Introduction
Scleractinian corals are holobionts comprised of the coral host and associated bacteria, archaea, fungi, viruses and microalgal dinoflagellates known as Symbiodinium (Rohwer et al., Reference Rohwer, Seguritan, Azam and Knowlton2002). Corals build the framework of the reef, which supports a high biodiversity of species (Knowlton et al., Reference Knowlton, Brainard, Fisher, Moews, Plaisance and Caley2010). The ability of corals to build reef structures critically relies on their interaction with Symbiodinium, which provide much of their metabolic requirements (Muscatine & Porter, Reference Muscatine and Porter1977). However, this symbiosis is sensitive to environmental perturbations, which can cause bleaching due to the degeneration or expulsion of symbionts from the coral host (Jokiel, Reference Jokiel2004).
Many environmental triggers are known to induce coral bleaching, chief among which is rising seawater temperature associated with the changing global climate (Hoegh-Guldberg et al., Reference Hoegh-Guldberg, Poloczanska, Skirving and Dove2017; Hughes et al., Reference Hughes, Kerry, Álvarez-Noriega, Álvarez-Romero, Anderson, Baird, Babcock, Beger, Bellwood, Berkelmans, Bridge, Butler, Byrne, Cantin, Comeau, Connolly, Cumming, Dalton, Diaz-Pulido, Eakin, Figueira, Gilmour, Harrison, Heron, Hoey, Hobbs, Hoogenboom, Kennedy, Kuo, Lough, Lowe, Liu, McCulloch, Malcolm, McWilliam, Pandolfi, Pears, Pratchett, Schoepf, Simpson, Skirving, Sommer, Torda, Wachenfeld, Willis and Wilson2017). Increase in sea surface temperature (SST) may have contributed to the decline in coral cover within the Coral Triangle, a region in the Indo-Pacific with exceptional marine biodiversity (Carpenter et al., Reference Carpenter, Abrar, Aeby, Aronson, Banks, Bruckner, Chiriboga, Cortés, Delbeek, Devantier, Edgar, Edwards, Fenner, Guzmán, Hoeksema, Hodgson, Johan, Licuanan, Livingstone, Lovell, Moore, Obura, Ochavillo, Polidoro, Precht, Quibilan, Reboton, Richards, Rogers, Sanciangco, Sheppard, Sheppard, Smith, Stuart, Turak, Veron, Wallace, Weil and Wood2008). This region is known to experience pronounced increases in SST during phases of the El Niño Southern Oscillation (ENSO) (McPhaden et al., Reference McPhaden, Zebiak and Glantz2006), which can also trigger widespread coral bleaching.
Field and laboratory experiments confirm that corals exhibit different responses to thermal stress, with branching corals being more affected by bleaching events than massive corals (Marshall & Baird, Reference Marshall and Baird2000). These results were confirmed in a laboratory simulation experiment where branching corals, such as Acropora spp. and Pocillopora damicornis, exhibited greater susceptibility to bleaching at 32°C compared with massive corals, including P. decussata, P. lutea and Montipora sp. (Li et al., Reference Li, Yu and Shi2008). Variation in the bleaching response among colonies within a species can also arise. For example, colonies of the massive coral Porites from the Palm Islands in the central Great Barrier Reef were more susceptible to bleaching than were Porites from Magnetic Island during a 1998 bleaching event (Marshall & Baird, Reference Marshall and Baird2000). One factor that might contribute to these variable responses is the association of the coral host with Symbiodinium of different thermal tolerances (Rowan, Reference Rowan2004). Indeed, corals inhabiting areas with highly variable temperature, nutrient and light conditions are dominated by thermally tolerant clade D Symbiodinium (Howells et al., Reference Howells, Berkelmans, van Oppen, Willis and Bay2013; Keshavmurthy et al., Reference Keshavmurthy, Meng, Wang, Kuo and Yang2014; Silverstein et al., Reference Silverstein, Cunning and Baker2015).
Despite advances in the study of coral and microalgal symbiont responses to elevated temperature, corals from regions within the Coral Triangle remain underexplored. To address this deficiency, we examined the symbiont associates and individual thermal stress responses of six scleractinian species from the reefs of Bolinao, north-western Philippines.
Materials and methods
Coral collection and acclimatization
Three colonies each of Acropora digitifera, Acropora millepora, Acropora tenuis, Favites colemani, Montipora digitata and Seriatopora caliendrum were collected in November 2016 from various sites within the Bolinao-Anda Reef Complex where each coral genus is abundant: Acropora (16′17.287N 120′00.448E; ~5–9 m depth), Favites (16′18.623N 120′01.790E; ~3–5 m depth), Montipora (16′26.513N 119′56.494E; ~1–2 m depth) and Seriatopora (16′22.293N 120′00.228E; ~4–6 m depth). Based on regular monitoring by the Bolinao Marine Laboratory, sea surface temperatures within the reef complex range from 25–32°C with annual mean temperature of 28.89 ± 0.90°C. Sample collection was conducted with the permission of the Philippines Department of Agriculture Bureau of Fisheries and Aquatic Resources (DA-BFAR Gratuitous Permit No. 0102-15). Colonies at least 10–15 m apart horizontally were collected to minimize genotypic similarity, although validation of genotypes was not conducted. Corals were fragmented into 2.5–5.0 cm long nubbins (~70–80 fragments from each of 3 colonies per species) and pre-conditioned for 2 weeks in outdoor tanks with running seawater maintained at 28 ± 1°C and illumination under low photosynthetic photon flux density of ~80–90 µmol m−2 s−1 on a 12:12 light-dark cycle. Fragments were tagged to keep track of their colony of origin. Healed fragments were then allowed to acclimatize for 2 weeks in indoor experimental tanks with running seawater maintained at 28 ± 1°C and illumination of ~80 µmol m−2 s−1 on a 12:12 light-dark cycle.
