004). Moreover, statolith diameter has also been successfully used as a proxy of age in the planktonic tunicate Oikopleura vanhoeffeni (Choe & Deibel, Reference Choe and Deibel2009). If S. officinalis statolith experiences daily increments throughout its life, measuring dome diameters could be another way to separate the two annual cohorts.
In cephalopod species, rearing experiments have shown that maturation is driven by different factors including photoperiod, food availability and temperature (Mangold, Reference Mangold and Boyle1987). Cephalopods are known not to store ingested energy in their tissues and as such individuals, particularly females, need a regular food availability in order to reach maturity as observed for Sepioteuthis australis (Ho et al., Reference Ho, Moltschaniwskyj and Carter2004) and Loligo vulgaris (Moreno et al., Reference Moreno, Pereira and Morais da Cunha2005). Thus, the variability observed in the female GSI could be explained by food availability experienced by each specimen. In male specimens, the lower amount of energy dedicated to reproduction allows more energy for somatic growth and therefore explains both the low variability of the GSI and a larger DML than in females. It is also worth noting that, within this study, the size-at-maturity plots for males are quite homogeneous and do not reveal subgroups maturing at different sizes as previously described for other cephalopod species (Castro et al., Reference Castro, Guerra, Jardón and Boucaud-Camou1991; Coelho et al., Reference Coelho, Quintela, Bettencourt, Olavo and Villa1994; Moreno et al., Reference Moreno, Morais da Cunha and Pereira1994; Boyle & Rodhouse, Reference Boyle and Rodhouse2005). Finally, the English Channel warming (Hawkins et al., Reference Hawkins, Southward and Genner2003; Saulquin & Gohin, Reference Saulquin and Gohin2010) leads to the modification of species communities by increasing abundance of warm water species and decreasing abundance of cold water species. Such modifications could also influence the English Channel population of S. officinalis by modifying the competition and the predator-prey relationships.
Temperature is a key parameter influencing cephalopod life cycles, from egg development to reproduction (Rodhouse et al., Reference Rodhouse, Symon and Hatfield1992; Boyle & Pierce, Reference Boyle and Pierce1994; Robin et al., Reference Robin, Roberts, Zeidberg, Bloor, Rodriguez, Briceño, Downey, Mascaró, Navarro, Guerra, Hofmeister, Barcellos, Lourenço, Roper, Moltschaniwskyj, Green and Mather2014). An increase in environmental temperature generally accelerates the somatic growth of cephalopods, shortening the life cycle duration by accelerating maturation (Moreno et al., Reference Moreno, Azevedo, Pereira and Pierce2007). This is a well-known feature in the life cycle of S. officinalis which shortens from the north of its distribution area (English Channel) to the south (West African coasts; Mangold-Wirz, Reference Mangold-Wirz1963; Mangold, Reference Mangold1966; Guerra & Castro, Reference Guerra and Castro1988; Coelho & Martins, Reference Coelho, Martins and Boucaud-Camou1991). The warming of the English Channel (Hawkins et al., Reference Hawkins, Southward and Genner2003; Saulquin & Gohin, Reference Saulquin and Gohin2010) could be the cause of appearance of a GIB composed of males and females and the observation of a lower size-at-maturity (on average 12.16 cm for males and 12.41 cm for females in 2010 and 2011 respectively) than that observed by Dunn (Reference Dunn1999a) (14.6 cm for males and 16.4 cm for females). Further investigations are required to investigate if this size-at-maturity variability could be correlated with environmental indices (such as NAO index) and whether GIB is actually involved in reproduction by analysing the sperm reservoirs of females to understand whether cross-mating between generations exists (Gauvrit et al., Reference Gauvrit, Pinczon Du Sel, Blanc and Daguzan1998). Warming of the English Channel could also lead to an extension of the reproduction season allowing the more precocious hatchlings sufficient time to mature prior to the following spring.
