INTRODUCTION
Sipunculans are among the most dominant macroboring fauna living inside dead coral skeletons in coral reefs around the world (Rice & Macintyre, Reference Rice, MacIntyre, Rutzler and MacIntyre1982; Hutchings, Reference Hutchings1986; Glynn, Reference Glynn and Birkeland1997). The Sipuncula, which are exclusively marine and widely distributed in soft and hard substrata (Rice, Reference Rice1976) from tropical to polar regions (Murina, Reference Murina, Rice and Todorovic1975), are vermiform animals, also called peanut worms. A great proportion of sipunculans are tropical species accounting for more than 60% of the total, followed by temperate and polar species (Murina, Reference Murina, Rice and Todorovic1975). According to the most comprehensive taxonomic study on sipunculans, the group is composed of two classes, four orders, six families, 17 genera and more than 160 species and subspecies (Cutler, Reference Cutler1994). The body is divided in two main parts: a trunk without segmentation and a retractable introvert, usually with a tentacular arragement at the most distal part. Sipunculans present a variety of epidermal structures such as papillae, hooks, and shields that are distinctive characters of the group, and are basically the only external morphological features used for identification (Rice, Reference Rice, Rice and Todorovic1975; Cutler, Reference Cutler1994).
Sipunculans are ecologically important in marine sediments since they are responsible for the bioturbation of organic matter and serve as a food source for higher trophic levels (Murina, Reference Murina1984; Cutler, Reference Cutler1994). They are especially common in coral reef environments where they occupy a wide variety of habitats such as sand flats, coral boulders and rubble, and in the majority of the cases they bore into the coral reef framework (Rice & Macintyre, Reference Rice, MacIntyre, Rutzler and MacIntyre1982; Hutchings, Reference Hutchings1986; Glynn, Reference Glynn and Birkeland1997). The sipunculans are one of the most abundant groups found in dead coral skeletons in terms of biomass and number of organisms (Hutchings, Reference Hutchings1986). They have an important ecological role in coral reefs because of their capacity of in situ degradation of calcium carbonate, thus, being an important component in the process of bioerosion (Rice & Macintyre, Reference Rice, MacIntyre, Rutzler and MacIntyre1982; Hutchings, Reference Hutchings1986; Peyrot-Clausade & Brunel, Reference Peyrot-Clausade and Brunel1990; Peyrot-Clausade et al., Reference Peyrot-Clausade, Hutchings and Richard1992). The recruitment is mostly via pelagic larvae that metamorphose into the reef matrix where the animal develops and grows (McCloskey, Reference McCloskey1970). The rate of recruitment depends on different biotic and abiotic factors, and should be considered variable in space and time (Davies & Hutchings, Reference Davies and Hutchings1983; Hutchings et al., Reference Hutchings, Kiene, Cunningham and Donnelly1992). Some studies have determined that the degree of sipunculan infestation (including other macroborers such as polychaetes) has a direct relationship with the physical characteristics of the coral skeleton, such as hardness and morphology, cover of epifauna and algae, the degree of succession in the coral skeleton (Hutchings, Reference Hutchings, Cameron, Cambell, Cribb, Endean, Jell, Jones, Mather and Talbot1974; Hutchings & Peyrot-Clausade, Reference Hutchings, Peyrot-Clausade, Choat, Barnes, Borowitzka, Joll, Davies, Flood, Hatcher, Hopley, Hutchings, Kinsey, Orme, Pichon, Sale, Sammarco, Wallace, Wilkinson, Wolanski and Bellwood1988; Perry, Reference Perry1998), and water quality among others (Hutchings & Peyrot-Clausade, Reference Hutchings and Peyrot-Clausade2002).
