INTRODUCTION
The Falkland Islands are situated in the south-west Atlantic 500 km from the coast of Argentina and 1600 km north of the Antarctic Circle. The archipelago consists of two main islands, East and West Falkland, and 778 smaller ones. Biogeographically the Falkland Islands are considered to be a part of the Magellanic Region (Hedgpeth, Reference Hedgpeth1969).
The archipelago is in the path of the northward flowing coldwater Falkland Current which originates from the Antarctic Circumpolar Current (ACC) (Peterson & Whitworth III, Reference Peterson and Whitworth III1989). To circumvent the Falkland Islands, the Falkland Current splits into two main northward flowing streams, the weaker western branch and the stronger eastern branch (Bianchi et al., Reference Bianchi, Massonneau and Olevera1982). When the Falkland Current meets the continental slope to the south of the Falkland Islands, it causes a strong upwelling of the Sub-Antarctic Superficial Water Mass. The north-western waters of the Falkland Islands are dominated by the Argentine Drift which is cold temperate (Zyrjanov & Severov, Reference Zyrjanov and Severov1979). These two current systems split the marine environment in the Falklands into two different ecological regions with the north-western areas dominated by temperate waters and the south-eastern areas dominated by colder sub-Antarctic waters (Arkhipkin, Brickle and Laptikhovsky, unpublished data).
The sponges of the shallow waters of the Falkland Islands are poorly known. Only three previous expeditions have studied the sponge fauna, namely the Scotia Expedition (1903–1904) (Topsent, Reference Topsent1915), the Swedish Antarctic Expedition (1901–1903) (Burton, Reference Burton1934), and the Discovery Expeditions (1925–1929) (Burton, Reference Burton1932). The sixty stations at which sponges were obtained by these expeditions ranged from 0–313 m in depth, however only 30% were from shallow infralittoral and circalittoral coastal sites (up to 50 m) with the majority being deeper offshore sites. Sampling was carried out by a variety of remote methods including dredging, otter trawling, beam trawling and tow nets.
Recent work has revealed the potential for diving surveys in studying sponge biodiversity (Boury-Esnault, Reference Boury-Esnault1971; Wiedenmayer, Reference Wiedenmayer1977; Pansini, Reference Pansini, Vacelet and Boury-Esnault1987; Picton & Goodwin, Reference Picton and Goodwin2007; Goodwin & Picton, Reference Goodwin and Picton2009), particularly in areas where many species are small and in habitats which are difficult to sample by other means (Vacelet & Perez, Reference Vacelet and Perez1998). Sampling by SCUBA diving enables the study of bedrock habitats and encrusting species which are likely to be undersampled by remote methods and consequently has the potential to significantly increase the number of species recorded (Picton & Goodwin, Reference Picton and Goodwin2007; Goodwin & Picton, Reference Goodwin and Picton2009). Additionally SCUBA diving surveys enable the in situ appearance of species to be recorded providing information of great use to field surveyors.
MATERIALS AND METHODS
Specimens were collected by SCUBA diving. Sponges were selected by eye: the divers attempted to sample species that looked different from those previously sampled during the dive. The aim was to sample as many different species as possible, rather than gaining any quantitative information. Once selected, three photographs of each specimen were taken in situ using housed digital SLR cameras (Nikon D70 and Nikon D300 in Subal housings with Ikelite DS125 substrobe and SB800 flash units both with 60 mm macro lenses). A small piece (approximately 1 cm2 of tissue) was then removed. After collection the samples were transferred to 95% ethanol for storage.
Tissue slides were prepared by sectioning a very thin portion of tissue at a 90o angle through the sample. This was then dehydrated in absolute ethanol for four minutes and placed in clove oil for a further four minutes to clarify the tissue before being mounted on a microscope slide in Canada balsam. A coverslip was then placed on the slide and they were then kept at 50oC for at least 48 hours to allow the mountant to dry. Spicule preparations were prepared by dissolving the tissue in a drop of concentrated nitric acid directly on a microsope slide. The slide was heated over a spirit burner to aid the reaction. Once the acid had burnt off, the remaining spicules were rinsed in water and ethanol and then mounted in Canada balsam as above.
The tissue slide was used primarily for identification to genus level. Spicule measurements were taken from the spicule preparations; at least 20 spicules of each type were measured using ProgRes® CapturePro 2.7 Software (Jenoptik Optical Systems, Jena, Germany). Type material is in the zoology collections of the Ulster Museum, National Museums Northern Ireland. Material in these is indicated by BELUM (Belfast Ulster Museum) Mc (Porifera collections).
Information on extant species was obtained from the World Porifera Database (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez, Hajdu, Pisera, Vacelet, Manconi, Schoenberg, Janussen, Tabachnick and Klautau2008). Type specimens were examined from several collections; those examined are listed in the text, institutional abbreviations used are as follows: BMNH, Natural History Museum, London; NMSZ, Zoology Collections of the National Museums of Scotland.
The study sites
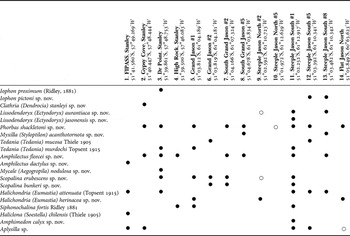
Sponge samples were taken from four sites around Stanley (East Falkland) and nine around the Jason Islands (north-west Falkland Islands) (Figure 1; Table 1). For safety, dive depths were limited to 20 m because of the lack of a recompression chamber. Dive site names correspond to dive numbers from the Shallow Marine Surveys Group Jason cruise, sponge samples were not collected on all survey dives. The survey was part of the Stanley based Shallow Marine Surveys Group's exploration of the inshore marine environment of the Falkland Islands.

Fig. 1. The survey area. (A) Sites near to Stanley on East Falkland; (B) sites on the Jason Islands, West Falkland.
Table 1. Sponge species present at sites surveyed. Site numbers correspond to site positions on Figure 1. • Indicates a specimen collected, ○ species recorded from photographic record only.
The Jason Islands lie to the north-west of West Falkland and are thus subject to the warmer waters of the Argentine Drift. The waters surrounding them are extremely productive and testament to this are the large numbers of globally important nesting seabirds there such as the black-browed albatross (Thalassarche melanophris (Temminck, 1828)), with Steeple Jason being home to the world's largest colony. Steeple Jason, Grand Jason and Clarke's Islet are nature reserves owned by the Wildlife Conservation Society, New York. The remainder of the islands in the group are National Nature Reserves owned by the Falkland Islands Government.
RESULTS
SPECIMENS
All samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4736) Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57°48.753′W; water depth: 10–12 m); collected by C. Goodwin and J. Jones, 19 October 2008; (BELUM Mc4739) Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57°48.753′W; water depth: 12 m); collected by C. Goodwin and J. Jones, 19 October 2008.
EXTERNAL MORPHOLOGY (FIGURE 2A)
Yellow crust with distinct surface channels, epibiotic on scallops.

Fig. 2. Iophon proximum (Ridley, Reference Ridley1881). (A) Sponge covering scallop; (B) spicules. Scale bar 10 µm.
SKELETON
Choanosome: reticulation of acanthostyles, bundles 2–3 spicules thick. Ectosome: palisade of strongyles fanning out to create a tangential surface layer. Microscleres present throughout sponge tissue.
SPICULES (FIGURE 2B)
(1) Acanthostyles: fairly abruptly pointed, slightly curved, entirely spined with small spines apart from a small area at the tip, 105–195 µm.
(2) Tylostrongyles: slightly curved with micro-spined ends, 120–160 µm (most 155–160 µm).
(3) Anisochelae: 15 µm.
(4) Bipocilles: 7.5 µm.
REMARKS
Originally described from Magellan Strait, Chile (Ridley, Reference Ridley1881). Also known from Argentina, (Burton, Reference Burton1940; Cuartas, Reference Cuartas1992, Reference Cuartas, Boschi and Cousseau2004; Schejter et al., Reference Schejter, Calcinai, Cerrano, Bertolino, Pansini, Gilberto and Bremec2006), Chile (Sarà, Reference Sarà1978), South Africa (Lévi, Reference Lévi1963) and the Falkland Islands (Burton Reference Burton1932, Reference Burton1934).
TYPE MATERIAL
Holotype: (BELUM Mc4819) sample in 95% ethanol, tissue section and spicule preparation on slides; Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 5, Falkland Islands (51°03.392′S 61°10.341′W; water depth: 13 m); collected by C. Goodwin and J. Jones, 31 October 2008.
Paratype: (BELUM Mc4822) sample in 95% ethanol, tissue section and spicule preparation on slides; Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 8, Falkland Islands (51°03.482′S 61°10.342′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 31 October 2008.
ETYMOLOGY
Named for Bernard Picton, Curator of Marine Invertebrates at National Museums Northern Ireland and fellow sponge taxonomist.
EXTERNAL MORPHOLOGY (FIGURE 3A)
Thick, bright custard yellow, crust with large oscules and circular sub-dermal spaces visible on its surface, giving a porous appearance.

