INTRODUCTION
The invertebrate marine communities include thousands of species that play important roles in determining biodiversity patterns (Hendrickx et al., Reference Hendrickx, Brusca, Cordero and Ramírez2007). The tropical communities are recognized as the most diverse but, paradoxically, they are also the least understood (Brehm et al., Reference Brehm, Homeier and Fiedler2003) because studies of diversity at large spatial scales have rarely been carried out in these regions. This is clear in the Mexican Pacific coasts, where the regional distribution patterns of invertebrates, especially polychaetes, have seldom been studied (Brusca, Reference Brusca1980; Hernández-Alcántara et al., Reference Hernández-Alcántara, González-Ortiz and Solís-Weiss1994, Reference Hernández-Alcántara, Salas-de León, Solís-Weiss and Monreal-Gómez2013, Reference Hernández-Alcántara, Salas-de León, Solís-Weiss and Monreal-Gómez2014; Hernández-Alcántara & Solís-Weiss, Reference Hernández-Alcántara and Solís-Weiss1999, Reference Hernández-Alcántara and Solís-Weiss2005). However, before more irreversible damage occurs due to climate change and other anthropogenic factors, biodiversity studies in tropical ecosystems are necessary to understand better those marine environments and generate appropriate conservation programmes and strategies for the sustainable exploitation of natural resources.
The gradients in species richness have been extensively studied because of their significance in understanding ecological and evolutionary processes (Colwell et al., Reference Colwell, Rahbek and Gotelli2004). In particular, the latitudinal gradient of diversity is one of the most conspicuous patterns, and the decreasing number of species from low (equator) to high (poles) latitudes is well documented for terrestrial taxa, but these latitudinal changes appear to be less consistent in the marine environments (Gray, Reference Gray1997, Reference Gray2002; Currie & Kerr, Reference Currie and Kerr2008; Hernández-Alcántara et al., Reference Hernández-Alcántara, Salas-de León, Solís-Weiss and Monreal-Gómez2013).
The Gulf of California is well known as one of the marine regions with the highest biodiversity worldwide (Roberts et al., Reference Roberts, McClean, Veron, Hawkins, Allen, McAllister, Mittermeier, Schueler, Spalding, Wells, Vynne and Werner2002), including almost 5000 macroinvertebrate species (Brusca & Findley, Reference Brusca, Findley, Hendrickx, Brusca and Findley2005). Its significant length (over 1000 km) and orientation (roughly from north to south) allows a meaningful analysis of the diversity patterns along a cline of 9° latitude of many animal groups, in this case, the polychaetes. The polychaetes are one of the most abundant and diverse macroinvertebrate groups worldwide, from the intertidal zone down to the deep sea, and are an essential component in structuring the benthic communities (Mackie & Oliver, Reference Mackie, Oliver and Hall1996).
An essential condition to examine variations of biodiversity, is integrating the existing information on species distribution in a determined region (Carr, Reference Carr2012). Up to now, nearly 860 species of polychaetes have been reported from the Gulf of California. However, their latitudinal variation of diversity is still far from being understood. Despite their high diversity, the study of ecological and biogeographic issues regarding them has been hampered by the lack of information on the local distribution of species, which is essential to understand the mechanisms and processes that structure their diversity patterns. Likewise, the seasonal changes of the polychaete fauna in the Gulf of California have been poorly explored: for example, in the study of one of the most abundant polychaete groups in the Mexican Pacific, the Spionida (Hernández-Alcántara & Solís-Weiss, Reference Hernández-Alcántara and Solís-Weiss2005), it was shown that the species distribution varied seasonally, mainly due to temperature changes. For this reason, in this study we present a first estimate on the spatial variation of density, number of species and species turnover of polychaetes along the soft bottoms of the eastern and south-western end coasts of the Baja California Peninsula. The spatial trends were examined in two ways: (1) by evaluating the patterns of density and species richness, and defining the species assemblages formed by the polychaetes collected in the course of three oceanographic expeditions; and (2) by characterizing the latitudinal variation of the species composition using beta-diversity, with bands of 1° latitude, building a comprehensive dataset which includes the distribution records of all polychaete species reported in published taxonomic and ecological surveys.
MATERIALS AND METHODS
Study area
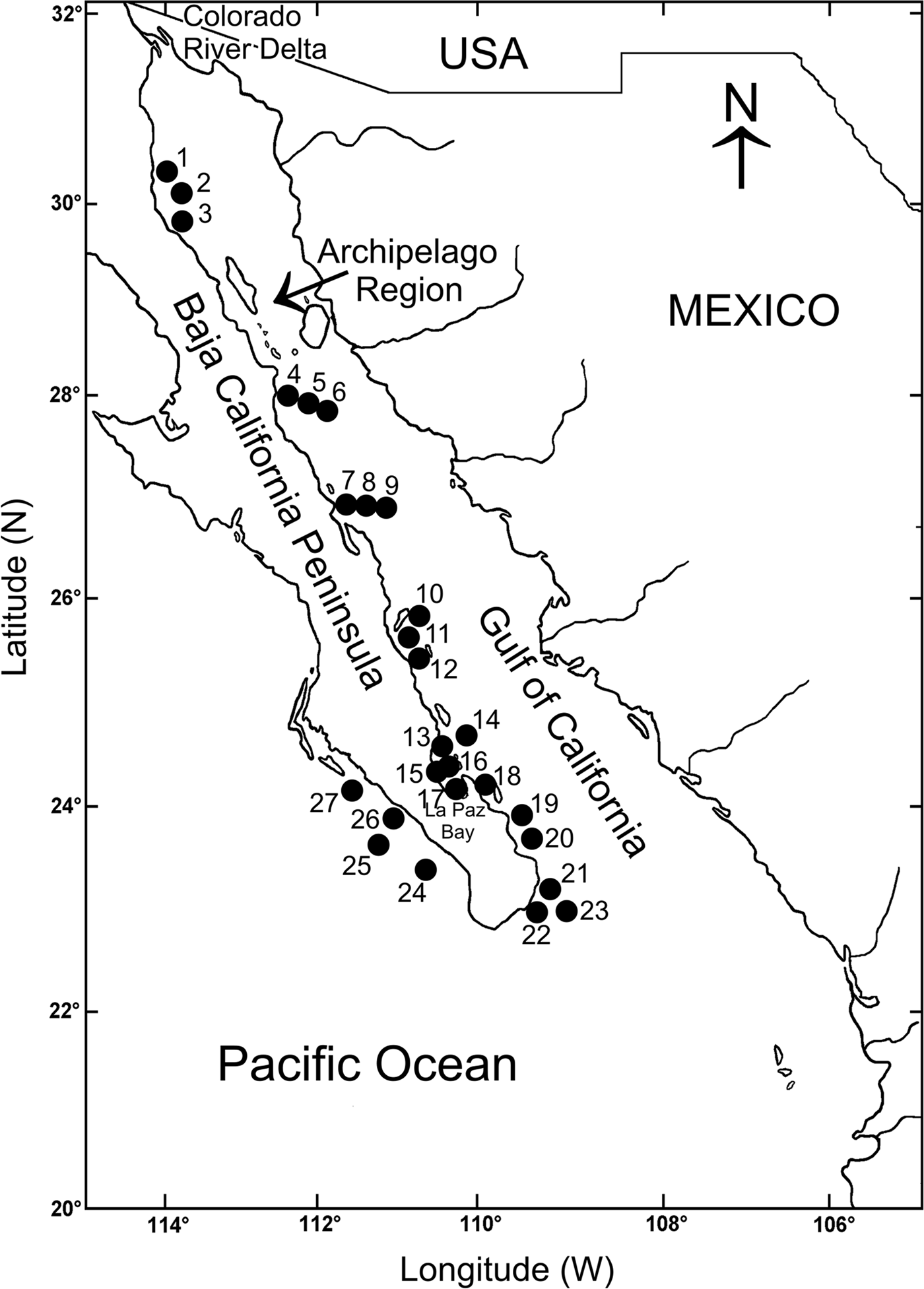
The Baja California Peninsula (23°–32°N 109°–115°W) is one of the longest in the world, around 1200 km long. It separates the Gulf of California from the Pacific Ocean (Brusca & Findley, Reference Brusca, Findley, Hendrickx, Brusca and Findley2005) (Figure 1). The western peninsular coasts are under the influence of the California Current, which is formed by a superficial current flowing southward, roughly following the coastline, and a sub-superficial counter current, which occasionally reaches the surface, giving the region a mostly warm-temperate climate (Parés-Sierra et al., Reference Parés-Sierra, López, Pavía and Lavín1997). The movement of northern waters southwards makes the coastal waters cooler than those located inside the Gulf of California at comparable latitudes. The eastern peninsular coasts are located inside the Gulf of California, which is a semi-closed sea, north-west–south-east oriented, more than 1100 km long and about 200 km wide on average (Figure 1). The circulation and thermohaline structure in the whole Gulf is the result of the seasonality of the main forcing agents, including the geostrophic circulation of the eastern tropical Pacific, the wind system and the heat and moisture fluxes (Lavín et al., Reference Lavín, Beier, Badan and Lavín1997). Due to this seasonal pattern, the variation in temperature, salinity, water masses and stored heat defines two main hydrodynamic seasons, winter-spring (November to May) and summer-autumn (June to October) (Lavín et al., Reference Lavín, Beier, Badan and Lavín1997). In winter-spring the surface circulation displays an anticyclonic gyre and a net surface outflow from the north through the archipelago, which is compensated by a permanent inflow close to the bottom on the western coast (Lavín & Marinone, Reference Lavín, Marinone, Velasco Fuentes, Sheimbaum and Ochoa2003).
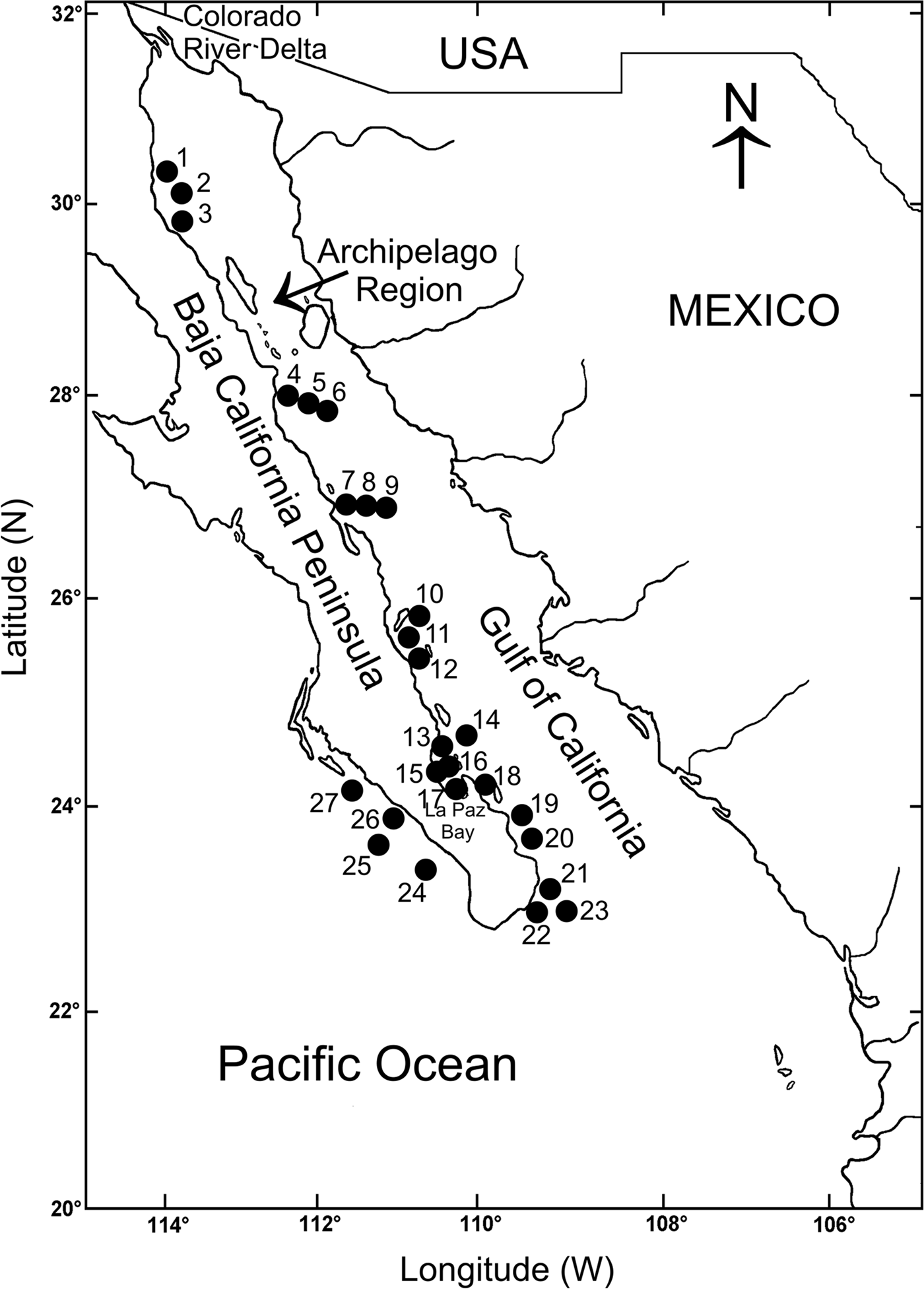
Fig. 1. Location of the study area showing the sampling stations on the Baja California Peninsula shelf.