Comprehensive studies about macroborers, which emphasize the role of sipunculans in coral reef substratum, have been conducted in the Indo-Pacific (Peyrot-Clausade & Brunel, Reference Peyrot-Clausade and Brunel1990; Hutchings et al., Reference Hutchings, Kiene, Cunningham and Donnelly1992; Peyrot-Clausade et al., Reference Peyrot-Clausade, Hutchings and Richard1992; Hutchings & Peyrot-Clausade, Reference Hutchings and Peyrot-Clausade2002), and the Caribbean region (Rice, Reference Rice, Rice and Todorovic1975, Reference Rice1976; Rice & Macintyre, Reference Rice, MacIntyre, Rutzler and MacIntyre1982; Schulze & Rice, Reference Schulze and Rice2004; Schulze, Reference Schulze2005). To our knowledge there are no studies about sipunculans for the Caribbean coast of Colombia. Nevertheless, one publication about cryptobiota exists, which mentions some specimens at the family level and some at genus level (Moreno-Forero et al., Reference Moreno-Forero, Navas and Solano1998). Rice (Reference Rice, Rice and Todorovic1975) conducted one of the most complete studies in the Caribbean including 11 stations in the Southern region such as Venezuela and Curacao. The purpose of the present study is to characterize the sipunculan community living inside massive coral skeletons of Diploria strigosa (Dana, 1846), Montastraea cavernosa Linnaeus, 1767 and Montastraea annularis (Ellis & Solander, 1786) in the Santa Marta area of Colombia, and to determine if there are differences in the structure of the sipunculan community between the different coral species. This work represents the first detailed study of the sipunculan community from coral reefs around Santa Marta, and contributes to the knowledge about the diversity of macroborers in the area.
MATERIALS AND METHODS
Study site
This study took place in a patchy reef near Santa Marta, Colombia (11°10′44″–11°13′24″N and 74°14′43″W), which consists of sponge dominated coral communities that develop mainly on metamorphic rocks (Díaz et al., Reference Díaz, Escobar and Velásquez1990). The region is influenced by a seasonal upwelling process, lowering the seawater temperatures with increments in salinity (Arévalo-Martínez & Franco-Herrera, Reference Arévalo-Martínez and Franco-Herrera2008), and receives a strong input from inland waters and sediments from the Ciénaga Grande de Santa Marta, the Magdalena River and a number of rivers from the Sierra Nevada de Santa Marta (Ramírez, Reference Ramírez1990; Franco, Reference Franco2005). It is possible to find a reef development with Diploria Milne-Edwards & Haime, 1848, Colpophyllia natans (Houttuyn, 1772) and Montastraea de Blainville, 1830 as predominant species.
Field sampling
Using SCUBA, samples were collected in nearby coral reefs around Santa Marta (Gaira Bay) between March and April 2005 (Figure 1). Dead portions of corals Diploria strigosa (N = 14), Montastraea cavernosa (N = 15), and Montastraea annularis (N = 14) that at the time of the collection looked superficially similar (covered with filamentous algae) (Figure 2), were sampled at 10 m depth. To determine the coral species, each sample was superficially scraped in situ to observe basic structures like shape and size of the calyx, which were later corroborated in the laboratory. Coral species identification was performed according to Reyes & Santodomingo (Reference Reyes and Santodomingo2002) taking into consideration the following characteristics: Diploria strigosa has long continuous valleys of 6–9 mm wide (Veron, Reference Veron2000) with 15–20 septa cm−2 (Reyes & Santodomingo, Reference Reyes and Santodomingo2002); the colonies are hemispheric meandroids (Veron, Reference Veron2000). Colonies of Montastraea cavernosa form massive boulders and domes (Veron, Reference Veron2000) with corallites protruding from the skeleton. It has rounded calyces of 5–7 mm in diameter with 48 septa; 24 of them complete and connected to the columella (Reyes & Santodomingo, Reference Reyes and Santodomingo2002). Montastraea annularis grows in clusters of thick columns with dome-like tops, and the corallites are somehow projected from the coral matrix with calyces between 2 and 2.7 mm in diameter. It has 24 septa per calyx and 12 are connected to the columella (Reyes & Santodomingo, Reference Reyes and Santodomingo2002).

Fig. 1. Study site in the Santa Marta region. Arrows indicate the sampling location.

Fig. 2. Skeletal samples analysed. (A–B) Montastraea annularis (Ellis & Solander, 1786) samples; (C–D) Diploria strigosa (Dana, 1846); (E–F) Montastraea cavernosa Linnaeus, 1767.
