INTRODUCTION
Glass sponges (class Hexactinellida) of the north-eastern (NE) Pacific, in a restricted area of interest (AOI) for this report (Figure 1—benthos offshore of Washington and Alaska, USA, and British Columbia, Canada), have been the subject of a moderate literature. The first descriptions were those of the common rossellid, Rhabdocalyptus dawsoni (as Bathydorus) and the common aphrocallistid Aphrocallistes vastus Schulze, Reference Schulze1886 (as A. whiteavesianus) by Lambe (Reference Lambe1893). Shortly thereafter Lambe (Reference Lambe1894) described Staurocalyptus dowlingi (as Rhabdocalyptus). The majority of the presently known species of the area were reported or described by Schulze (Reference Schulze1899) from the exploratory USFS ‘Albatross’ cruises, including as new species, Hyalonema ovuliferum, Holascus undulatus, Rhabdocalyptus tenuis (as Acanthosaccus), Farrea aculeata and one he previously described from Japan, Heterochone calyx Schulze, Reference Schulze1886 (as Chonelasma). He also described many other species then found only north or south of the AOI, but later reported by others as occurring within the AOI. Wilson & Penney (Reference Wilson and Penney1930) described a new subspecies, Rhabdocalyptus dawsoni alascensis from lower Alaska. In his checklist of the fauna of the wider region (California to northern Alaska), Austin (Reference Austin1985) gave the first report of Hyalonema populiferum Schulze, Reference Schulze1899, Farrea occa Bowerbank, Reference Bowerbank1862, Leucopsacas scoliodocus Ijima, Reference Ijima1898, Acanthascus cf. cactus Schulze, Reference Schulze1886, Staurocalyptus fasciculatus Schulze, Reference Schulze1899, and Staurocalyptus solidus Schulze, Reference Schulze1899, occurring in the AOI. Farrow et al. (Reference Farrow, Syviski and Tunnicliffe1983) added Acanthascus platei Schulze, Reference Schulze1899, as occurring in the AOI. Occurrence within the AOI of Rhabdocalyptus asper Schulze, Reference Schulze1899, was reported by Tunnicliffe et al. (Reference Tunnicliffe, Juniper and de Burgh1985), and both Rhabdocalyptus heteraster Okada, Reference Okada1932, and Staurocalyptus fuca Tabachnick, Reference Tabachnick1989, were described from there by Tabachnick (Reference Tabachnick1989). The new sceptrulophoran without sceptrules, Asceptrulum axialis, was described as a new genus and species in the AOI by Duplessis & Reiswig (Reference Duplessis and Reiswig2004). Finally, existence of a new undescribed species of Heterochone (sp. nov. A, in preparation) on the offshore Gulf of Alaska seamounts was reported by Schuchert & Reiswig, Reference Schuchert and Reiswig2006. Many other references to undescribed or partially described ‘sp.’ cannot be accorded credence for taxonomic purposes, but they indicate that many hexactinellid species of the area remain unknown. Interspersed in time between the above reports are the large number of publications dealing with the hexactinellid species either serving as construction agents or associated components of the glass sponge reefs of the British Columbia shelf. The species considered to be valid in these reports sum to a total of 21 species or subspecies of Hexactinellida, known or certain to be described within the AOI.

Fig. 1. Map of collection sites of new hexactinellid species: (A) Farrea omniclavata sp. nov.; (B) Farrea truncata sp. nov.; (C) Chonelasma oreia sp. nov.; (D) Amphidiscella lecus sp. nov.; (E) Oopsacas olypicus sp. nov.; (F) Acanthascus malacus sp. nov. Seamount names are shown.
A synopsis of hexactinellid species for a sub-area of the AOI offshore or bordering British Columbia posted online at the Royal British Columbia Museum (RBCM) taxonomic keys website (Reiswig & Frey, Reference Reiswig and Frey2012) includes 12 of the above species plus two not yet described or formally named; those two species are described and named here. A major extension of the known hexactinellid fauna of the AOI by 29%, and, of course, significant for the world inventory of hexactinellids, is made here in describing six species new to science.
MATERIALS AND METHODS
Specimens reported here became available through a variety of routes: some were discovered during investigation of old unidentified specimens in the RBCM collections. Some were sent directly from other institutions for identification, or indirectly through another taxonomic specialist. Some were sent from cruise participants seeking identifications of their recent catch. They were all collected from deep water (702–2438 m) by either trawl, manually operated submersible vehicle (MOV) or remotely operated submersible vehicle (ROV) off Cape Flattery, Washington State, USA, through offshore waters of British Columbia, Canada, and along the chain of seamounts of the Gulf of Alaska (Figure 1).
When first encountered, each specimen was digitally photographed; ‘pics’ (small pinches with fine forceps) were taken from dermal and atrial surfaces, dehydrated, cleared and whole-mounted on microscope slides in Canada balsam. Larger samples of dermal surface, atrial surface and body wall were digested in hot nitric acid (93°C) for two hours. The resulting cleaned skeletal frameworks, where present, were rinsed in distilled water and either mounted with epoxy on aluminium stubs for scanning electron microscopy (SEM), or dissected, dried and mounted in Canada balsam on microscope slides for light microscopy (LM). Spicules in suspension were isolated from the nitric acid using several techniques. Large spicules, where present, were individually picked from the diluted acid solution under a dissecting microscope using forceps or pipette and transferred to distilled water in embryo dishes; they were ultrasonically cleaned for two minutes to remove adherent clay particles, rinsed several times in distilled water and either mounted on scanning electron microscope stubs, or onto 9 mm square coverslips which, after drying, were epoxied to stubs. Smaller spicules in acid suspension were filter-captured and post-rinsed on either 10 mm diameter ion-etched 0.22 µm pore diameter polycarbonate filters for SEM or 25 mm diameter compressed nitrocellulose fibre filters for LM. After drying, filters with spicules for SEM were attached to aluminium SEM stubs by double-sided tape; filters with spicules for LM were cleared in xylene and mounted on microscope slides in Canada balsam. All SEM stub preparations were sputter-coated with gold-palladium and viewed in either a Hitachi S-3400N or S-4800 SEM or both.
Measurements of spicules were made using compound or dissecting LM linked to a computer-digitizer by drawing tube (camera lucida) and Sigma-Scan© version 3.92 software. Relative abundance of spicule types was estimated by categorizing individual spicules sequentially encountered in systematic scans of LM preparations—usually 100–200 spicules. Potential bias due to sampling location on the specimen and preparation procedure was minimized by qualitative assessment of preparations from three locations and use of filtration for spicule collection, thereby avoiding differential loss of spicules associated with alternate procedures involving repeated sedimentation or centrifugation or blotting. Data reported in tables is given as mean ± standard deviation (range; number of measurements); data reported in text position is given as minimum–mean–maximum ± standard deviation (number of measurements). Restricted synonomies are given only where changes of names or diagnoses are made. The new specimens are deposited in the collections of the Royal British Columbia Museum, Victoria, BC, Canada (RBCM), the Smithsonian Institution, Washington, DC, USA (USNM), or California Academy of Sciences, Invertebrate Zoology, San Francisco, CA, USA (CASIZ). Other institution abbreviations: Monterey Bay Research Institute, Moss Landing, CA, USA (MBARI); Olympic Coast National Marine Sanctuary, Port Angeles, WA, USA (OCNMS).
RESULTS
SYSTEMATICS
Class HEXACTINELLIDA Schmidt, 1870
Subclass HEXASTEROPHORA Schulze, Reference Schulze1886
Suborder HEXACTINOSIDA Schrammen, 1903
Family FARREIDAE Gray, 1872
Genus Farrea Bowerbank, Reference Bowerbank1862
Farrea omniclavata sp. nov.
(Figures 2 & 3; Table 1)
TYPE MATERIAL
Holotype: RBCM 011-00039-001, MOV ‘Pisces IV’, dive 1156, 21 September 1982, Brown Bear Seamount, 46°02.4′N 130°12′W, 702 m, 70% ethanol.
Paratypes: RBCM 012-00192-001, trawl, September 1981, Cobb Seamount, 46°44′N 130°47′W, 800 m, 70% ethanol; RBCM 010-00309-003, CCGS ‘W.E. Ricker’ trawl, 9 March 2002, west of north end Queen Charlotte Island, 53°59.37–54°00.34′N 133°55.94–55.47′west, 1171–1196 m, 40% isopropanol; RBCM 010-00260-008, CCGS, W.E. Ricker trawl, 9 March 2001, south-west of Barkley Sound, west Vancouver Island, 48°19.947–412′N 126°23.746–22.459′W, 1160–1175 m, 40% isopropanol.
DIAGNOSIS
Farrea with clavate clavules as the only clavule type. Microscleres are mainly oxyhexasters, with a few onychohexasters and very few oxydiasters.
DESCRIPTION
General body form of mature specimens is a hemispherical mass of branching tubes to at least 15 cm overall diameter (Figure 2A) and probably larger. Tube diameter (mid-wall to mid-wall) is 8.0–20.1–30.3∀5.7 mm (N = 42). Distance between branching points is only measureable by careful dissection of a large intact specimen which was not available; estimates from specimen fragments and in situ images suggest it is about the same as tube diameter, resulting in a closely packed mass where individual tubes are difficult or impossible to follow for significant distance. Wall thickness, including the dermal clavules with varying projection distances, is 1.2–1.3–1.7 mm. Tube walls are not channelized and no associations with other organisms was detected. Distributed offshore on two seamounts and inshore along the British Columbia upper continental slope (Figure 1A) from 702–1196 m depths.