Fig. 3. Iophon pictoni sp. nov. (A) External appearance; (B) spicules: (a) acanthostyle; (b) tylostrongyle; (c) anisochelae. Scale bars 10 µm.
SKELETON
Choanosomal skeleton: isodictyal reticulation of bundles of acanthostyles. Spurred anisochelae scattered throughout the choanosome. Ectosomal skeleton: tylostrongyles with spined ends in erect brushes and forming surface mesh.
SPICULES (FIGURE 3B)
(1) Acanthostyles: 134(150)162 by 5.4(8.2)11.2 µm.
(2) Tylostrongyles 140(168)175 by 4.0(6.9)8.9 µm.
(3) Anisochelae 15(19)21 µm.
.
REMARKS
This species can be distinguished from most other Iophon species present in this area by the absence of bipocilles and the small size of the acanthostyles (Table 2). The only other species lacking bipocilles is Iophon timidum Desqueyroux-Faúndez & Van Soest, Reference Desqueyroux-Faúndez and van Soest1996, but this is a massive spherical sponge and possesses larger acanthostyles and two categories of anisochelae.
Table 2. Iophon species of the south-west Atlantic, Chile and the Antarctic. Information from type descriptions, Desqueyroux-Faúndez & Van Soest (Reference Desqueyroux-Faúndez and van Soest1996), Rios (Reference Rios2006) and Ríos et al. (Reference Ríos, Cristobo and Urgorri2004).

SPECIMENS
Holotype: (BELUM Mc4714) sample in 95% ethanol, tissue section and spicule preparation on slides. Gypsy Cove, Falkland Islands (51°40.447′S 57°48.444′W; water depth 8 m) collected by C. Goodwin and J. Jones, 15 October 2008.
COMPARATIVE MATERIAL EXAMINED
Stylostichon tuberculata Burton Reference Burton1934, holotype, Station 39 Swedish Antarctic Expedition, BMNH 33.3.17.158.
Microciona basispinosa Burton Reference Burton1934, holotype, Station 57 Swedish Antarctic Expedition, BMNH 33.3.17.39a.
EXTERNAL MORPHOLOGY (FIGURE 4A)
Pale yellow thick crust with lobed surface.

Fig. 4. Clathria (Dendrocia) tuberculata (Burton, Reference Burton1934). (A) External appearance; (B) spicules: (a) large style; (b) small acanthostyle; (c) toxa. Scale bars 10 µm.
SKELETON
Choanosomal skeleton: plumose fibres cored with styles with very sparsely spined heads, echinated by secondary spined acanthostyles. Ectosomal skeleton: columns of large smooth styles (of the same type as those in the choanosome) fan to form brushes at the surface.
SPICULES (FIGURE 4B)
(1) Large acanthostyles: 215(337)416 by 6.7(8.7)11.5 µm, lightly spined on head but otherwise smooth.
(2) Small acanthostyles 70(110)155 by 7.3(11.1)13.5 µm, all spined, head slightly tylote.
(3) Oxhorn type toxas 28(35)43 µm some quite thick.
REMARKS
Burton originally described this species as Stylostichon tuberculata (1934) as having a plumose choanosomal skeleton and a dermal skeleton formed by a dense palisade of small subtylostyles set at right angles to the surface. He stated there were three classes of spicules but that intermediates were found and their being one class was not improbable: measurements from the type confirmed the presence of only one class. The form and sizes of the spicules (large acanthostyles (270–410 µm), small acanthostyles (90–180 µm) and toxa 35µm are similar to our specimen). The form of Burton's specimens, thick lobed crusts, is similar to ours.
The presence of toxas would preclude inclusion in Stylostichon. Koltun (Reference Koltun1976) considered this species to be the same as Microciona basispinosa Burton Reference Burton1934 and synonomized both with Clathria (Microciona) antarctica (Topsent, 1917). This was followed by Hooper (Reference Hooper1996), although he stated that synonomy of C. tuberculata was not confirmed as it had not been possible to re-examine the type specimen. However, C. tuberculata differs from both these species in that it does not possess a separate category of ectosomal megascleres, its acanthostyle heads are only marked by a slight constriction, and the majority of the choanosomal styles are smooth with only a small number bearing almost invisible spines. In contrast, M. antarctica possesses acanthostyles with distinctive rounded heads which are neatly spined in the basal half with small spines, and a separate category of ectosomal spicules. Given the difference in spicule size reported by Hooper (Reference Hooper1996), synonomy of C. basispinosa with C. antarctica should be revisited.
The sub-genus Clathria (Dendrocia) Hallmann, 1920 is defined by the lack of a specialized class of ectosomal spicules. There are only seven species in the sub-genus, all of which are endemic to temperate Australian waters (Hooper, Reference Hooper1996). The majority of these species only possess chelae as microscleres, the only other species with toxa is Clathria (Dendrocia) scabida (Carter, 1885) but this also has three categories of chelae (Hooper, Reference Hooper1996).
Previous records from Port William and Berkeley Sound in the Falkland Islands in 40 and 16 m (Burton, Reference Burton1934).
TYPE MATERIAL
Holotype: (BELUM Mc4804) sample in 95% ethanol, tissue section and spicule preparation on slides; Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands, (51°02.252′S 61°12.917′W; water depth 13–20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
ETYMOLOGY
Named for the type locality, the Jason Islands, West Falkland.
EXTERNAL MORPHOLOGY (FIGURE 5A)
Massive, pale yellow lump with obvious channels on surface.

Fig. 5. Lissodendoryx (Ectyodoryx) jasonensis sp. nov. (A) External appearance showing channels; (B) spicules: (a) large acanthostyle; (b) small acanthostyle; (c) tornote; (d) chelae. Scale bars: 10 µm.
SKELETON
Choanosomal skeleton: reticulation of large and small acanthostyles, chelae scattered throughout. Ectosomal skeleton a palisade of tornotes.
SPICULES (FIGURE 5B)
(1) Large acanthostyles sparsely but entirely spined, 274(315)476 by 5.1(10.3)15.1 µm.
(2) Small entirely spined acanthostyles with thick spines 99(128)147 by 8.9(13.5)17.2 µm.
(3) Tornotes: inequiended 167(202)234 by 2.3(4.2)5.7 µm.
(4) Chelae 25(32)38 µm.
REMARKS
This sub-genus is characterized as a Lissodendoryx which possesses echinating acanthostyles (Van Soest, Reference Van Soest, Hooper and van Soest2002a). There are comparatively few species of Lissodendoryx (Ectyodoryx) from this region (Table 3). This species can be distinguished from all Lissodendoryx (Ectyodoryx) species present in the region apart from L. patagonica (Ridley & Dendy, Reference Ridley and Dendy1886) by the absence of sigmata. Lissodendoryx patagonica has much larger acanthostyles, chelae and tornotes.
Table 3. Lissodendoryx (Ectyodoryx) species from the south-west Atlantic, Chile, Antarctic and South Africa. Information from type descriptions and (indicated by1) Rios (Reference Rios2006).

TYPE MATERIAL
Holotype: (BELUM Mc4794) sample in 95% ethanol, tissue section and spicule preparation on slides. Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
Paratype: (BELUM Mc4836) sample in 95% ethanol, tissue section and spicule preparation on slides. Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 8, Falkland Islands (51°03.482′S 61°10.342′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 31 October 2008.
COMPARATIVE MATERIAL EXAMINED
Hymedesmia areolata (Thiele, Reference Thiele1905). Tissue section slide prepared from the type specimen (Berlin Museum). Collected Calbuco, Chile. BMNH 08.9.24.164a.
ETYMOLOGY
From the Latin ferruginis meaning the colour of rust.
EXTERNAL MORPHOLOGY (FIGURE 6A)
Thin rusty orange crust with large pore sieves. Crater-like pore sieves with pronounced raised rims. These are very dense over sponge surface with rims almost touching.

Fig. 6. Phorbas ferrugineus sp. nov. (A) Surface showing large pore sieves; (B) spicules: (a) large acanthostyle; (b) small acanthostyle; (c) ectostomal tornote; (d) palmate chelae. Scale bars: 10 µm.
SKELETON
Choanosomal skeleton: plumose columns of large acanthostyles heavily echinated by small acanthostyles. The columns are very densely packed in the tissue so the arrangement appears almost reticulate. Ectosomal skeleton: ascending columns of tornotes, 10–15 spicules thick. Layer of chelae present at sponge surface, also present throughout tissue in small numbers.
SPICULES (FIGURE 6B)
(1) Large acanthostyles 225(266)293 by 9.6(12.0)17 µm. Spined with large spines one-half to two-thirds of the way up the shaft with the tip of the shaft usually being smooth, although in some spicules a few scattered individual spines may be present. Head not tylote.
(2) Small acanthostyles 121(141)158 by 5.5(8.2)12.3 µm. Entirely spined with large spines. Head not tylote. Ends come to an abrupt point. Majority have a bend in the shaft slightly up from the head.
(3) Ectosomal spicules: fat tornotes with smoothly tapering points 245(289)316 by 3.7(6.0)8.4 µm.
(4) Chelae: chelae with a palmate appearance (alae fused onto shaft, shaft straight) in a single size category 20(22)24 µm.
REMARKS
The only Phorbas species from the region with similar sized spicules and tornotes as ectosomal spicules is Phorbas areolata (Thiele, Reference Thiele1905) (Table 4). However, this differs in having chelae with a wide semi-circular shaft, fatter and more fusiform ectosomal tornotes, and large acanthostyles which taper gradually and tend to be entirely spined.
Table 4. Phorbas species from the south–west Atlantic, Chile, Antarctic and west coast of South Africa. Information from type descriptions and Rios & Cristobo (Reference Rios and Cristobo2007).

