INTRODUCTION
Sponges do not have organs, including gonads. Therefore, their gametes originate from somatic cells, mainly choanocytes and archaeocytes (Ereskovsky, Reference Ereskovsky2010). As in other animals, oogenesis in sponges is a complex multi-step process that forms a nutrient-rich cell containing all the materials needed to initiate and maintain the metabolism of the future individual (Maldonado & Riesgo, Reference Maldonado and Riesgo2008; Gilbert, Reference Gilbert2010). Hence, egg production is an energetically expensive process, mainly in the vitellogenic phase, when the future oocyte must accumulate nutrients through one of these three processes: (1) authosynthesis – when the own oocyte produces its yolk; (2) heterosynthesis – when the yolk is transferred to the oocyte; or (3) mixed – a combination of the other two. Considering the evolutionary importance probably associated with oogenesis, it should be better investigated, mainly in ancient animals such as sponges (Ramirez-Llodra, Reference Ramirez-Llodra2002).
In calcareous sponges (Calcarea, Porifera), knowledge on oogenesis is still fragmentary. In the subclass Calcinea, for example, the origin of the oocytes is still unresolved (Ereskovsky, Reference Ereskovsky2010). While for some authors oocytes may derive from a lineage of porocytes-pinacocytes (Borojevic, Reference Borojevic1969), for others they can derive from amoebocytes (Minchin, Reference Minchin and Boutan1900; Hadzi, Reference Hadzi1917; Tuzet, Reference Tuzet1948; Johnson, Reference Johnson1979) or from choanocytes (e.g. Sarà, Reference Sarà1955). Independently of their origin, the oocyte seems to grow in the mesohyl without undergoing successive divisions (Sarà, Reference Sarà1955; Ereskovsky, Reference Ereskovsky2010).
Another under-studied topic is the nutrition of the oocytes, as no ultrastructural or experimental approach has been used to date to describe vitellogenesis in Calcinea. It has been assumed that young oocytes perform amoeboid movements and actively phagocytize several types of somatic cells, including choanocytes, choanocyte-derived cells, eosinophilic amoebocytes and hyaline amoebocytes (Sarà, Reference Sarà1955; Johnson, Reference Johnson1979; Ereskovsky, Reference Ereskovsky2010). In some species, oocytes phagocytize some of these cells and migrate into a special temporary structure, the nests (from French – ‘nids’), which are complex and anarchic tissues specifically involved in the nutrition of the oocytes and embryos during their development (Borojevic, Reference Borojevic1969). To date, nests were observed only in the calcareous sponges Ascandra falcata Haeckel, 1872, Ascandra minchini Borojevic, 1966, and Ascandra contorta (Minchin, 1905) (Borojevic, Reference Borojevic1969).
Considering the lack of knowledge on the oogenesis of Calcinea and recently published phylogenetic trees of the calcinean order Clathrinida (Rossi et al., Reference Rossi, Russo, Solé-Cava, Rapp and Klautau2011; Klautau et al., Reference Klautau, Azevedo, Cóndor–Luján, Rapp, Collins and Russo2013), we investigated the oogenesis of three Clathrinida species belonging to two different evolutionary lineages using light and electron microscopy. We compared our present findings with others previously reported in the literature, looking for aspects of the oogenesis in the light of evolution (Rossi et al., Reference Rossi, Russo, Solé-Cava, Rapp and Klautau2011; Klautau et al., Reference Klautau, Azevedo, Cóndor–Luján, Rapp, Collins and Russo2013).
MATERIALS AND METHODS
Sampling sites
The present study was carried out in Cabo Frio and Arraial do Cabo, located at the eastern region of Rio de Janeiro state, Brazil (Figure 1A). This region is characterized by an upwelling event, which usually occurs during the austral spring and summer seasons, decreasing the water temperature to ~14°C (Fernandes et al., Reference Fernandes, Quintanilha, Monteiro-Ribas, Gonzalez-Rodriguez and Coutinho2012). Specimens of Clathrina aurea Solé-Cava, Klautau, Boury-Esnault, Borojevic & Thorpe, 1991 were collected in a small cave located at the south eastern face of Papagaios Island, Cabo Frio (22.897°S 41.982°W, Figure 1B). Specimens of Borojevia aspina (Klautau, Solé-Cava & Borojevic, Reference Klautau, Solé-Cava and Borojevic1994) and B. brasiliensis (Solé-Cava, Klautau, Boury-Esnault, Borojevic & Thorpe, 1991) were collected in shafts and ropes hanging from a fluctuating structure located close to a mussel farm at the entrance of Forno Beach bay (22.967°S 42.007°W, Figure 1C).

Fig. 1. Sampling site and the Clathrinida species investigated in the present study. (A) Map showing the sampling sites: (B) Papagaios Island and (C) Forno Beach, in Cabo Frio and Arraial do Cabo, respectively. (D, E) In situ photographs of the investigated species: (D) Clathrina aurea (insert: regular triactine), (E) Borojevia aspina (insert: apical actine [arrow] with the vestigial spines typical of this species), and (F) B. brasiliensis (insert: apical actine [arrow] with a row of spines typical of this species).
Studied species
The three species investigated here are endemic from the Brazilian coast (Muricy et al., Reference Muricy, Lopes, Hajdu, Carvalho, Moraes, Klautau, Menegola and Pinheiro2011). Clathrina aurea is characterized by a yellow cormus with several simple oscular openings (Figure 1D). The skeleton is formed by a single category of triactines, and the specimens are usually found on caves and crevices of the rocky shores along the Brazilian coast (Klautau & Valentine, Reference Klautau and Valentine2003). Borojevia aspina and B. brasiliensis occur in sympatry. They present a similar morphology (Figure 1E, F) and were already considered cryptic species, but a study with allozymes (and later on with DNA sequences) showed that they are different species (Klautau et al., Reference Klautau, Solé-Cava and Borojevic1994, Reference Klautau, Azevedo, Cóndor–Luján, Rapp, Collins and Russo2013). Both species present white cormi composed of thin, anastomosed tubes with water collecting tubes. Their skeleton is formed by tripods, triactines and tetractines. Besides the size of the spicules, the main difference between these species is the abundance and organization of spines on the apical actine of the tetractines (Figure 1E, F). All these species are common in the study region and were collected by scuba or snorkelling in depths of 1–12 m.