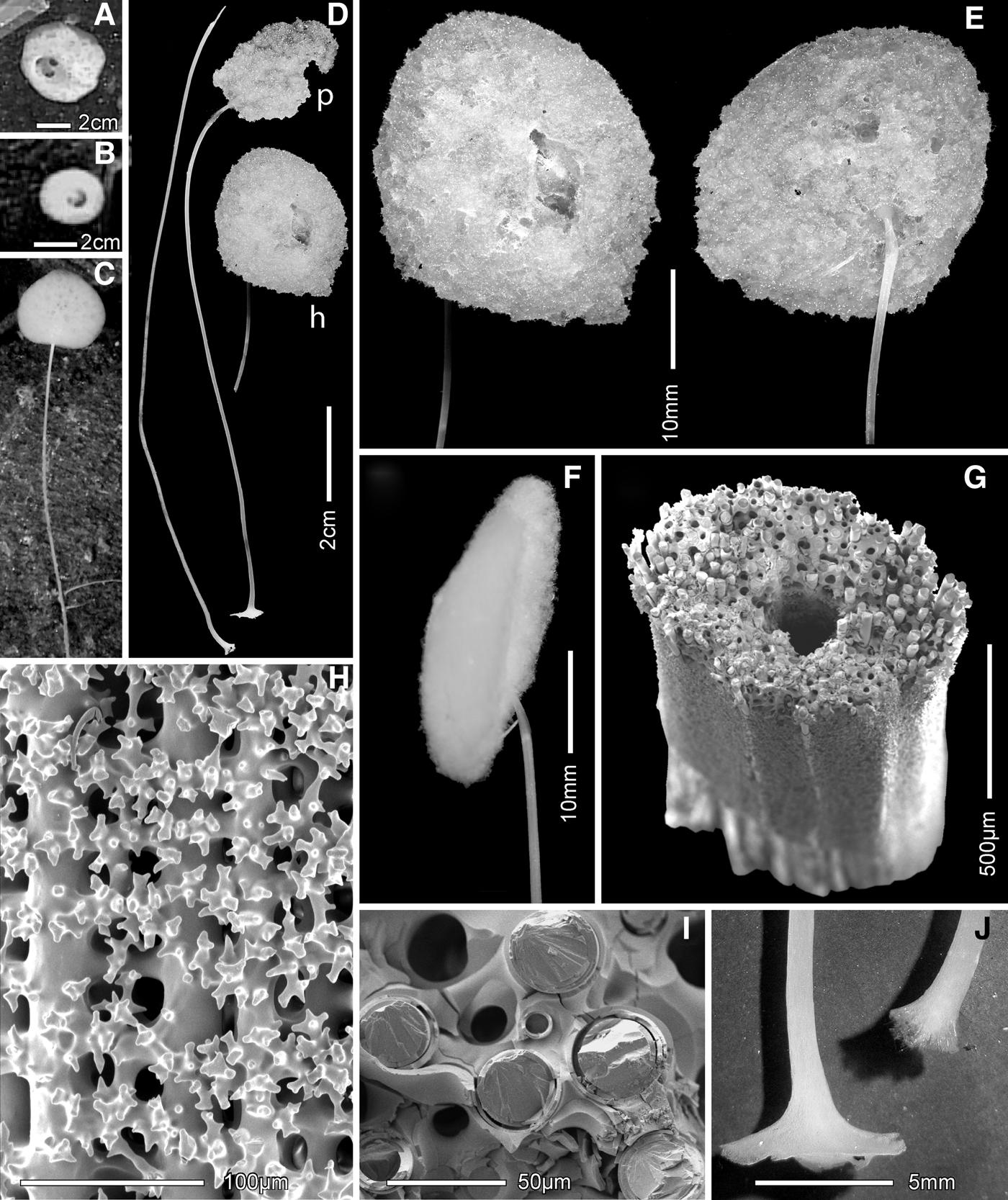
Fig. 2. Farrea omniclavata, sp. nov., body and framework: (A) an uncollected specimen from the type locality presumed to be this species; (B) paratype RBCM 012-001920001; (C) the collected holotype fragments; (D) one-layered farreoid dictyonal framework scanning electron microscopy (SEM); (E) magnified view of framework showing spurs and smooth beams (SEM); (F) framework in longitudinal section, dermal side on left (SEM); (G) backlit view of preserved dermal surface with projecting clavules. C–G from holotype; in situ image courtesey of Dr Verena Tunnicliffe.
The skeletal framework (for data see Table 1) is a typical farreoid dictyonal type, mostly one dictyonal layer thick (Figure 2D–F) but a second layer is appended on the dermal side in older areas. Beams are smooth and the rectangular, often square, meshes are relatively large, sides average 649 m. Spurs are long and slightly curved on the atrial side, but shorter and often broken on the dermal side (Figure 2F). Small oxyhexactins appended to the framework are common on the two nearshore paratypes but absent from the offshore specimens.
Table 1. Skeletal framework and spicule dimensions of Farrea omniclavata, sp. nov., holotype, RBCM 011–00039–001 (dimensions in µm).

Megascleres are surface pentactins, clavate clavules and uncinates. Dermal pentactins are mostly finely rough while atrial pentactins are mostly sparse-macrospined (Figure 3A) but there is overlap between the two populations; ray tips are rounded and slightly clavate. A few pentactins with short and very swollen clavate rays (Figure 3A left) are found among the normal forms on both surfaces. Since dimensions of pentactins on the two sides do not differ, they are combined in Table 1. Clavules are all clavate, without head spines or shaft thorns. In offshore specimens the clavule heads smoothly increase in diameter to a hemispheric cap; they are entirely rough except for a smooth patch on the head top (Figure 3B) although rare small clavules may be entirely smooth. Clavules of the two nearshore slope specimens are also entirely clavate, but about half of them have a slight constriction with a noticeable inflated upper cap (Figure 3C). Clavules are abundant on the dermal side where they project in bundles of 4–5 for each pentact far beyond the pentactins (Figure 2G); clavules are very sparse with patchy occurrence on the atrial surface. Uncinates (Figure 3D) are moderate in size with well developed brackets and barbs. Two distinct size-classes were found in one paratype (RBCM 012-001920001), but not in the other specimens.