TYPE MATERIAL
Holotype: (BELUM Mc4720) sample in 95% ethanol, tissue section and spicule preparation on slides. Gypsy Cove, Falkland Islands (51°40.447′S 57°48.444′W; water depth: 8–10 m); collected by C. Goodwin and J. Jones, 15 October 2008.
Paratypes: all samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4723) Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57° 48.753′W; water depth 10–12 m); collected by C. Goodwin and J. Jones, 16 October 2008. (BELUM Mc4726) Doctor's Point, Stanley, Falkland Islands (51° 39.861′S 57°48.753′W; water depth 10–12 m); collected by C. Goodwin and J. Jones 16 October 2008. (BELUM Mc4727) Doctor's Point, Stanley, Falkland Islands (51° 39.861′S 57°48.753′W; water depth 10–12 m); collected by C. Goodwin and J. Jones 16 October 2008. (BELUM Mc4731) Doctor's Point, Stanley, Falkland Islands (51° 39.861′S 57°48.753′W; water depth 10–12 m); collected by C. Goodwin and J. Jones 16 October 2008. (BELUM Mc4746) Shallow Marine Surveys Group Jason Islands Cruise, Grand Jason Station 1, Falkland Islands (51° 03.812′S 61°04.189′W; water depth: 20 m); collected C. Goodwin and J. Jones, 26 October 2008. (BELUM Mc4772) Shallow Marine Surveys Group Jason Islands Cruise, South Grand Jason Station 2, Falkland Islands (51°04.16′6S 61°07.324′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 27 October 2008. (BELUM Mc4805) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 13 m); collected by C. Goodwin and J. Jones, 30 October 2008. (BELUM Mc4813) Shallow Marine Surveys Group Jason Islands Cruise, South Grand Jason Station 5, Falkland Islands (51°03.392′S 61°10.341′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 31 October 2008. (BELUM Mc4831) Shallow Marine Surveys Group Jason Islands Cruise, Flat Jason North, Falkland Islands (51°05.849′S 60°53.623′W; water depth: 11 m); collected by C. Goodwin and J. Jones, 1 November 2008. (BELUM Mc4832) Shallow Marine Surveys Group Jason Islands Cruise, Flat Jason North, Falkland Islands (51°05.849′S 60°53.623′W; water depth: 11 m); collected by C. Goodwin and J. Jones, 1 November 2008.
COMPARATIVE MATERIAL EXAMINED
Anchinoe antarctica (Hentschel, Reference Hentschel1914) sensu Burton, Reference Burton1940. Collected from south-east coast of South America, Buenos Aires Museum (16482), 33.6.1–.98. BMNH 33.6.10.98.
ETYMOLOGY
Named for Sir Ernest Shackleton, Antarctic Explorer, in recognition of the Shackleton Scholarship Fund's support of this research.
EXTERNAL MORPHOLOGY (FIGURE 7A)
Thin peach crust with many inflated pore sieve regions, combining inhalant ostia and exhalent oscules. Pore sieves cover the majority of the surface of the sponge and are occasionally adjoining. Sponge often forms quite large patches, up to 30 cm in diameter.

Fig. 7. Phorbas shakletoni sp. nov. (A) External appearance; (B) skeleton; (C) spicules: (a) large acanthostyle; (b) small acanthostyle; (c) strongyle. Scale bars 10 µm.
SKELETON (FIGURE 7B)
Typical Phorbas skeleton with long columns of large acanthostyles echinated by smaller acanthostyles. Sponge thickness up to 3000 µm. In thinner individuals <1500 µm the organization appears hymedesmoid with a basal layer of acanthostyles in which the small acanthostyles are more frequent. The ascending columns of strongyles are 10–20 spicules thick and divide into smaller columns towards the surface, eventually forming a palisade of individual spicules supporting the pore sieves.
SPICULES (FIGURE 7C)
(1) Large acanthostyles: 284(322)353 by 7.0(8.9)12.1 µm, head slightly tylote, tips come to an abrupt point. Most spined only around the head region, but some are spined for up to half their length. The spines are very small, in contrast with those present on the small acanthostyles.
(2) Small acanthostyles: 147(163)182 by 7.7(11.8)15.3 µm spined all over with large recurved spines. The head is not tylote but is often marked by a slight constriction of the shaft above it and denser spination than the rest of the shaft. The tip does not taper gradually but comes to an abrupt point.
(3) Ectosomal spicules: fusiform anisostrongyles 240(290)388 by 4.9(6.0)7.3 µm.
REMARKS
A range in skeletal form from Hymedesmia to Phorbas has been reported for other Hymedesmia and Phorbas species—e.g. the common Mediterranean Phorbas tenacior (Topsent, 1925). The external morphology, spicule form and size-range are the same in all these specimens. They vary only in the fact that the thinner specimens have a Hymedesmia (Stylopus) skeleton and the thicker have Phorbas architecture. In our view this species should be assigned to Phorbas.
There are no Hymedesmia (Stylopus) species with spicules corresponding to this species and there are very few species known from this region. Of those described from the South Atlantic, Hymedesmia (Stylopus) antarctica (Hentschel, Reference Hentschel1914) has polytylote strongyles and only one category of acanthostyles. Hymedesmia (Stylopus) fristedti (Topsent, Reference Topsent1916) has large acanthostyles up to 650 µm and tornotes as ectosomal spicules.
The majority of Phorbas species from this area have microscleres (Rios, Reference Rios2006). The two that do not are P. longurioides (Burton, Reference Burton1932) and P. antarctica (Hentschel, Reference Hentschel1914). Phorbas longurioides (Burton, Reference Burton1932) has large acanthostyles which are entirely spined and tornotes as ectosomal spicules. Phorbas antartica (Hentschel, Reference Hentschel1914) was originally described from the Antarctic as Hymedesmia dermata var. antarctica Hentschel, Reference Hentschel1914 with large acanthostyles 352–480 µm, small acanthostyles 128–144 µm, and ectosomal strongyles 296–328 µm. Burton (Reference Burton1940) named a specimen from Mar del Plata, Argentina as Anchinoe antartica (Hentschel, Reference Hentschel1914). It was not possible to obtain the type of Hymedesmia dermata var. antarctica (Hentschel, Reference Hentschel1914) as it is missing from the collection in Berlin. However, a specimen of Burton's Phorbas antarctica was examined and found to differ from this species in the possession of spicules of a smaller size-range (large acanthostyles 305–315 µm, small acanthostyles 140–160 µm, ectosomal spicules 300–320 µm), small acanthostyles with distinctive very large recurved spines, and a much thicker structure with small acanthostyles echinating a greater length of the ascending columns. There are several other species in the family Hymedesmiidae with the specific name antarctica and these need to be redescribed and evaluated in future studies as their taxonomy is confused.
TYPE MATERIAL
Holotype: (BELUM Mc4778) sample in 95% ethanol, tissue section and spicule preparation on slides. Shallow Marine Surveys Group Jason Islands Cruise, South Grand Jason Station 5, Falkland Islands (51°04.678′S 61°03.834′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 27 October 2008.
Paratype: (BELUM Mc4827) sample in 95% ethanol, tissue section and spicule preparation on slides. Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 8, Falkland Islands (51°03.482′S 61°10.342′W; water depth 18–22 m); collected by C. Goodwin and J. Jones, 28 October 2008.
COMPARATIVE MATERIAL EXAMINED
Hymedesmia tenuissima Thiele, Reference Thiele1905 spicule preparation and co-type BMNH 1931.3.27.13.
Hymedesmia laevis Thiele, Reference Thiele1905, tissue section. Falkland Islands, Discovery Sponges BMNH 28.2.15.411a.
ETYMOLOGY
From the Latin acantho, meaning spiny for its spined tornotes, unusual in this family.
EXTERNAL MORPHOLOGY (FIGURE 8A)
Thick pale yellow crust with a few large oscules; irregular radiating channels arise from the edges of these. Sponge produced a lot of slime when collected.