Sampling design and specimens’ preparation
For C. aurea, at least 10 specimens (fragments or the whole individual, depending on the size of the sponge) were collected monthly from September 2008 to March 2010. For B. aspina and B. brasiliensis, the sampling was made irregularly from January 2010 to June 2011 (January, February, March, May, July, November and December 2010, and January, February, March, and June 2011). We collected 10 specimens with similar phenotypes, but as these species are not readily recognized in the field, the number of specimens varied depending on the month in which the sponges were sampled.
All fragments were removed from their substrate using a thin forceps. Soon after sampling, the specimens were put in a bowl containing seawater and were split into three parts: a fragment for histological procedures – fixed in Bouin's solution; a fragment for electron microscopy procedures – fixed in a 2.5% glutaraldehyde solution (buffered with sodium cacodilate 0.2 M, and 0.2 µm filtered seawater, 1:4:5 parts, respectively); and a third fragment for species identification – fixed in ethanol 93%.
In the laboratory, the fragments for histology and electron microscopy were decalcified in 5% ethylenediaminetetraacetic acid (EDTA), pH 7.0, for 24 h. Then, they were rinsed six times in filtered seawater in order to remove the excess of EDTA solution. Tissue samples were processed to light and electron microscopy as described previously by Lanna & Klautau (Reference Lanna and Klautau2010).
Ancestral character state reconstruction
The tentative reconstruction of the ancestral state of the vitellogenesis pathway and of the oocyte development location was made in Mesquite 3.0 (Maddison & Maddison, Reference Maddison and Maddison2014). We collected data on the reproductive traits of Calcinea species and a few Calcaronea species (as outgroup) in the literature and from non-published results to construct a matrix with states of these traits (Table 1). Both traits were considered to be binary (Table 1) for the current analysis, because ‘autosynthesis’ (for the vitellogenesis pathway) and ‘follicles’ (for oocyte development location) were not present in any of the selected species. Species relying on the phagocytosis of trophocytes, nurse cells or any other somatic cells were considered to take the heterosynthesis pathway for their vitellogenesis (Eckelbarger, Reference Eckelbarger1994). The ancestral reconstruction was made based on a simplified tree modified from Voigt et al. (Reference Voigt, Wülfing and Wörheide2012) and Klautau et al. (Reference Klautau, Azevedo, Cóndor–Luján, Rapp, Collins and Russo2013) considering only the species that had already had aspects of their oogenesis investigated. The character ancestral state was recovered using both the unordered Parsimony Ancestral State (MP) and the Maximum likelihood (ML). The ML reconstructions were performed using the MK1 model of evolution.
Table 1. Summary of the species included for the ancestral character state reconstruction of oogenesis traits of Clathrinida.

RESULTS
Clathrina aurea
Individuals of C. aurea undergoing oogenesis were seldom observed. Only six of the almost 170 analysed specimens presented some stages of oogenesis. Specimens undergoing oogenesis were observed from November 2008 to January 2009 and in June 2009 (Figure 2A), without any synchronism at the individual or the population level. The oogenesis was restricted to some tubes of the sponges. This characteristic makes the location of the oocytes difficult in SEM and TEM, complicating the description of its oogenesis on an ultrastructural level. Therefore, the description provided here for this species is based mainly on light microscopy analysis. Although it was not possible to be sure about the origin of the oocytes, they may have derived from hyaline amoebocytes found in the mesohyl. These amoebocytes were constantly present in the tubes of the sponges throughout the year, but became more abundant in the reproductive specimens. In their somatic phase, these cells measured ~8.5 µm in diameter, presented a spherical nucleolated nucleus (~3.8 µm diameter), and a hyaline cytoplasm (Figure 2B), with several clear vesicles, and some electron dense phagosomes (Figure 2C). Later on, the hyaline amoebocyte accumulated chromidial bodies in its cytoplasm and inner nuclear envelope, and the nucleus started to swell (Figure 2E). At this stage, the oocytes increased, attaining ~15 µm, presented a large nucleus (~9 µm) and nucleolus (2.5 µm), and the cytoplasm became more basophilic (Figure 2E). Oocytes grew in the thin mesohyl, reaching ~30 µm, and tended to cease their movements. At this stage, the nucleus presented a well-marked nucleolus (~4.5 µm) and heterochromatin close to the nuclear envelope. The vitellogenic oocyte cytoplasm became more granulated with clear hydrophobic granules (Figure 2F). Phagocytosis of somatic cells was never observed in this species and there was no special structure involved in the nutrition of C. aurea oocytes.

Fig. 2. Some aspects of the oogenesis of Clathrina aurea (B, D–F: light microscopy [LM]; C: transmission electron microscopy [TEM]). (A) Frequency of oogenic individuals during the studied period. (B) Presence of hyaline amoebocytes (ha) in oogenic specimens. (C) Detail of the ultrastructure of the cytoplasm of a hyaline amoebocyte (insert) showing the abundance of clear vesicles (cv), mitochondrion (m) and some electron dense phagosomes (ph). (D) Hyaline amoebocyte (ha) that can differentiate into an oocyte. (E) Pre-vitellogenic oocyte with large nucleolated (nu) nucleus (n) and a thin granular cytoplasm. (F) Vitellogenic oocyte surrounded by spherical choanocytes in a contracted tube. ch, choanocytes; cy, cytoplasm; n, nucleus; nu, nucleolus; pi, pinacocytes.