Fig. 3. Farrea omniclavata, sp. nov., spicules (scanning electron microscopy): (A) surface pentactins, with macrospined form, microspined form (behind) and short-ray clavate form to left; tangential and proximal ray tips are magnified; (B) clavate clavule, whole and magnified head side and end views; (C) semi-constricted head form of clavate clavule occurring only in nearshore specimens; (D) uncinate, whole and enlarged middle segment; (E) oxyhexaster, whole and enlarged terminal ray tuft; (F) onychohexaster, whole and enlarged part of terminal ray tuft. All except C from holotype; C from paratype RBCM 010-00309-003.
Microscleres are mainly oxyhexasters (97%), with a few onychohexasters (3%) and very few oxydiasters (<0.5%). Oxyhexasters (Figure 3E) have relatively long smooth primary rays, each of which supports 3–4–5 shorter, slightly and finely spined terminals ending in curved sharp tips. Onychohexasters (Figure 3F) likewise have relatively long smooth primary rays, each supporting 2–3–5 shorter finely spined terminal rays which end in a splay of 3–5 recurved claws. The rare oxydiasters have the characters of the oxyhexasters, but development of primary rays and branching is restricted to one axis.
ETYMOLOGY
The species name, omniclavata, refers to the condition that all clavules are clavate.
REMARKS
Clavate clavules are fairly common in the genus Farrea, occurring in two species, F. lendenfeldi Ijima, Reference Ijima and Weber1927, F. spirifera Ijima, Reference Ijima and Weber1927, and seven of the ten subspecies of F. occa Bowerbank, Reference Bowerbank1862. They have never been reported to occur as the only clavule type in any of these, most commonly co-occurring with pileate clavules as the dominant form. Among these Farrea bearing clavate clavules, none of them has been reported with onychohexasters. These very clear differences justify recognition of these NE Pacific specimens as a new species, here named Farrea omniclavata sp. nov. Although there are some differences between the inshore slope and offshore seamount specimens in clavule form as noted above, they all share the two major features—all clavules are clavate and microsclere complements are the same. It is very likely that the two populations are genetically isolated but the morphological differences are not presently considered to be of sufficient degree to warrant recognition of a different species.
TYPE MATERIAL
Holotype: CASIZ 191783, MBARI ROV ‘Tiburon’ from RV ‘Western Flyer’, dive 875, 9 August 2005, Axial lava ponds, Axial Seamount, 45.5669°N 130.0379°W, 2311 m, 70% ethanol.
DIAGNOSIS
Farrea with all clavules truncate in form. Microscleres are mainly discohexasters, with a few hemidiscohexasters and very few discodiasters.
DESCRIPTION
Body form of the single known specimen is a sparsely branched bush, the initial branching occurring just above the basal disc attached to volcanic basalt. Some of the approximately eight ruffled branches in the in situ image (Figure 4A) clearly arise by secondary branching, indeed the system of branching may be entirely dichotomous but pattern is not determinable from limited available images. The largest branch is 42 cm in length. Branch margins extend laterally as an undulated fringe curled back alternately and regularly above and below the branch axis as ruffles or frills, alternating also on opposite sides of branches, with a periodicity of 2.2–3.1–3.7 mm. This results in branches approximating an open double tube, about 6.7 cm wide and 4.8 cm high in middle areas. The greatly damaged collected fragments (Figure 4B) confirm that there is no fusion of body wall and no tubular elements. Wall thickness, including the dermal clavules with varying projection distances, is 0.65–1.06–2.55 mm (N = 36); walls are not channelized and association with other organisms was not noted. The species is known only from the type location.

Fig. 4. Farrea truncata, sp. nov., body and framework: (A) the holotype in situ, brown parts are dead; white triangles highlight 29 cm spaced laser beams; (B) fragments of the collected holotype; (C) one and two layered framework viewed from atrial side; (D) close up of framework and spurs; (E) transverse section of framework with spurs mostly intact; dermal side up, margin to left: (F) close-up of a beam showing sparse low microspines. All from holotype; in situ image courtesey of The Monterey Bay Aquarium Research Institute.
The skeletal framework (Table 2) is a typical farreoid dictyonal type, one dictyonal layer thick in the marginal fringes but a second layer is appended on the dermal side in older areas (Figure 4C–E). Beams have sparse, low, blunt-tipped spines scattered irregularly (Figure 4F). Longitudinal beams are both longer and thicker than transverse beams. Spurs are long, thin, sharply pointed and straight but slightly bent towards the growth margin (Figure 4E); they do not differ on the two sides of the framework. Small appended oxyhexactins are not present.
Table 2. Skeletal framework and spicule dimensions of Farrea truncata, sp. nov., holotype, CASIZ 191783 (dimensions in µm).

Megascleres are surface pentactins, truncate clavules and uncinates. Pentactins of the dermal and atrial sides do not differ; their tangential rays are coarsely macrospined on the outer and lateral surfaces and taper to bluntly-pointed tips (Figure 5A). Proximal rays are generally longer and may be over twice the length of tangential rays. The most common clavules (clavule 1) have truncate heads with very small, smooth, nearly flat top without a distinct cap; the margin is irregularly serrate with both small and medium-size teeth (Figure 5B, right). Neck and shaft are entirely rough down to the sharply pointed proximal end. About 25% of the clavules (clavule 2) have slightly inflated caps (Figure 5B left) and can be called truncate–pileate. Both clavule forms, considered to be slight variations of a single type, occur equally on both sides of the body in the normal position projecting above the pentactin surfalia. Uncinates are large, with both brackets and barbs well developed (Figure 5C).