By means of hammer and chisel coral samples were taken randomly along several 50 m belt transects after preliminary identification of coral species. A chunk of approximately 1 dm3 (Peyrot-Clausade, Reference Peyrot-Clausade, Cameron, Cambell, Cribb, Endean, Jell, Jones, Mather and Talbot1974; Hutchings, Reference Hutchings1981) of dead coral was extracted from the reef matrix and taken to the surface in plastic bags, where they were fixed with 10% buffered formaldehyde, for a posterior extraction and analysis of the sipunculans in the laboratory. Specimens were preserved in 70% ethanol. Because of the irregularity of the coral samples, the volume was recorded by measuring the changes in water displacement, introducing each portion of coral skeleton in a 4000 ml beaker. Using hammer and chisel, dead coral samples were broken up in small pieces where the extraction was performed. After this, sipunculans embedded in the coral matrix were extracted with fine-tipped forceps to minimize damage. Some specimens (particularly small ones) were destroyed in the extraction process and were not quantified; however this accounts for less than 5% of the total individuals. Every species had several relaxed individuals showing the entire introvert, on which the full identification was done. When specimens were contracted, only other characteristics, such as the type of band muscles and/or characteristics on the anal shield and papillae, were taken in consideration. The identification was performed according to Cutler (Reference Cutler1994) and one individual of each species was sent for comfirmation to Dr G. Kawauchi, a specialist taxonomist for the group.
Sample treatment
To carry out the description of the sipunculan community, a list of the species found for this study was created for each skeletal substrate where the absolute abundance (number of organisms), relative abundance (%), and mean density (organisms dm−3) ± standard error (SE) were calculated. To characterize the assemblage of sipunculans between coral skeletons, species richness (S), Shannon–Wiener diversity (H′Ln), and Pielou evenness (J′) were calculated with PRIMER®V5 (Clarke & Warwick, Reference Clarke and Warwick2001). For all the calculations the samples were categorized according to the coral skeleton (D. strigosa, M. cavernosa, M. annularis). To test for statistical differences in abundance, species richness, and diversity, one-way ANOVAs were applied after data met the assumptions of homogeneity of variances (tested by Bartlett's test) and normality (as shown by the Kolmogorov–Smirnov test with Lilliefor's correction). For evenness, arcsin square root transformation was applied to the raw data as recommended for percentages and proportions (Quinn & Keough, Reference Quinn and Keough2002; Zar, Reference Zar2010). Data of dead coral samples (1 dm3), within species, were analysed as replicates. Values were considered statistically significant when P < 0.05.
RESULTS
General overview
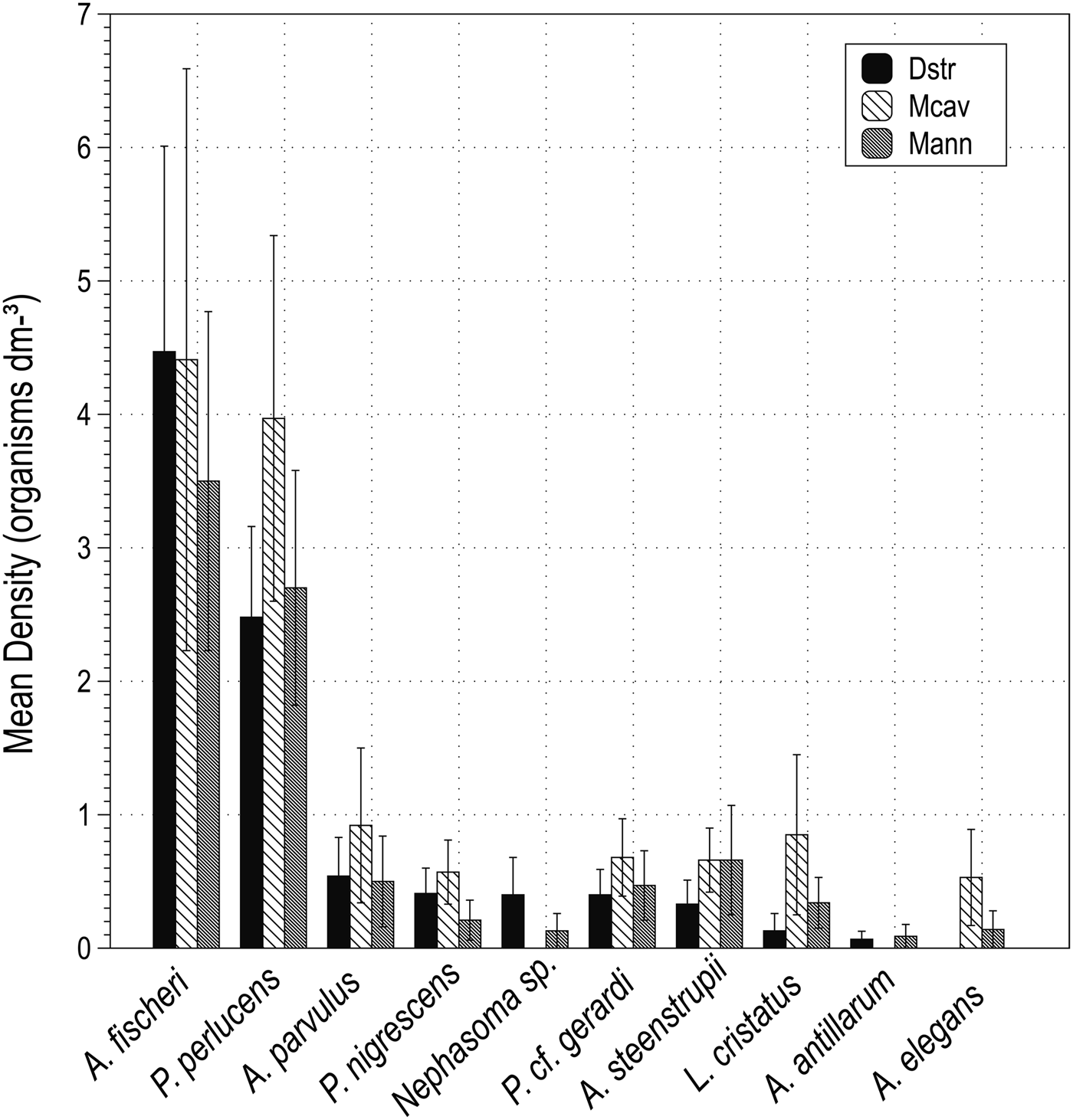
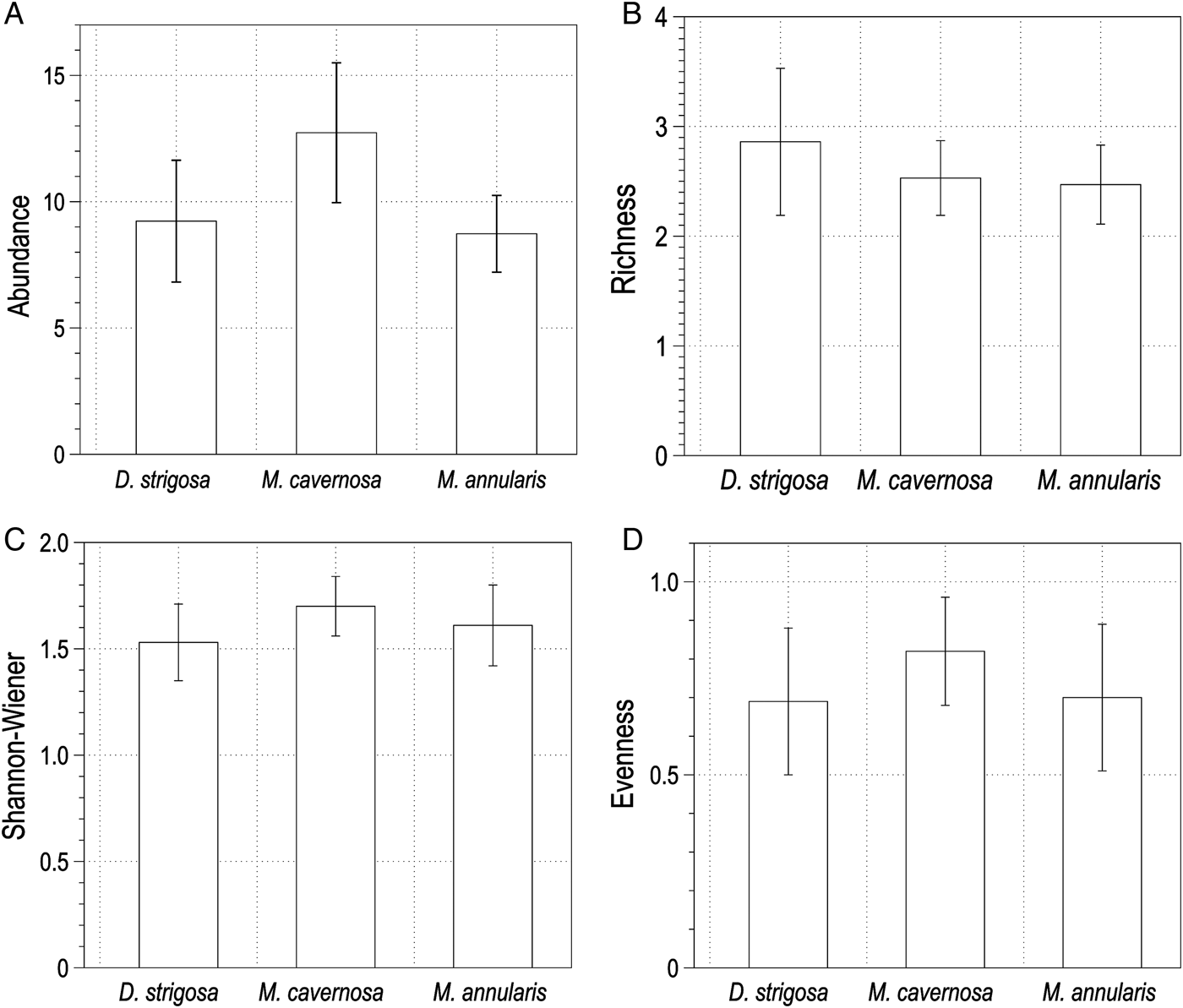
A total of 381 sipunculans (10.69 ± 1.00 organisms dm−3) living inside dead skeletons of Dipoloria strigosa, Montastraea cavernosa and Montastraea annularis in approximately 36 dm3 were found. Sipunculans were distributed in four families and ten species. Aspidosiphon fischeri ten Broeke, 1925 and Phascolosoma perlucens (Baird, 1868) were the most abundant and frequent species, followed by Aspidosiphon parvulus Gerould, 1913, Aspidosiphon steenstrupii (Diesing, 1859), Phascolion cf. gerardi Rice, 1993, Lithacrosiphon cristatus (Sluiter, 1902), Phascolosoma nigrescens (Keferstein, 1865), Aspidosiphon elegans (Chamiso & Eysenhardt, 1891), Nephasoma sp. and Antillesoma antillarum (Grube & Oersted, 1858). Values of the total number of organisms, mean density ± SE and frequency of occurrence are given in Table 1. Dead skeletons of M. cavernosa had the greatest abundance with 148 sipunculans (38.85%) with a mean density of 12.63 ± 2.78 organisms dm−3, and were distributed in three families and eight species. Organism abundance ranged between two and 38 individuals across samples and the species