INTRODUCTION
Seagrass beds constitute a conspicuous feature of sheltered soft-shores worldwide, and are amongst the most productive marine communities (Duarte & Chiscano, Reference Duarte and Chiscano1999; Mateo et al., Reference Mateo, Cebrian, Dunton, Mutchler, Larkum, Orth and Duarte2006). As a result, the ecology of seagrass beds and their associated macrofaunal communities has attracted considerable attention in recent decades (Kikuchi, Reference Kikuchi, Phillips and McRoy1980; Hemminga & Duarte, Reference Hemminga and Duarte2000). Research to date suggests that the presence of seagrass positively influences macrofaunal abundance, biomass, and diversity through: (1) enhanced particulate organic matter (POM) deposition and retention within vegetated beds; (2) enhanced larval settlement; (3) provision of greater surface area and habitat complexity; (4) provision of food resources; and (5) protection from predators (Fonseca et al., Reference Fonseca, Fisher, Zieman and Thayer1982; Heiss et al., Reference Heiss, Smith and Probert2000; Kharlamenko et al., Reference Kharlamenko, Kiyashko, Imbs and Vyshkvartzev2001; Gacia et al., Reference Gacia, Duarte, Marba, Terrados, Kennedy, Fortes and Tri2003; Atilla et al., Reference Atilla, Fleeger and Finelli2005; Bos et al., Reference Bos, Bouma, de Kort and van Katwijk2007).
Seagrass beds are under increasing pressure from environmental stressors such as eutrophication, increased sediment loads, and chemical contaminants (Ralph et al., Reference Ralph, Tomasko, Moore, Seddon, Macinnis-Ng, Larkum, Orth and Duarte2006). The loss of seagrass beds from coastal ecosystems is likely to have a major impact on the structure and functioning of associated invertebrate communities (Kenworthy et al., Reference Kenworthy, Wyllie-Echeverria, Coles, Pergent, Pergent-Martini, Larkum, Orth and Duarte2006); however, some components of seagrass-associated fauna, such as meiofauna, are poorly known. Most meiofaunal studies have focused on epiphytic meiofauna (Novak, Reference Novak1982; Hall & Bell, Reference Hall and Bell1993; De Troch et al., Reference De Troch, Fiers and Vincx2001a; Da Rocha et al., Reference Da Rocha, Venekey, Bezerra and Souza2006), and a few have investigated the meiofauna living in seagrass bed sediments (Bell et al., Reference Bell, Walters and Kern1984; Giere, Reference Giere2009). Nematodes, which typically dominate meiofaunal communities (Heip et al., Reference Heip, Vincx and Vranken1985), are poorly represented in studies of seagrass-associated meiofauna (e.g. Fisher, Reference Fisher2003), even though they may play an important role in the energetics of these ecosystems (Danovaro et al., Reference Danovaro, Gambi and Mirto2002).
Several authors have argued that the high bacterial standing stock and/or organic detritus content of seagrass bed sediments have a positive influence on meiofaunal abundance and biomass (Castel et al., Reference Castel, Labourg, Escaravage, Auby and Garcia1989; Danovaro, Reference Danovaro1996; Danovaro & Gambi, Reference Danovaro and Gambi2002). Experimental evidence has also linked changes in nematode abundance with changes in the amount of fine organic particles associated with the presence of seagrass cover (Edgar, Reference Edgar1999). The high abundances of small predators such as shrimps and juvenile flatfish found within seagrass beds may, on the other hand, have adverse effects on meiofaunal abundance (Decho et al., Reference Decho, Hummon and Fleeger1985). The shallower redox potential discontinuity (RPD) layer sometimes associated with the high organic load of seagrass bed sediments could affect the vertical distribution of meiofauna by restricting their distribution to oxygenated surface sediments (Barron et al., Reference Barron, Marbaa, Terrados, Kennedy and Duarte2004). The concentration of meiofauna near the sediment surface could, in turn, make them more susceptible to predation (Coull & Bell, Reference Coull, Bell and Livingston1979; Sogard, Reference Sogard1984).
Meiofaunal diversity and community structure are likely to be affected by the presence of seagrass. Seagrass may affect meiofaunal composition through its effect on sediment characteristics (Ndaro & Olafsson, Reference Ndaro and Olafsson1999), organic content (Castel et al., Reference Castel, Labourg, Escaravage, Auby and Garcia1989), exposure to currents (Steyaert et al., Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003), and the availability of food sources such as bacteria and benthic microalgae (Danovaro & Gambi, Reference Danovaro and Gambi2002; Fisher, Reference Fisher2003; Fisher & Sheaves, Reference Fisher and Sheaves2003). Meiofaunal assemblages may also be influenced by the complex structure of seagrass rhizomes and the release of oxygen and dissolved organic matter in the sediments by the roots (Osenga & Coull, Reference Osenga and Coull1983; Marba et al., Reference Marba, Holmer, Gacia, Barron, Larkum, Orth and Duarte2006).