Borojevia aspina and B. brasiliensis
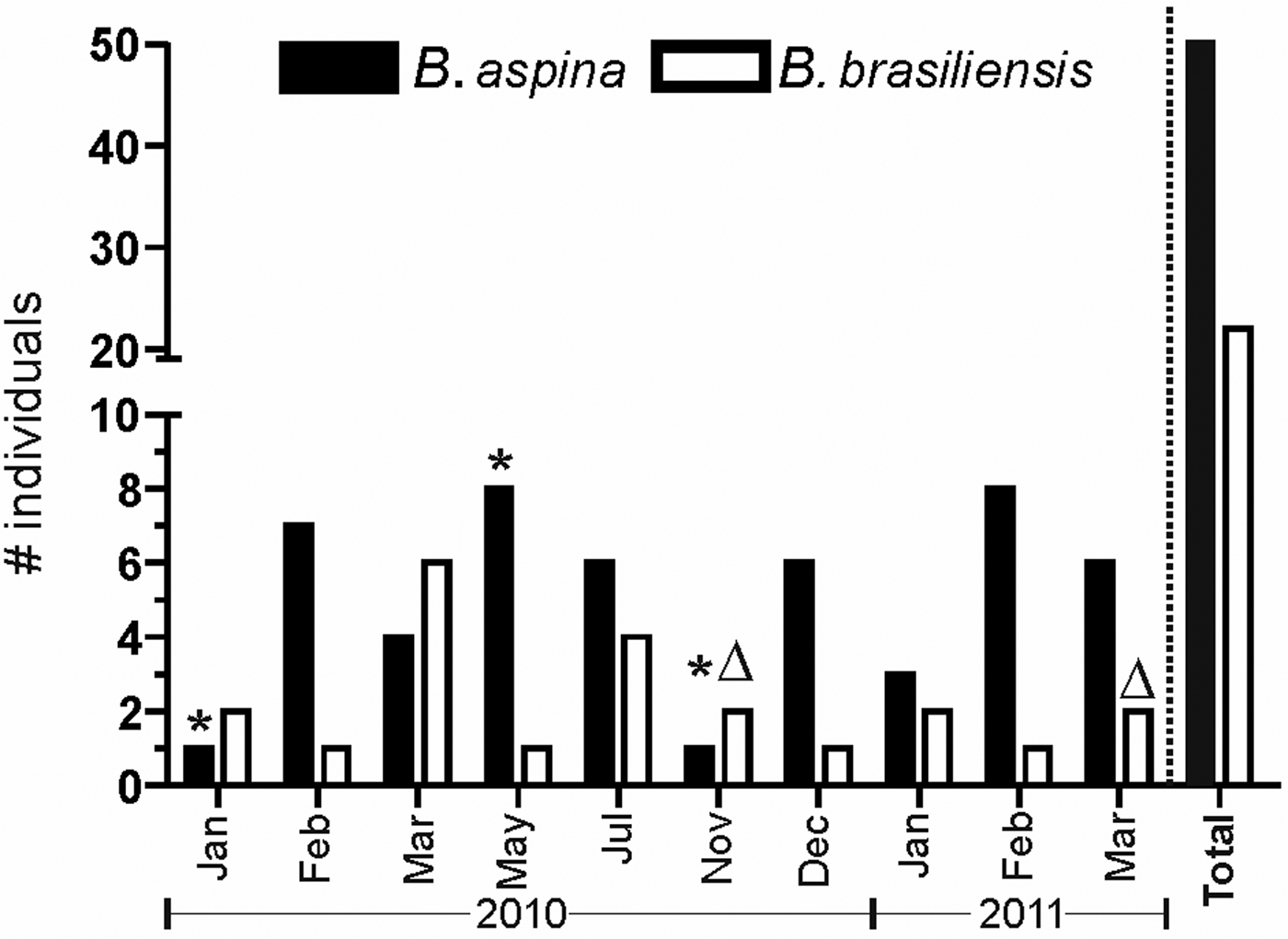
Differently from C. aurea, the specimens of B. aspina and B. brasiliensis were collected irregularly between January 2010 and March 2011. As the external morphology of these species are very alike and are also similar to other Clathrinida spp. occurring in the same region, the number of sampled individuals varied throughout the studied period, but B. aspina was constantly more abundant than B. brasiliensis (Figure 3). During the studied period, we observed that the population of both species at Forno Beach presented only a few individuals undergoing oogenesis. Borojevia aspina showed oogenesis in January, May and November 2010 (one individual in each month), while B. brasiliensis presented a single individual undergoing oogenesis in November 2010 and another in March 2011. Therefore, as C. aurea, these Borojevia spp. also presented scant reproduction, at least during the investigated period.

Fig. 3. Total number of specimens of Borojevia aspina (black bars) and B. brasiliensis (white bars) collected each month during this study, and occurrence of oogenesis for both species (*, B. aspina; Δ, B. brasiliensis). The ‘Total’ bars indicate the total number of specimens collected during the whole study.