Thermal stress experiments
Thermal stress experiments were conducted in 40 l tanks with constantly aerated, 10 µm-filtered flow-through seawater. Two independent replicate tanks were used for each temperature treatment. Seawater was pumped into each tank from chilled reservoirs maintained at 27°C and the temperature in individual experimental tanks was adjusted using submersible thermostat heaters. Flow rate was ~5–8 l h−1 and additional mixing within the tanks was provided by 600 l h−1 pumps. All setups received illumination under low photosynthetic photon flux density of ~80 µmol m−2 s−1 on a 12:12 light-dark cycle to avoid light stress. Light and temperature in each experimental tank was monitored using submersible loggers (Onset HOBO). Approximately 60–70 coral fragments (3–4 fragments from each of 3 colonies per species or a total of 10–12 fragments per species) were quickly transferred into each experimental tank set at either elevated temperature (32 ± 1°C, treatment) or ambient temperature (28 ± 1°C, control). Although this thermal shock treatment does not approximate what happens in nature, it allows investigation of the robustness of each species by emphasizing differences in acute temperature shock response, as has been done in other studies (Putnam et al., Reference Putnam, Edmunds and Fan2010; Meyer et al., Reference Meyer, Aglyamova and Matz2011; Barshis et al., Reference Barshis, Ladner, Oliver, Seneca, Traylor-Knowles and Palumbi2013; Parkinson et al., Reference Parkinson, Banaszak, Altman, LaJeunesse and Baums2015; Hou et al., Reference Hou, Xu, Su, Wu, Cheng, Wang, Zhou and Wang2018). Measurement of photochemical efficiency and collection of fragments for quantification of microalgal symbiont density were performed after 4, 24, 48 and 72 h of exposure.
Physiological measurements
Temperature stress in corals is readily detectable as a change in the photosynthetic fitness of associated algal symbionts measured using non-invasive pulse-amplitude modulated (PAM) fluorometry (Ralph et al., Reference Ralph, Hill, Doblin, Davy, Woodley, Downs, Bruckner, Porter and Galloway2015). The dark adapted photochemical efficiency (Fv/Fm) of coral fragments was measured using a diving PAM (Walz, Germany) at least 1 h after the onset of the dark phase of the light cycle. Two PAM measurements were taken from each of 5 coral fragments, taking care to include one to two randomly selected fragments from each of 3 colonies per species (N = 5 fragments per species per treatment). To quantify symbiont density, three coral fragments, one from each of 3 colonies per species (N = 3 fragments per species per treatment), were sacrificed at every sampling point and incubated for 1 h in 50 ml of 1 M NaOH to separate symbionts from the host tissues. The resulting cell suspension was spun at 4000 rpm for 15 min and the pellet washed twice with distilled water. Symbiont cells were resuspended in 30 ml distilled water before counting on a Neubauer hemocytometer (LeGresley & McDermott, Reference LeGresley, McDermott, Karlson, Cusack and Bresnan2010). Cell counts were taken four times for each coral fragment. The average cell count for each fragment was normalized to the total surface area of each fragment as determined using the wax dipping method (Veal et al., Reference Veal, Carmi, Fine and Hoegh-Guldberg2010).
DNA extraction, PCR and sequencing
DNA was extracted from symbionts dissociated from host tissues using the Hot-Shot method (Truett et al., Reference Truett, Heeger, Mynatt, Truett, Walker and Warman2000). PCR amplification of the internal transcribed spacer 2 (ITS2) region was performed using the primers ITSintFor2 and ITS2CLAMP (LaJeunesse & Trench, Reference LaJeunesse and Trench2000). PCR reactions contained 1 µl of crude DNA template, 1× PCR buffer, 1.5 mM MgCl2, 200 µM dNTP, 100 nM ITSintFor2, 300 nM ITS2CLAMP and 0.5 units of Taq DNA Polymerase (Invitrogen). PCR was carried out on a FlexCycler with a touchdown amplification protocol consisting of an initial denaturing step of 94°C for 3 min, 21 cycles of 94°C for 30 s, 62°C for 40 s and 72°C for 30 s with annealing temperature decreasing by 0.5°C at each cycle, followed by 15 cycles of 94°C for 30 s, 52°C for 40 s and 72°C for 30 s, and a final extension at 72°C for 10 min. PCR products were resolved on a 30–60% linear gradient of urea and formamide with 8% acrylamide (Sampayo et al., Reference Sampayo, Dove and Lajeunesse2009). Gels were run in 1× TAE at 60 V for 16 h at 60°C in a denaturing gradient gel electrophoresis (DGGE) apparatus (C.B.S. Scientific). After electrophoresis, the gel was stained with SYBR Gold nucleic acid stain in 1× TAE buffer, rinsed, and photographed. Distinct bands were excised from the gel and placed in 30 µl nuclease free water overnight to diffuse the DNA. The eluted DNA was re-amplified using the primers ITSintFor2 and ITSrev (LaJeunesse & Trench, Reference LaJeunesse and Trench2000) for direct sequencing at First Base, Malaysia. Sequences were deposited in NCBI under accession numbers MG662689–MG662692.
Phylogenetic analysis
Microalgal symbiont ITS2 sequences obtained from DGGE were aligned to selected sequences from GeoSymbio (Franklin et al., Reference Franklin, Stat, Pochon, Putnam and Gates2011) using MUSCLE (Edgar, Reference Edgar2004). Poorly aligned sequences were trimmed using Gblocks v0.91b (Talavera et al., Reference Talavera, Castresana, Kjer, Page and Sullivan2007). A total of 283 bp were retained after trimming. Phylogenetic analysis was conducted using maximum likelihood based on the Kimura 2-parameter model. Initial trees for the heuristic search were obtained automatically by applying neighbour-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with superior log-likelihood value. Phylogenetic analysis was conducted using MEGA6 with 1000 bootstrap replicates (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013).