Fig. 8. Myxilla (Styloptilon) acanthotornota sp. nov. (A) External appearance; (B) spicules: (a) large acanthostyle; (b) small acanthostyle; (c) ectosomal spined tornote; (d) sigma; (e) chelae. Scale bars 10 µm.
SKELETON
Plumose columns of large acanthostyles echinated by a smaller category of acanthostyles. Ectosomal layer of spined tornotes. Microscleres scattered throughout the sponge.
SPICULES (FIGURE 8B)
(1) Large acanthostyles: 217(254)287 µm by 11.1(13.2)16.0 µm. Head not tylote. Bent 1/3 of way up the shaft. Entirely spined with small spines.
(2) Small acanthostyles: 107(125)147 µm by 7.4(10.2)14.1 µm. Head not tylote. Entirely spined with small spines.
(3) Ectosomal tornotes: 176(199)221 µm by 5.8(7.3)9.0 µm. Fusiform and the majority slightly bent in the middle. Sparsely spined all over with small spines.
(4) Anchorate chelae in two categories: 14(17)21 µm and 24(29)33 µm. Myxillid type with sharply pointed teeth.
(5) Sigmas: 33(46)59 µm.
REMARKS
Hymenancora tenuissima (Thiele, Reference Thiele1905), originally described as Hymedesmia tenuissima, has tornotes of a very similar form, entirely covered with small spines. We prepared fresh tissue sections from the type specimen which revealed that it has a plumose skeletal architecture rather than hymedesmoid architecture and therefore believe it should be reclassified as Myxilla (Styloptilon) tenuissima. This species has large acanthostyles 212(243)275 by 15.1(20.5)29.4 µm, small acanthostyles 87(113)170 by 10.1(13.2)17.5 µm, lightly spined tornotes 181(194)217 by 5.9(8.5)10.8 µm, chelae 13(16)19 and 27(32)35 µm, and sigmas 41(45)50 µm. The size-range of its spicules is similar to Myxilla (Styloptilon) acanthotornota. However, all spicules are much more robust. This is particularly noticeable in the large acanthostyles which average 20.2 µm diameter compared to 13.2 µm in the new species. The spination on the large acanthostyles is less even in M. tenuissima with more spines near the head. The small acanthostyles are also more heavily spined. Lévi (Reference Lévi1963) identified a South African specimen as the same as Thiele's, designating it Ectomyxilla tenuissima. However, this has large acanthostyles measuring up to 420 µm and therefore could possibly represent a separate species of Myxilla (Styloptilon) with spined tonotes, although re-examination of the type would be necessary to confirm skeletal architecture.
There are currently two other recognized species in the genus: the type species Myxilla (Styloptilon) ancoratum (Cabioch, Reference Cabioch1968) and Myxilla (Styloptilon) anchoratum (Bergquist & Fromont, 1988). From examination of Burton's Falkland specimen of Hymedesmia laevis it would seem that this should also be redesignated as Myxilla (Styloptilon) as it possesses plumose skeletal architecture and anchorate chelae. These three species are distinct from Myxilla (Styloptilon) acanthotornota as they do not possess spined tornotes.
SPECIMENS
All samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4760) Shallow Marine Surveys Group Jason Islands Cruise, Grand Jason Station 2, Falkland Islands (51°03.819′S 61°04.181′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 26 October 2008. (BELUM Mc4814) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 5, Falkland Islands (51°03.392′S 61°10.341′W; water depth: 13 m); collected by C. Goodwin and J. Jones, 31 October 2008. (BELUM Mc4750) Shallow Marine Surveys Group Jason Islands Cruise, Grand Jason Station 1, Falkland Islands (51° 03.812′S 61°04.189′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 26 October 2008. (BELUM Mc4800) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61° 12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
EXTERNAL MORPHOLOGY (FIGURE 9A)
Very thick beige crust with surface formed into low mounds and ridges. Large oscules present on top of mounds or ridges, frequently forming lines of oscules on the surface, with rims almost touching.

Fig. 9. Tedania (Tedania) mucosa (Thiele, Reference Thiele1905). (A) External appearance; (B) spicules: (a) tornote; (b) style; (c) large onychaete; (d) small onychaete. Scale bars 10 µm.
SKELETON
Choanosome: bundles of styles form a reticulate skeleton, bundles of onychaetes ascend through the tissue, loose onychaetes also present. Ectosomal skeleton: layer of tornotes upright with points towards surface, also bundles of onychaetes.
SPICULES (FIGURE 9B)
(1) Styles: 254(290)318 by 6.4(9.3)13.2 µm, rather fat with abrupt points, majority slightly curved.
(2) Ectosomal tornotes: 208(229)247 by 3.8(6.1)8.7 µm, with mucronate ends.
(3) Onychaetes: 2 categories: 52(65)83 and 190(209)240 µm.
REMARKS
Desqueyroux-Faúndez & Van Soest (Reference Desqueyroux-Faúndez and van Soest1996) reassessed the genera Tedania and Trachytedania, and reclassified the species present into three sub-genera: Tedania (Tedaniopsis) with long styles 300–700 µm, Tedania (Tedania) with short styles 150–300 µm and mucronate tornotes, and Tedania (Trachytedania) with smooth or spined short styles 150–300 µm and oxeote or mucronate tornotes. The redescription of Tedania (Trachytedania) was based on the fact that no basal acanthostyles, the characterizing feature of the genus, could be found in the type species. Trachytedania has since been re-established as a valid genus by Cristobo & Urgorri (Reference Cristobo and Urgorri2001) who re-examined the type and located basal acanthostyles. The genus is consequently restricted to species which possess basal acanthostyles, as per the original description. Therefore T. mucosa should presumably now be reassigned to Tedania (Tedania). However, another feature of the genus is possession of a hymedesmiid skeletal arrangement which is not clearly demonstrated in the type species. The genus clearly requires further revision, which is beyond the scope of this study; one solution may be to combine the sub-genera into a single large genus.
Tedania and Trachytedania from this region are listed in Table 5. The sub-genus Tedania (Tedaniopsis) is not included, as these species possess long choanosomal styles >350 µm (Van Soest, Reference Van Soest, Hooper and van Soest2002b), whereas all the specimens collected in this study had styles <350 µm.
Table 5. Tedania (Tedania) and Tedania (Trachytedania) species of the south-west Atlantic, Chile and Antarctic. Information from type descriptions and Desqueyroux-Faúndez & Van Soest (Reference Desqueyroux-Faúndez and van Soest1996).

Our specimens are a good match for the descriptions given by Thiele (Reference Thiele1905) and Desqueyroux-Faúndez & Van Soest (Reference Desqueyroux-Faúndez and van Soest1996). Other records are from deep waters of the Argentine coast (Bertolino et al., Reference Bertolino, Schejter, Calcinai, Cerrano, Bremec, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007), and the Argentine coast (Burton, Reference Burton1934, Reference Burton1940; Cuartas, Reference Cuartas1986, Reference Cuartas1992; Desqueyroux-Faúndez & Van Soest, 1996), Strait of Magellan (Sarà et al., 1992), Chilean coast and the Falkland Islands (Desqueyroux, 1972).
SPECIMENS
(BELUM Mc4740) sample in 95% ethanol, tissue section and spicule preparation on slides. Doctor's Point, Stanley, Falkland Islands (51°39.861S′ 57°48.753′W; water depth: 10–12 m); collected by C. Goodwin and J. Jones, 19 October 2008. (BELUM Mc4808) sample in 95% ethanol, tissue section and spicule preparation on slides. Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 13 m); collected C. Goodwin and J. Jones, 30 October 2008.
COMPARATIVE MATERIAL EXAMINED
Tedania murdochi (Topsent, Reference Topsent1915) section and spicule preparation prepared from part of holotype. NMSZ.1921.143.1410.
EXTERNAL MORPHOLOGY (FIGURE 10A)
Large pale to dark grey chimney-like mounds with oscules on the tops of the chimneys.