Fig. 5. Farrea truncata, sp. nov., spicules (scanning election microscopy): (A) macrospined surface pentactins, with enlarged ray ends, of dermal (front) and atrial sides are indistinguishable; (B) truncate clavules, type 1 (centre whole and magnified parts, right) with barely inflated discoid cap bordered by marginal teeth; type 2 (magnified, top left) has a more inflated cap; (C) uncinate, whole and magnified anterior segment; (D) discohexaster with large reclined spines on terminal rays, with magnified terminal end; (E) discodiaster, a variation of the basic style exhibited in the discohexaster. All from holotype.
Microscleres are mainly discohexasters (97%), with a few hemidiscohexasters (3%) and very few discodiasters (<0.5%). The discohexasters have very small terminal discs and could be called onychohexasters with very regular, short claws (Figure 5D). The very short, stout primary rays each support 2–5 coarsely barbed, stout terminal rays ending in small discs with 3–6 marginal teeth. Hemihexasters are very similar but differ in having only 1 terminal ray on at least one primary ray. Discodiasters (Figure 5E) have only two opposite primary rays, usually of very unequal length. Each bears 3–7 terminal rays similar to those on discohexasters.
ETYMOLOGY
The species name, truncata, refers to the truncate condition of the clavules, similar in form to pileate clavules with the top broken off at the lower neck.
REMARKS
The truncate clavules found in this species are unique; they are unlike any clavule form previously described in the genus (see survey in Lopes et al., Reference Lopes, Hajdu and Reiswig2011). The other spicule types are not special and similar forms occur in many other Farrea species. The sparsely spined framework is a relatively uncommon feature among the known members of the genus Farrea. The distinct clavule form is sufficient to qualify this specimen from Axial Seamount as a new species, designated here as Farrea truncata sp. nov.
The body form of F. truncata, a sparsely-branching group of ruffled fronds, is common along the Pacific Coast of North America. A search made by L. Lundsten of the video records of MBARI's submersibles for occurrence of similar body form resulted in 864 individual records (L. Lundsten, personal communication), very few accompanied by collected vouchers. One specimen from Monterey Bay, Central California, with very similar body form, differs from F. truncata in the form of every spicule category. Thus, while some of the recorded occurrences of stalked ruffled sponges may represent F. truncata, determination of these imaged specimens to species level on the basis of body form alone can be done only in very special situations where the benthic megafauna is well known and a single body form can be related directly to one species only.
Family EURETIDAE Zittel, 1877
Subfamily CHONELASMATINAE Schrammen, 1912
Genus Chonelasma Schulze, Reference Schulze1886
Chonelasma oreia sp. nov.
(Figures 6 & 7; Table 3)
Synonymy. Chonelasma sp. Reiswig & Frey, Reference Reiswig and Frey2012.
TYPE MATERIAL
Holotype: USNM 1199192, MOV ‘Alvin’ from RV ‘Atlantis’, dive 4042, 18 August 2004, Giacomini Seamount, Gulf of Alaska, 56°22.930′N 146°21.989′W, 1726 m.
Paratypes: USNM 1199193, MOV ‘Alvin’ from RV ‘Atlantis’, dive 4031, 7 August 2004, Dickens Seamount, Gulf of Alaska, 54°30.853′N 136°54.561′W, 852 m. USNM 1199194, MOV ‘Alvin’ from RV ‘Atlantis’, dive 4032, 8 August 2004, Welker Seamount, Gulf of Alaska, 55°03.595′N 140°18.895′W, 751 m. USNM 1199195, MOV ‘Alvin’ from RV ‘Atlantis’, dive 4035, 11 August 2004, Welker Seamount, Gulf of Alaska, 55°03.987′N 140°24.499′W, 1122 m. RBCM 011-00072-001, FV ‘Ocean Pearl’, trawl set 24, 7 April 2004. Bowie Seamount, Gulf of Alaska, 53°20.4′N 135°32.8′W, 768 m.
DIAGNOSIS
Chonelasma with funnel-form body. Megascleres are dermal and atrial pinular hexactins, two forms of subtylote scopules and uncinates. Microscleres include a variety of oxy-tipped forms and a strongyle-tipped microhexaster; disco-tipped microscleres are absent.
DESCRIPTION
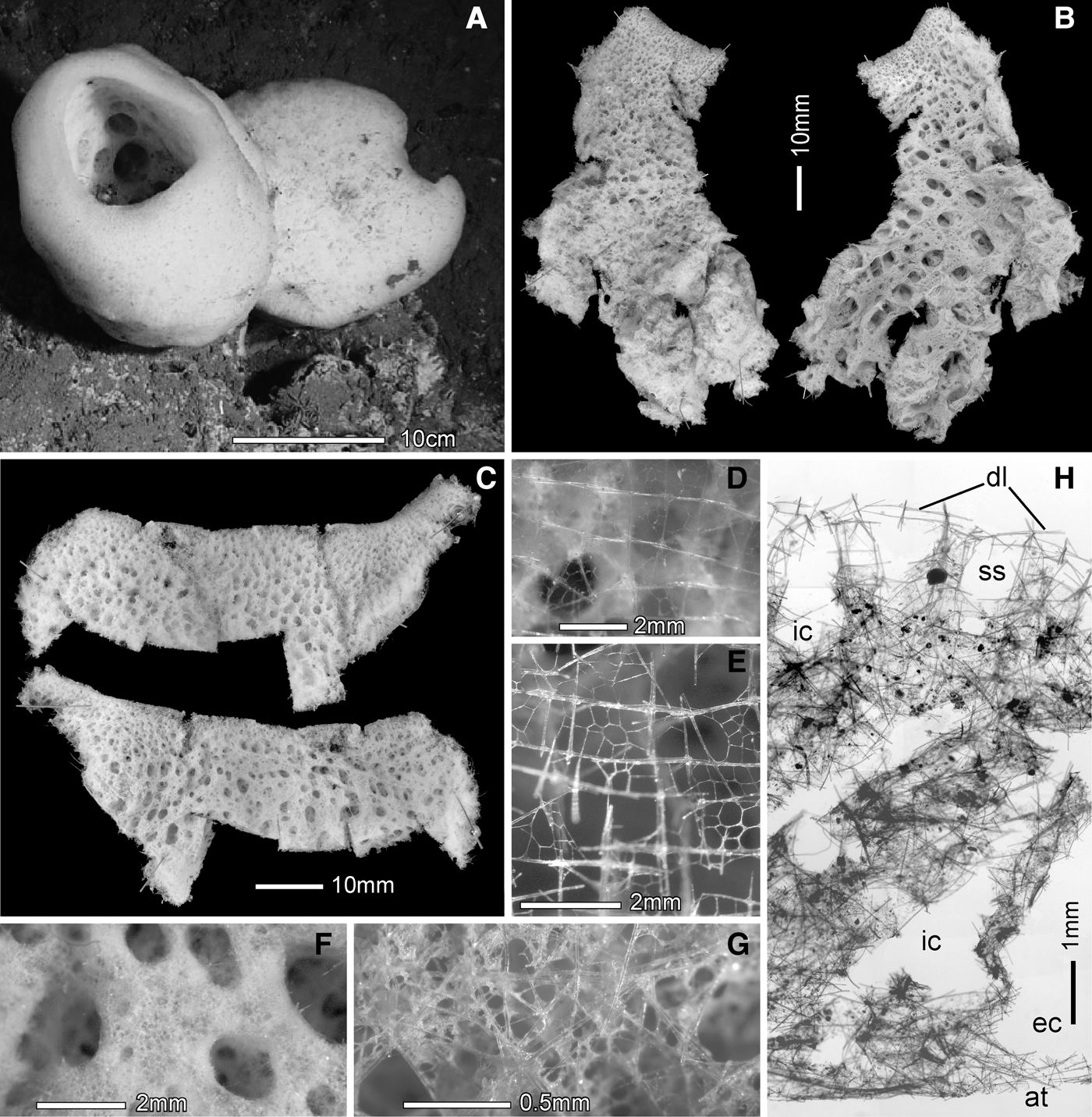
Body form is an upright funnel or cone with a large round terminal osculum; the oscular margin in young specimens is curled inwards (Figure 6A) but in larger (older) specimens it is either vertical and aligned with the growth direction (Figure 6B) or, more commonly, reflexed over 90° and extended horizontally or slightly downwards as a broad skirt (Figures 6C–D). Laser point pairs enabling estimation of body size are not clear in any of the in situ images of this species, but comparing the specimens in Figure 6B–D with other benthic fauna suggests their major dimensions are in the neighbourhood of one metre. All collected material are plate-like fragments of the funnel wall or skirt; dimensions vary from 4.0–8.0–12.5 cm; thickness varies from 2.6–9.6–20.1 mm. Texture is hard, rigid and brittle; the fragments are not deformable. The outer (dermal) surface (Figure 6E upper) is ornamented with two cycles of ridges and depressions; the larger cycle has ridges 2–5 mm high, circumscribing shallow centimetre-size depressions (diameters 3.5–12.0–22.0 mm). The smaller cycle has general dimensions of 2 mm. The entire dermal surface is bounded by an unfused quadrate spicule lattice with mean mesh size of 231 µm (range 162–286 µm) (Figure 6H), through which the smaller cycle depressions and openings into inhalant canals are visible. The inner (atrial) surface (Figure 6E lower) has large apertures of the exhalant canals on a smooth surface, but low longitudinal ridges are present in the larger fragments. A quadrate spicule lattice of loose spicules also covers the entire atrial surface but it is so closely attached to the underlying tissues that it is usually evident only where it spans across the apertures of exhalant canals (Figure 6G). Deck photographs show that living tissues were quite inflated with very smooth surfaces compared to the aspects of preserved fragments. Freshly collected specimens from Welker and Dickens Seamounts were orange in colour while that from Giacomini Seamount was beige. The species is attached by relatively small basal disc to hard substrate on the Gulf of Alaska seamount chain from Bowie to Giancomini (Figure 1), at depths of 751–1726 m.

Fig. 6. Chonelasma oreia, sp. nov., body and framework: (A) smallest paratype USNM 1199194, Welker Seamount; (B) paratype USNM 1199195, Welker Seamount; (C) holotype USNM 1199192, Giacomini Seamount; (D) paratype USNM 1199193, Dickens Seamount; (E) dermal surface (above) with ridge/pit pattern over several dimensional cycles; atrial surface (below) and large caliber longitudinal folds devoid of finer sculpting; (F) dermal lattice spanning over smallest pits; (G) atrial lattice fitted closely to the atrial surface but spans across exhalant openings; (H) openings of epirhyses on cleaned dermal framework; (I) openings of aporhyses on cleaned atrial framework; (J) closer view of dermal epirhyses and framework dictyorhyses; (K) closer view of atrial aporhyses and framework dictyorhyses; (L) longitudinal section of framework composed of three layers (marked on right by black and white bars); a thick dermal cortex, a low density middle (primary) layer, and thin but high density atrial cortex; septa indicated by arrows; (M) magnified view of beams and meshes of the middle layer. In situ images are courtesy of NOAA, WHOI, the Alvin Group and the 2004 GOA Expedition science party. All other images from holotype.
Table 3. Skeletal framework and spicule dimensions of Chonelasma oreia, sp. nov., holotype, USNM 1199192 (dimensions in µm).