Nematodes provide a good model for investigating the impact of seagrass on the structure of invertebrate communities due to their high diversity and sensitivity to environmental conditions (Bongers & Ferris, Reference Bongers and Ferris1999; Moreno et al., Reference Moreno, Vezzulli, Marin, Laconi, Albertelli and Fabiano2008). The small size and limited dispersal capabilities of nematodes also make them ideal organisms for studying changes in environmental conditions over small spatial scales. Studying sites over small spatial scales (which differ mostly in the presence or absence of seagrass cover) may provide more meaningful comparisons than studies comparing sites further apart, which may be subject to contrasting environmental conditions (e.g. hydrology and water depth) (Mills & Berkenbusch, Reference Mills and Berkenbusch2009). In addition, the effect of seagrass cover on nematode community structure and diversity can be studied both horizontally (metre scale) and vertically (centimetre scale) (e.g. Steyaert et al., Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003).
Nematodes can be assigned to feeding types based on buccal structures (Moens & Vincx, Reference Moens and Vincx1997), allowing the effect of seagrass on benthic trophic pathways to be evaluated. Several authors have reported high abundance of microbial- and deposit-feeding nematodes in seagrass meadows, which suggests that detritus is an important food for meiofauna of vegetated sediments (Hopper & Meyers, Reference Hopper and Meyers1967; Danovaro & Gambi, Reference Danovaro and Gambi2002; Fisher, Reference Fisher2003; Fisher & Sheaves, Reference Fisher and Sheaves2003). This is in agreement with the suggestion that seagrass cover enhances the input of detritus to the benthos relative to bare sediments (Marba et al., Reference Marba, Holmer, Gacia, Barron, Larkum, Orth and Duarte2006). Few studies, however, have compared the diet of meiofauna in seagrass beds and adjacent unvegetated sediments (Leduc et al., Reference Leduc, Probert and Duncan2009).
The paucity of data on the distribution of meiofauna in seagrass beds and adjacent unvegetated sediments makes any generalization about the effects of seagrass cover on the ecology of meiofauna difficult. In the present study, the abundance and biomass of meiofauna in an intertidal Zostera muelleri meadow and adjacent unvegetated sediments in Papanui Inlet, southern New Zealand, were compared to test whether the presence of seagrass has a positive effect on meiofaunal abundance and biomass. In addition, nematode community structure, diversity, and feeding groups were compared between habitats, and at different sediment depths, to investigate the effect of Z. muelleri on nematode species distribution and feeding ecology. This investigation was part of an integrated study on the role of meiofauna in the energy flows of intertidal benthic communities (Leduc et al., Reference Leduc, Probert and Duncan2009).
MATERIALS AND METHODS
Study location
The study was carried out in Papanui Inlet, southern New Zealand, as described in Leduc et al. (Reference Leduc, Probert and Duncan2009). Papanui Inlet is an unpolluted sheltered inlet with an area of 3.5 km2; tides are semidiurnal with a mean tidal range of 1.15 m (Albrecht & Vennell, Reference Albrecht and Vennell2007). Most of the inlet is exposed at low tide and consists of a patchwork of unvegetated sediments and Zostera muelleri beds. Three 10 × 10 m sampling sites were used during the study: an unvegetated site (45°50′53.1″S 170°42′40.9″E), a sparsely vegetated site (1250 shoots m−2; 45°50′46.8″S 170°42′35.1″E) and a densely vegetated site (8920 shoots m−2; 45°50′48.1″S 170°42′35.7″E). The sites were within 150 m of each other, and situated at least 30 m from the edge of the Z. muelleri meadow. The unvegetated site and the densely vegetated site were sampled in June 2005 and January 2006. The sparsely vegetated site was added in January 2006 to provide comparison between densely and sparsely vegetated habitats.
Sampling
Sediment characteristics were measured in June 2005 and January 2006. Sediment samples (N = 5) were obtained using randomly allocated cores (2.6 cm diameter) taken to a depth of 5 cm. Samples were split into 0–2 and 2–5 cm depth fractions and stored in dark plastic containers. Separate cores were taken for water and organic matter content, sediment granulometry and pigment analyses.
Meiofauna samples (N = 3–4) were obtained using randomly allocated cores (2.6 cm diameter) to a depth of 5 cm in June 2005 and to a depth of 10 cm in January 2006. Samples were split into 0–2 and 2–5 cm depth fractions in June 2005 and 0–2, 2–5, and 5–10 cm depth fractions in January 2006. Seagrass blades were cut at the sediment surface prior to taking the cores at the vegetated sites. An aluminium casing with handles was used to help penetration of the core into the sediment at the densely vegetated site, which was characterized by a dense seagrass rhizome mat. Meiofauna samples were stained with rose Bengal and fixed in warm (70°C) 5% formalin.
Laboratory procedures
Sediment water content was determined by weight loss after drying at 60°C for 48 hours, and organic content was measured by loss on ignition of dried samples at 550°C for 5 hours. Sediment granulometry was determined by wet (silt fraction) and dry sieving (sand fraction) of fresh sediment samples no more than 2 days after collection (Bale & Kenny, Reference Bale, Kenny, Eleftheriou and McIntyre2005). Chlorophyll-a and phaeophytin were extracted by boiling homogenized and freeze-dried sediment samples in 90% ethanol. The extract was analysed using a spectrophotometer (Beckman DU-70) and included an acidification step to separate degradation products from chlorophyll-a (Sartory, Reference Sartory1982).