Fig. 10. Tedania (Tedania) murdochi (Topsent, Reference Topsent1915). (A) External appearance; (B) spicules: (a) style; (b) tornote; (c–e) onychaetes. Scale bars: 10 µm.
SKELETON
Choanosome: bundles of styles form a reticulate skeleton, bundles of onychaetes ascend through the tissue, loose onychaetes also present. Ectosomal skeleton: layer of tornotes upright with points towards surface, also bundles of onychaetes.
SPICULES (FIGURE 10B)
(1) Styles: 205(222)240 by 7.1(9.3)12.1 µm. Rather fat with an abrupt point, the majority slightly curved.
(2) Ectosomal tornotes: 197(212)224 by 4.3(5.5)6.9 µm, majority anisotornotes with one end slightly fatter and more rounded.
(3) Onychaetes: 2 categories: 37(48)60 and 107(138)174 µm.
REMARKS
Trachytedania has recently been re-established as a valid genus by Cristobo & Urgorri (Reference Cristobo and Urgorri2001) who re-examined the type and located basal acanthostyles (see above). The genus is consequently restricted to species which possess basal acanthostyles, as per the original description. Currently Tedania (Tedania) murdochi is listed as a synonym of Trachytedania spinata but, as it does not possess basal acanthostyles, under this classification they are in separate genera and therefore cannot be synonyms (Cristobo & Urgorri, Reference Cristobo and Urgorri2001). Ideally these genera require further revision. However, even if the division of the genera is incorrect, the presence of acanthostyles would separate the two species. Species from the genera Trachytedania and Tedania (Tedania), which have been reported from this area, are compared in Table 5. The type locality for this species is Stanley in the Falkland Islands. Spicule form and size-range are a good match for the type specimen. Measurements from type: styles 215(228)239 by 5.7(9.1)11.5 µm, tornotes 189(220)233 by 3.9(5.8)7.4 µm, onychaetes 39(48)67 and 98(129)161 µm.
SPECIMENS
All samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4715) Gypsy Cove, Stanley, Falkland Islands (51°40.447′S 57°48.444′W; water depth: 10 m); collected by C. Goodwin and J. Jones, 15 October 2008. (BELUM Mc4719) Gypsy Cove, Stanley, Falkland Islands (51°40.447′S 57°48.444′W; water depth: 10 m); collected C. Goodwin and J. Jones, 15 October 2008. (BELUM Mc4722) Doctor's Point, Stanley, Falkland Islands (51° 39.861′S 57°48.753′W; water depth: 12 m); collected by C. Goodwin and J. Jones, 16 October 2008. (BELUM Mc4724) Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57° 48.753′W, water depth 12 m); collected by C. Goodwin and J. Jones, 16 October 2008. (BELUM Mc4733) Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57°48.753′W; water depth: 12 m); collected by C. Goodwin and J. Jones, 16 October 2008. (BELUM Mc4741) High Rock, Stanley, Falkland Islands (51°39.500′S 57°46.083′W; water depth 10–12 m); collected by C. Goodwin and J. Jones, 21 October 2008. (BELUM Mc4742) High Rock, Stanley, Falkland Islands (51°39.500′S 57°46.083′W; water depth 10–12 m); collected by C. Goodwin and J. Jones, 21 October 2008. (BELUM Mc4770) Shallow Marine Surveys Group Jason Islands Cruise, South Grand Jason Station 2, Falkland Islands (51°04.166′S 61°07.324′W; water depth: 20 m.); collected by C. Goodwin and J. Jones, 27 October 2008. (BELUM Mc4773) Shallow Marine Surveys Group Jason Islands Cruise, South Grand Jason Station 5, Falkland Islands (51°04.678′S 61°03.834′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 27 October 2008. (BELUM Mc4798) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008. (BELUM Mc4820) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 8, Falkland Islands (51°03.482′S 61°10.342′W; water depth: 13–20 m); collected by C. Goodwin and J. Jones, 31 October 2008.
ETYMOLOGY
Named for the survey vessel for the sampling trip to the Jason Islands, the 65 ft yacht the ‘Golden Fleece’ owned by the Poncet family.
EXTERNAL MORPHOLOGY (FIGURE 11A)
Creamy yellow cushion with obvious oscules on the tips of small raised projections and channels on surface of sponge. Typically 10–15 cm maximum diameter although some specimens can be much larger.

Fig. 11. Amphilectus fleecei sp. nov. (A) External appearance; (B) skeleton; (C) spicules: (a) style; (b) chelae. Scale bars 10 µm.
SKELETON (FIGURE 11B)
Ascending columns of styles (2–3 spicules thick) interconnected by single spicules. Ends of columns fan out to form brushes at the surface.
REMARKS
Amphilectus Vosmaer, 1880 is currently defined as an Esperiopsidae with a ladder-like skeleton of ascending and interconnecting spicules tracts. Usually the microscleres are only small palmate microchelae and no sigmas are present, the megascleres being small styles under 400 μm. Esperiopsis is used in the sense of Burton (Reference Burton1929) for species conforming to Esperia villosa; these have an anastomosing plumoreticulate skeleton of thick spicule tracts of large mycalostyles. Microscleres can include up to three categories of palmate isochelae and two categories of sigmas. The genus Amphilectus is considered by many authors to be a synonym of Esperiopsis and species within these genera require thorough revision (Van Soest & Hadju, Reference Van Soest, Hadju, Hooper and van Soest2002). For this reason we have considered species of both Amphilectus and Esperiopsis occurring in this area (Table 6). Amphilectus fleecei sp. nov. differs from the majority of other species present in this region in the small size-range of its acanthostyles and small size of its chelae. The spicule dimensions are similar to specimens of Amphilectus fucorum (Esper, 1794) recorded by Thiele (Reference Thiele1905) and Burton (Reference Burton1932) from the Falkland Islands. Our specimens are likely to be the same species as some of these specimens, although given the variation in external form, sampling location and spicule size Burton's specimens may be a complex of several species. However, A. fucorum is a well characterized European species with a distinctive bright orange colour (Ackers et al., Reference Ackers, Moss and Picton1992), quite different in appearance from our pale orange specimens. Thiele (Reference Thiele1905) and Burton (Reference Burton1932) were working from preserved specimens and therefore were not able to use external characters to aid in identification.
Table 6. Amphilectus and Esperiopsis species of the south-west Atlantic, Chile and Antarctic.

TYPE MATERIAL
Holotype: (BELUM Mc4702) specimen in 95% ethanol, tissue section and spicule preparation on slides. FIPASS, Stanley, Falkland Islands (51°41.560′S 57°49.269′W; water depth 6 m); collected by C. Goodwin and K. Neely, 14 October 2008.
ETYMOLOGY
From the Latin dactyl, meaning finger. Named for its growth form.
EXTERNAL MORPHOLOGY (FIGURE 12A)
Dichotomously branching orange sponge with slender, cylindrical branches.

Fig. 12. Amphilectus dactylus sp. nov. (A) External appearance; (B) skeleton (scale bar 100µm); (C) spicules. Scale bars 10 µm.
SKELETON (FIGURE 12B)
Plumo-reticulate disordered skeleton. No specialized ectosomal skeleton.
REMARKS
This species can be separated from other Esperiopsis and Amphilectus occurring in this area by the small size of its spicules and its branching form (Table 6).
TYPE MATERIAL
Holotype: (BELUM Mc4729) sample in 95% ethanol, tissue section and spicule preparation on slides, Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57°48.753′W; water depth: 12 m); collected by C. Goodwin and J. Jones, 16 October 2008.
COMPARATIVE MATERIAL EXAMINED
Mycale (Aegogropila) magellanica (Ridley, Reference Ridley1881). Slide and spicules preparation from specimen from the Scotia expedition. NMSZ.1921.143.1412.
ETYMOLOGY
From the Latin nodulus, diminutive of nodus, knot. Named for the lumpy projections on its surface.
EXTERNAL MORPHOLOGY (FIGURE 13A)
Massive pale cream sponge. Surface formed into a few large mounds, each bearing a large terminal oscule. Entire sponge surface bears small lumpy projections.

Fig. 13. Mycale (Aegogropila) nodulosa sp. nov. (A) External appearance; (B) spicules: (a) mycalostyle; (b) anisochelae; (c) sigma; (d) microxea. Scale bars 10 µm.
SKELETON
Choanosomal skeleton: irregular anastomosing tracts of styles. Microscleres scattered throughout.
Ectosomal skeleton: reticulation of mycalostyles. Surface tangential layer of mycalostyles forming a regular circular mesh. Mesh sides approximately 30 spicules thick and mesh apertures approximately 350 µm in diameter. The presence of an ectosomal mesh places the species in the subgenus Mycale (Aegogropila), as in Mycale (Mycale) the ectosomal skeleton is disordered.
SPICULES (FIGURE 13B)
(1) Mycalostyles: 326(374)415 by 8.0(11.3)14.9 µm.
(2) Anisochelae: 26(35)42 µm.
(3) Sigmas: 33 µm, very rare (but present in both the section and the SEM preparation).
(4) Microxeas: 2 categories: 17(27)40 and 5 µm (the latter very abundant). Some form trichodragmata.
REMARKS
Only two species of Mycale (Aegogropila) have been described from the South Atlantic and Antarctic: Mycale (Aegogropila) denticulata Bertolino, Calcinai & Pansini, Reference Bertolino, Calcinai and Pansini2009 and Mycale (Aegogropila) magellanica (Ridley, Reference Ridley1881). Mycale denticulata differs in possessing only chelae as microscleres. Mycale magellanica was originally described from the Magellan Strait and differs from our specimen in having longer styles (320–570 µm), larger chelae (44–53 µm), only one category of microxea (4–6 µm) and in lacking sigmas. Topsent (1913) collected four specimens he called M. magellanica from the Burdwood Bank in 102 m. These differ from the type specimens in having sigmas (35 µm), and in our opinion are likely to be an undescribed species. Topsent's specimens possess chelae of a similar size to our specimen (40–45 µm and 27–32 µm) and are also described as having abundant rhaphides (20–50 µm). However, they differ in having longer styles (320–570 µm) and lacking a second, smaller, class of oxeas. Burton (Reference Burton1934) compares several specimens of M. magellanica and, as he regards variation in microscleres in these specimens as unimportant, considers them all the same species. Revision of these specimens may reveal further species from this area.
TYPE MATERIAL
Holotype: (BELUM Mc4765) sample in 95% ethanol, tissue section and spicule preparation on slides. Shallow Marine Surveys Group Jason Islands Cruise, Grand Jason Station 2, Falkland Islands (51°03.819′S 61°04.181′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 26 October 2008.
Paratypes: all samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4766) Shallow Marine Surveys Group Jason Islands Cruise, South Grand Jason Station 2, Falkland Islands (51°04.166′S 61°07.324′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 27 October 2008. (BELUM Mc4792) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason North Station 5, Falkland Islands (51°01.972′S 61°12.629′W; water depth: 18–22 m); collected by C. Goodwin and J. Jones, 28 October 2008. (BELUM Mc4796) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
ETYMOLOGY
Named for Francis Bunker, marine biologist and algal taxonomist who provided laboratory facilities for part of this work.
EXTERNAL MORPHOLOGY (FIGURE 14)
Thin rusty orange encrusting sponge with distinctive spiky surface.