The fused skeletal framework is of fairly typical chonelasmoid form. The dense dermal cortex is penetrated by slightly elongate epirhyses about 1.5 mm in mean diameter (Figure 6H). The atrial cortex is penetrated by cylindrical aporhyses about 0.7 mm mean diameter but with great variation in size (Figure 6I). Exposed surfaces of both cortices are dominated by irregularly arranged triangular meshes (Figures 6J, K). Longitudinal section of cleaned fragments show the framework is constructed of three layers: a dermal cortex, an atrial cortex, and a much less dense middle (primary) layer with longitudinal strands and transverse septa with ~3.1 mm spacing (Figure 6L). Meshes in the middle layer are rectangular, elongate in the longitudinal direction (Figure 6M). Primary dictyonalia are mainly aligned in rounded surfaces mirroring the free growth edge, thereby producing the septal structures. Beams are predominately finely spined (rough) but most nodes and some beams are smooth. Spurs on both surfaces are similar in shape and size; they are slightly rough and digitate in form, often with small terminal swelling. No small oxyhexactins are attached to framework beams.
Megascleres are dermal and atrial pinular hexactins, two types of scopules and uncinates; regular mesohexactins occur very rarely in the dermal lattice. Dermal pinules (Figure 7A) are entirely rough with nearly cylindrical tangential rays ending in abruptly rounded or sharp tips. The pinulus is either ovoid or distally inflated and entirely covered in distally directed sharp scales. Proximal rays are normally tapered to a sharp tip, but some are short and terminally inflated and a few rare ones not encountered in the measurement data are rarely extremely long, to 700 µm (Figure 7C). Atrial pinules (Figure 7B) differ in pinulus shape; they are narrower and more spindle-shaped. The more common of the two scopules (88%) is designated type A (Figure 7D); this form has a circlet of 7–12 straight tines flaring out to form a funnel; tine tips appear subtylote due to a very slight subterminal swelling but the ultimate tips are better described as strongylote (Figure 7D left). Transition from shaft to head is abrupt, often with four lateral swellings. The less common scopule (12%), type B (Figure 7E), has 3–4–7 sturdy tines arising from a flattened neck to circumscribe a narrower, flattened funnel. Tine tips are strongylose and the shaft-neck transition is less abrupt and more inclined, and without basal swellings. Both types of scopules are entirely finely spined and have shafts tapering to sharp tips. Both scopule types are also absent from the bounding dermal and atrial lattices; they are choanosomal spicules here. Uncinates (Figure 7F) are extremely large (to nearly 8 mm) and anteriorly very bushy due to the great curvature of the barbs in that region. These are also unusual in the cylindrical nature of the posterior end which tapers significantly only in the last 10% of the spicule. Uncinates are mainly choanosomal spicules with anterior tips projecting vertically through the outer (dermal) body surface.

Fig. 7. Chonelasma oreia, sp. nov., spicules (scanning electron microscopy): (A) dermal pinular hexactins with enlarged ray ends; (B) atrial pinular hexactins; (C) rare pinular hexactin with very extended proximal ray (from paratype RBCM 011-00072-001); (D) scopule A, whole, and magnified head side and end views and enlarged tine; (E) scopule B, whole, and magnified head side and end views and enlarged tine; (F) uncinate, whole and magnified anterior segment; (G) hemioxyhexaster; (H) oxyhexactin; (I) hemioxypentaster; (J) hemioxydiaster; (K) oxyhexaster; (L) oxydiaster; (M) spriohemioxyhexaster; (N) microstrongylhexaster, whole and magnified terminal ray tuft. All from holotype unless otherwise indicated.
Microscleres consist of a great variety of oxy-tipped forms and a strongyle-tipped microhexaster; disco-tipped microscleres are absent. Treated here in sequence of abundance, hemioxyhexasters (Figure 7G) are the most common form (51% of oxy-tipped microscleres); 1–2 of the six primary rays support stout, strait, tapered, rough terminal rays. Terminal rays are longer than primary rays (1°/2° length = 0.46). Oxyhexactins (Figure 7H) are the second-most abundant microsclere (25%), similar to the former except that none of the six primary rays support terminal branches. Hemioxypentasters (Figure 7I), hemioxystaurasters, hemioxytriasters and hemioxydiasters (Figure 7J) (together 15%) have less than six primary rays, with at least one of them bearing terminal branches. Oxyhexasters (Figure 7K) are smaller and uncommon (3%); each of the six terminal ray bears 2–10 terminal rays. Oxydiasters (Figure 7L) are also uncommon (1.4%) with the two opposite primary rays bearing 2–6 terminal rays. Oxypentactins, oxytriactins, oxydiactins, spirohemioxyhexasters (Figure 7M) and irregular worm-like shapes are each rare (each <1%; together 4%) all have similar ray forms. Microstrongylhexasters (Figure 7N) have six short smooth terminal rays, each bearing 8–12 short, curved rough terminal rays which end in tips that are simply rounded, flattened or parabolic, but not inflated.
ETYMOLOGY
The species name, oreia, is derived from Greek oreiosi meaning ‘of the mountains’, with reference to the exclusive occurrence of the species on seamounts.
REMARKS
Of the nine presently listed species of Chonelasma, dermal spicules are most commonly pentactins (six species) but three species have pinulate hexacins as dermalia like the present form—C. doederleini Schulze, Reference Schulze1886, from Japan, C. hamatum Schulze, Reference Schulze1886, from north of New Zealand, and C. chathamense Reiswig & Kelly, Reference Reiswig and Kelly2011 from east of New Zealand. These three species all have exclusively discohexasters as microscleres, in contrast to the almost exclusive oxy-tipped microscleres in the new form. These comparisons show that the form described above is a new species, here designated as C. oreia sp. nov.
Order LYSSACINOSIDA Zittel, 1877
Family EUPLECTELLIDAE Gray, 1867
Subfamily BOLOSOMINAE Tabachnick, Reference Tabachnick, Hooper and van Soest2002
Genus Amphidiscella Tabachnick & Lévi, Reference Tabachnick and Lévi1997
Amphidiscella lecus sp. nov.
(Figures 8 & 9; Table 4)
Synonymy. Amphidiscella sp. Reiswig & Frey, Reference Reiswig and Frey2012.
TYPE MATERIAL
Holotype: CASIZ 191781, ROV ‘Tiburon’ from RV ‘Western Flyer’, dive 882, 16 August 2005, North Flows of CoAxial Segment, Juan de Fuca Ridge off Washington State, USA, 46.528178°N 129.57099°W, 2436 m, formalin > ethanol.
Paratype: CASIZ 191782, same dive and general location, 46.528116°N 129.57105°W, 2438 m, formalin > ethanol. Other material. In situ high resolution digital image of a third specimen, same dive, date, location but coordinates not recorded.
DIAGNOSIS
Amphidiscella with discoid body on a long very thin stem. Microscleres include large discohexasters 51–151 µm in diameter, amphidiscs, tetradiscs, floricomes, codonhexasters and spiroxyhexasters.
DESCRIPTION
Both the collected and uncollected specimens are lollipop-like or stiptate in body form, having a soft ovoid or flattened body attached at the top of a long thin stiff stem (Figure 8A–C). Both type specimens imaged in situ have moderate size oscular openings in the centre of the upper side of the body (Figure 8A, B), but after collection and preservation the aperture is either completely collapsed (paratype) or nearly closed (holotype) (Figure 8D–F). The body of the holotype was 4.6 × 4.8 cm in diameters with an osculum 1.7 cm in diameter; in the preserved sample the body dimensions are 2.8 × 3.4 × 1.0 cm with an osculum 0.9 cm in diameter. Assuming height of body to have been about the same as diameters, these data suggest that the preserved state is 12% of the live in situ body volume. The paratype body was calculated to have been 3.1 × 3.5 cm diameter with a 1.0 cm diameter osculum; in its preserved state it is 2.0 × 2.6 cm with an imperceptible osculum. Shrinkage has been about the same in both specimens. Connection between body and stem differs in the three sponges; it emanates abruptly from the edge of the body disc in the paratype (Figure 8Dp) and from the back-centre of the disc opposite the osculum in the holotype (Figures 8E, F) and the uncollected specimen (Figure 8C). The outer surfaces of the very soft, delicate bodies are felt-like, without perceptible prostalia. A dermal lattice is probably present but it is not visible in the wet state. The stems of the holotype and paratype are 18.4 and 11.3 cm long, respectively, and 0.8–0.9 mm in diameter in both. The stem is hollow, the axial tube being ~0.2 mm in diameter. The external stem surface is sculpted by ~10–12 flat longitudinal facets (Figure 8G); the entire external surface is ornamented in finer scale by a uniform coating of short, sharp branching spines (Figure 8H) from the secondary silica layer investing the main diactin spicules. The constituent diactin spicules of the stem are absolutely longitudinal in orientation; no spicules are in direct contact with each other (Figure 8I). These diactins are rigidly joined by perforate transverse tabulae laid down at regular intervals as secondary silicification. The tabulae and fluid spaces between them are open externally and easily visible at moderate magnification through the spiny external stem surface (Figure 8H). No free spicules are associated with any part of the stem and the stems themselves are completely clean and uncolonized by foreign organisms. Basally the stems are attached by small discs (Figure 8J) to recently erupted (three year old) pillow lava at depths of 2436–2438 m.