richness ranged between 1–5 species. Skeletons of D. strigosa contained a total of 121 sipunculans (31.76%) with a mean density of 9.92 ± 2.04 organisms dm−3, and were distributed in four families and nine species. Organism abundance ranged between one and 28 individuals across samples and the species richness ranged between one and seven species. Skeletons of M. annularis contained a total of 112 organisms (29.39%) with a mean density of 9.38 ± 1.48 organisms dm−3 distributed in four families and 10 species. Organism abundance ranged between one and 22 individuals across samples and the species richness ranged between one and five species. Figure 3 shows the mean densities (sipunculans dm−3) for each species in each skeletal substrate. A one-way ANOVA showed that there were no significant differences in the abundance (F2,40 = 0.23, P = 0.79) or species richness (F2,40 = 0.18, P = 0.83) among coral skeletons (Figure 4A, B). Skeletons of M. cavernosa had the highest Shannon–Wiener diversity index values (H′ = 1.70, confidence interval 1.57–1.84 nits), followed by M. annularis (H′ = 1.61, confidence interval 1.42–1.80 nits), and D. strigosa (H′ = 1.53, confidence interval 1.35–1.71 nits). The evenness was slightly higher in skeletons of M. cavernosa (J′ = 0.82) than M. annularis (J′ = 0.70) and D. strigosa (J′ = 0.69) but it had the lowest species richness (S = 8). Likewise, no significant differences were found for these indices among coral skeletons (Shannon–Wiener: F2,30 = 0.106, P = 0.89; Pielou: F2,30 = 0.644, P = 0.53) (Figure 4C, D).

Fig. 3. Values of density (organisms dm−3) for each species found in skeletons of Diploria strigosa (Dstr) (Dana, 1846), Montastraea cavernosa (Mcav) Linnaeus, 1767, and M. annularis (Mann) (Ellis & Solander, 1786). Values are expressed as mean ± standard error.

Fig. 4. Values for (A) abundance, (B) species richness, (C) diversity of Shannon–Wiener, and (D) evenness of Pielou of the sipunculans found in the three skeletal substrates. Mean ± standard error in (A) and (B), mean ± confidence intervals in (C) and (D).