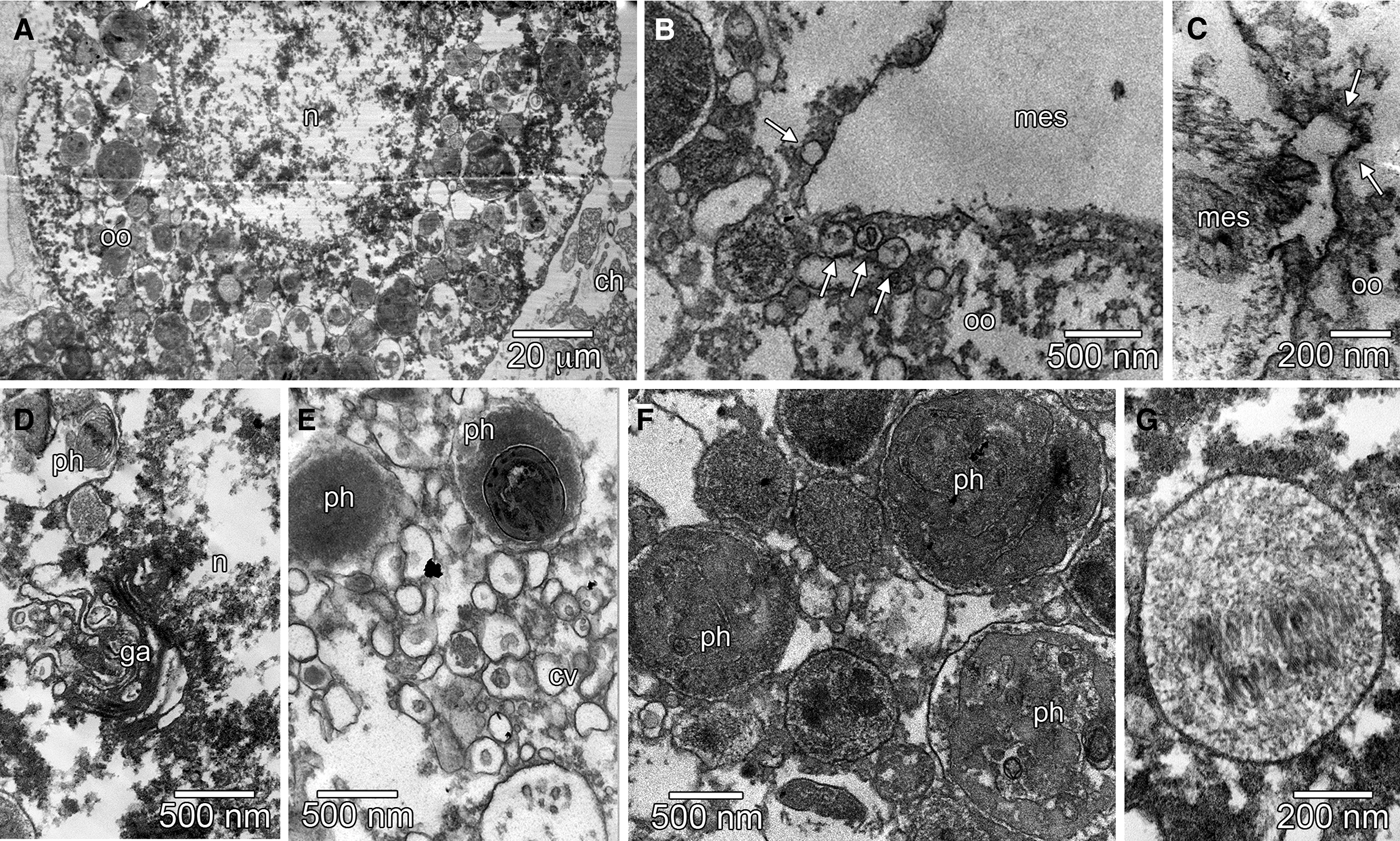
In Borojevia aspina the origin of the oocytes could not be confirmed, but they seemed to derive also from hyaline amoebocytes found in the mesohyl. These cells were usually spherical, measuring ~8–10 µm, with a large nucleus-cytoplasm ratio. The nucleus presented a large nucleolus and heterochromatin was spread throughout the nuclear matrix. Its cytoplasm lacked large electron dense inclusions, and presented several small clear vesicles (probably sections of the endoplasmic reticulum). Larger phagosomes could be observed only in a region close to the nucleus (Figure 4A). The density of these cells increased in the specimens undergoing oogenesis.

Fig. 4. Oogenesis of Borojevia aspina (A–D: TEM; E, G: scanning electron microscopy [SEM]; insert A, F, and H: LM). (A) Hyaline amoebocyte (ha) showing the nucleus (n) with heterochromatin dispersed in the nuclear matrix, and cytoplasm filled with small clear vesicles (cv) and some phagosomes (ph) (insert: light microscopy of a hyaline amoebocyte (ha) showing the nucleolated nucleus). (B) Pre-vitellogenic oocyte (oo) showing the nucleus (n) with a large nucleolus and cytoplasm with several spherical homogeneous electron dense inclusions (hi). (C) Detail of the space between the pre-vitellogenic oocyte (oo) and the basis of the adjacent choanocyte (ch) showing several small clear vesicles (arrow). (D) Detail of the pre-vitellogenic oocyte (oo) periphery showing the presence of bacteria (b) next to the oolemma. (E, F) Vitellogenic oocytes (oo) in the mesohyl (mes) contacting the adjacent choanocytes (ch). (G) Oocyte in the end of the vitellogenesis with a granular cytoplasm and pseudopodia (arrowhead). (H) Spherical oocyte (oo) in the end of vitellogenesis showing a large nucleolated (nu) nucleus (n) and granular cytoplasm. es, spongocoel.
The oocytes developed in the mesohyl. Its nucleus became larger, dispersing the heterochromatin, and in the cytoplasm, some spherical homogeneous electron dense inclusions were accumulated, characterizing the pre-vitellogenic stage (Figure 4B). After starting vitellogenesis, the oocyte grew between the choanoderm and the pinacoderm, always projecting several pseudopodia (Figure 4E–G). During this phase, the oocyte reached its maximum length (~60 µm), becoming elongated, with a large central nucleus and a very granular cytoplasm (Figure 4E–G). At the end of the vitellogenesis, the oocyte increased in volume, becoming spherical or ovoid, with a large nucleus containing a compartmentalized nucleolus, and a densely granular cytoplasm (Figure 4H). Some Golgi apparatuses with secretory vesicles and some phagosomes were observed in the perinuclear region (Figure 4I).
The oocytes of B. aspina seemed to obtain their nutrients by the exchange of small vesicles, probably containing nutritive substances, with adjacent choanocytes (Figure 4C) and amoeboid cells (nurse cells) that kept contact with the oocytes during their whole development (Figure 4G). Nevertheless, during the entire vitellogenesis, the choanocytes surrounding the oocytes did not suffer modifications (Figure 4E, F). During the beginning of the vitellogenic phase, some bacteria were observed close to the oolemma (Figure 4D), but they were not found being endocytosed or digested by the oocyte. During the maturation phase, the oocytes were surrounded by choanocytes and increased in volume, reaching ~70 µm (Figure 4H). In the meantime, the number of phagosomes, showing different stages of digestion, also increased in the cytoplasm (Figure 5A). The oolemma presented many indentations during this stage (Figure 5B, C), indicating that pinocytosis may play a role in the nutrition of the gamete. We could observe different Golgi apparatuses close to the nuclear envelope (Figure 5D). The Golgi apparatuses seemed to produce many clear vesicles that contacted the phagosomes. Early phagosomes presented an electron dense membranous content (Figure 5E, F), which underwent some modifications to produce the yolk inclusions with a condensed fibrous content (Figure 5G). These yolk inclusions were scattered in the ooplasm, indicating that the oocytes of B. aspina were isolecithal (Figure 5A).