Fig. 8. Amphidiscella lecus, sp. nov., body and framework: (A) holotype in situ; (B) paratype in situ; (C) third uncollected specimen in situ; (D) holotype (h) and paratype (p) preserved in ethanol; broken stem of holotype on left; (E) two faces of discoid holotype body; (F) edge view of holotype; (G) oblique view of cleaned holotype stem segment (scanning electron microscopy (SEM)); (H) external ornamentation of stem segment in G (SEM); (I) close-up of the face of a stem perforate tabula joining the diactins (SEM); (J) basal attachment discs of the paratype (left, complete) and holotype (right, partial). In situ images courtesey of The Monterey Bay Aquarium Research Institute.
Skeleton of the soft body consists of a network of choanosomal hexactine, pentactine, stauractine (rare) and diactine megascleres without detectable organization. Surface megascleres are pentactins and pinular hexactins in about equal proportions (1.06:1.00, 365 spicules assayed). Atrial megasclere types do not differ from samples taken from the choanosome. Mesoxyhexactins and the great variety of microscleres are distributed rather evenly throughout the soft body.
Megascleres. Surface pinular hexactins (Figure 9A) have short finely spined distal pinular rays, slightly spined and usually ending in pointed tip, only occasionally clavate with a round tip. All other rays are finely spined and end in abrupt sharp tips, but the proximal third of all rays are smooth. The proximal ray is always much longer than tangential rays. Surface and choanosomal pentactins (Figure 9B) do not differ; their rays are similar to those of surface hexactins, finely spined in distal 2/3, abrupt pointed tips. The proximal ray is commonly curved away from its origin perpendicular to the tangenial rays. Choanosomal hexactins (Figure 9C) typically have rays of unequal length, one often shorter, and one to three rays are usually strongly curved. Ray ornamentation and tip form are the same as previously described megascleres. The few choanosomal stauractins (Figure 9D) are larger than the other megascleres but do not differ otherwise. Small mesoxyhexactins (Figure 9E) are similar to the larger choanosomal hexactins but their fair abundance and restricted size-range support their recognition as a distinct spicule class rather than immature stages of the latter. Body diactins (Figure 9F) are fairly common; they are cylindrical, slightly curved, have two or four swellings at the spicule centre and end in slightly inflated rough tips. Stem diactins could only be measured for width in their broken cross-sections (Figure 8I); data are presented in Table 4.

Fig. 9. Amphidiscella lecus, sp. nov., spicules (scanning electron microscopy): (A) surficial pinular hexactin with enlarged distal, tangential and proximal ray ends; (B) surficial pentactin; (C) choanosomal hexactin; (D) rare choanosomal stauractin; (E) choanosomal mesohexactin; (F) choanosomal diactin, whole and enlarged segments; (G) amphidisc; (H) end view of amphidisc umbel; (I) tetradisc; (J) spherical discohexaster, whole and enlarged terminal ray end; (K) floricome, whole and enlarged partial terminal ray tuft; (L) codonhexaster; (M) spiroxyhexaster parts, a centrum, terminal ray and enlarged ends of terminal ray. All from holotype.
Table 4. Spicule dimensions of Amphidiscella lecus, sp. nov., holotype, CASIZ 191781 (dimensions in µm).

Microscleres include mainly amphidiscs, tetradiscs, discohexasters, floricomes, codonhexasters and spiroxyhexasters. The amphidiscs (Figure 9G, H) are small and smooth, with umbels of 10–12 sharp pointed teeth and two short knobs emanating from the spicule centre as reduced transverse rays. Tetradiscs (Figure 9I) are entirely similar but transverse rays with umbels are fully developed. Small numbers of pentadiscs and hexadiscs are present and are not figured. Spherical discohexasters (Figure 9J) are large and entirely smooth; they have short primary rays, each bearing 5–7 straight terminal rays ending in hemispherical caps with 7–12 sharp strong marginal teeth. A very few discohexactins (not figured) of the same size and terminal disc form were found in both type specimens. Floricomes (Figure 9K) are small and extremely delicate spicules; each primary ray supports a whorl of 11–12 sigmoid terminal rays ending in asymmetric caps with 2–5 sharp teeth on the outward facing side. The terminal rays bear two ragged rows of recurved thorns on their outer surfaces but the inner surfaces are smooth. Sperical codonhexasters (Figure 9L) have relatively short umbels for this spicule class. Each moderately long primary ray carries a single whorl of five curved terminals and a central straight terminal, each ending in an umbel with 10–12 long pointed teeth. Spiroxyhexasters (Figure 9M), a new term introduced here for this distinctive spicule type, are invariably broken and occur as centra and scattered terminal rays. The very finely rough primary rays each retain 5–7 short bases of the terminal rays; the terminal ray bases are all about the same length and terminate in flat facets, suggesting that uniform breakage is a design component of the spicule's structure. The terminal rays spiral clockwise about 1.5 revolutions and end in sharp points; they are completely covered with small reclined thorns which are slightly larger along the outer surface of the spiral.
ETYMOLOGY
The species name, lecus, derived from Greek lecos refers to the discoid form of the body.
REMARKS
With its typical euplectellid dermal sword-shaped hexactins and spectactular stalked body form, this sponge belongs in the subfamily Bolosominae. The combination of microscleres places it in the genus Amphidiscella, of which three valid species are known. The new form differs from A. caledonica Tabachnick & Lévi, Reference Tabachnick and Lévi1997 (South-western Pacific and South Atlantic), and A. atlantica Tabachnick & Collins, Reference Tabachnick and Collins2008 (North Atlantic) in having large spherical discohexasters which are absent in both of those species. It differs from A. monai Tabachnick & Lévi, Reference Tabachnick and Lévi1997 (North Atlantic) in size of the floricome, 79–104 µm diameter in the latter versus 32–43 µm diameter in the new form. Two species, A. monai and A. atlantica also have spiroxyhexasters which were inappropiately called ‘sigmatocomes’ in their descriptions. True sigmatocomes have very thin, ‘S’ shaped terminal rays that are planar and end in sharp tips; spiroxyhexasters have thick spiral terminal rays ending in sharp tips. In low magnification planar view, they appear grossly similar but careful focusing at high magnification in LM and normal viewing in SEM allow easy distinction between the two spicule types. The NE Pacific form is clearly distinct from all known members of the genus Atlantisella, and is here designated a new species named Atlantisella lecus sp. nov. It is very likely that the three known specimens of this species are all young stages since they are one of the first macrofaunal elements to colonize recently extruded pillow lava in this region (D. Clague, personal communication); in more mature stages of this species, as yet unknown, body form may become more cup-like, the common mature form of other species of the genus. The tabulate form of the stem is apparently unique among Hexactinellida, although details of few stalks are yet known. Absence of fouling on the stem suggests that it is actively defended by living tissues of the sponge residing on and within it. Attempts to obtain proof of living tissue by dissolution of silica of stem samples with HF produced a mixed fibrous residue that could be hexactinellid syncytial tissue, but it did not settle the issue.
Family LEUCOPSACIDAE Ijima, Reference Ijima1903
Genus OOPSACAS Topsent, Reference Topsent1927
Oopsacas olympicus sp. nov.
(Figures 10 & 11; Table 5)
TYPE MATERIAL
Holotype: RBCM 012-00233-001, coll. OCNMS EPI-193, ROV ‘Ropos’ from CCGS ‘John P. Tully’, dive 1161, 11 July 2008, Juan de Fuca Canyon, South-west of Cape Flattery, Washington, USA, 47°51.4128′N 125°28.3602′W, 924 m, ethanol.
DIAGNOSIS
Oopsacas with saccate body form. Megascleres are highly diverse and include thick-rayed dermal pentactins, thick-rayed choanosomal hexactins and stauractins, irregular tetractins and triactins. Thin rayed choanosomal megascleres are hexactins, diactins and pentactins. Microscleres are robust spherical discohexasters, discasters and thin spherical onychohexasters.
DESCRIPTION
Body form is a sac with a large atrium, very thick body wall and large terminal osculum with sharp inturned margin. The in situ image (Figure 10A) contains the holotype (left) and a second specimen developed from a closely settled larva; other lower-angle images show the attachments of the two specimens are at least 2 cm apart. Attachment to hard substrate is probably by basidictyonal network, but this was not collected. The collected upper part and one side of the type specimen was divided into two nearly equal fragments on shipboard in preparation for freezing, one longitudinal with a small part of the oscular margin (Figure 10B) and one transverse, around the remaining oscular margin (Figure 10C). The external dermal surface is completely smooth with inhalant canals visible in both living and preserved states through a very thin surface lattice (Figure 10D, E); they increase about 7× in diameter from margin to mid-body. The atrial surface is composed of a flat smooth surface interrupted by large open apertures of exhalant canals that increase ca 31× in diameter from oscular margin to the base of the atrium (Figure 10E). The surface between the exhalant apertures is an irregular network of fine openings without a regular lattice (Figure 10G). A section through the body wall (Figure 10H) shows the major elements and large internal spaces. Texture is very soft and delicate; the specimen collapses when removed from water. Dimensions of the holotype are 17 cm height, 15.0 × 16.6 cm diameters (slightly flattened), 9.8 cm osculum diameter; those of the uncollected specimen are 19.9 cm in height, 20.2 cm in diameter, 9.1 cm in oscular diameter. Body wall is maximally 4.2 cm in situ and only 1.9 cm thick in the preserved specimen. Inhalant canals are 0.38–1.20–2.54 mm in diameter and exhalant canals are 0.36–2.40–11.27 mm in diameter in the preserved specimen. The species is known only from the type location South-west of Cape Flattery, Washington, in 924 m depth.