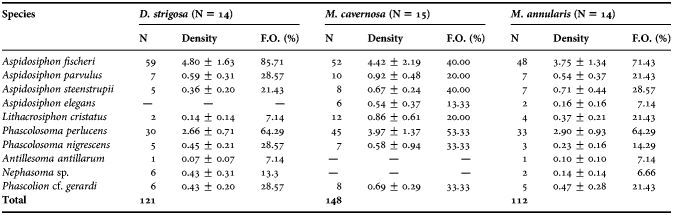
Table 1. Total species list with the values of the total abundance (N), density (organisms dm−3 ± standard error), and frequence of ocurrence (F.O. %) found in each skeletal substrate.

Species characterization
ASPIDOSIPHON FISCHERI
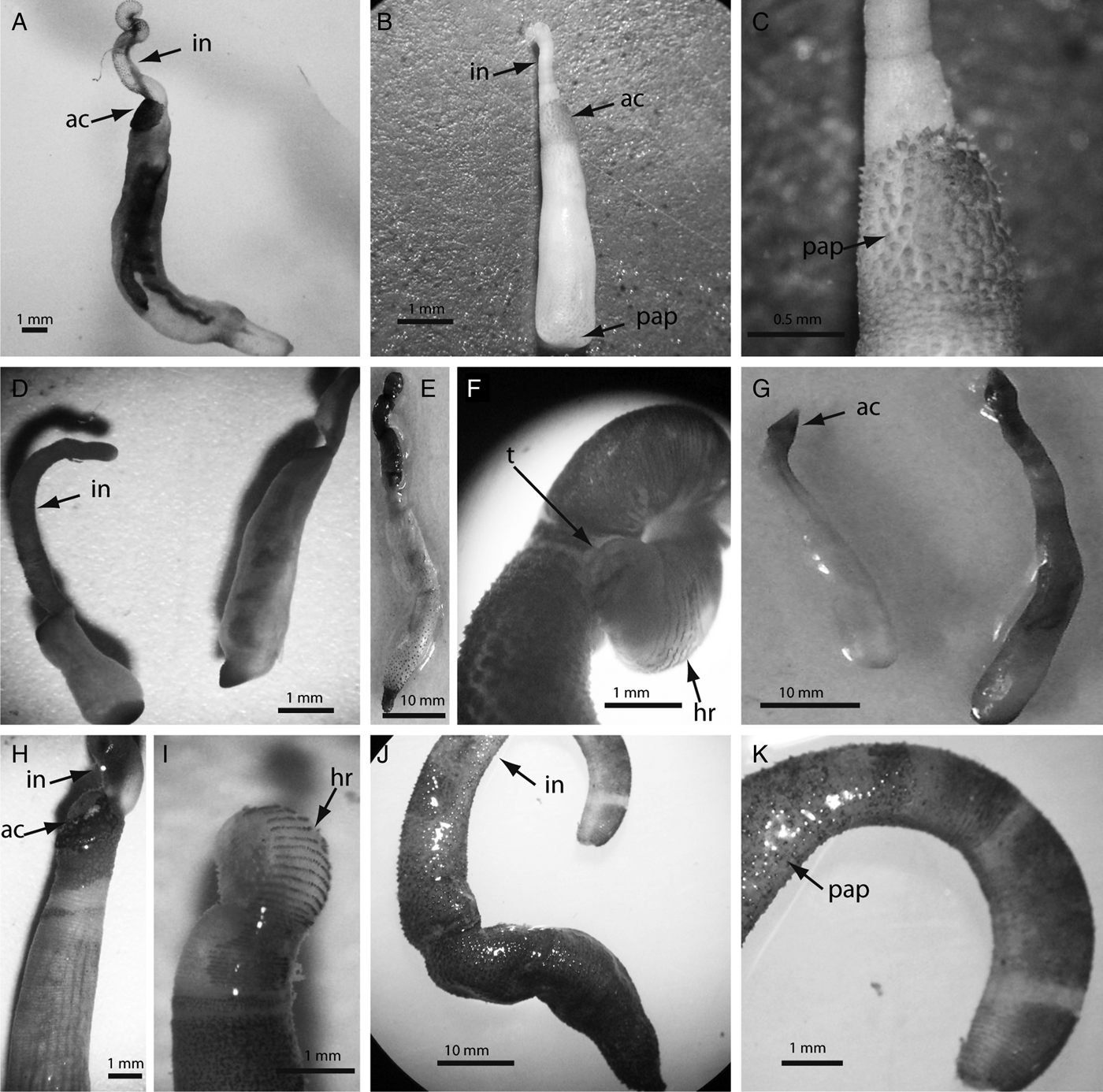
This was the most abundant and frequent species in the study. It was more abundant and frequent in skeletons of D. strigosa > M. annularis > M. cavernosa (Table 1). Aspidosiphon fischeri is commonly found in shallow coral rock in the southern Caribbean boring into coral skeletons (Cutler, Reference Cutler1994) (Figure 5D). Material examined: UNIANDES-IM 1685, south-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi.