Fig. 14. Scopalina bunkeri sp. nov. external appearance.
SKELETON
Columns of long styles in tissue very heavy in spongin. Few spicules present. The tips of the columns project from the surface creating spikes.
SPICULES
Styles: 694–1742 µm.
REMARKS
The low number of spicules present in the samples collected and the presence of abundant spongin is typical of the genus. There are only eight described species of Scopalina, none of which have been recorded from the South Atlantic. Scopalina bunkeri sp. nov. are distinguished from Scopalina erubuscens sp. nov. by the presence of much longer styles, creating a more prominently spiked surface.
TYPE MATERIAL
Holotype: (BELUM Mc4725) sample in 95% ethanol, tissue section and spicule preparation on slides, Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57°48.753′W; water depth: 12 m); collected by C. Goodwin and J. Jones, 16 October 2008.
Paratypes: all samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4728) Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57°48.753′W; water depth: 12 m); collected by C. Goodwin and J. Jones, 16 October 2008. (BELUM Mc4759) Shallow Marine Surveys Group Jason Islands Cruise, Grand Jason Station 2, Falkland Islands (51°03.819′S 61°04.181′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 26 October 2008. (BELUM Mc4793) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
ETYMOLOGY
From the Latin erubescens, meaning blushing or turning red. Named for its pale peach colour.
EXTERNAL MORPHOLOGY (FIGURE 15)
Thick pale pink crust with connulose surface and obvious oscules. Patches can reach a large size, some attaining over 50 cm in diameter.

Fig. 15. Scopalina erubescens sp. nov. external appearance.
SKELETON
Columns of styles 15–20 spicules in diameter. Columns embedded in thick spongin fibre. Tissue very dense with spongin—hard to see spicules in tissue sections.
SPICULES
Styles: 331(395)459 by 9.4(13.0)15.6 µm.
REMARKS
The low number of spicules present in the samples collected and the presence of abundant spongin is typical of the genus. There are only eight described species of Scopalina, none of which have been recorded from temperate waters. Scopalina erubescens sp. nov. differs from Scopalina bunkeri sp. nov. in that its styles are much shorter.
SPECIMENS
All samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4706) FIPASS, Stanley, Falkland Islands (51°41.560′S 57°49.269′W; water depth: 6 m). Collected by C. Goodwin and K. Neely, 14 October 2008. (BELUM Mc4732) Doctor's Point, Stanley, Falkland Islands (51°39.861′S 57°48.753′W; water depth: 10 m); collected by C. Goodwin and J. Jones, 16 October 2008. (BELUM Mc4802) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
COMPARATIVE MATERIAL EXAMINED
Eumastia attenuata (Topsent, Reference Topsent1915) from the Bruce Scottish National Antarctic Expedition collection. Possible syntype. Tissue section and spicule preparation from specimen. NMS.Z.1921.143.1426.
EXTERNAL MORPHOLOGY (FIGURE 16A)
Massive sponge with distinctive lemon yellow colour. Surface covered with tassel-like projections up to 1 cm in length, though frequently shorter. Internal supporting spicule columns clearly visible through surface of projections. Occasional large oscules present. Sponge can be very large, up to 50 cm in diameter, but smaller patches also observed.

Fig. 16. Halichondria (Eumastia) attenuata (Topsent, Reference Topsent1915). (A) External appearance; (B) skeleton of papillae; (C) oxeas.
SKELETON (FIGURE 16B)
Papillae: core of oxeas in centre of papillae with thinner columns of oxeas radiating out to support ectosome. The ectosome is a mesh of crossing oxeas.
Choanosomal skeleton: confused mass of oxeas.
SPICULES (FIGURE 16C)
Oxeas: 349(417)537 by 5.8(9.7)14.7 µm, abruptly pointed ends.
REMARKS
Preserved specimen very similar in appearance to the type. Spicule measurement range slightly larger than those reported for the type (330–480 µm) but spicules identical in form. Type location: Stanley, Falkland Islands. Also recorded from the Falklands from Berkeley Sound and Port Louis from depths of 0–16 m by Burton (Reference Burton1932, Reference Burton1934) and from South Georgia Island (Burton, Reference Burton1934). There is an additional record from Bransfield Strait in Antarctica (Campos et al., Reference Campos, Mothes and Veitenheimer Mendes2007). However, this specimen did not possess the well developed papillae and specialized ectosomal skeleton characteristic of Halichondria (Eumastia). Additionally, although the spicule size-range is similar, the form of the oxeas differs in that they taper smoothly to conical points rather than being abruptly pointed and are straight rather than bent. Therefore this identification is doubtful.
SPECIMENS
All samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4749) Shallow Marine Surveys Group Jason Islands Cruise, Grand Jason Station 1, Falkland Islands (51°03.812′S 61°04.189′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008. (BELUM Mc4738) High Rock, Stanley, Falkland Islands (51°39.500′S 57°46.083′W; water depth: 16 m); collected by C. Goodwin and J. Jones, 21 October 2008. (BELUM Mc4751) Shallow Marine Surveys Group Jason Islands Cruise, Grand Jason Station 1, Falkland Islands, (51°03.812′S 61°04.189′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 26 October 2008. (BELUM Mc4803) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Site 2, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
EXTERNAL MORPHOLOGY (FIGURE 17A)
White to pale purple branching sponge. Each branch in the form of a hollow tube bearing a terminal oscule. Tubes frequently constricted at points along their length. Some individuals consist of just a single unbranched tube. Fibre mesh visible below sponge surface. Texture hard and fibrous.

Fig. 17. Siphonochalina fortis (Ridley, Reference Ridley1881). (A) External appearance; (B) choanosomal skeleton; (C) ectosomal skeleton; (D) oxea.
SKELETON (FIGURE 17B&C)
Choanosome: square mesh of small oxeas. Primary columns 10–20 spicules thick, secondary columns unispicular. Ectosomal skeleton a unispicular irregular square mesh.
SPICULES (FIGURE 17D)
Small oxea: 47(59)71 by 2.0(5.7)8.1 µm. Ends abruptly pointed.
REMARKS
Originally described from Portland Bay, near Madre-de-Dios Island, Chile (Ridley, Reference Ridley1881), also recorded from the coast of Argentina (Burton, Reference Burton1940; Cuartas, Reference Cuartas1991, Reference Cuartas, Boschi and Cousseau2004) and in the Falkland Islands' Port William, Port Albemarle and William Scoresby Stations 72 (north-east of East Falkland), 83 (west of George Island, East Falkland), 84 (west of Sea Lion Island) and 86 (south of Falkland Islands) (Burton, Reference Burton1932, Reference Burton1934). Depth-range of previous Falkland records from 17–147 m.
SPECIMENS
All samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4704) FIPASS, Stanley, Falkland Islands (51°41.560′S 57°49.269′W; water depth: 6 m); collected by C. Goodwin and K. Neely, 14 October 2008. (BELUM Mc4707) FIPASS, Stanley, Falkland Islands (51°41.560′S 57°49.269′W; water depth: 6 m); collected by C. Goodwin and K. Neely, 14 October 2008. (BELUM Mc4708) FIPASS, Stanley, Falkland Islands (51°41.560′S 57°49.269′W; water depth: 6 m); collected by C. Goodwin and K. Neely, 14 October 2008
EXTERNAL MORPHOLOGY (FIGURE 18A)
Creamy white sponge approximately 10–15 cm diameter with tubular growths up to 5 cm in length arising horizontally and vertically from a common base. Large oscules at the tip of some of the projections. Surface conulose with ectosomal mesh clearly visible.