Fig. 10. Oopsacas olympicus, sp. nov., body and framework: (A) holotype (left) and uncollected specimen in situ; (B) two sides of the larger fragment of the collected holotype, oscular margin at top, dermal view (left) and atrial view (right); (C) two sides of the smaller fragment of the collected holotype, oscular margin at top, dermal view (above), atrial view (below); (D) dermal spicule lattice (wet); (E) dermal spicule lattice (dry); (F) atrial surface with large open exhalant canals; (G) enlarged view of the flat part of the atrial surface between exhalant canals; (H) thick transverse section through the body wall showing dermal lattice (dl), subdermal spaces (ss), inhalant canals (ic), exhalant canal (ec) and atrium (at). In situ image courtesy of The Olympic Coast National Marine Sanctuary.
Table 5. Spicule dimensions of Oopsacas olympicus , sp. nov., holotype, RBCM 012-00233-001 (dimensions in µm).

The main skeleton is a network of loose siliceous spicules that forms fairly regular cubic meshes in the outer half of the choanosome but gradually loses regularity towards the atrial surface (Figure 10H). The dermal lattice supported by robust pentactins overlies a widespread subdermal space. The ordered frame of the outer choanosome is formed by arrangement of regular hexactins. The more confused spicule arrangement in the inner choanosome results from addition of diactine and hexactine megascleres with thin, sinuous rays.
Megascleres are highly diverse and assigned to the following categories with some difficulty. Thick-rayed (>30 µm thickness) megascleres (12% of total) have tapered rays; they include dermal pentactins (7%), choanosomal hexactins (5%), choanosmal stauractins, irregular tetractins and triactins (together 0.3%). Thin-rayed (~15 µm thickness) megascleres (88% of total) have cylindrical rays and are all choanosomal; they include hexactins (48%), diactins (38%) and pentactins (2%). The stout pentactins (Figure 11A) are dermalia; they are smooth with slightly inflated rough rounded tips; the proximal ray varies greatly in length. The stout choanosomal hexactins (Figure 11B) are likewise smooth, with rough slightly inflated sharp pointed tips; they form the regular cubic array in the outer choanosome. Stout choanosomal stauractins, irregular tetractins and triactins (Figure 11C) have identical ray form and similar dimensions to those of the surface pentactins and choanosomal hexactins. Thin-rayed hexactins (Figure 11D) are highly variable in form; rays are often curved, sometimes sinuous, and variable in length. Rays are smooth except for the inflated, rounded or sharp-pointed tips. Thin rayed pentactins (Figure 11E) and long diactins are similar in ray form. Some diactins are relatively short but there is no basis for recognizing them as a separate spicule population. Attempts to identify a specific spicule type as atrialia failed. It is concluded here that any and all choanosomal spicule types may occur on the atrial surface and serve the function of atrialia.

Fig. 11. Oopsacas olympicus, sp. nov., spicules (scanning electron microscopy): (A) dermal pentactins, whole and magnified ray ends; (B) stout choanosomal hexactins, whole and magnified ray end; (C) stout choanosomal stauractin, irregular tetractin and triactin; (D) thin choanosomal hexactins, whole and enlarged ray ends; (E) thin choanosomal pentactin, whole and enlarged spicule centre; (F) choanosomal diactins, whole and enlarged end and middle segments; (G) stout spherical discaster, whole and enlarged terminal ray end; (H) thin onychohexaster, whole and enlarged terminal ray end.
Microscleres are robust spherical discohexasters and discasters (Figure 11G) and thin spherical onychohexasters (Figure 11H) that probably represent different maturation stages of a single spicule category with different levels of ray thickening. All have similar dimensions, similarly rough terminal rays and similar numbers of terminal marginal teeth (5–10). They are listed separately here since spicule form is distinct for the two types, but they are probably produced by the same genetic programme but with differential timing of ‘cease silica deposition’ instruction. There are some but few intermediate spicules, less than 5%.
ETYMOLOGY
The species name, olympicus, refers to the location of collection of the specimen, off the Olympic Penninsula of Washington State.
REMARKS
This species has no hypodermalia and agrees with the diagnosis of family Leucopsacidae (Tabachnick, Reference Tabachnick, Hooper and van Soest2002). Within that family, it conforms to the diagnosis of Oopsacas in having only pileate discohexasters or their immature onychoid stages as microscleres. That genus presently has only two recognized species, O. minuta Topsent, Reference Topsent1927 from the Mediterranean Sea and O. stellata (Ijima, Reference Ijima and Weber1927) from Indonesia. The new NE Pacific form differs from both of those species in having much larger discohexasters, mean 104 versus 45–55 and 45–86 µm diameters in those, respectively and much larger surface pentactins, mean 628 versus 190 and 145–300 µm ray length in those, respectively. The species Chaunoplectella spinifera Ijima, Reference Ijima1903 from Japan, does not agree with the diagnosis of the genus in which it is presently placed; none of the variety of discohexasters or onychohexasters are anchorate in form and it lacks sigmatocomes, which are admittedly not a necessity for admittance to Chaunoplectella, but which are characteristic of the only other and type species of the genus. The species is here transferred to Oopsacas as O. spinifera (Ijima, Reference Ijima1903). The new NE Pacific form differs from O. spinifera in having only a single class of spherical discohexaster (and its onychoid stage) while the latter species has four distinct types of pileate discohexasters and onychohexasters, of which two are spherical and two are stellate. These comparisons certify that the NE Pacific form is a member of a new species, here designated as Oopsacas olympicus sp. nov.
Family ROSSELLIDAE Schulze, 1885
Subfamily ACANTHASCINAE Schulze, 1897
Genus ACANTHASCUS Schulze, Reference Schulze1886
Acanthascus malacus sp. nov.
(Figures 12 & 13; Table 6)
TYPE MATERIAL
Holotype: RBCM 010-00190-005, CCGS ‘W.E. Ricker’, Station TC2006-013, 11 October 2006, off north-western tip Vancouver Island, British Columbia, Canada, 50°43.2–42.7′N129°25.0–23.8′W, 1748–1788 m
DIAGNOSIS
Acanthascus with dermal and atrial regular and pinular hexactins. Microscleres are mainly discoctasters of medium to large size, 156–235 µm diameter, with very short primary rays, and large oxyhexasters, 131–208 µm diameter; microdiscohexasters are absent.
DESCRIPTION
The single specimen was collected by trawl and is severely damaged. It consists of flat, 5-mm-thick, broken fragments (Figures 12 A, B) which are greatly filled with sediment and retain the dermal spicule lattice only in some places. Body form of the original specimen was presumably sac-shape. Holotype fragments range in dimensions from 3.2 × 4.2 to 8.8 × 21.3 cm. No prostalia are present on the outer body surfaces. In places with little damage, the external surface is relatively smooth to the naked eye (Figure 12C); where the dermal lattice is rubbed off, apertures to vertical inhalant canals, 1.4–2.3–3.6 mm diameter (N = 33), are easily visible. The internal atrial surface (Figure 12D) is covered with apertures of the vertical exhalant canals, 0.7–2.4–4.8 mm in diameter (N = 37). Where the dermal lattice is preserved (Figure 12 E, F), the meshes are square with 2–4 tangential rays of hexactine dermalia composing the 138–196–257 um long mesh sides (N = 36). There is no similar lattice on the atrial surface (Figure 12G); atrial hexactins are packed without obvious order on the flat margins between apertures. Osculum size, shape and oscular margin details are not available. Texture is very soft and fragile; color is brownish green (deep-water mud colour). Known distribution is restricted to the continental shelf edge west of north Vancouver Island and Queen Charlotte Islands, NE Pacific Ocean (Figure 1F), at depths of 1748–1788 m.