Fig. 5. Photographs of the most representative sipunculans found in the present study: (A) Aspidosiphon elegans (Chamiso & Eysenhardt, 1891), arrows indicate the position of the anal shield (ac) and introvert (in); (B) Aspidosiphon parvulus Gerould, 1913, arrows pointing at the anal shield (ac), introvert (in) and papillae in the caudal shield (pap); (C) close-up of anal shield of A. parvulus, arrow indicates the conical papillae (pap); (D) Aspidosiphon fischeri ten Broeke, 1925; (E) Phascolosoma perlucens (Baird, 1868); (F) close-up of P. perlucens introvert with arrows pointing at the distal tentacles (t) and hooks disposed in rings (hr); (G) Lithacrosiphon cristatus (Sluiter, 1902); (H) Aspidosiphon steenstrupii (Diesing, 1859); (I) close-up of A. steenstrupii introvert, arrow indicates the introvert with the arrange of hooks in rings (hr); (J) Phascolosoma nigrescens (Keferstein, 1865), arrow indicates the introvert; (K) P. nigrescens introvert with arrow pointing at the dark papillae.
ASPIDOSIPHON PARVULUS
This was the third most abundant species. It was more abundant in skeletons of M. cavernosa > M. annularis = D. strigosa, and more frequent in skeletons of D. strigosa (Table 1). All bore into coral skeletons (Cutler, Reference Cutler1994) (Figure 5B, C). Material examined: UNIANDES-IM 1687. South-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi.
ASPIDOSIPHON STEENSTRUPII
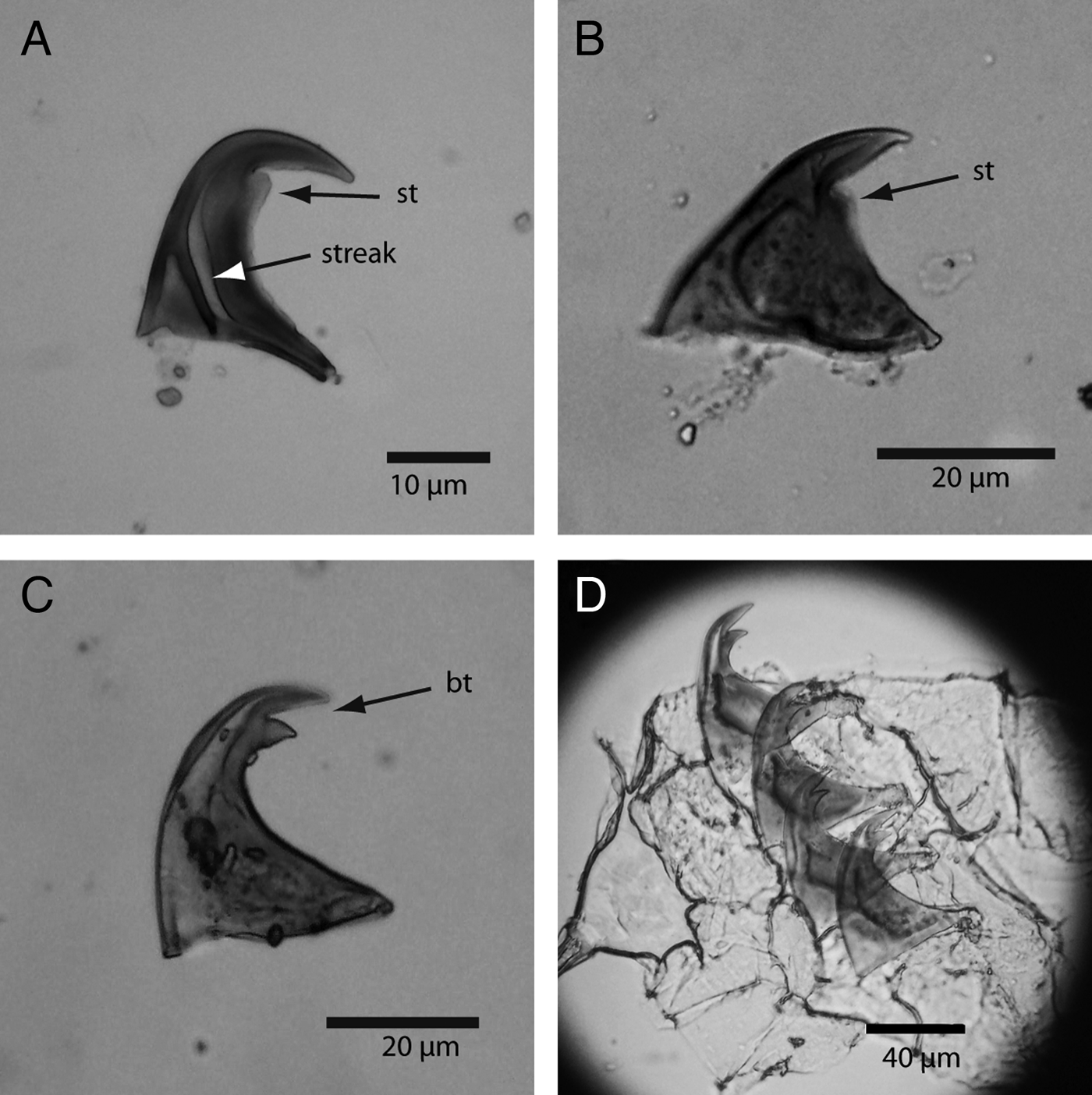
This was the fourth most abundant species of the study. It was more abundant in skeletons of M. annularis > M. cavernosa > D. strigosa, and more frequent in skeletons of M. cavernosa (Table 1). Aspidosiphon steenstrupii has a distinctive anal shield where it is common to see externally deposited calcium carbonate, and it has bidentate hooks disposed in rings, which are between 30 and 70 μm tall (Cutler, Reference Cutler1994) (Figures 5H, I and 6D). It has a circumtropical distribution and bores into coral skeletons (Cutler, Reference Cutler1994). Material examined: UNIANDES-IM 1686. South-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi.