Statistical analysis
Data were tested for homogeneity of variances using Levene's test and normality using the Kolmogorov–Smirnov test. Fv/Fm data did not pass normality tests, even after data transformations, and were thus subjected to the non-parametric Friedman repeated measures test to analyse changes in Fv/Fm through time per temperature treatment for each species. Differences in Fv/Fm between temperature treatments were examined using a Mann–Whitney U test for each species. The Kruskal–Wallis test was also conducted to examine the differences in Fv/Fm among species for each temperature treatment. Differences in microalgal density between temperature treatments across time points were analysed using repeated measures ANOVA (analysis of variance) for each species. Separate ANOVA tests were conducted to clearly see the difference in microalgal density between temperature treatments for each species. Significant ANOVA results were further tested using a post-hoc Tukey's HSD test to compare pairwise differences in microalgal density between temperature treatments or between time points. A Bonferroni correction was applied on P-values to account for the increase in statistical error in multiple comparisons (Zar, Reference Zar1984). To determine which species had a significant association between Fv/Fm and microalgal density, Spearman correlation tests were conducted. All tests were conducted in Statistica 6.
Results
Identification of microalgal symbiont ITS2 types
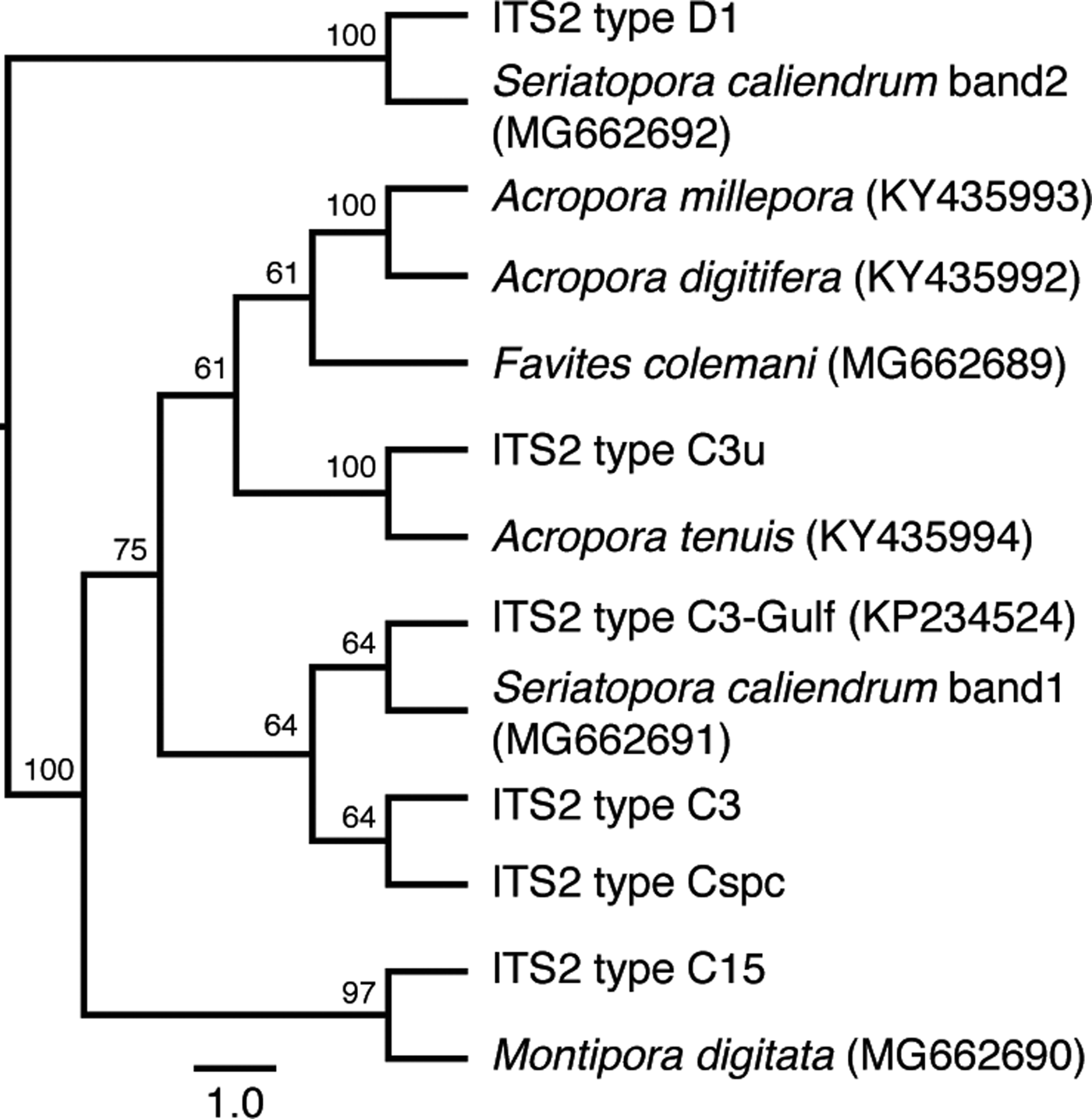
Sequencing of the major ITS2 DGGE bands from each coral species revealed that the dominant symbiont in the three acroporids and in F. colemani was most closely related to ITS2 type C3u (Figure 1). Montipora digitata hosted ITS2 type C15 while S. caliendrum hosted an ITS2 type similar to C3-Gulf (Hume et al., Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015) and D1. Multiple lighter bands appearing above the prominent bands in DGGE are likely to be heteroduplexes or artefacts of PCR (LaJeunesse, Reference LaJeunesse2002). No other phylotypes were identified using DGGE.