In the S. officinalis population of the English Channel, Challier et al. (Reference Challier, Pierce and Robin2006) showed that recruitment in the trawling fishery occurs throughout the year at a constant age leading to the conclusion that S. officinalis hatches throughout the year. GIB observed in this study could thus be individuals which had hatched early in the reproduction season and which had had enough time before offshore migration to start maturation and finish it before the end of next spring.
The English Channel has long been exploited by both French and UK fishers. Trawling started 200 years ago and fishing pressure has regularly increased since the beginning of the 20th century (Hawkins et al., Reference Hawkins, Southward and Genner2003; Guenette & Gascuel, Reference Guenette and Gascuel2012; Gras, Reference Gras2013). High fishing pressure greatly impacts demersal communities and can modify life history traits by favouring specimens which mature faster or reach maturity at a smaller size or lower age (Shin et al., Reference Shin, Rochet, Jennings, Field and Gislason2005; Kantoussan et al., Reference Kantoussan, Ecoutin, Fontenelle, Thiaw, Tito de Morais and Laë2009). This phenomenon has been observed in a range of N-E Atlantic stocks (Shin & Rochet, Reference Shin and Rochet1998; Grift et al., Reference Grift, Rijnsdorp, Barot, Heino and Dieckmann2003; Engelhard & Heino, Reference Engelhard and Heino2004; Olsen et al., Reference Olsen, Heino, Lilly, Morgan, Brattey, Ernande and Dieckmann2004) and could also have influenced the English Channel population of S. officinalis, which has been fully exploited since the beginning of the 1990s (Royer et al., Reference Royer, Pierce, Foucher and Robin2006; Gras et al., Reference Gras, Roel, Coppin, Foucher and Robin2014), leading to the appearance of a GIB and a potential decreasing trend in size-at-maturity. Modifications in demersal community assemblages caused by high fishing pressure could have modified the prey availability for S. officinalis. Maturation efficiency in cephalopods, which could be unable to store energy in their tissue (Mangold, Reference Mangold and Boyle1987; Moreno et al., Reference Moreno, Pereira and Morais da Cunha2005) could therefore be impacted.
Sepia officinalis is one of the top predators of the English Channel ecosystem (Araújo et al., Reference Araújo, Mackinson, Ellis and Hart2005). In contrast to other top predators, S. officinalis has a short lifespan and as such, the consequences of any regime shift can be easily observed in the short term and could thus provide a useful indicator of climate change. According to Jackson et al. (Reference Jackson, Kurtz and Fisher2000), an indicator of climate change must meet four criteria: (i) relevance to assessment and ecological functions, (ii) feasibility of data collection, (iii) response to variation and (iv) interpretation and utility of data to highlight the changing environment. Although S. officinalis is a non quota species (Pierce et al., Reference Pierce, Allcock, Bruno, Bustamante, Gonzalez, Guerra, Jereb, Lefkaditou, Malham, Moreno, Pereira, Piatkowski, Rasero, Sanchez, Santos, Santurtun, Seixas, Sobrino and Villanueva2010), two scientific bottom trawl surveys sample it each year (Carpentier et al., Reference Carpentier, Martin and Vaz2009; Gras et al., Reference Gras, Roel, Coppin, Foucher and Robin2014) and maturity stages can be easily determined using the international WKMSCEPH scale (ICES, 2010). This could be complemented by data collected at landing sites throughout the year according to the Data Collection Framework (DCF) programme. Sepia officinalis therefore meets the four criteria listed above and should be considered as an indicator for environmental shift.
ACKNOWLEDGEMENTS
We thank Jéhane Lepoittevin, Said Slimane-Moussa, Olivier Goetz, Florentin Bezin and Béatrice Adeline for their participation in dissecting and preparing the samples as well as the two reviewers for their valuable comments to improve this manuscript.
FINANCIAL SUPPORT
The study was carried out under the EU funded project ‘CRESH’ (under the Interreg IV A France-Manche-England programme).