Data sources
A database was built with information on the distribution and abundance of the polychaetes collected exclusively on soft bottoms from the continental shelf of the eastern and at the south-western end of the Baja California Peninsula. We selected 27 stations sampled during three oceanographic expeditions, entirely carried out during the winter-spring season (expedition Cortes 2: March 1985; expedition Mazcab 3: May 2001; expedition GC06–1: May 2006). The stations were exclusively sampled on soft bottoms, two or three replicates were taken at each station from expedition Cortes 2 with a Smith-McIntyre grab (0.1 m2), and with a Reineck box-core (0.042 m2) during the expeditions Mazcab 3 and GC06–1. The scarce number of studies on the ecology of the polychaetes in the Tropical Eastern Pacific has limited the knowledge on the latitudinal patterns of diversity of this abundant and diverse group of invertebrates. However, the samples taken during different scientific expeditions, and their geographic position, prompted us to try and carry out a first estimate on the latitudinal changes in the number of species along the littoral region of the Baja California Peninsula.
At each station depth was recorded and temperature, salinity and dissolved oxygen were measured close to bottom. Temperature and salinity were measured with a Neil Brown CTD, and dissolved oxygen was calculated by the Winkler method (Strickland & Parsons, Reference Strickland and Parsons1977).
The collected sediments were washed through a 0.5 mm mesh to separate the macrofauna, following standard methodology; the biological material was fixed in 4% formaldehyde and preserved in alcohol at 70%. The polychaetes were identified to species, catalogued and deposited in the Colección Nacional de Anélidos Poliquetos at the Instituto de Ciencias del Mar y Limnología (ICML) and Universidad Nacional Autónoma de México (UNAM) (CNAP–ICML, UNAM; DFE.IN.061.0598).
Additionally, and with the goal of examining the latitudinal changes in the species composition at a landscape scale, we complemented the list of polychaetes identified by us with an exhaustive compilation of all species reported in taxonomic and ecological studies from 1901, when Gravier (Reference Gravier1901) recorded the first polychaete from the Gulf of California (as Platynereis polyscalma (Chamberlin, Reference Chamberlin1919)). The compiled information was taken from primary data sources and the database was built incorporating the geographic position of all species distributed on the continental shelf. The validity of names and synonymies of all species were also verified with recent systematic reviews and with the World Polychaeta database (Read & Fauchald, Reference Read and Fauchald2016). The correct taxonomic identification of specimens and their geographic position are important to know their spatial distribution, but in the Baja California Peninsula littoral areas, the species reported in the literature cannot be taxonomically re-examined. So, several taxa might need to be confirmed, for example if the name used is one of a species described from very distant areas or different habitats. In those cases, the records were regarded as doubtful and they were not included in the diversity analysis.
Data analysis
For each station, the average density per species (ind. 0.1 m−2) was assessed and its variation throughout the study area was examined. To detect if any faunal spatial pattern was present in the study area, an analysis of the resemblance among the sampling sites, based on their composition and density of species, was carried out using a non-metric multidimensional scaling method (nMDS) with the Bray–Curtis similarity index and a four-root data transformation was applied to reduce the contribution of abundant species (Clarke et al., Reference Clarke, Gorley, Somerfield and Warwick2014; Clarke & Gorley, Reference Clarke and Gorley2015). The faunal groups were significantly separated by the ‘similarity profile’ (SIMPROF) permutation test, which accompanies the clustering method, defining group structures for which there is some statistical support. That is, it looks for statistically significant evidence of internal group structure in the full set of samples that are submitted to it (1000 permutations; P < 0.1%), under the assumption that the samples are a priori unstructured (Clarke & Gorley, Reference Clarke and Gorley2015). A shade plot helped us to identify the species contributing to differences among the well-defined groups, which showed the distribution of density of the 35 more important species. All previous analyses were carried out using the software Plymouth Routines in Multi-Variate Ecological Research (PRIMER v.7) (Clarke & Gorley, Reference Clarke and Gorley2015).
The terms associated with diversity and their spatial scales have been used in different ways in the literature and have quite often been misunderstood (Halffter & Moreno, Reference Halffter, Moreno, Halffter, Soberón, Koleff and Melic2005); thus, it is important to indicate that in this study the diversity is expressed as the number of species per station. However, as the number of samples varied among stations, individual-based rarefaction curves were estimated to a common number of 100 individuals per station with the Sanders (Reference Sanders1968) modified by Hurlbert (Reference Hurlbert1971) method. This technique provides strong estimates of species richness when the number of samples is different or they have different sizes (Sanders, Reference Sanders1968; Hurlbert, Reference Hurlbert1971; Gotelly & Colwell, Reference Gotelly and Colwell2001; Colwell et al., Reference Colwell, Rahbek and Gotelli2004). To assess the latitudinal trends and the differences among stations along the Baja California Peninsula shelf, the number of species was correlated with the environmental parameters.
To compare the species richness values among the faunal groups detected, a one-way analysis of similarity (ANOSIM) was carried out, testing the null hypothesis that there are no differences among the assemblages (Clarke & Gorley, Reference Clarke and Gorley2015). The degree of separation of the groups was estimated in a range of R = 0 (groups indistinguishable from one another) to R = 1 (no similarity between groups) (Clarke, Reference Clarke1993). Models of linear regression (P < 0.05) were used to evaluate the relationships among the hydrographic parameters and the latitudinal changes along the coastal Baja California Peninsula.
The changes in species composition (beta-diversity) at landscape scale were based on a comprehensive compilation of all polychaete species reported in the study area. The aim of this section was to assess the species turnover in the Baja California Peninsula shelf, but a previous estimation of the total number of species likely to occur in this marine area is important to support the analysis on the latitudinal changes in species composition. The process to determine the total number of species from primary data sources can be different according to the characteristics of the study area, but also through the attributes of the database (Soberón et al., Reference Soberón, Jiménez, Golubov and Koleff2007). In the Baja California Peninsula coasts the data were compiled from different sources, which included all types of samples. This could bias the trends detected in the turnover species. To reduce the influence of these differences in sampling, we only compiled information on species distributed on soft bottoms but also, due to the irregular and different collection efforts carried out in the primary sources, we estimated the number of species partitioning the geographic region in bands of 1° of latitude. We then assessed the total richness with the ICE and Chao2 procedures (Colwell & Coddington, Reference Colwell and Coddington1994; Lee & Chao, Reference Lee and Chao1994; Soberón et al., Reference Soberón, Jiménez, Golubov and Koleff2007). Many methods have been proposed to estimate ‘true values’ from sampled primary data (Soberón et al., Reference Soberón, Jiménez, Golubov and Koleff2007). Among them, it has been shown that the ICE estimator is relatively unaffected by the sample size (Chazdon et al., Reference Chazdon, Colwell, Denslow, Guariguata, Dallmeir and Comiskey1998; Soberón et al., Reference Soberón, Jiménez, Golubov and Koleff2007), but also that the Chao2 estimator can be better in terms of bias and accuracy (Walther & Moore, Reference Walther and Moore2005). Therefore, we used both indicators to compare their predictions on our heterogeneous database. These estimators were computed with the software EstimateS v.9.1.0 (Colwell, Reference Colwell2013).
The latitudinal changes in the species composition along the study area was evaluated with the β T-diversity index proposed by Wilson & Schmida (Reference Wilson and Schmida1984) (β T = (g(H) + l(H))/2α), where g(H) is the number of species gained along the gradient H, l(H) is the number of species lost along H, and α is the average number of species found within the samples. This beta index used the presence/absence of species to measure the species replacement, calculating the species newly encountered (gain) or lost along latitudinal bands of 1°. Values of β T-diversity close to 1 indicate larger differences in the composition of species between two compared latitudinal bands (Wilson & Schmida, Reference Wilson and Schmida1984; Gray, Reference Gray2000; Hernández-Alcántara, Reference Hernández-Alcántara2002).
To detect whether the variation in beta-diversity was essentially a property of the compiled dataset, due to sampling effort differences among the latitudinal bands, we re-calculated the beta-diversity separating the species located in three or more latitudinal bands and those species occurring only on 1 or 2 bands. In the latitudinal bands with lower sampling intensity, the species with small distribution intervals should be less represented than those species located all along the study area, modifying the original beta-diversity pattern.
RESULTS
Composition and number of species at local scale
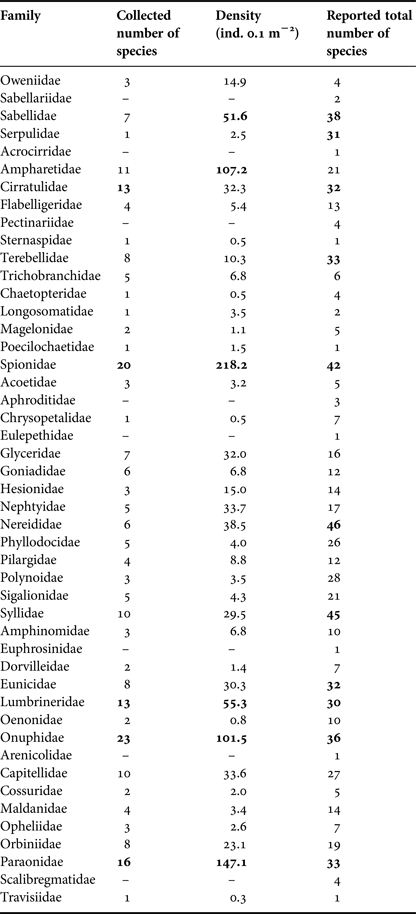
In all, 2858 specimens belonging to 39 families and 231 species of polychaetes were collected and identified. The families Onuphidae, Spionidae, Paraonidae, Cirratulidae and Lumbrineridae represented 36.8% of all the polychaete fauna, with more than 12 species each. The first four families were also the most abundant, accounting for almost half of all individuals (47.8%) with values above 100 ind. 0.1 m−2 each while the Lumbrineridae had only 55.3 ind. 0.1 m−2. On the other hand, 23 families (60.5%) were represented by fewer than five species each, and 23 families had less than 15 ind. 0.1 m−2 each (Table 1).
Table 1. Species richness and average density (ind. 0.1 m−2) per family across the sampling stations, and total number of species of all polychaetes reported from the Baja California Peninsula shelf. The highest values are indicated in bold.