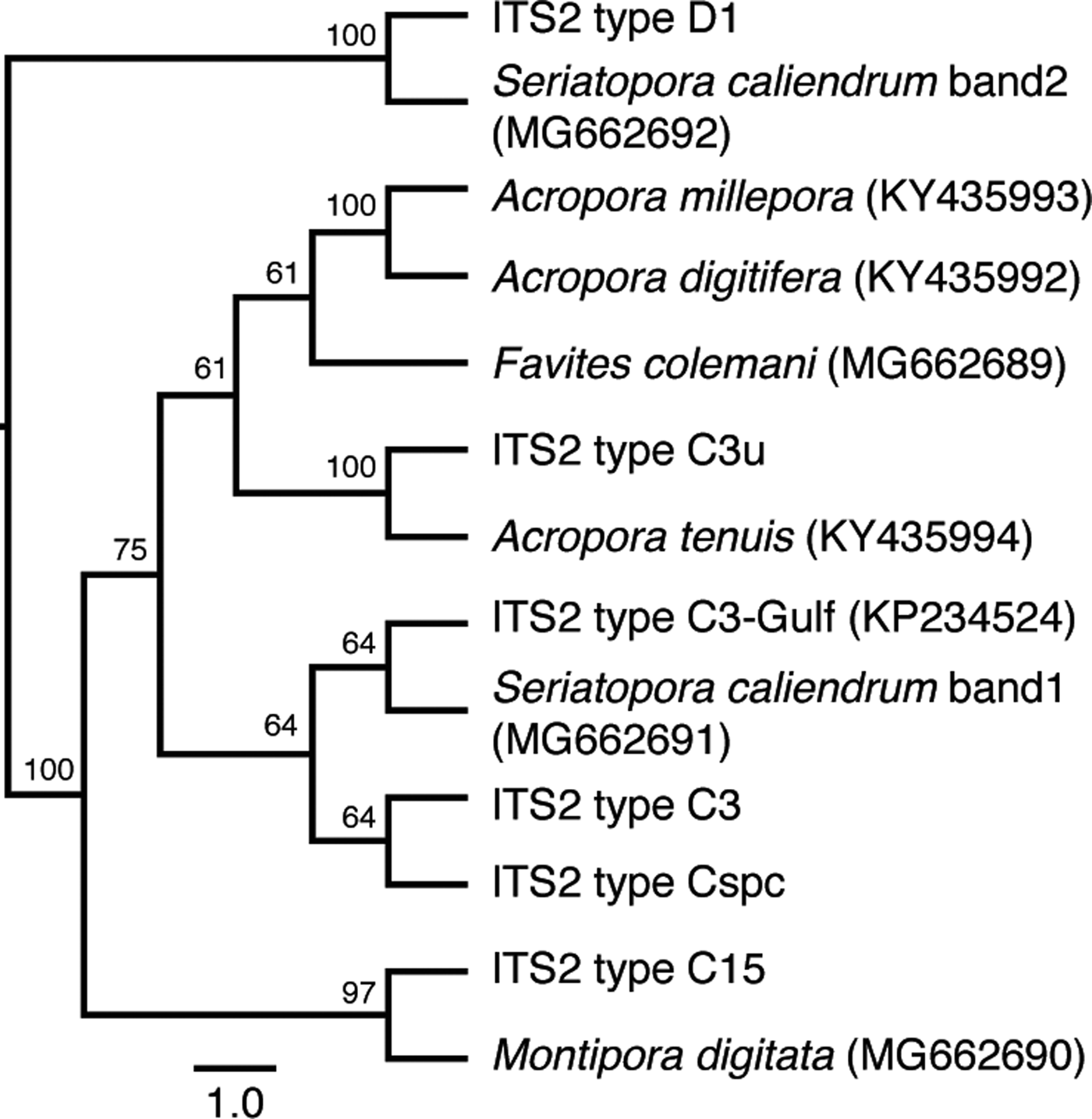
Fig. 1. Genetic identification of microalgal symbionts in colonies of six coral species at the Bolinao-Anda Reef Complex, Bolinao, Pangasinan, Philippines. Cladogram shows the sequence similarity of ITS2 bands from DGGE to known ITS2 types in GeoSymbio. NCBI accession numbers are shown in parentheses. Tree topology is based on maximum likelihood. Numbers on the branches indicate bootstrap values.
Effect of thermal stress on photochemical efficiency
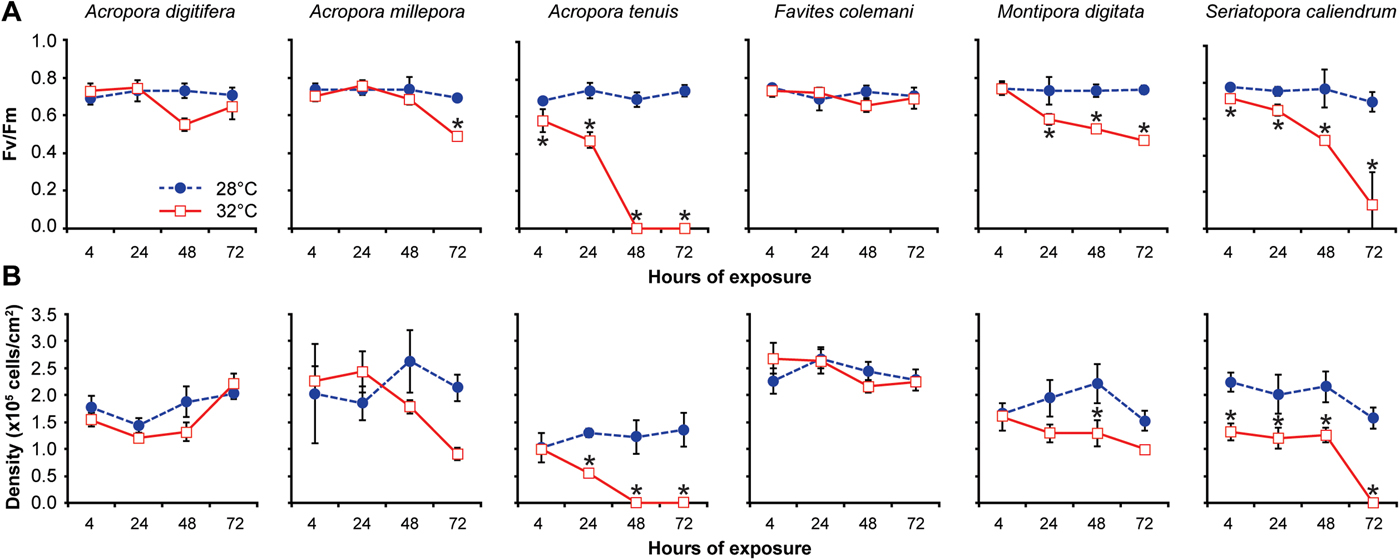
Coral fragments maintained at the control temperature exhibited a dark-adapted photochemical efficiency (Fv/Fm) of 0.72 ± 0.02 (mean ± SD) for all species (Figure 2A). Sustained exposure of corals to elevated temperature resulted in a significant reduction in Fv/Fm over several days for some species (Table 1). Acropora tenuis showed the most immediate response, with Fv/Fm values dropping to 0.47 ± 0.04 after 24 h and then to 0.0 after 48 h, when fragments exhibited complete bleaching (Figure 2A). Montipora digitata also showed a significant decrease in Fv/Fm, reaching 0.52 ± 0.06 in 24–72 h. Seriatopora caliendrum revealed a gradual decrease in Fv/Fm values, dropping to 0.48 ± 0.02 after 48 h and then to 0.13 ± 0.18 after 72 h (Figure 2A). For A. millepora, a significant decrease in Fv/Fm to 0.49 ± 0.02 was observed only at 72 h. Favites colemani and A. digitifera showed a decrease in Fv/Fm at 48 h, but this was not significant relative to controls and appeared to recover by 72 h (Figure 2A).