Fig. 5. TEM of the oogenesis of Borojevia aspina. (A) Vitellogenic oocyte (oo) with finely granular nucleus (n) and cytoplasm filled with fibrous inclusions and phagosomes. (B) Large indentation in the oolemma with some clear vesicles (arrows) in the cytoplasm of the oocyte (oo). (C) Detail of an indentation in the plasma membrane (arrows) of an oocyte (oo). (D) Perinuclear (n) region of a vitellogenic oocyte containing a developed Golgi apparatus (ga) and some phagosomes (ph). (E) Ooplasm with several clear vesicles (cv) and some phagosomes (ph). (F) Ooplasm containing phagosomes (ph) with amorphous membranous substances. (G) Fibrous inclusion. ch, choanocytes; mes, mesohyl.
As in the previous species, in Borojevia brasiliensis it was also not possible to be sure about the origin of the oocytes. However, small hyaline amoebocytes measuring ~7.5 µm and with a high nucleus-cytoplasm ratio were more abundant in the few reproductive specimens. The cytoplasm of this cell was heterogeneously filled with small clear vesicles, some phagosomes with electron dense material, several mitochondria, and Golgi apparatuses (Figure 6A, B). The nucleus of the hyaline amoebocyte was spherical, presenting a finely granulated chromatin and a large central nucleolus (Figure 6A, B). The oocyte started its vitellogenesis and grew in the mesohyl, always keeping contact with the choanoderm (Figure 6C–F, H–I). We could not observe the fully developed oocyte, but the larger oocytes observed measured ~30–40 µm. These oocytes presented a large nucleus (with ~12 µm) with a large (~4 µm) acentric nucleolus with the heterochromatin mainly found close to the inner nuclear envelope (Figure 6C, G). Golgi apparatuses were not numerous in the oocytes of this species and did not seem to be actively secreting transport vesicles (Figure 6G). The ooplasm was packed with phagosomes and fibrous inclusions randomly distributed, characterizing an isolecithal egg (Figure 6G).

Fig. 6. Oogenesis of Borojevia brasiliensis (A, B, G–N: TEM; C: LM; D–F: SEM). (A) Hyaline amoebocyte (ha) localized in the mesohyl (mes). (B) Detail of the cytoplasm of the hyaline amoebocyte showing several mitochondria (m), clear vesicles (cv) and phagosomes (ph). (C) Oocyte (oo) in the mesohyl (mes) with large nucleolated (nu) nucleus (n) and a hyaline amoebocyte (ha) located close to the oocyte. (D) Oocyte (oo) during vitellogenesis with long pseudopodium (ps) below the choanocytes (ch). (E) Spherical oocyte (oo) overlaid by choanocytes (ch) and in contact with a modified choanocyte (mch). (F) Detail of the contact between the modified choanocyte (mch) and the oocyte (oo) highlighted in (E). The modified choanocyte presented a basal projection (arrow) that increases the contact between this cell and the oocyte (oo). Cytoplasm of the oocyte with fibrous inclusions (fi). (G) Vitellogenic oocyte (oo) with finely granular chromatin dispersed in the nuclear matrix (nucleolus is not shown) and the heterogeneous cytoplasm showing the Golgi apparatus (g), phagosomes (ph) and fibrous inclusions (fi). Note the presence of a constriction at the distal region of the cytoplasm indicating the formation of a pseudopodium (ps). (H) General view of the vitellogenic oocyte (oo) overlaid by choanocytes (ch). Note the presence of the modified choanocyte (mch) with the basal projection (arrow) in close contact with the oocyte. (I) Membrane continuities (arrow) and vesicles (ve) in the space between an oocyte (oo) and basal projection of a modified choanocyte (mch). (J) Bacteria (b) close to the ooplasm (arrowhead) suggesting phagocytosis. (K–N) Development of the fibrous yolk inclusion: (K) Early phagosome (eph), (L) Late phagosome (lph) showing an amorphous electron dense material, (M) Early fibrous yolk inclusions (efi) presenting an electron dense centre and fibrous content loosely distributed, (N) Fibrous yolk inclusion (fi) fully formed located close to the nucleus (n). ch, choanocytes; ext, external region of the sponge; g, Golgi apparatus; mes, mesohyl; n, nucleus; spc, spongocoel.
During the vitellogenic phase, the oocyte of B. brasiliensis projected several pseudopodia directed into the choanoderm. These projections seemed to increase the contact between the oolemma and the choanocytes’ bases, but phagocytosis of choanocytes was never observed. In addition to the oocyte’ projections, some choanocytes seemed to modify their original shape, producing an apparently permanent basal projection (Figure 6E–F, H–I). The basal projection was found in a few modified choanocytes, and apparently aided in the nutrition of the oocyte, as vesicles located in the narrow space between the two cells and some cell membrane continuities were observed (Figure 6F, I). A narrow space with several small vesicles was also constantly observed between the non-modified choanocytes and the oocyte (Figure 6H), and it is possible that these vesicles were related to the nutrition of the gamete. As in B. aspina, the oolema of B. brasiliensis oocytes presented many indentations (Figure 6J), probably enrolled in pinocytosis. The ooplasm contained phagosomes with different stages of digestion (Figure 6H). The main nutritional reserve (yolk) of this species started with the phagosomes with membranous and amorphous content surrounded by small vesicles with clear (transparent) content (Figure 6K). After that, the content inside the phagosome became more electron dense and amorphous (Figure 6L). Then, the amorphous substance was gradually modified into a fibrous material (Figure 6M). The final and most abundant form of yolk was a compacted fibrous inclusion limited by membrane (Figure 6N).