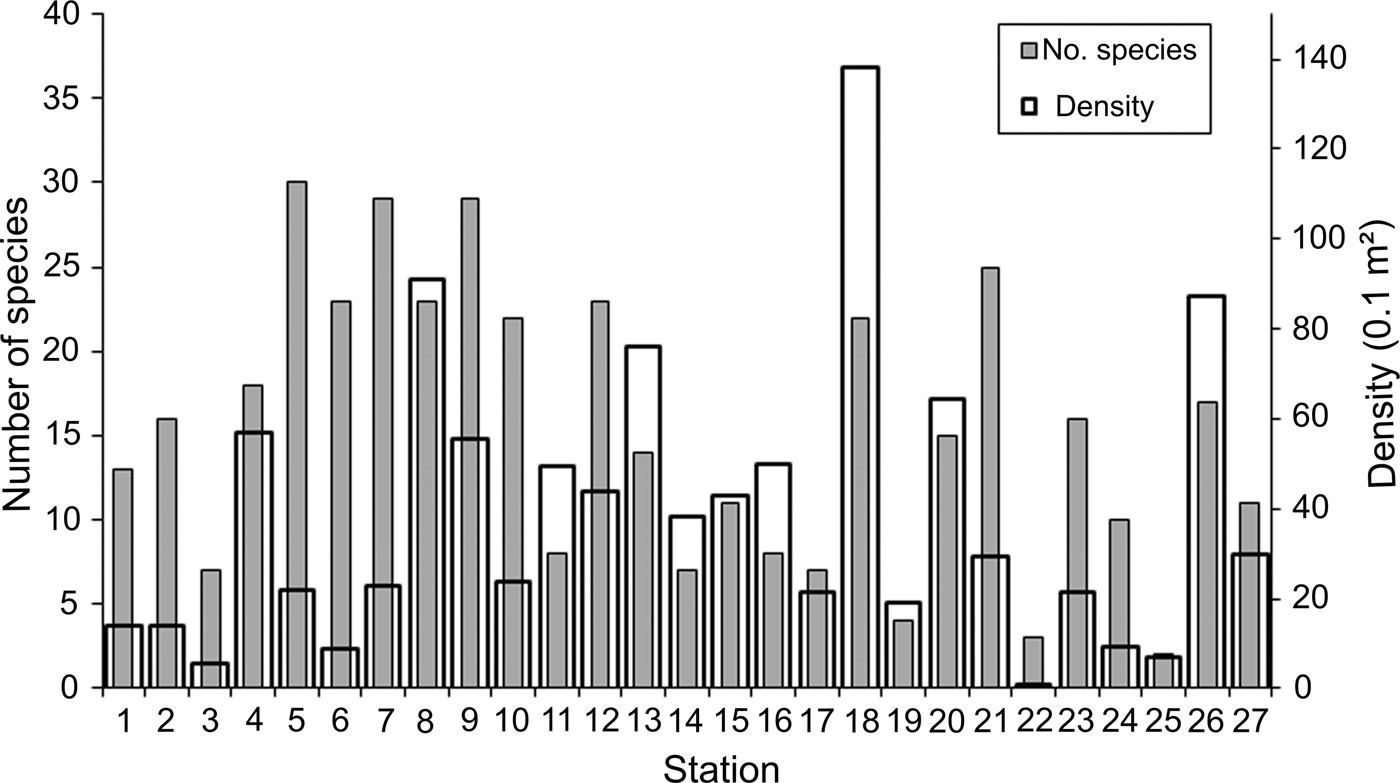
Density and number of species widely varied among the sampling stations: 0.8–138.1 ind. 0.1 m−2 (mean = 37.5 ind. 0.1 m−2; SD = 31.9 ind. 0.1 m−2) and 2–30 species station−1 (mean = 15 species station−1; SD = 8.4 species station−1). Stations with higher densities were irregularly distributed along the study area, although most of them were located in the middle Gulf, mainly stations 8 (91.0 ind. 0.1 m−2) and 9 (55.7 ind. 0.1 m−2), and around La Paz Bay, stations 13 (76.2 ind. 0.1 m−2) and 18 (138.1 ind. 0.1 m−2); this last station presented the highest density value (Figure 2). Except for station 4 (57.0 ind. 0.1 m−2), northwards, the density declined around the archipelago region, while at the southern end of the peninsula and the Pacific coasts of Baja California, except for station 26 (87.5 ind. 0.1 m−2), the number of individuals dropped significantly at average levels of 16.4 ind. 0.1 m−2.
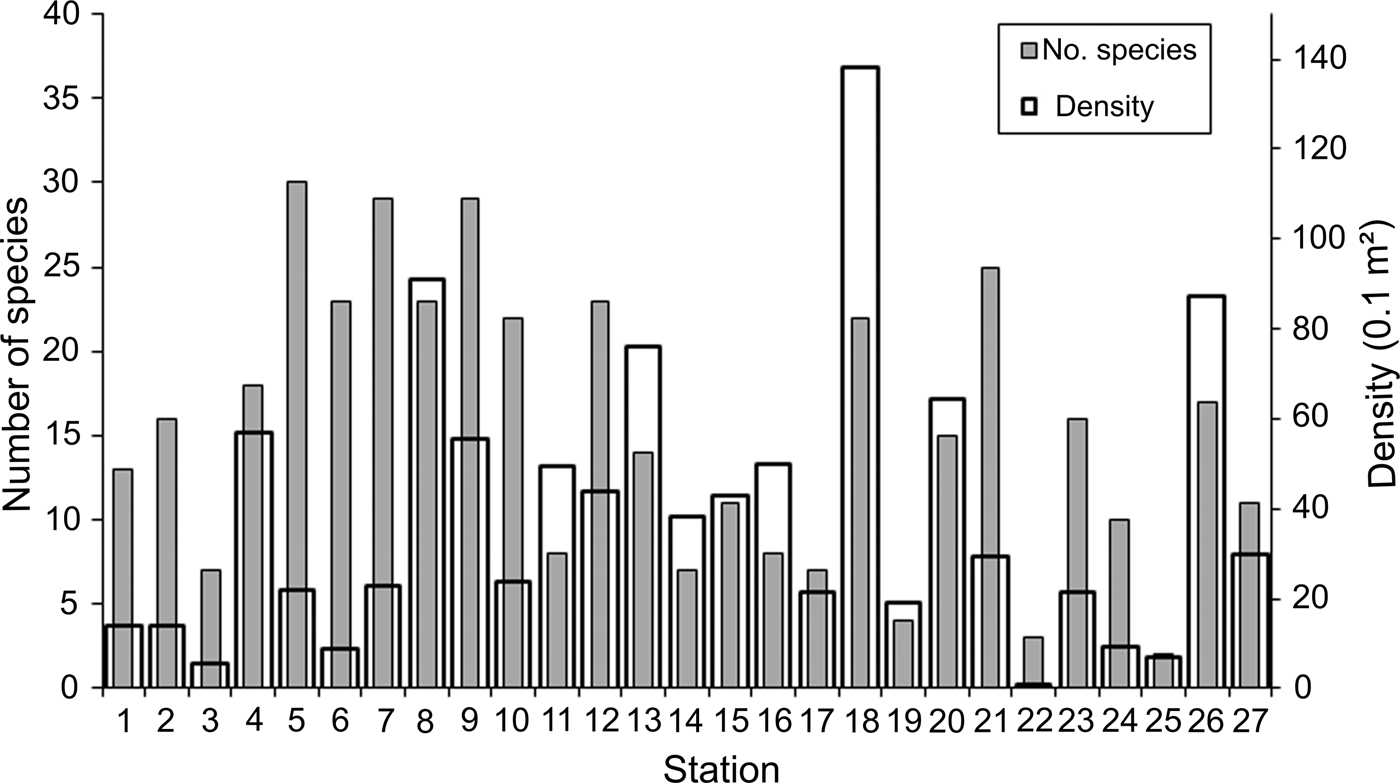
Fig. 2. Spatial distribution of the number of species and density (ind. 0.1 m−2) per sampling station.
In contrast to the density distribution, the highest number of species was found at the southern archipelago region: stations 4, 5 and 6 with 18–30 species. In the middle Gulf some of the more abundant stations also harboured a diverse fauna: stations 7, 8 and 9 with 23–29 species (Figure 2). Although the number of species was widely variable, in general, their values gradually declined towards the northern and southern Gulf, and at the Pacific coasts. However, towards the southern region, this decrease in the number of species was not observed in several localities: station 18 around La Paz Bay, station 21 from the southern end peninsula and station 26 from the western coast, with 22, 25 and 17 species, respectively.
Environmental trends
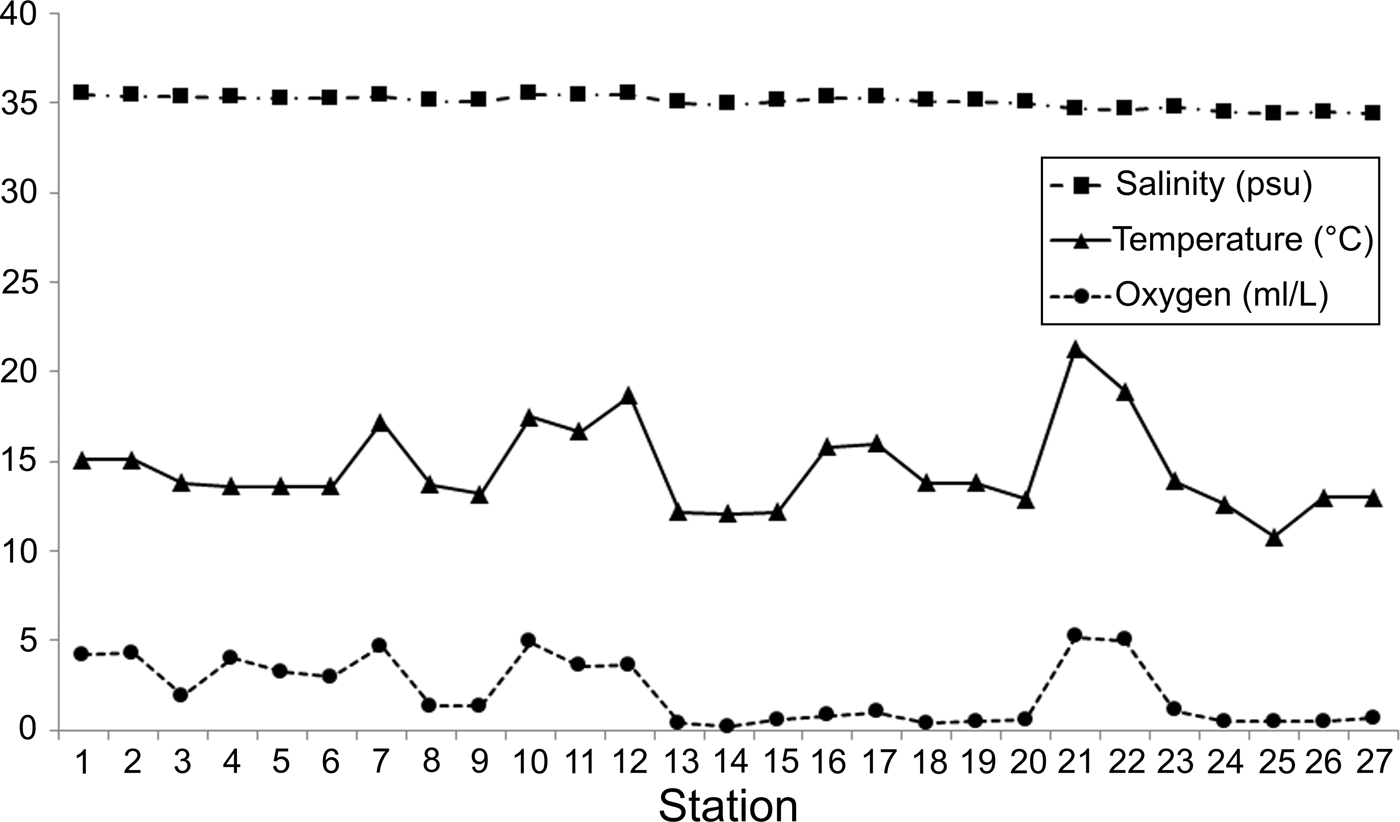
The environmental factors varied little along the littoral Baja California Peninsula (Figure 3). The salinity levels were very homogeneous throughout the continental shelf (mean = 35.07; SD = 0.35 psu) and their values decreased only in the Pacific coasts (34.40–34.50 psu); its spatial variation was not significantly correlated with the other environmental variables (r < 0.4). Temperature (mean = 14.60; SD = 2.45°C) and dissolved oxygen (mean = 2.15; SD = 1.79 ml l−1) displayed the largest differences along the study area and were significantly correlated (r = 0.763); that is, in zones with higher temperatures, greater oxygen concentrations were also recorded. Temperature and dissolved oxygen values increased mainly in the middle Gulf and at the southern end of the peninsula, but low oxygen concentrations were detected around La Paz Bay and in the Pacific coasts (around 0.5 ml l−1).