Fig. 12. Acanthascus malacus, sp. nov., body and framework: (A) main holotype fragments, dermal surfaces; (B) main holotype fragments, atrial surfaces; (C) dermal surface in closer view; (D) atrial surface in closer view; (E) dermal spicule lattice (dry); (F) dermal spicule lattice mounted in balsam; (G) atrial surface with open exhalant canal aperture.
Table 6. Spicule dimensions of Acanthascus malacus, sp. nov., holotype, RBCM 010–00190–005 (dimensions in µm).

The skeleton is entirely composed of loose spicules; diactins, occurring either singly or in bundles, are the only choanosomal megascleres; they are distributed without detectable pattern. The only organized skeletal element is the dermal lattice described above. Atrialia are restricted to the atrial lining but do not form an organized lattice. All categories of microscleres are evenly distributed through the body wall.
Megascleres are dermal and atrial hexactins and choanosomal diactins. Dermalia occur in two forms, regular (77%) and pinular (23%) hexactins (Figure 13A) with intermediates rather rare. Rays are slightly tapered and rough on the distal 2/3; tips are mostly bluntly-rounded, but pinular and proximal rays are abruptly pointed. Atrialia are similar, also occurring in the same two forms, regular (14%) and pinular (86%) hexactins (Figure 13B). Two classes of choanosomal diactins are recognizable, thick (~43 µm) and thin (~14 µm) classes (Figure 13C). Both are mostly smooth and cylindrical with rough ends and either rounded or abruptly pointed tips; a central swelling is either barely detectable or absent. The larger diactins serve as hypodermalia and hypoatrialia. Proper pentactin megascleres are either absent or so rare that they are not significant skeletal elements. In a digest of a 1.5 cm2 peel of a minimally disturbed dermal surface, five macropentactins were found, but all rays were broken. They are considered either foreign in origin or, if proper, too rare to form a hypodermal support grid; their condition and the method of specimen collection supports the probability that they are of foreign origin.

Fig. 13. Acanthascus malacus, sp. nov., spicules (scanning electron microscopy): (A) regular and pinular hexactine dermalia, whole and enlarged ray ends; (B) regular and pinular hexactine atrialia; (C) choanosomal diactins, whole and enlarged middle segment and ends; (D) discoctaster, whole and enlarged terminal ray tuft and end of one terminal ray; (E) discoctaster centra, regular (left) and irregular (right); (F) oxyhexaster, whole and enlarged terminal ray end. All from holotype.
Microscleres are mainly discoctasters and oxyhexasters; microdiscohexasters are not present. Discoctasters (Figure 13D) occur throughout the wall in a relatively narrow size-range. Each smooth primary ray supports 3–7–9 finely rough terminal rays that curve slightly outward to form a spread bouquet. Primary and terminal (secondary) rays are about equal in length (mean 1°/2° length is 1.07). Terminal ray ends have a tall, marginally serrate disc. The centrum is commonly of the normal discoctaster form with six short interradial knobs (Figure 13E left) but a significant proportion, ~ 20%, have irregular centra which bear from 1 to 15 short curved finger-like processes projecting directly from the centra between the reduced primary ray bases (Figure 13E right). Primary rays in these ‘irregulars’ often number more than eight and some primary rays bear only a single terminal ray. These are clearly terminal rays in which branching and bundling of primary rays during early discoctaster development failed to progress correctly. Oxyhexasters (Figure 13F) are moderately large; their short smooth primary rays support 2–4 terminal rays ornamented with large reclined spines.
ETYMOLOGY
The species name, malacus, is derived from Greek, malakos meaning soft and squidgy, as reference to the delicate and flacid texture of the specimen.
REMARKS
Presence of discoctasters places this sponge in the subfamily Acanthascinae. Lack of a hypodermal pentactin layer relegates it to genus Acanthascus. The presence of hexactin dermalia in the new form differs from all six presently accepted valid species of Acanthascus. Pentactine dermalia occur in A. alani Ijima, Reference Ijima1898, A. mitis Koltun, Reference Koltun1967, A. platei Schulze, Reference Schulze1899, and A. sacculus Hernandez, Reference Hernandez1932, while stauractin dermalia dominate in A. cactus Schulze, Reference Schulze1886, and A. pachyderma Okada, Reference Okada1932. Other additional differences from the known species involve presence of pinular atrialia (somewhat similar spicules known only for A. pachyderma), absence of microdiscohexasters (present in five species) and size and shape of discoctasters (similar only to A. alani in this character). Based upon these differences, it is clear that the new specimens cannot be assigned to any known species and are thus recognized as members of a new species, here erected as Acanthascus malacus sp. nov.
DISCUSSION
This report adds six new hexactinellid species to the 21 previously known from the restricted part of the NE Pacific AOI. Government programmes and conservation groups have identified this area as needing increased basic knowledge for management of the coral-sponge communities (F&O Canada, 2010; NOAA, 2010; McGillivary, 2011) and call for increased funding to support new sampling programmes. These suggestions are not, however, accompanied by a proposal for support of taxonomic specialists to identify the new collections. More importantly, the persons behind formulation of these programmes seem completely unaware of the large collections from this targeted area presently stored on institution shelves as ‘unidentified’ material, or worse yet, recently disposed of due to financial restrictions on such institutions (e.g. Biocuration Facility at Oregon State University, general collections of Michigan State University Museum, collections of Amhurst College). Thankfully there are still thousands of unidentified hexactinellid specimens from this area in institution collections awaiting examination, but there is no evidence of serious interest or even awareness of them or their potential value by agencies dispersing government funding. Allocation of ship time and support of technical advancement continue to be preferred funding directions, with the result that institutions end up with the cost of storing even more unidentified specimens and government agencies end up with cartons of new videotape showing organisms that are either unknown, or, more often, erroneously identified by unqualified students or technical staff. This AOI certainly contains at least 10 more species of hexactinellids, and possibly as many as are presently known (100% more), but with the current focus of funding decisions, they may never be described. Morphological taxonomy is neither attractive to such committees nor an activity that committee members' own students are able to perform within these time constraints. Classical morphological taxonomy is still required for revealing the composition of coral-sponge communities.
ACKNOWLEDGEMENTS
I thank the scientist parties, pilots and crews of [the ships] CCGS ‘W.E. Ricker’, CCGS ‘John P. Tully’, RV ‘Western Flyer’, RV ‘Atlantis’ and submersibles ‘Ropos’, ‘Alvin’ and ‘Tiburon’, whose skills and patience resulted in the collection of these exciting new animals. Help with obtaining access to specimens and their data was gratefully received from George Schmahl, Lonny Lundsten, David Clague, William Austin, Jackson Chu, Ed Bowlby, Mary-Sue Broncatto, Mellissa Frey and Moretta Frederick. Assistance with SEM was provided by Brent Gowan and Elaine Humphrey. Cedric Littlepage helped immensely with choosing and correct species names. I also thank Dorte Janussen and an unidentified referee for suggestions that improved this paper.
FINANCIAL SUPPORT
Cruises on which specimens were collected were funded by NOAA's Office of Ocean Exploration, The Natural Sciences and Engineering Council of Canada, Fisheries and Oceans Canada and The Monterey Bay Aquarium Research Institute. Processing of samples for description was funded by The Royal British Columbia Museum, Victoria, BC, Canada.