Evolutionary aspects of oogenesis traits
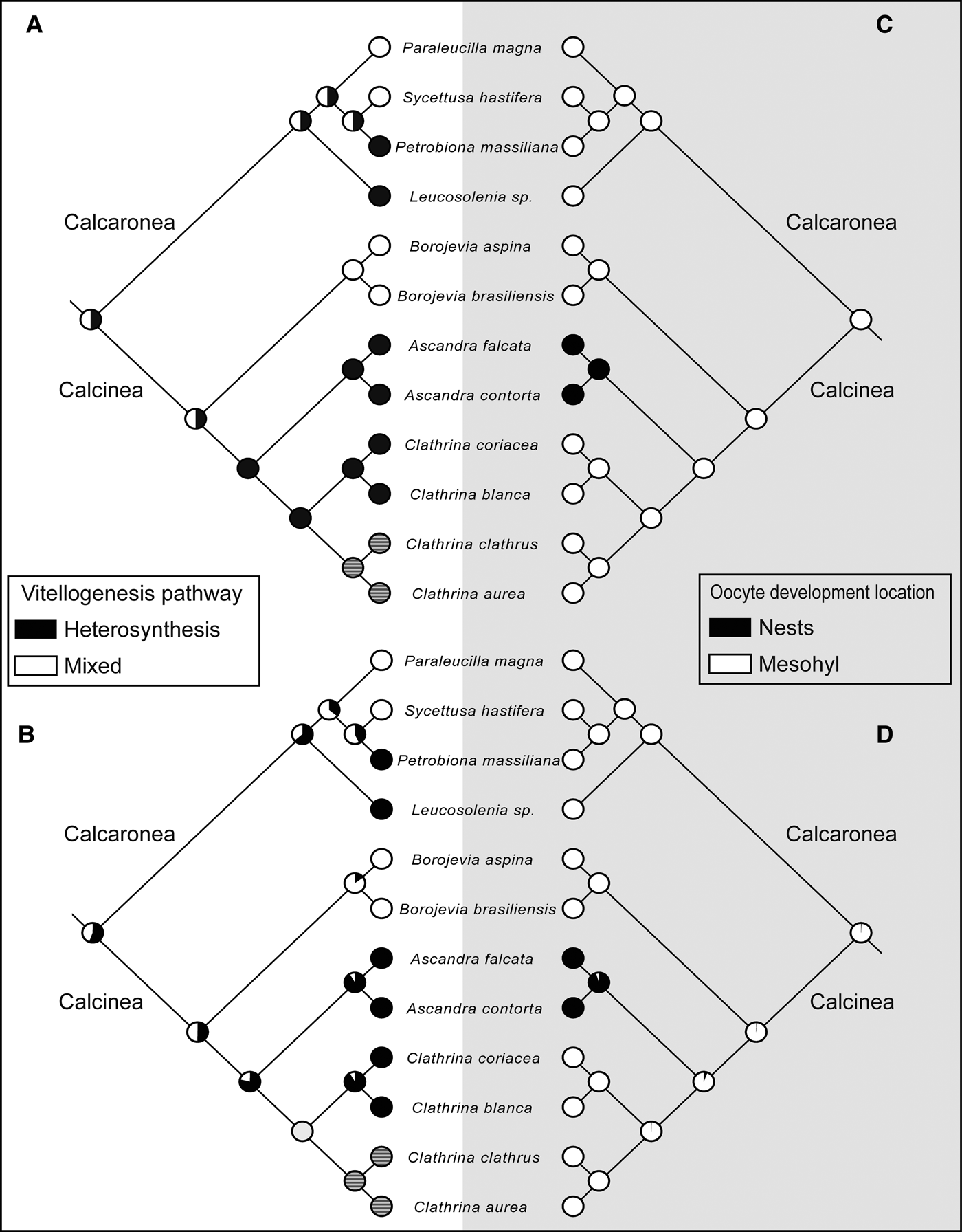
The MP and ML reconstructions provided similar results. The ancestral state of vitellogenesis for Calcarea and for Calcinea was equivocal for both MP and ML analyses. However, for Borojevia the ancestral state recovered was the mixed pathway, while for Ascandra it was heterosynthesis. For the genus Clathrina, this characteristic was dubious, with MP pointing to the heterosynthesis and ML to the mixed pathway, probably because of the lack of knowledge on the vitellogenesis pathway of the yellow clathrinas – C. aurea and C. clathrus (Figure 7A, B).

Fig. 7. Reconstruction of the ancestral condition of the vitellogenesis pathway (A, B) and the oocyte development location (C, D) for Calcarea. (A and C) Maximum parsimony analysis, (B and D) Maximum likelihood analysis. Dashed circles mean unknown conditions.
The majority of the species of Clathrinida, and also of Calcaronea, do not present any special structure for egg development. Therefore, the ancestral condition in the class Calcarea was probably the oocyte development location in the mesohyl, without any special structure. The only exception is the genus Ascandra, which is the single lineage in which nests were developed (Figure 7C, D).
DISCUSSION
Sperm cells have never been described in Calcinea (Ereskovsky, Reference Ereskovsky2010) and, accordingly, in the present work we did not observe any indication of spermatogenesis. We could not observe embryonic cleavages, or larva, oogenesis being the single reproductive aspect observed for these species. In fact, the oogenesis was restricted only to a few individuals that do not seem to dedicate a large effort in producing a high number of oocytes. The oogenesis process of the three studied species was similar, with oocytes apparently originating from hyaline amoebocytes and yolk production, at least in Borojevia spp., being made in a mixed pathway without the development of any special structure to nourish the egg.
The rarity of oocytes in the three studied species was unexpected. Generally in calcareous sponges the reproductive effort (fecundity) is elevated, and reproductive elements can be observed throughout the sponge body (Lanna et al., Reference Lanna, Paranhos, Paiva and Klautau2014 and references therein). In calcineans, the high number of reproductive elements observed in some species seems to be associated with the formation of nests or follicles surrounding the oocytes (Dendy, Reference Dendy1913; Borojevic, Reference Borojevic1969; Ereskovsky & Willenz, Reference Ereskovsky and Willenz2008). Species that do not form any special structure, such as nests and follicles, to mature their oocytes (e.g. Clathrina blanca (Miklucho-Maclay, 1868), C. coriacea (Montagu, 1814) and C. clathrus (Schmidt, 1864)) showed a reduced number of oocytes (Johnson, Reference Johnson1979, Gaino et al., Reference Gaino, Pansini, Pronzato, Cicogna, Reitner and Keupp1991). The same was observed here. Clathrina aurea, B. aspina and B. brasiliensis showed no nests or follicles and produced a low number of reproductive elements.