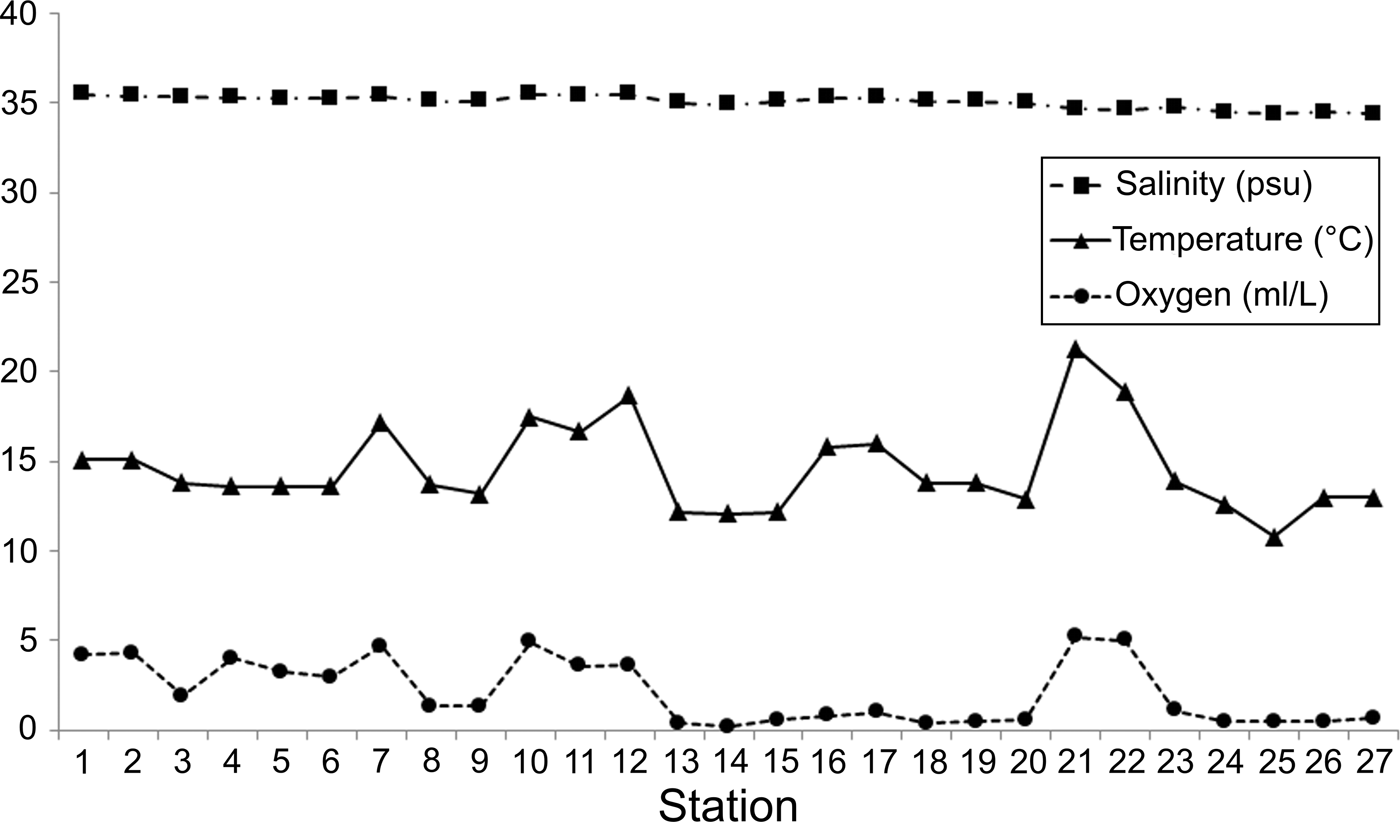
Fig. 3. Spatial variation of salinity, temperature and dissolved oxygen among the sampling stations.
Although there were variations in temperature and dissolved oxygen, in general, the environmental conditions were relatively homogeneous along the coastal Baja California Peninsula. This abiotic pattern caused by the salinity (R 2 = 0.10), temperature (R 2 = 0.05) and oxygen (R 2 = 0.14) cannot significantly explain the latitudinal changes detected in the number of species.
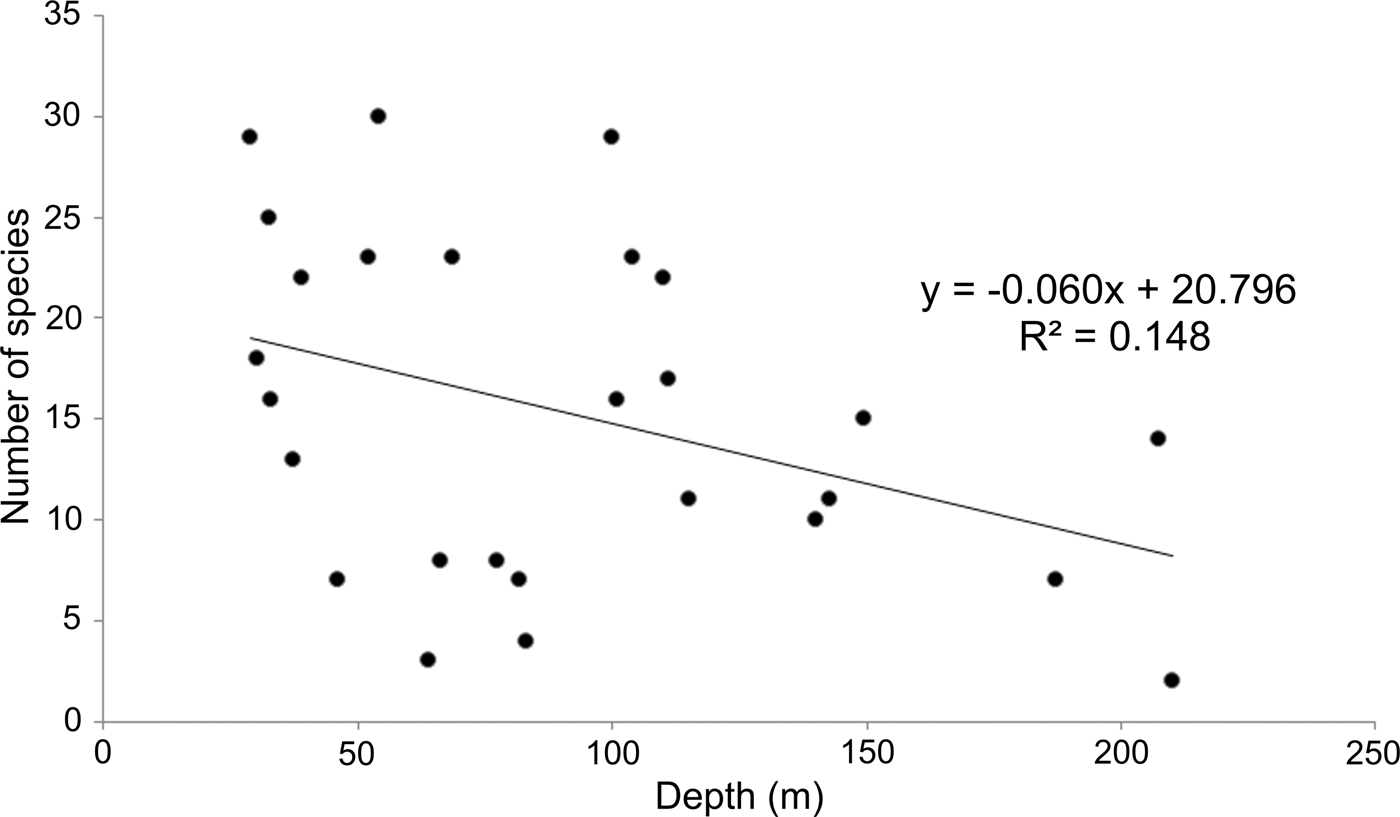
Since sampling took place along the continental shelf, depth values varied as a consequence (30–210 m). As a result, it was necessary to examine whether these depth differences could be related to the observed number of species along the peninsula (Figure 4). Although the stations with the higher number of species (>20 species) were all located at less than 110 m, six of the less diverse stations (<8 species) were distributed between 46 and 80 m. On the other hand, only three stations were sampled around 200 m, but their richness values did not show any clear pattern either: two of them had 2–7 species, but in the other, 14 species were identified; the average over all of the study area was 15 species station−1. The distribution of stations according to their number of species and depth presented a wide dispersion (Figure 4), indicating that depth could not explain the observed changes in the number of species (R 2 = 0.148).
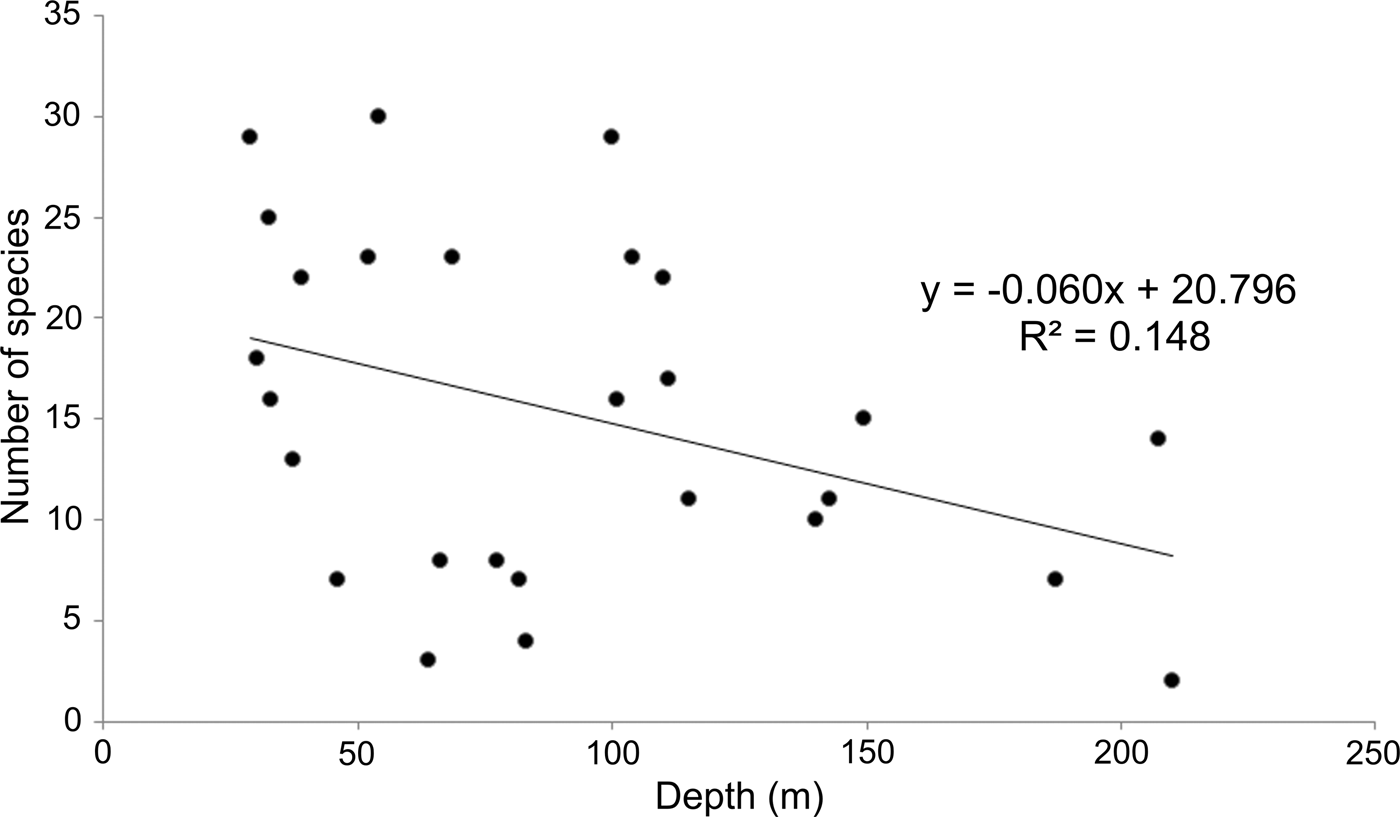
Fig. 4. Relationship between depth and number of species by sampling station.