Meiofauna samples were washed on a 500 µm sieve to remove large particles and macrofauna, and on a 45 µm sieve to retain meiofauna. Meiofauna were extracted from the sieved sediments by Ludox flotation, transferred to pure glycerol, and mounted onto permanent slides (Somerfield & Warwick, Reference Somerfield and Warwick1996). Meiofauna were counted and identified to major taxa under a compound microscope (100× magnification). A sub-sample of 100 individuals (or all individuals if fewer were present in the sample) of each taxon was randomly selected and used for biomass determination. Meiofaunal biomass was measured using video image analysis (Grove et al., Reference Grove, Probert, Berkenbusch and Nodder2006). Body volumes were converted to dry weight by assuming a relative density of 1.13 and a dry:wet weight ratio of 0.25 (Feller & Warwick, Reference Feller, Warwick, Higgins and Thiel1988). Foraminiferans and soft-bodied taxa such as turbellarians are not quantitatively extracted using the Ludox method and were excluded from the analysis.
In January 2006 at least 150 nematodes (or all individuals if fewer were present in the sample) from each site and depth fraction (0–2, 2–5 and 5–10 cm) were randomly selected and mounted separately for community structure analysis. Three replicates were analysed for each site and depth combination. Specimens were identified to genus/putative species using the descriptions by Platt & Warwick (Reference Platt and Warwick1983, Reference Platt and Warwick1988) and Warwick et al. (Reference Warwick, Platt and Somerfield1998), as well as the primary literature. Nematodes were assigned to feeding groups based on their buccal structures using the modified classification of Wieser (Reference Wieser1953) proposed by Moens & Vincx (Reference Moens and Vincx1997): microvores (M), deposit feeders (DF), epistrate feeders (EF), ciliate feeders (CF), facultative predators (FP) and predators (P).
Data analysis
Data were assessed for normality and homogeneity of variance using the Anderson–Darling normality test and Levene's test, respectively (Quinn & Keough, Reference Quinn and Keough2009). When necessary, data were log(x + 1)-transformed to meet assumptions for parametric analyses. Sediment characteristics within each depth fraction and sampling time were compared using t-tests (June 2005) and one-way ANOVA with Tukey's post-hoc test (January 2006).
Nematode, copepod, and total meiofaunal abundance and biomass in the top 5 cm of sediments were compared between the unvegetated and densely vegetated sites and between sampling times (June 2005 and January 2006) using two-way ANOVA. A comparison of nematode density (ind. cm−3) was carried out on the January 2006 data across all three sites and sediment depths using two-way ANOVA with a split-plot design (replicates nested within sites but not within depths) (Steyaert et al., Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003). Nematode diversity indices (i.e. Hill's diversity N1, species evenness J′, and expected number of species in a sample of 50 individuals ES(50)) were compared between sites and sediment depths in the same way. Depth-integrated diversity indices in January 2006 were compared between sites using one-way ANOVA. Nematode community structure from the three sites and sediment depths (January 2006) was compared by constructing Bray–Curtis similarity matrices from square-root transformed relative abundance data in PRIMER v6 (Clarke, Reference Clarke1993). Differences in community structure were tested using two-way ANOSIM.
RESULTS
Sediment characteristics
Sediment at the study sites consisted of well-sorted fine sand (Table 1). Sediments at the densely vegetated site had higher silt, water, organic matter content, and phaeophytin concentrations than at the unvegetated site (P < 0.05). Differences were more pronounced in January 2006 than June 2005. Sediment characteristics at the sparsely vegetated site were intermediate between those of the unvegetated and densely vegetated sites. The depth of the redox potential discontinuity (RPD) layer was deeper than 10 cm at the unvegetated and sparsely vegetated sites, and approximately 2 cm at the densely vegetated site.
Table 1. Sediment characteristics at the unvegetated, sparsely vegetated, and densely vegetated sites at Papanui Inlet in winter (June 2005) and summer (January 2006). Results are mean (SD) (N = 5). Within each sampling time, values followed by different letters were statistically different (P < 0.05) from corresponding values at the other site(s) for that sediment depth interval (t-test or one-way ANOVA with Tukey's post-hoc test).

Meiofaunal abundance and biomass
Mean meiofaunal abundance and biomass in the top 5 cm of sediments ranged from 2519 to 4979 ind. 10 cm−2 and from 251 to 528 mgDW m−2, respectively (Table 2). There was no significant difference in total meiofaunal abundance between sites or sampling time (two-way ANOVA, P > 0.05), but meiofaunal biomass was significantly higher at the densely vegetated site than at the unvegetated site (483 versus 294 mgDW m−2, P < 0.05; Table 3). Nematodes and copepods represented over 95% of meiofaunal abundance and biomass at the study sites; other taxa were therefore excluded from further statistical analysis. Nematode abundance and biomass were significantly higher at the densely vegetated site than at the unvegetated site (two-way ANOVA, P < 0.05), whereas copepod abundance and biomass showed the opposite trend (P < 0.001).
Table 2. Meiofaunal abundance (ind. 10 cm−2) and biomass (mgDW m−2) at the three study sites at Papanui Inlet. Results are mean (SD) (N = 3–4). Nd, no data.

Table 3. Two-way ANOVA testing for differences in meiofauna, nematode, and copepod abundance and biomass across the study sites and sampling times. Probability for main effects and interactions shown in bold type are significant at α = 0.05.