It is interesting that this scarcity of oocytes and other reproductive elements has also been observed in Clathrina clathrus, a sibling species of C. aurea (Rossi et al., Reference Rossi, Russo, Solé-Cava, Rapp and Klautau2011; Klautau et al., Reference Klautau, Azevedo, Cóndor–Luján, Rapp, Collins and Russo2013). After a long-term study, the period of sexual maturity of the former species could not be established (Gaino et al., Reference Gaino, Pansini, Pronzato, Cicogna, Reitner and Keupp1991). Actually, during that study, only a single individual of C. clathrus presented oocytes (E. Gaino, personal communication). However, it is unlikely that these yellow clathrinas species lack sexual reproduction, as a low number of clonal individuals of C. aurea were observed in another study (A. Padua & M. Klautau, unpublished). It is most possible that oogenesis in Clathrina and Borojevia occurs very quickly or is restricted to some tubes that escaped our observation. Therefore, we suggest that future studies dealing with this species should constrain the sampling periodicity to shorter intervals.
Origin of the oocytes
Our current data do not allow us to undoubtedly affirm which cell type is the precursor of the oocytes. Nonetheless, we are suggesting that oocytes may derive from hyaline amoebocytes, based on a set of characteristics of these cells: presence of a large spherical nucleus with a single large nucleolus, hyaline cytoplasm relatively clear of organelles, and high nucleus-cytoplasm ratio (Extavour & Akam, Reference Extavour and Akam2003). A strong point that could make our assumption invalid is that hyaline amoebocytes did not show specific markers of primordial germ cells, such as nuage (dense bodies used as ultrastructural markers found in the cytoplasm of germ cells; Extavour & Akam, Reference Extavour and Akam2003). However, this characteristic was also not observed in previous studies dealing with sponges and other non-model marine invertebrates (reviewed in Simpson, Reference Simpson1984; Extavour & Akam, Reference Extavour and Akam2003). Besides, the increase in the number of hyaline amoebocytes in specimens of C. aurea, B. aspina and B. brasiliensis undergoing oogenesis and the cytoplasm staining similar to oocytes could suggest that the hyaline amoebocytes are the precursors of the oogenesis in these species. A concurrent hypothesis is that the oocytes could be derived from choanocytes, as already observed in calcaroneans (e.g. Franzén, Reference Franzén1988; Ereskovsky, Reference Ereskovsky2010; Lanna & Klautau, Reference Lanna and Klautau2010). However, in those studies no ultrastructural or molecular markers were presented to confirm that choanocytes are, in fact, the precursors of the gametes. Some authors indicated that hyaline amoebocytes of calcareous sponges could be an autochthonous lineage (i.e. directly derived from larval cells) (Pavans-de-Ceccatty, Reference Pavans-de-Ceccatty1955; Vacelet, Reference Vacelet1964; Simpson, Reference Simpson1984), but the differentiation from an epithelial cell (pinacocyte or choanocyte) cannot be excluded.
Moreover, the hyaline amoebocytes in the current investigated species are probably not exclusively committed to a germ cell fate, but rather could acquire this characteristic during the development of the sponge. Thus, they can be considered as the archaeocytic lineage of these sponges (Simpson, Reference Simpson1984). Future studies searching for expression of genes of totipotent lineages expressed in demosponge archaeocytes and cnidarian I-cells (such as Piwi and Vasa) will help in the understanding of how germ cells arise in Calcinea.
The oogenesis of the three species occurs in the mesohyl without formation of special structures
The observed steps of the oogenesis of C. aurea, B. aspina and B. brasiliensis occurred in the mesohyl, without formation of any special structure. Actually, the presence of oocytes in these species does not seem to affect the adjacent choanocytes (except in B. brasiliensis, in which a choanocyte developed a basal projection next to the oocyte). In Calcinea, there are three locations for the oogenesis: (i) oocytes develop in the mesohyl directly under the choanoderm, without affecting the morphology of the surrounding choanocytes (e.g. the present species); (ii) oocytes are covered by a follicle, which will surround individually each oocyte/embryo (e.g. Ascaltis gardineri (Dendy, Reference Dendy1913) and Clathrina arnesenae Rapp, 2006 (Dendy, Reference Dendy1913; Ereskovsky & Willenz, Reference Ereskovsky and Willenz2008)); and (iii) oocytes mature inside nests (e.g. Ascandra falcata, A. minchini, A. contorta (Borojevic, Reference Borojevic1969)). The reconstruction of the ancestral conditions of the oogenesis of Calcinea should be taken cautiously due to the lack of knowledge on the reproduction of this subclass. However, our phylogenetic character mapping analysis showed that the ancestral egg development location in Calcarea seems to be the mesohyl, directly under the choanoderm. However, as the whole oogenesis of C. aurea and B. brasiliensis was not completely observed, we cannot ignore the possibility of follicle formation at the end of oogenesis, as previously observed in other calcineans (Ereskovsky & Willenz, Reference Ereskovsky and Willenz2008).
Nests are anarchic tissues derived from choanocyte tubes, which are composed of several phagocytes in degeneration involved in the nourishment of eggs, embryos and early larvae (Borojevic, Reference Borojevic1969). This structure was found in three species of the genus Ascandra (A. minchini, A. falcata and A. contorta) (Borojevic, Reference Borojevic1969). Previously, A. contorta was placed in the genus Clathrina (Klautau et al., Reference Klautau, Azevedo, Cóndor–Luján, Rapp, Collins and Russo2013), indicating that nests were possibly a homoplastic characteristic of Clathrinida. Nevertheless, recently molecular data showed that C. contorta is in fact an Ascandra (Klautau et al., Reference Klautau, Azevedo, Cóndor–Luján, Rapp, Collins and Russo2013). Therefore, the emergence of this characteristic seems to be exclusive of the lineage leading to the genus Ascandra as presented here (Figure 7C, D).