Species assemblages
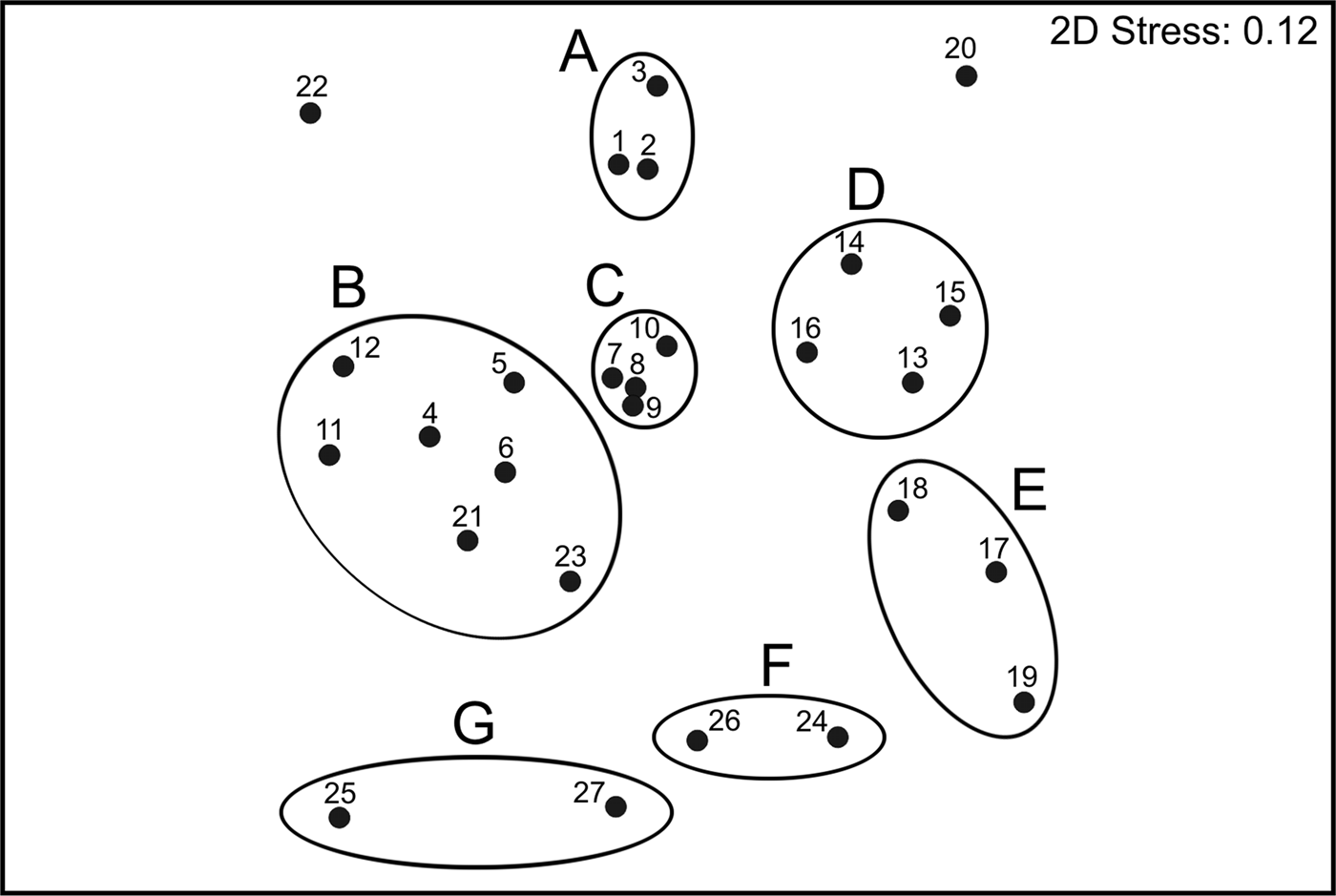
The ordination plot of the non-metric Multidimensional Scaling for the species composition of sampling sites produced seven main groups, which were supported by the SIMPROF test (Figure 5). Those seven groups were directly associated with their geographic position along the Baja California Peninsula shelf, and the visual representation of the data matrix, plotting the 35 most abundant and frequent species, confirmed the particularities among the different faunal sets found in each main group (Figure 6).
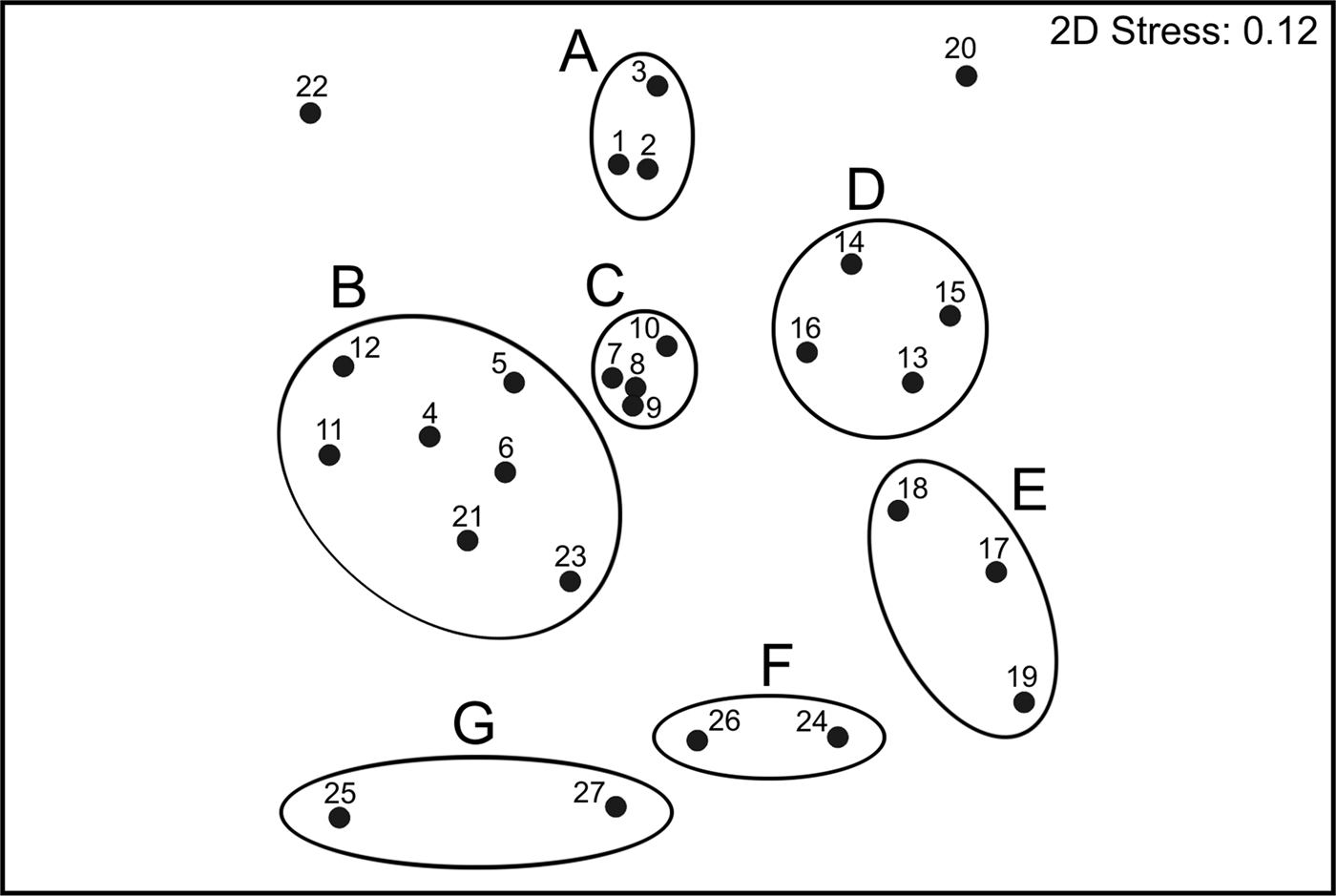
Fig. 5. Non-metric MDS ordination diagram of polychaete assemblages based on Bray–Curtis similarity matrix; the groups were significantly separated by the ‘similarity profile’ (SIMPROF) permutation test.

Fig. 6. Shade plot of stations/faunal assemblages based on the 35 most abundant and frequent polychaete species. The grey-scale intensity key has units square-root transformed to the original density measurements, the white cells represent the absence of the corresponding species and the full black cells the largest density in the whole matrix. Axis for stations is ordered by polychaete assemblages.
To the north of the study area (assemblage A), the fauna had fewer individuals (mean = 11.2 ind. 0.1 m−2) and species (mean = 12 species station−1), and Scoloplos texana Maciolek & Holland, Reference Maciolek and Holland1978 and Aglaophamus longicirrata Pérez-Torrijos, Hernández-Alcántara & Solís-Weiss, Reference Pérez-Torrijos, Hernández-Alcántara and Solís-Weiss2009, contributed the most to define the group (Figures 5 & 6). Although the stations included in assemblage B were abundant (mean = 33.2 ind. 0.1 m−2) and diverse (mean = 20 species station−1), they showed a disjunctive distribution inside the Gulf: around the archipelago region, at the north of La Paz Bay and at the end of the peninsula. This group was mainly defined by Glycera lapidum de Quatrefages, Reference de Quatrefages1866 and Notomastus magnus Hartman, Reference Hartman1947.
In the middle Gulf, assemblages C and D included stations with a high number of individuals (48.3 and 51.8 ind. 0.1 m−2 on average, respectively), but the number and type of species representing each group was totally different (Figures 5 & 6). Group C was the most diverse assemblage in the Peninsula, with 26 species station−1 on average, and Aricidea (Acmira) simplex Day, Reference Day1963, Paraprionospio pinnata (Ehlers, Reference Ehlers1901), Aglaophamus verrilli (McIntosh, Reference McIntosh1885) and Ceratocephale oculata Banse, Reference Banse1977 were the characteristic species. Group D was represented by only 10 species station−1 on average, with the stations distributed around La Paz Bay; this assemblage was represented by the motile polychaetes Aglaophamus verrilli and Scoletoma zonata (Johnson, Reference Johnson1901). South of La Paz Bay (assemblage E), the fauna was very abundant (mean = 59.5 ind. 0.1 m−2), but represented by fewer species (mean = 11 species station−1); this group was mainly characterized by the burrower Cirrophorus furcatus (Hartman, Reference Hartman1957).
The assemblages F and G only included stations located in the peninsular Pacific coasts, but displaying different distributional patterns. Group F had a higher number of individuals (mean = 48.5 ind. 0.1 m−2) and species (mean = 14 species station−1), and was characterized by the onuphids Diopatra obliqua Hartman, Reference Hartman1944, Mooreonuphis elsiae de León-González, Reference De Léon-González1994 and Mooreonuphis sp. 2, and the paraonid Aricidea (Acmira) lopezi rosea (Reish, Reference Reish1968). In contrast, group G had lower density values (mean = 18.5 ind. 0.1 m−2) and a lower number of species (mean = 7 species station−1) and was mostly represented by the tubicolous Eclysippe trilobata (Hartman, Reference Hartman1969).