Nematode abundance, diversity, and community structure
Two-way ANOVA revealed the presence of significant site, sediment depth and interaction effects on nematode density (expressed as ind. cm−3) in January 2006 (Table 4). Mean nematode density decreased with depth at all sites, but most markedly at the vegetated sites (Figure 1). Nematode density at 0–2 cm depth was 2–3 times greater at the vegetated sites than at the unvegetated site, but little between-site difference was observed at 2–5 and 5–10 cm depths.

Fig. 1. Mean (N = 3) nematode density and Hill's diversity (N1) at the three Papanui Inlet study sites and sediment depths, January 2006. Error bars are standard deviation from the mean.
Table 4. ANOVA tests for differences in nematode abundance (N), Hill's diversity (N1), species evenness (J′), and expected number of species (ES(50)) between sites and sediment depths at Papanui Inlet, January 2006. Probability for main effects and interactions shown in bold type are significant at α = 0.05.

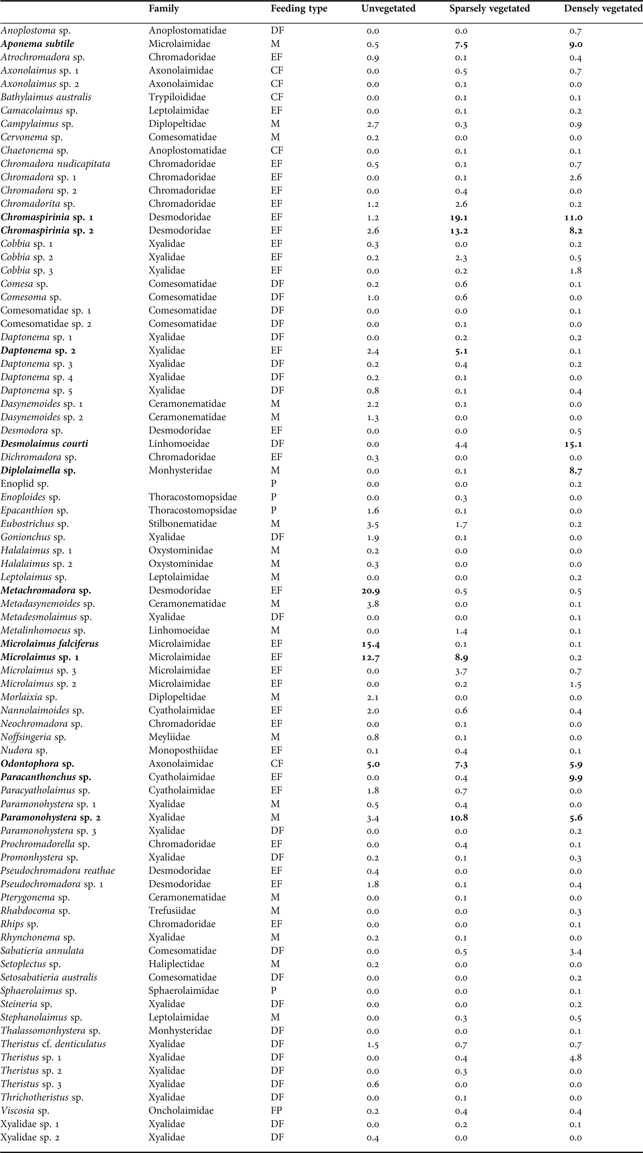
A total of 84 nematode species was identified: 45 at the unvegetated site, 59 at the sparsely vegetated site and 57 at the densely vegetated site. The unvegetated site had 10 species exclusive to that site while the sparsely and densely vegetated sites had 8 and 13 unique species, respectively. Two-way ANOVA comparing nematode diversity across sites and sediment depths revealed significant interaction effects for all diversity indices (Table 4). Diversity increased with depth at the unvegetated site, whereas the opposite trend was observed at the vegetated sites (Figure 1). There was no significant difference in depth-integrated diversity indices between sites (one-way ANOVA, P > 0.05).
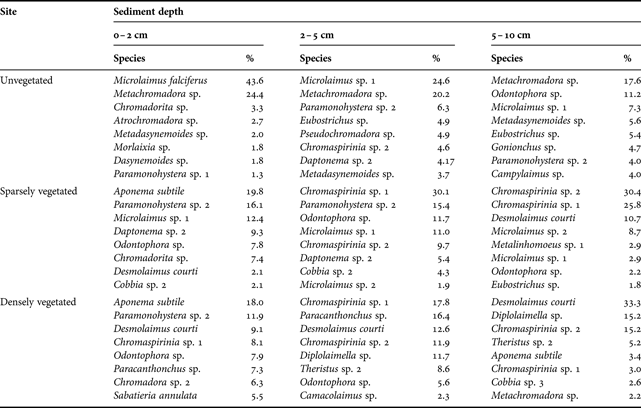
The nematode community at the unvegetated site was dominated by Metachromadora sp., Microlaimus falciferus and Microlaimus sp. 1 (Table 5 & Appendix). The most common species at the sparsely vegetated were Chromaspirinia sp. 1, Chromaspirinia sp. 2, Paramonohystera sp. 2 and Microlaimus sp. 1. At the densely vegetated site, Desmolaimus courti, Chromaspirinia sp. 1, Paracanthonchus sp. and Aponema subtile dominated. The two-dimensional MDS ordination plot and two-way ANOSIM showed a significant effect of site (R = 0.981, P = 0.1%) and depth (R = 0.781, P = 0.1%) on nematode community structure (Figure 2; Table 6). Pairwise comparisons showed significant differences between all sites and depths. Top (0–2 cm) and bottom (5–10 cm) layers were the most different, whereas middle (2–5 cm) and bottom layers were most similar. Pairwise comparisons, however, should be treated with caution since fewer than 4 replicates were compared (Clarke, Reference Clarke1993).