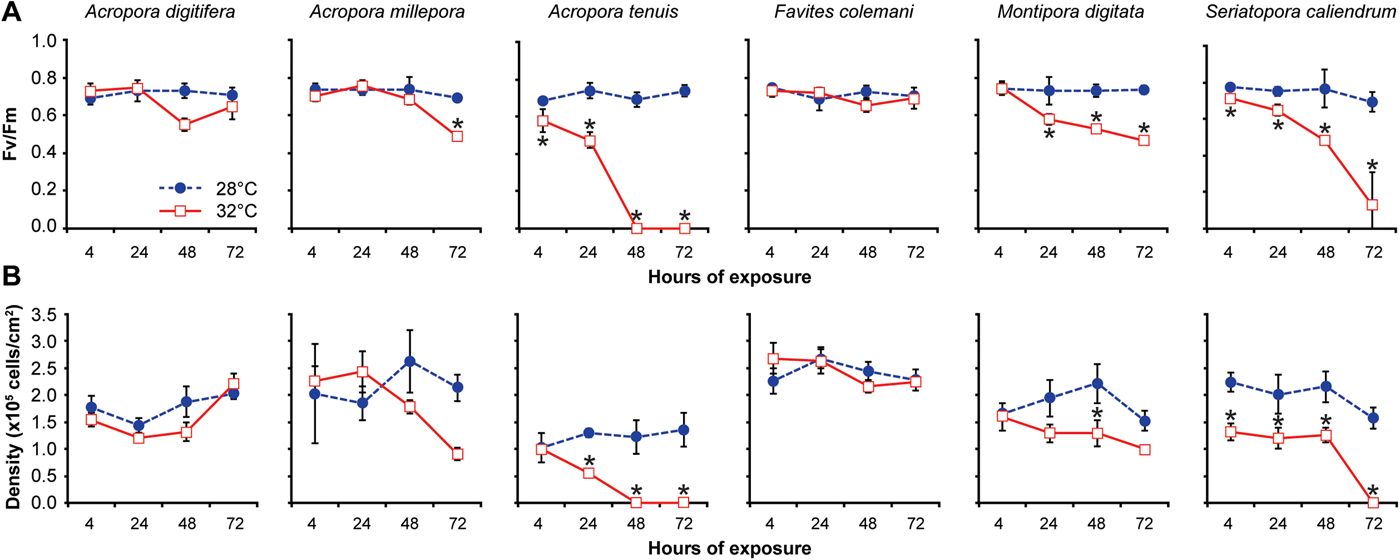
Fig. 2. Variation in the photochemical efficiency and density of microalgal symbionts among six coral species exposed to two treatments over 72 h. (A) Photochemical efficiency (Fv/Fm), and (B) Algal symbiont density (×105 cells per cm2 of coral surface area). Coral fragments of each species were subjected to a treatment temperature of 32°C (red-bordered squares) or a control temperature of 28°C (blue circles) for 4, 24, 48 and 72 h. Data are presented as means ± standard deviation of N = 3–5 coral fragments examined during each time period for each species. Asterisks indicate significant differences between treated (32°C) and control (28°C) values at each time point (Tukey's HSD test, P < 0.05).