Two stations from the southern Gulf were disconnected from any faunal assemblage: station 22 with only three species; and station 20 with 15 species, but dominated by species scarcely represented in other localities: Galathowenia pygidialis (Hartman, Reference Hartman1960), Ninoe marthae (Hernández-Alcántara, Hernández-Alcántara & Solís-Weiss, Reference Hernández-Alcántara, Pérez-Mendoza and Solís-Weiss2006) and Kinbergonuphis gorgonensis (Monro, Reference Monro1933).
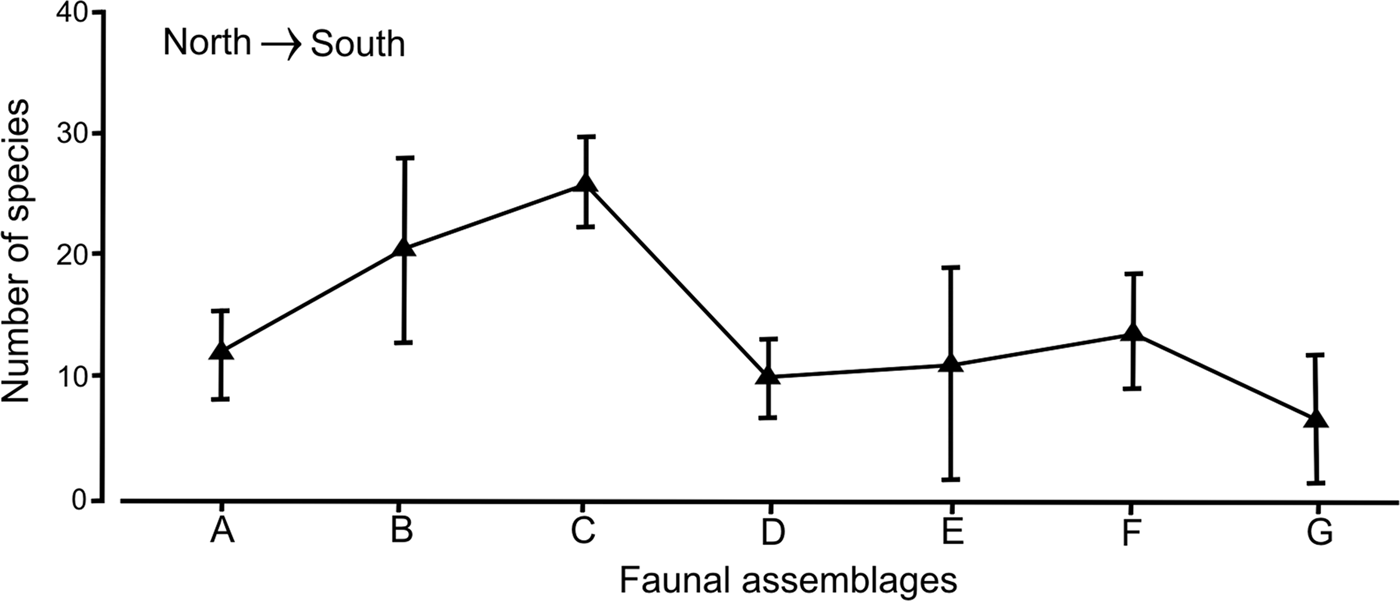
Although each faunal group had a distinct set of species and their differences along the peninsular coasts were clear, the variation in the number of species within the assemblages or groups was not significant (R = 0.283, P > 0.01). In fact, the faunal assemblages distributed in the middle Gulf: group C: A. (A.) simplex–P. pinnata–A. verrilli–C. oculata (mean = 26 species; SD = 3.78) and group B: G. lapidum–N. magnus (mean = 20 species; SD = 7.13), had the highest number of species, but their high values were not enough to separate them significantly from the other assemblages (Figure 7). The noticeable increase in variability of group B was directly related with the disjoint distribution of the stations, which were located around the archipelago and in the mouth of the Gulf.
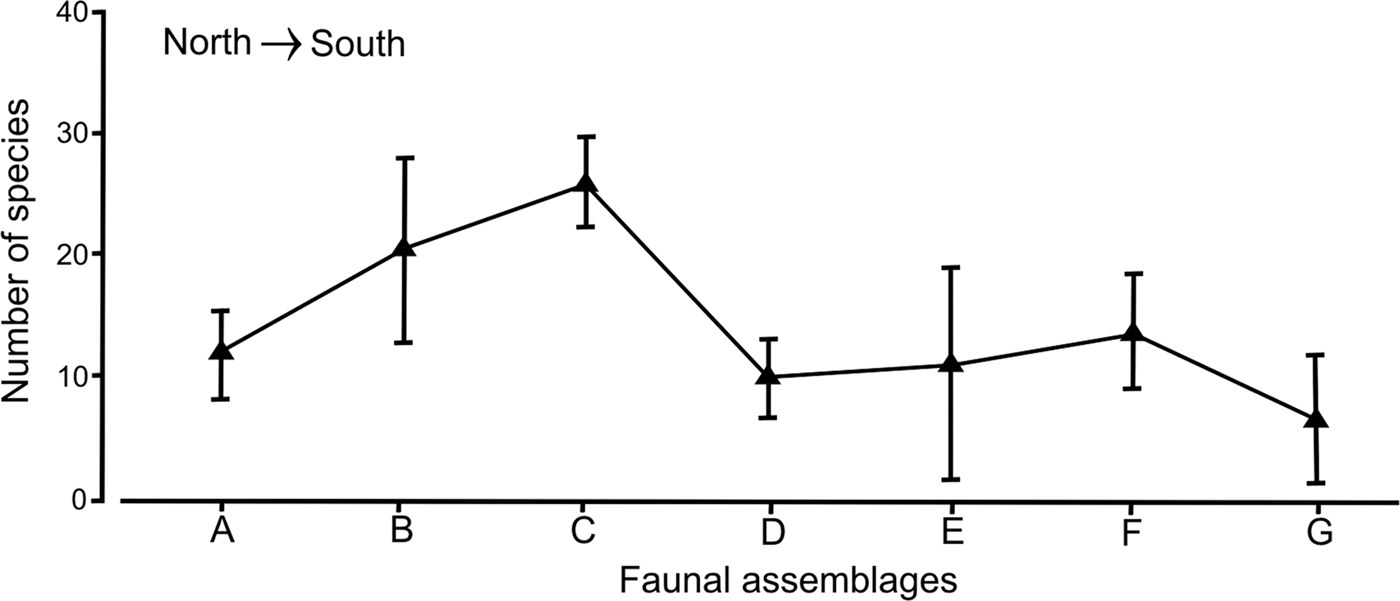
Fig. 7. Variability of the number of species for the polychaete assemblages found in the Baja California Peninsula shelf, in a north-south plane; the vertical bars are their standard deviations.
In group A: S. texana–A. longicirrata, from the northern Gulf, there were small differences in the number of species (mean = 12 species) with assemblages from the southern Gulf (10–11 species), but their variability ranges revealed different patterns. This assemblage A had low variation in the number of species (SD = 4.58), while the standard deviation values increased to 9.64 in group E (C. furcatus), located south of La Paz Bay (Figure 7). In the assemblages located in the Pacific coasts, an asymmetrical trend was detected, since group F (D. obliqua–M. elsiae–Mooreonuphis sp. 2–A.(A.) lopezi rosea) had on average up to 14 species with a standard deviation of 4.95, while in group G (E. trilobata), the number of species decreased to only 7 species on average, with an increase in its variability (SD = 6.36).
Beta-diversity along the Baja California Peninsula shelf
The compiled exhaustive database of polychaete species of the continental shelf of the Baja California Peninsula included 2033 records from 730 species belonging to 47 families. Since data come from different sources, which include all types of samples, it was necessary to estimate the number of species occurring there. The ICE estimator showed that 884 species could be present in the study area, while with the Chao2 estimator the number was 803 species. Both indexes showed that the polychaete species in the study area were relatively well known (>80%): the estimation of Chao2 was that fewer species are still unknown (73 species), while the ICE estimator indicated that it is necessary to collect a higher number of species (154 species).
The distribution of several families along the study area was different from those patterns observed at a local scale. The Onuphidae (36 species), Spionidae (42 species), Paraonidae (33 species), Cirratulidae (32 species) and Lumbrineridae (30 species) remained among the families with the highest number of species, but contrary to what was observed in the stations analysis, the Nereididae (46 species), Syllidae (45 species), Sabellidae (38 species), Terebellidae (33 species) and Serpulidae (31 species) showed higher numbers of species (Table 1). The background information on the compiled data can be observed at the family level in Figure 8, which indicates that the more diverse families do not display any distribution pattern and that their number of species only increased in some zones of the Gulf: nereidids, syllids and terebellids were more diverse around the 24 and 28° latitude bands, while sabellids and serpulids were better represented on the 28° and between the 24 to 26° latitude bands. These spatial changes can help us understand the beta diversity patterns, which indicate an increase in the number of species around the middle Gulf, but also a reduction in the species inhabiting the northern Gulf (29–31°) and around the Gulf's mouth (22°) (Figure 8).

Fig. 8. Shade plot of the number of species from the 25 most diverse families of polychaetes along the Baja California Peninsula coasts. The grey-scale intensity key has units linked with the number of species. The axis for latitude bands of 1° is ordered in a north-south plane.
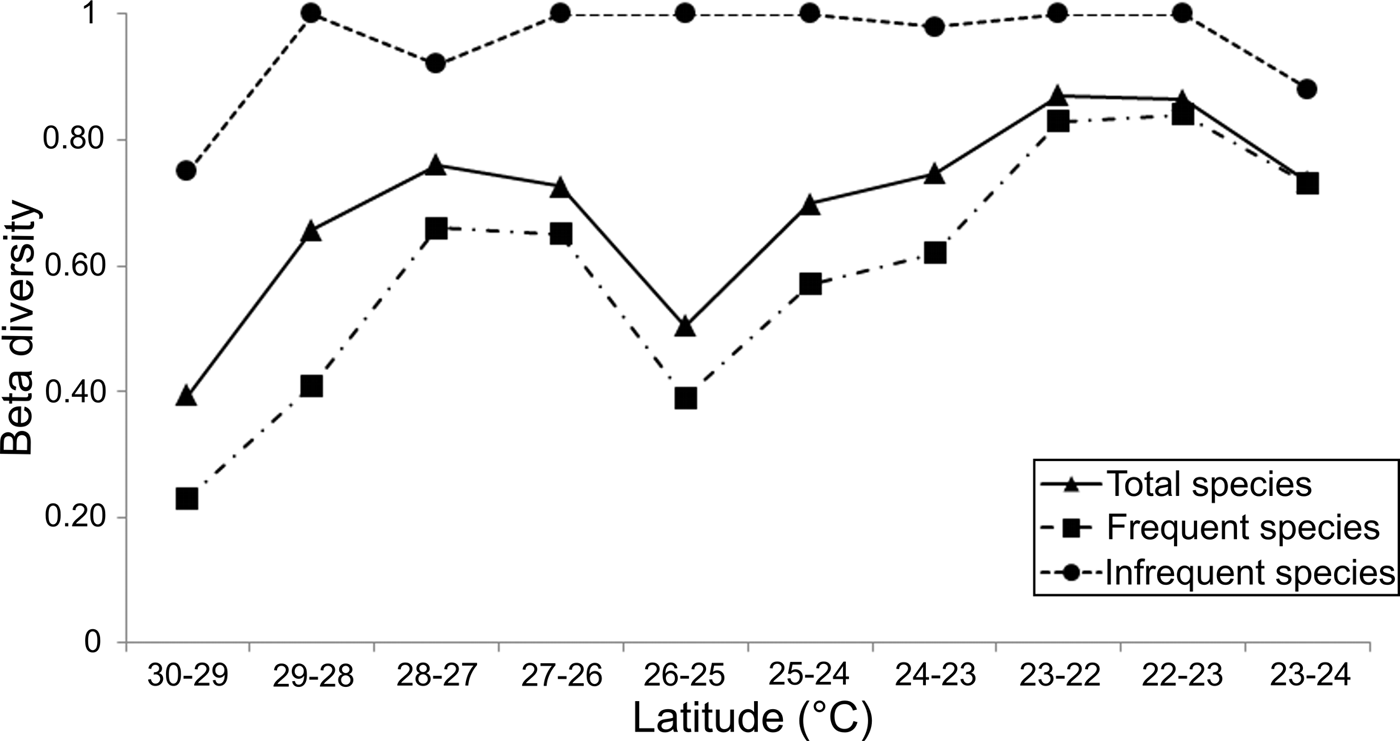
Although around 20% of the polychaete species could be unknown in the study area, the large variation in the β T-diversity values (0.39–0.87) indicated clear changes in the species composition along 1° latitude bands (Figure 9). Except at the northern end, where the lowest faunal changes were found (30–29° = 0.39), the β T-diversity values were higher than 0.5, reflecting faunal heterogeneity in the study area. To the south of the archipelago, the variation in species composition increased, but its latitudinal changes along the peninsula were very irregular. From 28–27° (0.76) a gradual drop in β T-diversity down to the 26–25° latitude band (0.5) was observed, due to the many species found there that were also distributed in the adjacent latitudinal bands.