Fig. 2. Two-dimensional multidimensional scaling configuration for nematode species abundance in Papanui Inlet, January 2006. T (top), M (middle), and B (bottom) refer to sediment depths 0–2, 2–5 and 5–10 cm, respectively.
Table 5. Percentage contribution of the eight most dominant nematode species to total nematode community composition at each site and sediment depth, January 2006.
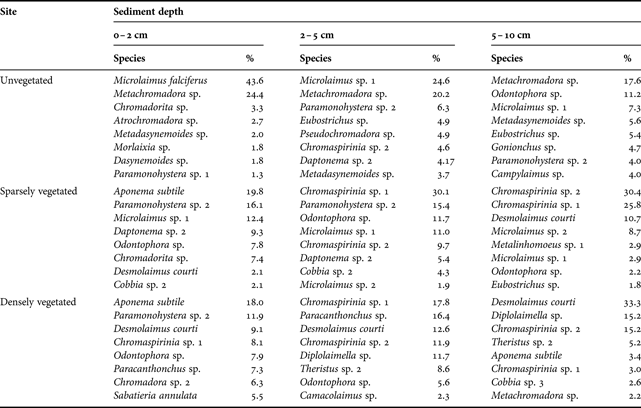
Table 6. Two-way ANOSIM results testing differences in nematode community structure between three sites (unvegetated, sparsely vegetated and densely vegetated) and sediment depths (bottom = 5–10 cm, middle = 2–5 cm, top = 0–2 cm), with details of global and pairwise comparisons; 999 permutations were run for each comparison. Results in bold are significant at α = 0.05 (global R value) or 0.017 (pairwise comparisons with Bonferroni corrections).

Nematode feeding groups
Predators and facultative predators represented less than 2% of feeding group composition at all sites and depths, and were excluded from the graphical representation for clarity. Epistrate feeders strongly dominated (84% of total) the surface nematode community at the unvegetated site (Figure 3). Epistrate feeders decreased in deeper sediment layers whereas deposit feeders and microvores increased. Microvores were the most common feeding group (34–38% of total) in surface sediments of both vegetated sites, followed by epistrate feeders (30–33%), deposit feeders (20–23%) and ciliate feeders (10%). Epistrate feeders were dominant in deeper sediment layers at the sparsely vegetated site, whilst microvores and deposit feeders decreased. This pattern was mostly a reflection of the greater abundance of Chromaspirinia spp. 1 and 2 in deeper sediments at that site. Epistrate feeders were more abundant at 2–5 cm depth at the densely vegetated site, but decreased again at 5–10 cm depth, where deposit feeders dominated.

Fig. 3. Composition of nematode feeding groups at the unvegetated, sparsely vegetated, and densely vegetated sites at different sediment depths, January 2006. M, microvores; DF, deposit feeders; EF, epistrate feeders; CF, ciliate feeders.
DISCUSSION
Sediment characteristics
Higher levels of organic matter, fine particles, chlorophyll-a, and phaeophytin in seagrass bed sediments are consistent with enhanced detritus deposition inside vegetated areas (Marba et al., Reference Marba, Holmer, Gacia, Barron, Larkum, Orth and Duarte2006). The input of seston to the sediment of Papanui Inlet, however, is likely to have been limited; analysis of the isotopic and fatty acid composition of sediments at the study sites suggests that other organic matter sources, such as macrophyte detritus and benthic microalgae, were the main contributors (Leduc et al., Reference Leduc, Probert and Duncan2009).
Between-site differences in sediment characteristics were most pronounced for the unvegetated and densely vegetated site, which suggests that the effect of Zostera muelleri on sediment characteristics (and associated benthic communities, see below) varies depending on shoot density (Webster et al., Reference Webster, Rowden and Attrill1998). Intertidal beds of Zostera muelleri have been shown to baffle currents and promote retention of fine particles (Heiss et al., Reference Heiss, Smith and Probert2000), despite the small length (about 12 cm) of the blades in this species. The shoot density (9000 m−2) recorded from the dense Z. muelleri site is close to the maximum densities reported for Zostera (Ismail, Reference Ismail2001; Lee et al., Reference Lee, Kim and Lee2006).
Meiofaunal distribution
The greater nematode abundance and biomass recorded inside relative to outside the Zostera muelleri bed in this study is consistent with earlier findings. The evidence available to date suggests that nematodes respond positively to the finer, organically-rich sediments associated with seagrass meadows (Castel et al., Reference Castel, Labourg, Escaravage, Auby and Garcia1989; Danovaro, Reference Danovaro1996; Edgar, Reference Edgar1999; Danovaro et al., Reference Danovaro, Gambi and Mirto2002), as observed in the present study.