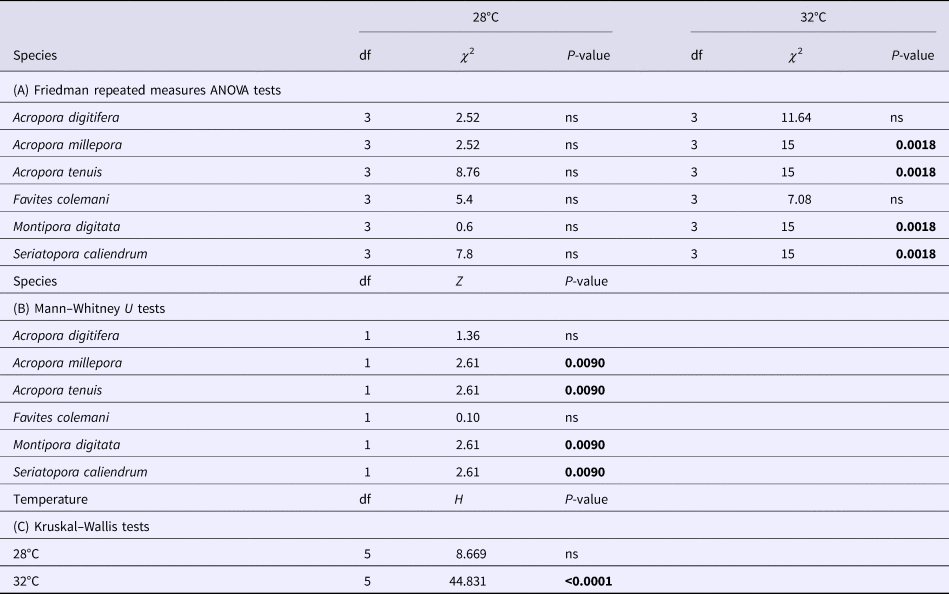
Table 1. Summary results of non-parametric tests of variation in photochemical efficiency (Fv/Fm) between two temperature treatments (28 and 32°C) across four time points (4, 24, 48, 72 h) in each of six coral species
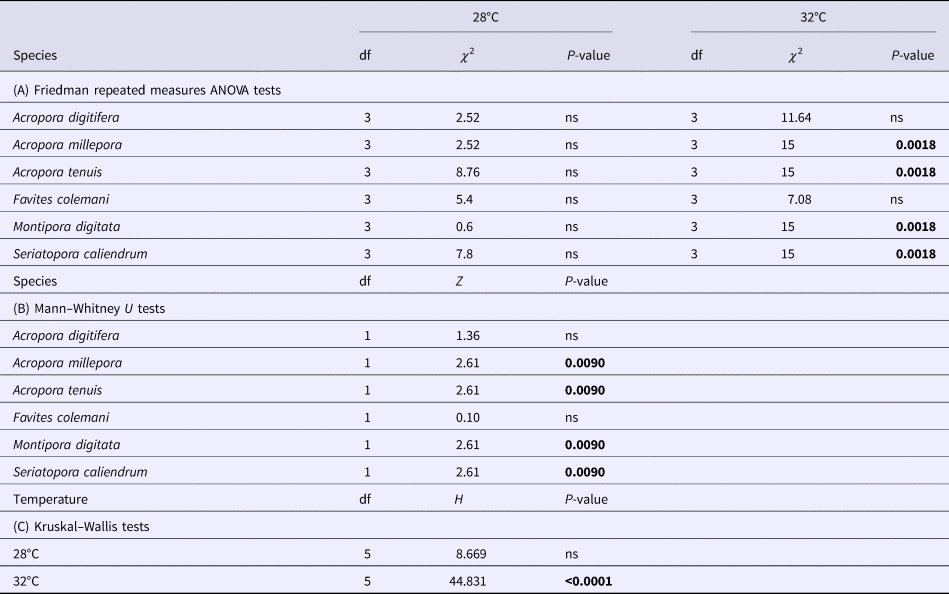
(A) Friedman repeated measures ANOVA tests of changes across time points (4, 24, 48 and 72 h) in each of the two temperature treatments (28 and 32°C) for each species. (B). Mann–Whitney U tests of differences between the two temperature treatments for each species at the 72 h time point. (C) Kruskal–Wallis tests of differences among the six species in each of the temperature treatments.
P-values in bold are significant at the 0.05 level after Bonferroni correction.
Effect of thermal stress on microalgal symbiont density
After the first 4 h at 28°C, the microalgal symbiont density already varied significantly among the six scleractinian coral species (one-way ANOVA: F = 334.6005; P < 0.001; Figure 2B). Acropora tenuis exhibited the lowest symbiont density, which differed significantly from that of F. colemani and S. caliendrum (Tukey's HSD test: pairwise comparisons between species, P < 0.05), but not from A. digitifera, A. millepora and M. digitata (Tukey's HSD test: pairwise comparisons between species, P > 0.05).
Prolonged exposure to elevated temperature resulted in a concomitant decrease in symbiont density for some coral species (Table 2). Acropora tenuis exhibited a significant reduction in symbiont density to a mean of 58% of that in the controls at 24 h (Tukey's HSD test: pairwise comparison between 28°C and 32°C, P < 0.05). This reduction in density was accompanied by visible bleaching, which progressed to a complete loss of symbionts by 48 h (Figure 2B). The symbiont density of A. millepora decreased to 57% of controls when exposed to elevated temperature, while M. digitata decreased to 34% of controls after 72 h, although both decreases were not significant (Figure 2B). Seriatopora caliendrum, on the other hand, retained a mean symbiont density of only 42% of controls after 4–48 h of elevated temperature exposure, which was significantly lower than that of the controls at all time points (Tukey's HSD test: pairwise comparisons between hours of exposure, P < 0.05; Figure 2B). Seriatopora caliendrum did not exhibit a sharp decline in symbionts until the 72 h timepoint, upon which bleaching was observed. In contrast, F. colemani and A. digitifera did not show a significant reduction in symbiont density from the control values, even after 72 h of exposure to elevated temperature (Tukey's HSD test: pairwise comparisons between hours of exposure, P > 0.05; Figure 2B).
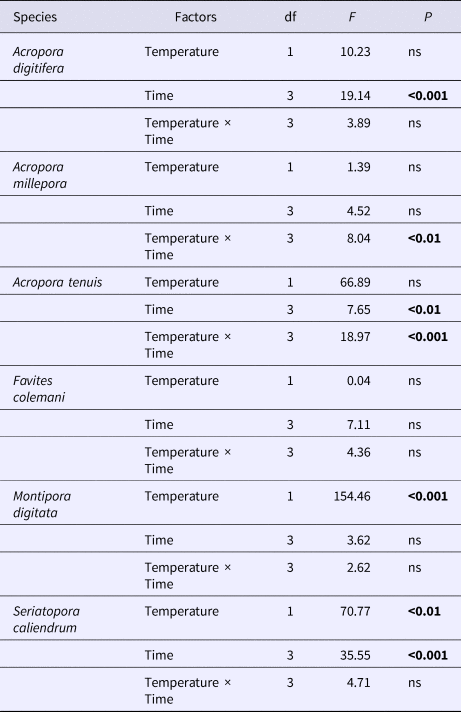
Table 2. Summary results of repeated measures ANOVA of microalgal symbiont density between two temperature treatments (28 and 32°C) across four time points (4, 24, 48 and 72 h) for each of six coral species
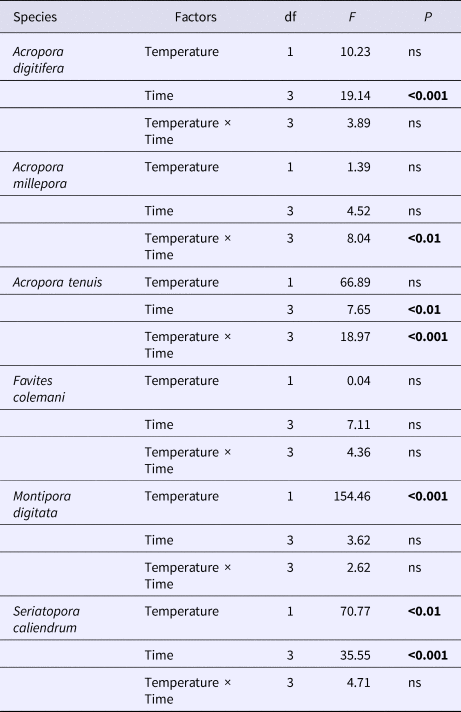
P-values in bold are significant at the 0.05 level after Bonferroni correction.