Oocyte nourishment and vitellogenesis
Sciscioli et al. (Reference Sciscioli, Scalera Liaci, Lepore, Gherardi and Simpson1991, Reference Sciscioli, Lepore, Gherardi and Scalera Liaci1994) highlighted that sponges have several methods of acquiring nutrients to produce energy storage structures in the ooplasm: phagocytosis of somatic cells, pinocytosis, assimilation of material from superficial cells, nurse cells and capture of symbiotic bacteria. In the present study, none of the three investigated species presented any special structure to nourish their eggs, neither phagocytized somatic cells (e.g. choanocytes or mesohyl amoebocytes) or bacteria. We were not able to observe the yolk structure and its development in C. aurea eggs in TEM, but the ultrastructure of the yolk inclusion was similar in both B. aspina and B. brasiliensis. The vitellogenesis of these Borojevia spp. was also similar, except for the presence of amoeboid nurse cells in B. aspina and the presence of modified choanocytes in B. brasiliensis. Borojevia aspina and B. brasiliensis yolk inclusions were derived from phagosome-like structures surrounded by clear vesicles, probably containing digestive enzymes. Phagosome-like structures that undergo drastic transformation leading to the production of the yolk inclusions seem to be a widespread characteristic of Porifera, indicating that the synthesis and storage of reserve material may follow similar metabolic processes in the entire phylum (Gaino & Sarà, Reference Gaino and Sarà1994).
Cytoplasmic inclusions with fibrous contents surrounded by membrane were considered to be the yolk inclusion in both B. aspina and B. brasiliensis eggs. Yolk with fibrous content seems to be the most common type observed in all Calcarea studied so far (Gallissian, Reference Gallissian1981; Gaino et al., Reference Gaino, Burlando and Buffa1987; Lanna & Klautau, Reference Lanna and Klautau2010) and could be observed even in later stages of the development of several species of Calcinea and Calcaronea (Borojevic, Reference Borojevic1969; Ereskovsky & Willenz, Reference Ereskovsky and Willenz2008; Ereskovsky, Reference Ereskovsky2010; Lanna & Klautau, Reference Lanna and Klautau2012). Gallissian (Reference Gallissian1981) observed that the ooplasm of Grantia compressa (Fabricius, 1780) was rich in carbohydrates other than glycogen, but the proper nature of this yolk type is still unknown. In fact, little is known about the biochemical and molecular components of the yolk in Porifera, and future studies should focus on this issue in order to improve our knowledge about the vitellogenesis of sponges. The fibrous yolk inclusion is exclusive to Calcarea (see Ereskovsky, Reference Ereskovsky2010), but inclusions containing loose and disorganized fibrous material are found in some oviparous demosponges, usually at the cortical region of the egg (Sciscioli et al., Reference Sciscioli, Scalera Liaci, Lepore, Gherardi and Simpson1991, Reference Sciscioli, Lepore, Gherardi and Scalera Liaci1994).
The events that occur during oogenesis are similar across all invertebrates, but some processes, such as vitellogenesis, are phylogenetically constrained. Vitellogenesis is the synthesis and accumulation of ooplasmic reserves (yolk) in the oocytes. The amount and the size of the eggs produced by an organism are intimately related to life-history strategies, as the combination of these two parameters represents the amount of energy that has been allocated to the next generation (Ramirez–Llodra, Reference Ramirez-Llodra2002). Heterosynthetic yolk production is usually related to opportunistic species, as a rapid vitellogenesis is necessary due to a non-predictable environment, meanwhile autosynthetic yolk production is usually observed in species with longer oogenesis time span that live in stable environments (Ramirez–Llodra, Reference Ramirez-Llodra2002; Eckelbarger, Reference Eckelbarger2005 and references therein). A third vitellogenic pathway is the combination of both autosynthetic and heterosynthetic pathways, and is termed a mixed pathway (Ramirez–Llodra, Reference Ramirez-Llodra2002). In calcareans, Lanna & Klautau (Reference Lanna and Klautau2010) suggested that the vitellogenesis of Paraleucilla magna Klautau, Monteiro & Borojevic, 2004 is performed in a mixed pathway allowing the quick development of its oocytes. The same seems to happen with the Clathrinida species studied here. It is possible that choanocytes provide substances with low molecular weight (through clear vesicles) that are used in vitellogenesis, while the oocytes ingest by themselves substances of unknown nature, which are digested in their phagosomes.
Due to the limited information on the reproductive traits of Calcarea, we cannot confirm whether either the mixed or the heterosynthetic pathways were the ancestral character state in Calcarea. It still is necessary to investigate other species of both Calcinea and Calcaronea if we want to understand the evolution of the reproductive characters of the group. Nevertheless, based on previous and present studies, it is improbable that an autosynthetic pathway is the primitive condition in this group of sponges. This suggestion goes against the opinion that autosynthesis is the most primitive vitellogenic pathway found in Metazoa (Eckelbarger & Larson, Reference Eckelbarger and Larson1992), but reaffirms the view of Riesgo & Maldonado (Reference Riesgo and Maldonado2009) that the presence of the three types of vitellogenesis in sponges is probably not related to their phylogenetic position.
ACKNOWLEDGEMENTS
We would like to thank Vinicius Padula, Juliana Bahia, Roberta Cavalcanti, Eduardo Rezende and his crew of LitoralSub dive centre from Cabo Frio (RJ), Nenem and Nido from Arraial do Cabo (RJ), and our lab mates for their invaluable help in the fieldwork. We also thank the heads of the Laboratório de Ultraestrutura Celular Hertha Meyer (IBCCF) and he Serviço de Microscopia Eletrônica-CPqGM-FIOCRUZ Bahia, for their help in the preparation of the specimens and the use of the facilities for electron microscopy. We thank Inácio DS Neto for the borrowing of some fixatives.
FINANCIAL SUPPORT
Grants and fellowships were provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (E-26/111.541/2008), and the Brazilian National Council of Technological and Scientific Development (CNPq) (480368/2008-2; 302442/2011-1; 477227/2013-9). This work was part of the Doctorate Thesis of E. Lanna, presented to the Post-Graduation Program in Zoology of the Museu Nacional do Rio de Janeiro/UFRJ.