Most studies comparing the abundance of copepods inside and outside seagrass beds found higher copepod densities in vegetated than unvegetated areas (Ansari & Parulekar, Reference Ansari and Parulekar1994; Guerrini et al., Reference Guerrini, Colangelo and Ceccherelli1998; Ndaro & Olafsson, Reference Ndaro and Olafsson1999; De Troch et al., Reference De Troch, Gurdebeke, Fiers and Vincx2001b). Studies in intertidal habitats of New Zealand, however, have found that copepod densities were either the same (Iwasaki, Reference Iwasaki1993) or higher in bare sand relative to nearby Zostera muelleri beds (Hicks, Reference Hicks1986). It is possible that the small size and simple structure of Z. muelleri does not promote high copepod densities to the same extent as larger, more structurally complex seagrass species (Hicks, Reference Hicks1986; De Troch et al., Reference De Troch, Gurdebeke, Fiers and Vincx2001b). Moreover, shallow seagrass beds may not increase meiofaunal densities to the same extent as deeper seagrass beds (De Troch et al., Reference De Troch, Gurdebeke, Fiers and Vincx2001b). The most common species in intertidal soft shores of New Zealand, Parastenhelia megarostrum, reaches very high densities in unvegetated habitats (Hicks, Reference Hicks1984), although it is not clear why this species should prefer unvegetated sediments. It is possible that the presence of dense seagrass canopy prevents the growth of preferred food sources such as benthic microalgae (Leduc et al., Reference Leduc, Probert and Duncan2009). Alternatively, the shallower oxic zone associated with the greater amount of fine particles and detritus found in vegetated sediments may negatively impact populations of harpacticoid copepods (Wetzel et al., Reference Wetzel, Fleeger, Powers and Rabalais2001). Further research is required to better understand the mechanisms behind this unexpected pattern.
The mean copepod densities observed in the surface (0–2 cm) layer at the unvegetated site (463–1081 10 cm−2) are in the upper range of values recorded for Parastenhelia megarostrum (Hicks, Reference Hicks1984), and amongst the highest reported for harpacticoid copepods (Hicks, Reference Hicks1985). Similar abundances of 1727 and 1283 10 cm−2 were reported for Platychelipus littoralis on a mudflat in Southampton Water (Barnett 1970, cited in Hicks, Reference Hicks1984) and for Huntemannia jadensis on a sandy beach in Puget Sound (Feller, Reference Feller1980), respectively.
Meiofaunal biomass
Meiofaunal biomass was about 50% greater at the densely vegetated site than at the unvegetated site. This value, however, is relatively small compared to the 4- and 8-fold difference in organic matter and pigment content, respectively, between these sites. This discrepancy suggests that the trophic transfer efficiency between sediment organic matter and meiofauna is lower inside the seagrass bed than outside. Based on the organic carbon content of sediment and meiofauna at the study sites (Leduc et al., Reference Leduc, Probert and Duncan2009), the trophic transfer efficiencies (here calculated as the ratio of meiofauna carbon to sediment organic carbon) at the sparse and unvegetated sites are 3 and 5 times higher than at the dense seagrass site, respectively. The fatty acid profile of sediment organic matter at the densely vegetated site indicates that seagrass detritus (a refractory food source) is a major contributor, whereas benthic microalgae (a labile food source) may be more important at the sparsely vegetated and unvegetated sites (Leduc et al., Reference Leduc, Probert and Duncan2009). These findings are consistent with the suggestion that meiofaunal production in seagrass beds is limited by the amount of labile organic matter available (Danovaro, Reference Danovaro1996).
Meiofaunal biomass at the vegetated sites was low compared to published values from seagrass beds (≤0.5 versus 2–10 gDW m−2) (Tietjen, Reference Tietjen1969; Castel et al., Reference Castel, Labourg, Escaravage, Auby and Garcia1989; Danovaro & Gambi, Reference Danovaro and Gambi2002; Danovaro et al., Reference Danovaro, Gambi and Mirto2002). In addition, meiofaunal biomass represented <2% of macrofaunal biomass at the study sites (D. Leduc, unpublished data), which suggests that the contribution of meiofauna to secondary production was low. This is in contrast with studies reporting a substantial contribution of meiofauna to benthic energy flows in seagrass beds (Castel et al., Reference Castel, Labourg, Escaravage, Auby and Garcia1989; Danovaro & Gambi, Reference Danovaro and Gambi2002; Danovaro et al., Reference Danovaro, Gambi and Mirto2002). Meiofauna usually represent about 10% of macrofaunal biomass in shallow littoral sediments (Giere, Reference Giere2009), but values as low as 1–4%, and up to about 50%, have been reported (Warwick et al., Reference Warwick, Joint, Radford, Jeffries and Davy1979; Witte & Zijlstra, Reference Witte and Zijlstra1984). The ratio of macrofaunal to meiofaunal biomass tends to increase from muddy to sandy sediments (Castel et al., Reference Castel, Labourg, Escaravage, Auby and Garcia1989; Giere, Reference Giere2009). This pattern could be due to a generally lower abundance of nematodes (the dominant meiofaunal taxon) in coarser sediments (Heip et al., Reference Heip, Vincx and Vranken1985), whereas suspension feeding bivalves, which usually represent 30–60% of macrobenthic biomass in coastal sediments (Ricciardi & Bourget, Reference Ricciardi and Bourget1999), are usually more common in sandy sediments (Levinton, Reference Levinton1995). The venerid bivalve Austrovenus Stutchburyi, in particular, is known to reach very high densities (>1000 m−2) in sheltered soft shores of New Zealand (Larcombe, Reference Larcombe1971; Dobbinson et al., Reference Dobbinson, Barker and Jillett1989).