Discussion
Our results indicate that coral species from various sites around the Bolinao-Anda Reef Complex, north-western Philippines, vary in terms of the dominant microalgal symbiont type present within their tissues. The three acroporids and F. colemani hosted ITS2 type C3u, a host generalist that has also been detected in other corals in the western and north-eastern Indian Ocean (LaJeunesse et al., Reference LaJeunesse, Pettay, Sampayo, Phongsuwan, Brown, Obura, Hoegh-Guldberg and Fitt2010). Montipora digitata hosted ITS2 type C15, which is the same as the symbiont identified in M. digitata from other sites (Fisher et al., Reference Fisher, Malme and Dove2012; Krueger et al., Reference Krueger, Hawkins, Becker, Pontasch, Dove, Hoegh-Guldberg, Leggat, Fisher and Davy2015). Seriatopora caliendrum hosted two thermotolerant ITS2 types, C3-Gulf and D1. ITS2 type C3-Gulf was first detected in Red Sea corals (Hume et al., Reference Hume, D'Angelo, Smith, Stevens, Burt and Wiedenmann2015), while D1 has been found in corals that experience a high cumulative frequency of thermal stress anomalies (Stat & Gates, Reference Stat and Gates2011). These findings suggest that the examined corals may select for specific types of symbionts (Yamashita et al., Reference Yamashita, Suzuki, Kai, Hayashibara and Koike2014). It should be noted, however, that it is possible for corals to host other rare symbiont types that may contribute to the overall holobiont response but may not have been detected by DGGE.
Interspecies differences in thermal stress response were observed. Most of the corals were able to withstand sustained exposure to high temperature for up to 72 h, except for the thin branching corals, A. tenuis and S. caliendrum. These two corals showed the greatest reduction in photochemical efficiency and symbiont density, with bleaching after 48–72 h. Montipora digitata and A. millepora showed a less drastic reduction in photochemical efficiency and density, while A. digitifera and F. colemani were the least affected by sustained exposure to high temperature. Taking into consideration both photochemical efficiency and symbiont density changes over prolonged exposure to elevated temperature, the scleractinian corals of the Bolinao-Anda Reef Complex may be ranked in terms of decreasing thermal susceptibility in the following order: A. tenuis > S. caliendrum > M. digitata ≥ A. millepora>A. digitifera > F. colemani.
Interestingly, the temperature responses of the corals could not be directly correlated with the identity of their associated symbionts, nor with the morphology of the coral colony. The association of M. digitata with ITS2 type C15 was robust to heating, as has been reported by other studies (Fisher et al., Reference Fisher, Malme and Dove2012; Krueger et al., Reference Krueger, Hawkins, Becker, Pontasch, Dove, Hoegh-Guldberg, Leggat, Fisher and Davy2015). In contrast, the presence of two thermotolerant lineages in S. caliendrum did not translate to greater tolerance. This suggests that other factors may contribute to the overall response of the coral holobiont.
Amongst the corals hosting ITS2 type C3u, A. tenuis and A. millepora exhibited greater susceptibility to thermal stress compared with A. digitifera and F. colemani. In general, fast-growing branching corals are thought to be more susceptible to bleaching compared with slower-growing massive corals. This may be attributed to the accumulation of more harmful oxygen free radicals in organisms with higher metabolic rates (Lesser, Reference Lesser1997; Baird & Marshall, Reference Baird and Marshall2002). Variability in bleaching susceptibility has been reported in acroporid corals, which could be related to differences in the growth rate of the different branching morphologies (Dornelas et al., Reference Dornelas, Madin, Baird and Connolly2017; Hoogenboom et al., Reference Hoogenboom, Frank, Chase, Jurriaans, Álvarez-Noriega, Peterson, Critchell, Berry, Nicolet, Ramsby and Paley2017).
The examined coral species with finer branches, such as A. tenuis and S. caliendrum, were most susceptible to thermal stress compared with the other branching corals, A. millepora, M. digitata and A. digitifera, or to the massive coral, F. colemani. These differences may be due to differences in host morphology, tissue thickness and light scattering properties (Enríquez et al., Reference Enríquez, Méndez and Prieto2005). In general, acroporids and other branching corals have thinner tissues compared with massive corals (Loya et al., Reference Loya, Sakai, Yamazato, Nakano, Sambali and van Woesik2001). Thick tissue layers can expand and contract to provide a rapid and flexible means of regulating radiant flux and protecting symbionts via self-shading (Loya et al., Reference Loya, Sakai, Yamazato, Nakano, Sambali and van Woesik2001; Coles & Brown, Reference Coles and Brown2003). Thicker tissues may also translate to a higher density of microalgal symbionts, as observed for F. colemani, which may contribute to self-shading of symbiont cells and protection from bleaching. Studies on sea anemones and corallimorpharians further support the photoprotective role of thicker host tissues (Kuguru et al., Reference Kuguru, Winters, Beer, Santos and Chadwick2007; Dimond et al., Reference Dimond, Holzman and Bingham2012).