Fig. 18. Haliclona (Soestella) chilensis Thiele, Reference Thiele1905. (A) External appearance; (B) skeleton; (C) spicules.
SKELETON (FIGURE 18 B&C)
Choanosome: primary ascending tracts approximately 6–10 spicules in diameter joined by uni-paucispicular tracts of oxeas. Ectosome: rounded mesh of oxeas 4–6 spicules in width, each aperture approximately 300–350 µm in diameter.
SPICULES (FIGURE 18D)
Oxeas 130–200 µm, most about 170–200 µm. Fat oxeas with abrupt points. Majority are curved; the curve is most pronounced towards the middle of the shaft with the ends being straighter. This gives the impression of two bends along the shaft length.
REMARKS
Originally described from Calbuco, Chile (Thiele, Reference Thiele1905). Recorded from the Falkland Islands William Scoresby Station 84 (west of Sea Lion Island (74–75 m)) (Burton, Reference Burton1932) and Port Albemarle (15 m) (Burton, Reference Burton1934). Also known from South Shetland Islands and King George Islands (Antarctica) (Campos et al., Reference Campos, Mothes and Veitenheimer Mendes2007). Form similar to that pictured by Thiele (Reference Thiele1905) and Campos et al. (Reference Campos, Mothes and Veitenheimer Mendes2007), although in the smaller piece the tubular projections are not so obvious.
TYPE MATERIAL
Holotype: (BELUM Mc4799) sample in 95% ethanol, tissue section and spicule preparation on slides, Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason Station 2, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
Paratypes: all samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4801) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason Station 2, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 13 m); collected by C. Goodwin and J. Jones, 30 October 2008. (BELUM Mc4806) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason Station 2, Falkland Islands (51°02.25′2S 61°12.917′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 30 October 2008.
COMPARATIVE MATERIAL EXAMINED
Haliclona penicillata (Topsent, 1908) Discovery Sponges. BMNH 28.2.15.65a.
Haliclona bilamellata Burton, Reference Burton1932. Section and spicule preparation prepared from the type specimen. BMNH 28.2.15.121a.
EXTERNAL MORPHOLOGY (FIGURE 19A)
Pale orange, elongated, cup shaped sponge. Cup up to 15 cm in length. Surface pitted by numerous small oscules.

Fig. 19. Amphimedon calyx sp. nov. (A) External appearance; (B) skeleton; (C) spicules.
ETYMOLOGY
From the Latin calyx, meaning cup or goblet, named for its distinctive shape.
SKELETON (FIGURE 19B)
Plumo-reticulate with large fibres 6–10 spicules thick and smaller anastomosing fibres 2–3 spicules thick. No specialized ectosomal skeleton but ends of columns form brushes at the surface.
SPICULES (FIGURE 19C)
Oxeas: 229(259)289 by 1.4(5.8)9.2 µm. Two categories, one much thinner. Thicker ones in columns and thinner ones (1–2 µm) scattered in choanosome.
Thicker oxeas: bent centrally with each end tapering to a long fine point. Some are centrotylote.
Thin oxeas: bent centrally but this is less pronounced than in the thicker category. Ends taper to fine points. Some are centrotylote.
REMARKS
Of the Amphimedon species present in the region, this species may be distinguished by its distinctive external form and the presence of centrotylote oxeas. Amphimedon decuarta (Sarà, Reference Sarà1978) has similar sized spicules but these are style-like in form. Amphimedon anomala (Sarà, Reference Sarà1978) has spined tylostrongyles. Amphimedon minuta (Cuartas, Reference Cuartas1988) and Amphimedon tenera (Thilele, 1905) may be distinguished by their much smaller oxeas, measuring 60–110 µm and 130 µm respectively. Amphimedon paradisus (Desqueroux-Faúndez, Reference Desqueyroux-Faúndez1989) has much larger oxeas (179–290 µm). Amphimedon maresi (Sarà, Reference Sarà1978) and Amphimedon reticulosa (Thiele, Reference Thiele1905) are most similar, having oxeas 160–200 µm and 220 µm respectively. However, the former may be distinguished by its massive lobose form and lack of centrotylote oxeas, and the latter by its much smaller size (up to 5 cm), the abruptly pointed oxeas and the absence of centrotylote oxeas.
Given the confusion in the taxonomy of Haplosclerida, we also considered other haplosclerids recorded from the Falkland Islands (Burton, Reference Burton1932, Reference Burton1934). Haliclona bilamellata Burton, Reference Burton1932 has a very similar external appearance but has strong ascending fibres joined by single spicules. Haliclona penicillata (Topsent, 1908) possesses centrotylote oxeas but a typical Haliclona skeleton.
TYPE MATERIAL
Holotype: (BELUM Mc4754) specimen in 95% ethanol, tissue section and spicule preparation on slides, Shallow Marine Surveys Group Jason Islands Cruise, Grand Jason Station 1, Falkland Islands (51°03.812′S 61°04.189′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 26 October 2008.
Paratypes: all samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4783) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason North Station 2, Falkland Islands (51°02.592′S 61°10.723′W; water depth: 22 m); collected by C. Goodwin and J. Jones, 28 October 2008. (BELUM Mc4835) Shallow Marine Surveys Group Jason Islands Cruise, Flat Jason North, Falkland Islands (51°05.849′S 60°53.623′W; water depth: 11 m); collected by C. Goodwin and J. Jones 1 November 2008.
COMPARATIVE MATERIAL EXAMINED
Pachychalina magellanica (Thiele, Reference Thiele1905). Section of type specimen. Magellan Strait Chile. BMNH 08.9.24.175a
Dasychalina validissima (Thiele, Reference Thiele1905) Calbuco. Section, possibly from type specimen (not stated on slide). BMNH 08.9.24.178°.
ETYMOLOGY
From the Latin for hedgehog, erinaceus. Named for its lobose shape and long projections which give it a hedgehog-like appearance.
EXTERNAL MORPHOLOGY (FIGURE 20A)
Low white mound with surface covered with thin, finger-like projections. A few scattered, low oscules over its surface.

Fig. 20. Pachychalina erinacea sp. nov. (A) External appearance; (B) skeleton; (C) skeleton of papillae; (D) spicules.
SKELETON (FIGURE 20B&C)
The choanosomal skeleton is composed of anastomosing ascending columns of oxeas (6–10 spicules thick). There are many free oxeas in the tissue which make determining structure difficult. The terminal ends of the ascending columns form the papillae at the surface. Ectosomal skeleton strong mesh composed of ends of ascending fibres.
SPICULES (FIGURE 20D)
Bent oxeas: 114(170)206 by 3.7(6.5)8.3 µm.
REMARKS
The family Niphatidae Van Soest 1980 is defined as a Haplosclerida with a three-dimensional ectosomal skeleton of multispicular fibres (Desqueyroux-Faúndez & Valentine, Reference Desqueyroux-Faúndez, Valentine, Hooper and van Soest2002). Within the family, the genera Amphimedon, Dasychalina and Pachychalina are closely related. Amphimedon possesses a regular tangential ectosomal network and an optically smooth surface, Dasychalina has a spiny ‘aculeate’ surface and an ectosomal skeleton which is a network of fibres reinforced by a secondary subectosomal reticulation forming surface connules. Desqueyroux-Faúndez & Valentine (Reference Desqueyroux-Faúndez, Valentine, Hooper and van Soest2002) state that the presence of large oxeas >400 µm can also assist in distinguishing it from other species in the family. However, several species currently classified as Dasychalina have small oxeas. Pachychalina has an irregularly connulose to spiny surface with abundant aquiferous openings and no aculeations and a rather confused choanosomal skeleton (Desqueyroux-Faúndez & Valentine, Reference Desqueyroux-Faúndez, Valentine, Hooper and van Soest2002). Historically there has been much confusion in the definition of these genera with the creators of Dasychalina subsequently synonymizing it with Pachychalina as they realized the type species of Pachychalina had a spinose surface, the primary reason for the designation of Dasychalina (Ridley & Dendy, Reference Ridley and Dendy1887). We tentatively place this species in Pachychalina because of its small spicule size and confused skeleton, but these genera require further revision.
Pachychalina and Dasychalina species present in the area are Dasychalina magellanica (Thiele, Reference Thiele1905), Dasychalina validissima (Thiele, Reference Thiele1905) and Pachychalina glacialis (Burton, Reference Burton1934). Dasychalina validissima has oxeas of a similar size (250 µm), but these are much thicker and its skeleton is formed of very thick fibres (20–30 spicules thick). Dasychalina magellanica (Thiele, Reference Thiele1905) is very similar in skeletal structure to our specimen with ascending fibres with many confused spicules. The oxeas are also a similar length (190 µm), however, the ascending fibres terminate abruptly at the surface and it consequently lacks the connules characteristic of this species. Pachychalina glacialis (Burton, Reference Burton1934) was described from the Falkland Islands as Hoplochalina glacialis, but this is a branching species with a ‘hirsute’ rather than connulose surface.
SPECIMENS
All samples in 95% ethanol, tissue section and spicule preparation on slides. (BELUM Mc4713) FIPASS, Stanley, Falkland Islands (51°41.560′S 57°49.269′W; water depth: 6 m); collected by C. Goodwin and K. Neely, 14 October 2008. (BELUM Mc4718) Gypsy Cove, Stanley, Falkland Islands (51°40.447′S 57°48.444′W; water depth: 10 m); collected by C. Goodwin and J. Jones, 15 October 2008. (BELUM Mc4721) Gypsy Cove, Stanley, Falkland Islands (51°40.447′S 57°48.444′W; water depth: 10 m); collected by C. Goodwin and J. Jones, 15 October 2008. (BELUM Mc4807) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 1, Falkland Islands (51°02.252′S 61°12.917′W; water depth: 13 m); collected by C. Goodwin and J. Jones, 30 October 2008. (BELUM Mc4809) Shallow Marine Surveys Group Jason Islands Cruise, Steeple Jason South Station 5, Falkland Islands (51°03.392′S 61°10.341′W; water depth: 20 m); collected by C. Goodwin and J. Jones, 31 October 2008.
EXTERNAL MORPHOLOGY (FIGURE 21)
Distinctive bright yellow encrusting sponge with connulose surface, ranging in size from small patches on rock to large areas up to 40 cm in diameter.