Nematode distribution and community structure
Data on the vertical distribution of nematodes at the study sites show that the positive effect of seagrass on nematode abundance is mostly restricted to the upper 2 cm of sediment. Biotic factors such as the greater silt/clay content and greater amount of organic material available inside than outside the seagrass bed are likely to be important in promoting higher nematode abundance in vegetated areas (Edgar, Reference Edgar1999), but abiotic factors may also be involved. Strong hydrodynamic conditions, for example, may limit the abundance of nematodes in surface sediments of unvegetated, sandy habitats (Steyaert et al., Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003). More pronounced desiccation stress in unvegetated habitats may also induce a downward migration in some meiofaunal taxa (McLachlan et al., Reference McLachlan, Erasmus and Furstenberg1977). Low nematode abundance in the deepest (5–10 cm) sediment layer of the densely vegetated site relative to the other sites is likely to have been the result of anoxic conditions and high sulphide levels (Hendelberg & Jensen, Reference Hendelberg and Jensen1993; Wetzel et al., Reference Wetzel, Jensen and Giere1995; Steyaert et al., Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003). This is supported by the observation of a sharp transition between pale and black sediments at a depth of about 2 cm at the dense Z. muelleri sites, whereas no such transition was observed at the other two sites.
The surface (0–2 cm) nematode community at the unvegetated site was strongly dominated by Microlaimus falciferus and Metachromadora sp. (together representing 68% of total nematode abundance) and was characterized by low diversity. Nematode diversity at the vegetated sites, in contrast, was highest in surface sediments. Changes in nematode diversity are associated with a variety of factors such as sediment granulometry (Giere, Reference Giere2009; Steyaert et al., Reference Steyaert, Garner, van Gansbeke and Vincx1999, Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003), salinity (Soetaert et al., Reference Soetaert, Vincx, Wittoeck and Tulkens1995), disturbance (Austen et al., Reference Austen, Widdicombe and Villano-Pitacco1998), organic enrichment (Schratzberger & Warwick, Reference Schratzberger and Warwick1998) and the nature of organic matter (Danovaro & Gambi, Reference Danovaro and Gambi2002). Higher diversity near the surface of vegetated sites relative to the unvegetated site was probably the result of two main factors: (1) the provision of a sheltered environment by the seagrass blades compared to unvegetated sediments; and (2) the presence of organic particles of various origins and sizes, which would provide more opportunities for niche partitioning based on food and micro-habitat preferences (Edgar, Reference Edgar1999; Danovaro & Gambi, Reference Danovaro and Gambi2002). The low diversity at 0–2 cm depth at the unvegetated site could be explained by strong hydrodynamic and food-stressed conditions present near the surface (as suggested by low organic matter and silt/clay content). Low nematode diversity in surface sediments of an intertidal sandy site of the Westerschelde estuary was also ascribed to strong hydrodynamic conditions and tidal disturbance (Steyaert et al., Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003). The low diversity observed below 2 cm depth at the dense Z. muelleri site may be the result of low oxygen concentrations, which would allow only a limited number of physiologically-adapted species to survive.
Several common species that were most abundant in vegetated habitats, such as Aponema subtile, Sabatieria annulata, Desmolaimus courti, and Diplolaimella sp., belong to taxa that are often found in sediments rich in organic matter and/or low in oxygen (e.g. Villano & Warwick, Reference Villano and Warwick1995; Schratzberger et al., Reference Schratzberger, Warr and Rogers2006; Steyaert et al., Reference Steyaert, Moodley, Nadong, Moens, Soetaert and Vincx2007; Portnova, Reference Portnova2009). Species restricted to unvegetated sediment, such as Dasynemoides sp., Metadasynemoides sp., and, to a lesser extent, Microlaimus falciferus, Campylaimus sp., and Morlaixia sp., are characterized by ornamented cuticles. Species with thick and/or ornamented cuticles are common in sandy sediments (Heip et al., Reference Heip, Vincx and Vranken1985), probably because elaborate cuticular ornamentation aids locomotion and helps prevent mechanical damage in coarse, unstable sediments (Ward, Reference Ward1975). In addition, M. falciferus, which dominated surface nematode assemblages in unvegetated sediments, possesses numerous mucus-producing glands which may help anchor the animal to sediment particles and reduce desiccation stress at low tide (Turpeenniemi & Hyvarinen, Reference Turpeenniemi and Hyvarinen1996; Leduc & Wharton, Reference Leduc and Wharton2008). Overall, nematodes in vegetated sediments show a tendency for adaption to high organic matter input and low oxygen concentration whereas nematodes living in nearby unvegetated sediments are better adapted to live in strongly hydrodynamic conditions.