The Bolinao-Anda Reef Complex in north-western Philippines faces the South China Sea and is an area that experiences high temperature variation (Peñaflor et al., Reference Peñaflor, Skirving, Strong, Heron and David2009). Thus, it is likely that the corals that thrive here have adapted to recurrent thermal stress in the area (Yu, Reference Yu2012). Remarkably, M. digitata, which normally thrives on shallow reef flats that experience extreme temperature variation, exhibited greater tolerance to prolonged exposure to elevated temperature compared with the other corals with fine branching morphology. Its frequent exposure to high temperature and high light intensity in its shallow habitat may have contributed to this tolerance to sustained stress (Yap et al., Reference Yap, Espita, Montaño, Benjamin and Gomez2014). Other researchers have similarly demonstrated that corals grown at high temperature survive heat stress better than those grown at lower temperature (Middlebrook et al., Reference Middlebrook, Hoegh-Guldberg and Leggat2008; Howells et al., Reference Howells, Berkelmans, van Oppen, Willis and Bay2013). Pre-conditioning or exposure of corals to slow warming rates also leads to overall differences in the severity of bleaching responses exhibited during thermal stress events (Brown et al., Reference Brown, Dunne, Goodson and Douglas2002; Middlebrook et al., Reference Middlebrook, Hoegh-Guldberg and Leggat2008; Bellantuono et al., Reference Bellantuono, Hoegh-Guldberg and Rodriguez-Lanetty2011). This prior exposure may stimulate protective mechanisms, such as increased concentrations of certain pigments (Salih et al., Reference Salih, Larkum, Cox, Kühl and Hoegh-Guldberg2000; Dove et al., Reference Dove, Lovell, Fine, Deckenback, Hoegh-Guldberg, Iglesias-Prieto and Anthony2008; Middlebrook et al., Reference Middlebrook, Hoegh-Guldberg and Leggat2008), UV-protective compounds (Yakovleva et al., Reference Yakovleva, Bhagooli, Takemura and Hidaka2004; Baird et al., Reference Baird, Bhagooli, Ralph and Takahashi2009), enzymatic antioxidants (Richier et al., Reference Richier, Furla, Plantivaux, Merle and Allemand2005; Lesser, Reference Lesser2006), or expression of heat shock proteins (Rodriguez-Lanetty et al., Reference Rodriguez-Lanetty, Harii and Hoegh-Guldberg2009; DeSalvo et al., Reference DeSalvo, Sunagawa, Fisher, Voolstra, Iglesias-Prieto and Medina2010; Traylor-Knowles et al., Reference Traylor-Knowles, Rose, Sheets and Palumbi2017), which help reduce bleaching susceptibility.
Previous studies that used a short thermal shock regime to assay coral responses to thermal stress also reported variable temperature tolerance in different coral species. For example, P. damicornis and S. hystrix from Nanwan Bay in southern Taiwan did not exhibit significant changes in Fv/Fm after 12 h of exposure to thermal shock at 30, 28 and 21°C (Putnam et al., Reference Putnam, Edmunds and Fan2010). On the other hand, Galaxea fascicularis from Luhuitou, Hainan, China, showed symbiont density reduction, bleaching and tissue lysis after just 10 h at 32°C (Hou et al., Reference Hou, Xu, Su, Wu, Cheng, Wang, Zhou and Wang2018). A rapid increase in temperature from 29 to 34°C over 3 h also resulted in some bleaching in A. nana from Ofu Island in American Samoa (Bay & Palumbi, Reference Bay and Palumbi2015). Clearly, the responses of corals to stress are affected by many factors, such as host species, type of symbiont, coral colony morphology and environment, among others. Recent studies are also beginning to uncover variations in physiological response that may be governed by genotypic differences in corals hosting the same types of symbiont (Parkinson et al., Reference Parkinson, Banaszak, Altman, LaJeunesse and Baums2015).
The findings from these studies and others suggest that corals may have a multistage process for acclimation, with a rapid response mechanism to manage acute heat stress, which disrupts proteostasis and induces expression of protein folding chaperones and proteases (Oakley et al., Reference Oakley, Durand, Wilkinson, Peng, Weis, Grossman and Davy2017), as well as a slower response that changes background gene expression and physiological function to anticipate future stress events (Bay & Palumbi, Reference Bay and Palumbi2015). Thus, corals that regularly encounter thermal fluctuations in their natural habitat may already have higher baseline expression of transcripts encoding the proteins required for cellular repair and homeostasis (Barshis et al., Reference Barshis, Ladner, Oliver, Seneca, Traylor-Knowles and Palumbi2013), as well as gene regulatory mechanisms that allow the coral to mount a rapid cellular response in the event of acute thermal stress (Gajigan & Conaco, Reference Gajigan and Conaco2017). It would therefore be of interest to determine the gene expression dynamics that accompany the variable physiological responses of corals to environmental stress. Further investigations are also warranted to reveal how complementation between different coral host species and symbiont types contributes to the susceptibility or tolerance of the holobiont to environmental stressors.
In summary, we have shown differences in the response of different coral species to thermal stress through the use of photochemical efficiency and symbiont density as indicators of bleaching susceptibility. We also provide baseline information on the symbiont types present in common corals in this region. The findings highlight the role of the host in coral bleaching susceptibility and provide insights into how coral populations in the Coral Triangle region might respond to recurring thermal stress events.
Author ORCIDs
Jeric P. Da-Anoy, 0000-0002-9342-7200
Acknowledgements
The authors extend their gratitude to the staff of the Bolinao Marine Laboratory for assistance with coral collection and experiments.
Financial support
This study was funded by a research grant from the Department of Science and Technology Philippine Council for Agriculture, Aquatic, and Natural Resources Research and Development (DOST-PCAARRD) to CC. JPD was supported, in part, by a thesis grant from the University of the Philippines Marine Science Institute.