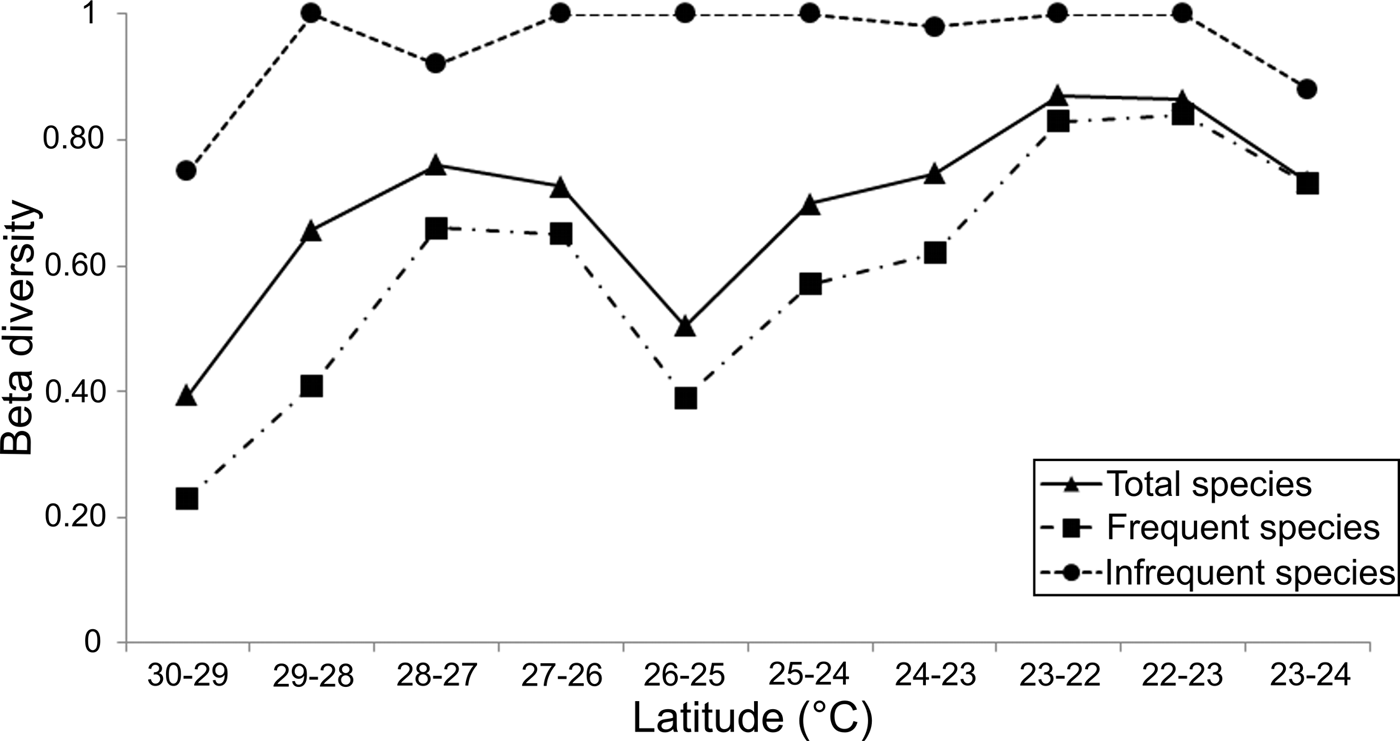
Fig. 9. Spatial pattern of beta diversity (Wilson–Schmida Index) in a north-south plane along the Baja California Peninsula shelf, the top curve (dotted line) represents the 415 infrequent species (located on 1 or 2 latitudinal bands). Downwards, the full line displays results from all analysed species (730), and the dashed line shows the variations of the 315 frequent species.
Following the faunal changes towards the Gulf's mouth, the β T-diversity displayed a continuous increase reaching its maximum value of 0.87 in the latitude band 23–22° (Figure 9). That is, the differences in species composition progressively increased towards south, since the species located inside the Gulf were different from those established around the mouth (22°). The notion that polychaetes living at the Gulf's mouth are different from those occurring in adjacent areas (Ekman, Reference Ekman1953) was confirmed when we compared its species composition with that recorded in the Pacific coasts. The β T-diversity between latitudinal bands 22–23° (western coasts) increased to 0.86, a value similar to the faunal changes observed between the 22° band (Gulf's mouth) and the 23° band inside the Gulf, which reached a value of 0.87. Both these measurements were the highest values of β T-diversity found along the Baja California Peninsula shelf. On the Pacific coasts, the differences in the species composition decreased northwards, reaching values of 0.73 in the 23–24° latitudinal band. This downward trend showed that the species composition changes around the southern end of the peninsula, because the species established in the Pacific Ocean coasts were also different from those recorded inside the Gulf.
The peninsula coasts were dominated by polychaetes (415 species) with restricted distribution (one or two latitudinal bands), while 315 species were located in three or more bands. Although the beta-diversity pattern generated from the species set with large distribution ranges recorded lower values, it followed a very similar trend to that displayed by all the species; only in the mouth of the Gulf and in the Pacific coasts were the beta-diversity values very close (Figure 9). On the other hand, the species with small distribution intervals produced a totally different beta-diversity pattern, since their values were always around 1. Decreases in beta-diversity were only found in the northern Gulf (30–29° = 0.75), in the vicinity of the archipelago region (28–27° = 0.92), and in the Pacific coasts (23–24° = 0.88).
Although the species with small distribution ranges were the dominant group (56.8%), most of them were not collected on adjacent latitudinal bands. The spatial distribution of these polychaetes increased differences in species composition along the peninsula, but its effect on beta-diversity values was very similar at each band. Therefore, the presence of these local species had no drastic effects on the complete beta-diversity pattern, modifying with similar intensity their values at each latitudinal band.
DISCUSSION
The relatively narrow continental shelf of the eastern and south-western end of the Baja California Peninsula with its extensive rocky shores and low input of sediment could restrict the establishment and good development of benthic soft bottom communities (Hernández-Alcántara, Reference Hernández-Alcántara2002). However, the number of species reported in the study area is quite high (730 species), but this could be expected in an area already known for its high invertebrate biodiversity (Roberts et al., Reference Roberts, McClean, Veron, Hawkins, Allen, McAllister, Mittermeier, Schueler, Spalding, Wells, Vynne and Werner2002; Brusca, Reference Brusca and Brusca2010). Also, even if in this study the number of species does not include the species recorded from the eastern coasts of the Gulf (where diversity is higher), or in the deep sea zones, the species richness of polychaetes only recorded for the continental shelf of the peninsula is still comparable to the number of species recorded in the whole Gulf of Mexico (829 species) (Reuscher & Shirley, Reference Reuscher and Shirley2014), in the Mediterranean Sea (876 species) (Castelli et al., Reference Castelli, Bianchi, Cantone, Çinar, Gambi, Giangrande, Sareri, Lanera, Licciano, Musco, Sanfilippo and Simonini2008), in the French Atlantic (934 species) (Dauvin et al., Reference Dauvin, Bachelet and Bellan2006) or on the Pacific coasts of Canada (791 species) (Carr, Reference Carr2012).
Though the recognition of the species richness patterns could be overlooked in a region so complex and rich in marine habitats as the Gulf of California, the distribution of the polychaete assemblages along the peninsula did show a latitudinal structure. To begin with, the environmental conditions were similar all over the area and the spatial variation of the number of species and the distribution of the faunal assemblages could not be associated with the salinity, temperature and dissolved oxygen values. The wind pattern in the Gulf is seasonal and during the winter-spring season, the winds from the north-west largely condition the circulation of the so-called ‘Gulf of California Water’, which is formed by evaporation processes and down-flowing along the eastern margin (Badan-Dangon et al., Reference Badan-Dangon, Dorman, Merrifield and Winant1991). The dominant presence of this water mass inside the Gulf meant that the environmental conditions barely changed along the peninsula and only slight disturbances in specific zones and on the Pacific coasts were observed. The depth variation too cannot be linked with the number of species, so that the faunal structure obtained could be the result of the influence of biotic factors or changes at the landscape scale. It appears then, that the topographic and hydrographic conditions on the Baja California Peninsula could play an important role in the changes in the composition and number of species.
In actual fact, the low concentrations of oxygen detected in the study area are not unusual, since the largest region of the world with hypoxia is found in the Eastern Pacific, including most of the Gulf of California (Diaz & Rosenberg, Reference Diaz and Rosenberg1995; Lluch-Cota et al., Reference Lluch-Cota, Aragón-Noriega, Arreguín-Sánchez, Aurioles-Gamboa, Bautista-Romero, Brusca, Cervantes-Duarte, Cortés-Altamirano, Del-Monte-Luna, Esquivel-Herrera, Fernández, Hendrickx, Hernández-Vázquez, Herrera-Cervantes, Kahru, Lavín, Lluch-Belda, Lluch-Cota, López-Martínez, Marinone, Nevárez-Martínez, Ortega-García, Palacios-Castro, Parés-Sierra, Ponce-Díaz, Ramírez-Rodríguez, Salinas-Zavala, Schwartzlose and Sierra-Beltrán2007). In the Gulf, observations made over 20 years showed that a large fringe located at or near the bottom had oxygen levels lower that 0.5 ml l−1 (Parker, Reference Parker1964; Lluch-Cota et al., Reference Lluch-Cota, Aragón-Noriega, Arreguín-Sánchez, Aurioles-Gamboa, Bautista-Romero, Brusca, Cervantes-Duarte, Cortés-Altamirano, Del-Monte-Luna, Esquivel-Herrera, Fernández, Hendrickx, Hernández-Vázquez, Herrera-Cervantes, Kahru, Lavín, Lluch-Belda, Lluch-Cota, López-Martínez, Marinone, Nevárez-Martínez, Ortega-García, Palacios-Castro, Parés-Sierra, Ponce-Díaz, Ramírez-Rodríguez, Salinas-Zavala, Schwartzlose and Sierra-Beltrán2007). The reduction of oxygen has also been observed in very shallow waters, at less than 100 m depth, in the southern Gulf (Hendrickx, Reference Hendrickx2001). In some areas, such as outside of La Paz Bay, the oxygen minimum zone can extend to 20–30 nautical miles, while in other areas, as in the southern end of the peninsula, the lower oxygen zone is narrower (<10 nautical miles). In contrast, the bottom of the northern Gulf is not affected by the hypoxia levels (Lluch-Cota et al., Reference Lluch-Cota, Aragón-Noriega, Arreguín-Sánchez, Aurioles-Gamboa, Bautista-Romero, Brusca, Cervantes-Duarte, Cortés-Altamirano, Del-Monte-Luna, Esquivel-Herrera, Fernández, Hendrickx, Hernández-Vázquez, Herrera-Cervantes, Kahru, Lavín, Lluch-Belda, Lluch-Cota, López-Martínez, Marinone, Nevárez-Martínez, Ortega-García, Palacios-Castro, Parés-Sierra, Ponce-Díaz, Ramírez-Rodríguez, Salinas-Zavala, Schwartzlose and Sierra-Beltrán2007).