Nematode feeding groups
The strong dominance of epistrate feeders in the top 2 cm of unvegetated sediments suggests that benthic microlagae were the most important food source at that site. Microvores were the dominant feeding group in the top 2 cm of vegetated sediments, but epistrate feeders, deposit feeders, and ciliate feeders were also common, suggesting a greater diversity of available food resources than in unvegetated sediments. Macrophyte detritus and benthic microalgae are both potential food sources at the vegetated sites. These results are consistent with the findings of a biomarker study carried out at the same sites in January 2006, which indicated that benthic microalgae and macrophyte detritus were the most likely food sources for nematodes at the unvegetated and vegetated sites, respectively (Leduc et al., Reference Leduc, Probert and Duncan2009). Isotopic and fatty acid biomarkers of copepods (which dominated meiofaunal production at the unvegetated site) and nematodes from the unvegetated site were also consistent with benthic microalgae as the main carbon source (Leduc, Reference Leduc2009; Leduc et al., Reference Leduc, Probert and Duncan2009).
The relative abundance of epistrate feeders at the unvegetated site declined gradually with depth, whilst microvores, ciliate feeders, and deposit feeders increased. This shift in the composition of trophic groups suggests that the contribution of benthic microalgae to the diet of nematodes declined in deeper sediment layers whereas other food sources, such as bacteria and protists, became more important. The greater dominance of epistrate feeders (mostly Chromaspirinia spp.) in deeper (2–10 cm) sediment layers of the vegetated sites was somewhat unexpected. Microalgae growing along macrofaunal burrows (as evidenced by an orange coloration of burrow linings, D. Leduc personal observation) may be an important food source for nematodes, which could explain subsurface maxima in the distribution of this trophic group. Both Chromaspirinia spp. have a slender shape (length to maximum width ratio >60), a common characteristic of nematode species living in thiobiotic environments (Giere, Reference Giere2009). It is therefore possible that these species live in close association with the seagrass roots in the thin oxygenated layer surrounding the rhizosphere. Some uncertainty remains with the classification of nematode feeding types, and Chromaspirinia spp. may rely on other food sources such as bacteria (Moens & Vincx, Reference Moens and Vincx1997; Koller et al., Reference Koller, Dworschak and Abed-Navandi2006), or even dissolved organic matter exuded by seagrass roots. The deepest sediment layer at the dense seagrass site was dominated by Desmolaimus courti, a deposit feeder. Species of this family have been found to be abundant in the oxic zone surrounding polychaete burrows (Wetzel et al., Reference Wetzel, Jensen and Giere1995). This habitat preference could explain the high variability in the abundance of this species (0–70 per core) in the deepest (5–10 cm) sediment layer of the dense Z. muelleri site.
The near absence of predators and facultative predators in this study contrasts with previous studies reporting high numbers of these feeding groups in seagrass beds (Hopper & Meyers, Reference Hopper and Meyers1967) and unvegetated sand (Steyaert et al., Reference Steyaert, Vanaverbeke, Vanreusel, Barranguet, Lucas and Vincx2003). It is possible that the sediment characteristics at the study sites are unfavourable to predators. For example, mean grain size below 180 µm, as reported in this study (125–164 µm), led to severely reduced predation by Enoploides longispiculosus (Gallucci et al., Reference Gallucci, Steyaert and Moens2005).
CONCLUSIONS
This study confirmed some of the trends often observed in studies of seagrass-associated benthic communities, i.e. that faunal biomass is greater inside than outside seagrass beds, that macrophyte detritus and associated microbiota are important food sources for invertebrates and that secondary production inside seagrass meadows is limited by the amount of labile organic matter available.
Other findings, however, appear to contradict some generally accepted tenets of seagrass community ecology. Meiofaunal biomass inside the Zostera muelleri meadow, for example, was low, indicating that the contribution of meiofauna to secondary productivity was limited. In addition, harpacticoid copepod abundance was highest in unvegetated sediments, which also contrasts with findings from previous studies. We did not observe trends in (depth-integrated) nematode diversity between vegetated and unvegetated habitats, although contrasting vertical diversity patterns were observed between vegetated and unvegetated sites. The near absence of predators at all study sites, as well as the high abundance of epistrate feeders in subsurface sediment inside the seagrass bed, is also intriguing. These discrepancies may indicate that the nature of seagrass–invertebrate interactions depends on habitat characteristics (e.g. intertidal versus subtidal, hydrodynamic conditions, sediment granulometry) and the identity and ecology of species considered. Nevertheless, our results show that the presence of Zostera muelleri creates complex 3-dimensional habitats characterized by markedly different invertebrate assemblages from surrounding unvegetated sediments, resulting in altered structure and function of sheltered soft-shore communities.
ACKNOWLEDGEMENTS
We would like to thank B. Dickson, K. Bonney, and D. Wilson from the Portobello Marine Laboratory for their technical assistance during this study. S. Kljucanin and N. Klein also provided valuable help in the field. We are grateful to two anonymous referees for their constructive criticisms on the manuscript. This study was funded by the Department of Marine Science, University of Otago and by a University of Otago Postgraduate Scholarship.
Appendix
Nematode species found at the unvegetated, sparsely vegetated and densely vegetated sites, Papanui Inlet, January 2006. Species abundance is expressed as % of total nematode abundance. Species representing ≥5% of abundance are in bold. CF, ciliate feeder; DF, deposit feeder; EF, epistrate feeder; FP, facultative predator; M, microvore; P, predator.