Fig. 21. Aplysilla sp. External appearance.
SKELETON
Spongin fibre skeleton only. Dichotomously branched fibres 100–130 µm in diameter.
REMARKS
Aplysilla species are defined by colour, conulation pattern, branching of the fibres and pigmentation of the fibres (Bergquist & Cook, Reference Bergquist, Cook, Hooper and van Soest2002). Of the ten currently valid species of Aplysilla only Aplysilla lendenfeldi Thiele, Reference Thiele1905 and Aplysilla sulphurea Schulze, 1878 have been recorded from this area. The type locality for A. lendenfeldi is Juan Fernandez Islands on the Pacific coast of Chile, and Thiele (Reference Thiele1905) recorded A. sulphurea from Chile in the Magellan Strait. Aplysilla sulphurea is synonymized with Aplysilla rosea (Barrois, 1876), but it is possible that Thiele meant A. sulfurea Schulze, 1878, a bright yellow species, type locality Adriatic Sea, which is common on European Atlantic coasts and in the Mediterranean. Unfortunately, Thiele worked on preserved material so details of colour of his specimens are not known. Given the distance from the type localities of these two species it is likely that Falkland Island specimens represent a new species. However, it has not yet been possible to examine the type of A. lendenfeldi, and consequently judgement is reserved.
DISCUSSION
The shallow water sponge fauna of the Falkland Islands was revealed by this study to include twelve species new to science. Knowledge of the Porifera of the south-west Atlantic is fragmentary with large areas having never been previously sampled (Lopez Gappa & Landoni, Reference Lopez Gappa and Landoni2005). Although the Falkland Islands are comparatively well sampled for this region, sampling has been largely from deep water, and the shallow water sponge fauna remains poorly understood. Many of the species described here were encrusting on bedrock, a habitat unlikely to have been sampled effectively by the previous surveys which sampled by dredging and trawling. Other studies have indicated that many of the encrusting sponges of shallow bedrock habitats remain undescribed, even in intensively studied areas (Picton & Goodwin, Reference Picton and Goodwin2007; Goodwin & Picton, Reference Goodwin and Picton2009). Worldwide, Porifera remain an understudied group with only approximately half of an estimated 15,000 species having been named (Hooper & Van Soest, Reference Hooper, Van Soest, Hooper and van Soest2002).
The previously known species reported in the Falklands have been recorded predominantly from southern Chile and Argentina with one species from each of South Georgia and the South Shetland Islands. No Antarctic species were reported from this study, although Halichondria (Eumastia) attenuata is known from South Georgia and Haliclona (Soestella) chilensis has been reported from the South Shetland Islands (Campos et al., Reference Campos, Mothes and Veitenheimer Mendes2007), both of which are below the Polar Frontal Zone. Burton (Reference Burton1932) recorded 51 species from the Falkland Islands, 29 of which were also found in the Antarctic, 23 off Graham Land, 19 from South Georgia, 24 from South America and 6 from Kerguelen. However, the majority of his samples were from deep water (>50 m) rather than the shallow coastal zone sampled here.
Depth has been shown to be a major influence on sponge community species composition in other areas (Voultsiadou, Reference Voultsiadou2005; Van Soest et al., Reference Van Soest, Cleary, De Kluijver, Lavaleye, Maier and van Duyl2007). It is possible that Antarctic water bodies influence the deeper water of the Falkland Islands but not the shallow water, accounting for the difference in fauna. Studies of a deepwater canyon (360 m) off Argentina have found a sponge fauna differing significantly from the shallow water habitats adjacent to it, including Antarctic species not known from the region (Bertolino et al., Reference Bertolino, Schejter, Calcinai, Cerrano, Bremec, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007), and the influence of Antarctic cold water currents have been suggested as a reason for the presence of Antarctic nudibranch species on the South American coastal slope (Schrödl, Reference Schrödl1999). However, further areas need to be sampled before firm conclusions can be drawn. The majority of the sampling for this study was at the Jason Islands, which are influenced by the cold temperate Argentine drift, in contrast to the south-eastern areas dominated by colder sub-Antarctic waters (Zyrianov & Severov, 1979). Additional sampling of contrasting biogoegraphical zones such as the south-east Falkland Islands, using the methodology employed by this study, will be useful in exploring the origins of the Falklands' shallow water sponge fauna and examining the effect of depth on species composition.
Biogeographically, the Falkland Islands are considered to be a part of the Magellanic Region, sharing a fauna with southern Patagonia (Hedgpeth, Reference Hedgpeth1969). All confirmed species of Falklands' Brachiopoda, Echinoidea, Asteroidea and freshwater fish are also found within the Magellanic/Patagonian region of South America, though some are also distributed more widely through the Southern Ocean (OBIS, 2010). This view has been supported by recent studies (e.g. Griffiths et al., Reference Griffiths, Barnes and Linse2009). However, studies on particular groups (e.g. shallow water (0–12 m) encrusting fauna (Barnes & De Grave, Reference Barnes and De Grave2001) and polychaetes (Montiel San Martin, Reference Montiel2005)) have demonstrated that there are distinct sub-regions within this province.
Southern Ocean biogeographical regions differ depending on the class of animal considered, with some groups, such as gastropods, demonstrating a much higher rate of endemism (Griffiths et al., Reference Griffiths, Barnes and Linse2009). Endemicity among Falkland Islands' shallow marine fauna varies from 0% (echinoids), to 6% (nudibranchs), to 15% (polychaetes) (Schrödl, Reference Schrödl1999; Montiel San Martin, 2005 ). The proportion of endemism is linked to reproductive mode, and consequently sponges with their limited dispersal capabilities could be expected to have a restricted distribution.
Halichondria (Eumastia) attenuata is a very conspicuous massively encrusting sponge and consequently likely to have been reported in surveys. The fact that it has only been found in the Falklands and South Georgia indicates that at least some of the fauna have a restricted distribution, and it is likely that some of the newly described species are endemic. Sponge larvae are relatively short lived, remaining in the plankton for between minutes and a few days (Maldonado, Reference Maldonado2006) and rarely appearing in off-shore plankton samples (Trégouboff & Rose, Reference Trégouboff and Rose1957). Consequently, most sponge species are thought to have limited dispersal capabilities and restricted distributions (Maldonado & Bergquist, Reference Maldonado, Bergquist and Young2002; Maldonado & Young, Reference Maldonado and Young1996). New molecular studies have further supported this view with ‘cosmopolitan species’ frequently demonstrated to be morphologically cryptic species complexes (e.g. Nichols & Barnes, Reference Nichols and Barnes2005). Re-examination of Falkland Islands sponge species using additional external appearance characters, and molecular techniques where necessary will accurately identify species and contribute to knowledge on Falkland sponge biogeography.
ACKNOWLEDGEMENTS
We would like to thank Amy Romanes, Susan Ware and Susan Chambers (National Museums of Scotland), Andreas Schmidt-Rhaesa (Zoological Museum University of Hamburg) and Clare Valentine, Andrew Cabrinovic and Emma Sherlock (Natural History Museum London) for providing access to their collections. Spicule comparison was greatly facilitated by the loan of a comparison microscope from Forensic Science Northern Ireland and provision of microscope access by Francis Bunker. Several researchers have provided comments on aspects of identification; we would like to particularly thank Eduardo Hadju, Rob Van Soest, Christine Morrow and Bernard Picton for their assistance. Special thanks is due to the other members of the Shallow Marine Surveys Group Jason Islands expedition team (Jude Brown, Steve Cartwright, Sarah Crofts, Wetjens Dimmlich, Vladimir Laptikhovsky, Dion Poncet and Vernon Steen). Support for travel to the Falkland Islands was provided by the Shackleton Scholarship Fund. Funding for the Shallow Marine Surveys Group Expedition work was provided by the the Overseas Territories Environment Programme (Grant FK501) and the Falkland Islands Government. Claire Goodwin was supported by additional funding from the Esmée Fairbairn Foundation/Scottish Natural Heritage and Countryside Council for Wales funded ‘Sponge Biodiversity of the UK’ project.





