In this study, several stations with the lowest concentrations of oxygen (<0.5 ml l−1) harboured very few polychaetes (2–7 species), while other stations with these minimum oxygen levels, included a moderately diverse fauna (14–22 species station−1). At present, it is difficult to formulate generalizations about the dwelling habitats of low-oxygen macrofauna (Levin et al., Reference Levin, Gage, Martin and Lamont2000), because further studies are needed in order to understand the relationships between the dissolved oxygen levels and the polychaetes that live under hypoxic conditions. These would have to include their metabolic, behavioural and morphological responses. However, even if the effects of the low oxygen concentrations on the presence and distribution of the local polychaete species have not yet been studied, it is known that, as a general rule, the polychaetes are better adapted to minimum oxygen levels than other invertebrates such as molluscs, crustaceans or echinoderms (Hendrickx & Serrano, Reference Hendrickx and Serrano2014), and that they can tolerate different levels of hypoxia (Lamont & Gage, Reference Lamont and Gage2000). There are even records of populations of spionids that permanently live under these conditions (Levin et al., Reference Levin, Gage, Martin and Lamont2000).
Although the northern Gulf is represented by only three stations, the reduction in the number of species and individuals in the S. texana–A. longicirrata assemblage could be associated with the presence of the archipelago. This physical barrier effectively separates the northern region from the rest of the Gulf, due to a combination of hydrological processes linked to the water circulation pattern, heat and moisture, seasonal fluxes in the presence of tidal stirring, plus winds and convective mixing (Paden et al., Reference Paden, Abbott and Winant1991). The influence of this group of islands on the dispersion and establishment of species in the northern region of the Gulf has already been documented (Salas-de León et al., Reference Salas-de León, Carbajal-Pérez, Monreal-Gómez and Gil-Zurita2011; Hernández-Alcántara et al., Reference Hernández-Alcántara, Salas-de León, Solís-Weiss and Monreal-Gómez2013) and is associated with the presence of shallow underwater edges and narrow channels through which the water circulation is disrupted (Lavín et al., Reference Lavín, Beier, Badan and Lavín1997).
South of the archipelago, we found the assemblages with the highest number of species (G. lapidum–N. magnus and A. (A.) simplex–P. pinnata–A. verrilli–C. oculata); the species included in both these groups were largely distributed in the central and southern regions of the Gulf. The net circulation in the Gulf of California, anticyclonic in winter-spring and cyclonic in summer-autumn, could influence the distribution of the organisms through larval dispersion, allowing species from the continental coasts or from the Tropical Pacific to settle in the middle region of the peninsula.
La Paz Bay, a highly productive area which plays an important ecological role as a feeding and protective habitat for commercially important species (Díaz-Uribe et al., Reference Díaz-Uribe, Arreguín-Sánchez and Cisneros-Mata2007), can also be a factor in helping to increase the number of species in the benthic ecosystem. However, clear differences were also observed between the type of species which live inside (A. verrilli–S. zonata group) and outside (C. furcatus group) the bay, due to the existing cyclonic eddy inside it (Duran-Campos et al., Reference Duran-Campos, Salas-de León, Monreal-Gómez, Aldeco-Ramírez and Coria-Monter2015), which can determine the distribution of the organisms, but also due to the existence of an underwater ‘threshold’ (or edge) found at the entrance of the bay (depth = 250 m). This edge seems to isolate the bay from the rest of the Gulf, as it restricts the water mass exchange and consequently the possible dispersion of organisms towards or from the Gulf of California (Molina-Cruz et al., Reference Molina-Cruz, Pérez-Cruz and Monreal-Gómez2002; Duran-Campos et al., Reference Duran-Campos, Salas-de León, Monreal-Gómez, Aldeco-Ramírez and Coria-Monter2015).
The southern end of the Baja California Peninsula shelf has been classified as a transitional zone between the warm water region from the Eastern Tropical Pacific and the cooler water region associated with the California Current (Brusca, Reference Brusca1980). Although the number of species was reduced in the western coasts of the peninsula, we could also note that the polychaete species established there were somewhat different from those recorded inside the Gulf. In contrast with the fauna with high tropical affinities living inside the Gulf, subjected to the influence of tropical and subtropical water masses (Lavín et al., Reference Lavín, Beier, Badan and Lavín1997), the polychaetes established on the Pacific shores can be associated with warm-temperate environments (Brusca, Reference Brusca1980). This is due to the California Current influence, which flows towards the equator following the coastal contours and is characterized by its low salinity and low temperature (Parés-Sierra et al., Reference Parés-Sierra, López, Pavía and Lavín1997). Differences between the fauna dwelling in the eastern and western coasts of the southern end of the peninsula have been reported in various groups of invertebrates (Brusca, Reference Brusca1980), such as the brachyurans (Garth, Reference Garth1960) or molluscs (Zamorano & Hendrickx, Reference Zamorano and Hendrickx2011), but up to now, the level of the exchange rates between the polychaetes that live inside and outside the Gulf of California had not been documented.
Although in the assemblages G. lapidum–N. magnus and A. (A.) simplex–P. pinnata–A. verrilli–C. oculata, located in the middle Gulf, the highest richness was reported, and towards the northern and southern ends of peninsula the faunal assemblages displayed a lower number of species, the differences in the number of species were not significant. That is, the differences between the faunal assemblages are linked with their species composition but not with the species richness. This relative homogeneity in the number of species along the peninsula is not consistent with the hypothesis that species richness will increase from high to low latitudes (Sanders, Reference Sanders1968; Gray, Reference Gray2001). In fact, the semi-enclosed basin shape of the Gulf of California, which restricts water exchanges with the Pacific Ocean, would lead us to expect a growth in diversity following a latitudinal north-to-south gradient due to limited genetic exchange occurring between the more isolated northern Gulf and the open Pacific Ocean (Brusca, Reference Brusca1980; Hendrickx, Reference Hendrickx1992). Those trends are already observed within the Stomatopoda (Hendrickx & Salgado-Barragán, Reference Hendrickx and Salgado-Barragán1991), Decapoda (Hendrickx, Reference Hendrickx1992) or Peracarida (Hendrickx et al., Reference Hendrickx, Brusca, Ramírez-Reséndiz and Hendrickx2002), but it seems that the polychaetes from the Baja California Peninsula shelf follow a different pattern.
The assemblages are sporadic arrangements whereby the species that become established and interact at a certain time depend upon the local evolutionary history that allowed them, or their precursors, to be established in a given area (Halffter & Moreno, Reference Halffter, Moreno, Halffter, Soberón, Koleff and Melic2005). For this reason, it was important to review the presence of all the polychaete species recorded in the study area and to examine the species turnover along the peninsula, in order to detect whether their latitudinal distribution revealed some recognizable pattern. The beta-diversity has been frequently used to evaluate variations in the composition of species between sites (Koleff et al., Reference Koleff, Gaston and Lennon2003; Legendre et al., Reference Legendre, Borcard and Peres-Neto2005), which, among other things, can help explain the local richness patterns (Guerra-Castro, Reference Guerra-Castro2012).
The variation in beta-diversity is linked to environmental changes at landscape scale (Halffter & Moreno, Reference Halffter, Moreno, Halffter, Soberón, Koleff and Melic2005), and the species exchange rate in the Baja California Peninsula shelf suggested the presence of four main faunal regions: the northern region of the Gulf, located between 31° and 28°N, with cold temperatures (around 15°C) and high salinities (>35.0) (Lavín et al., Reference Lavín, Beier, Badan and Lavín1997), linked with the lowest values of β T-diversity as a result of the high similarities among the species that live there. However, as we go towards the islands zone, the values of β T-diversity gradually increase, indicating that the archipelago, with its shallow underwater edges, its narrow channels and the formation of strong water mass gyres (Lavín et al., Reference Lavín, Beier, Badan and Lavín1997), could restrict species exchanges between the northern and central regions of the Gulf (Hernández-Alcántara et al., Reference Hernández-Alcántara, Salas-de León, Solís-Weiss and Monreal-Gómez2014).
In the central region (between 28° and 25°N), where temperatures are higher than 18°C and moderate tidal ranges are the norm (Thomson & Gilligan, Reference Thomson, Gilligan, Thomson, Findley and Kerstitch2000), we found one of the two highest values of β T-diversity. The gradual decrease of β T-diversity towards the south, reaching its minimum value of 0.5 at latitude 25°N (northern La Paz Bay), was associated with the fact that several species found in this latitudinal band also live in the adjacent areas. However, with the latitudinal variation of β T-diversity values, we could not detect the faunal differences between the polychaetes living inside and outside of La Paz bay, since this entire zone was included in the 24°N latitude band. Southward, differences in species composition among the adjacent latitudinal bands increased the β T-diversity until its highest value (0.87), precisely at the Gulf's mouth. This southern region is characterized by warm temperatures (>18°C) and is subjected to the confluence of different water masses, tropical and subtropical (Lavín et al., Reference Lavín, Beier, Badan and Lavín1997).
The fauna living at the southern end of the peninsula was clearly different from that found towards the north in the Gulf, but also from that of the Pacific coasts, where the β T-diversity values were high (0.86). The Gulf mouth is exposed to the constant influence of eddies and ocean front water masses with different hydrographic characteristics. This negatively affects the polychaete populations, because the tropical water masses present in the Gulf mouth are totally different from the warm and salty waters of the inner Gulf and the cold and low salinity waters of the Pacific coasts.
Since our measurements of β T-diversity were based on primary data sources, which were done with different sampling gears, it was important to evaluate the total number of species inhabiting the studied region to detect possible biases associated with those methodological differences. The ICE and Chao2 estimators indicated that the polychaete fauna from the peninsula coasts is relatively well known (>80%), but we could also observe that most species were not distributed along the whole region. The prevalence of species with small distribution ranges suggested that their sampling would be more random than species with a wide distribution, driving the β T-diversity changes. However, the effects of the infrequent species were similar at each latitudinal band, a result that suggests that the observed β T-diversity pattern could represent a suitable evaluation.
The beta-diversity values at landscape scale can be totally uncorrelated to species richness variation at local scale, and the way in which the regional processes can regulate the number of species in local communities is poorly understood as yet. In this study, we presented the first attempt to define the latitudinal variation of the diversity of the polychaetes along the eastern and south-western end coasts of the Baja California Peninsula shelf. Although more studies are necessary to verify the causes of the particularities found in their distribution, we can already indicate that the high values of β T-diversity were the result of the presence of a high number of species with short distribution ranges. However, it is not a simple task to predict the species distribution patterns either locally or regionally, especially if looking for consistency (Caley & Schluter, Reference Caley and Schluter1997). Nevertheless, the analysis of the latitudinal diversity patterns of one of the most abundant and diversified groups in the benthic systems (the polychaetes), in one of the most diverse and complex marine systems of the world (the Gulf of California), constitutes an important step to help establish the much-needed monitoring programmes and conservation measures to preserve biodiversity as much as possible along the whole Mexican Pacific.
ACKNOWLEDGEMENTS
We thank the participants in the research expeditions on board the RV ‘El Puma’ (UNAM), including the captain and crew, and Michel Hendrickx, head of the institutional project CORTES for inviting us to participate and collect part of the material used in this study.
FINANCIAL SUPPORT
The Instituto de Ciencias del Mar y Limnología of the Universidad Nacional Autónoma de México provided the financial support to undertake this study.