Fig. 6. Photomicrographs of hook types: (A) Phascolosoma perlucens (Baird, 1868) showing the characteristic rounded secondary tooth (st) and the streak; (B) Phascolosoma nigrescens (Keferstein, 1865), arrow pointing at the secondary tooth; (C) Aspidosiphon fischeri ten Broeke, 1925, arrow pointing at bidentate hook (bt); (D) row file of bidentate hooks of Aspidosiphon steenstrupii (Diesing, 1859).
ASPIDOSIPHON ELEGANS
Although this is one of the most common and widespread tropical sipunculans, it was under-represented in our study. Aspidosiphon elegans was present in three samples, and was absent from skeletons of D. strigosa. It appeared in two samples of M. cavernosa with three sipunculans in each sample, and in M. annularis we found two individuals in only one sample (Table 1). All specimens were small in size, smooth and white in colour with bidentate compressed hooks (Figure 5A). They are common dwellers of live and dead coral skeletons in shallow waters (Cutler, Reference Cutler1994). Material examined: UNIANDES-IM 1688. South-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi.
LITHACROSIPHON CRISTATUS
It was more abundant in skeletons of M. cavernosa > M. annularis > D. strigosa, and more frequent in skeletons of M. annularis and M. cavernosa than D. strigosa, which appears in only one sample (Table 1). It has an anterior, distinctive, calcareous cone-shaped anal shield that has parallel grooves, which is typical of the genus (Figure 5G). Lithacrosiphon cristatus has wide tropical distribution (Cutler, Reference Cutler1994), and has been collected from coral rock in shallow areas from different locations in the Caribbean (Rice, Reference Rice, Rice and Todorovic1975). Material examined: UNIANDES-IM 1689. South-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi.
PHASCOLOSOMA PERLUCENS
This was the second most abundant and frequent species. It was more abundant in skeletons of M. cavernosa > M. annularis > D. strigosa, and less frequent in M. cavernosa (eight samples) than D. strigosa and M. annularis (nine samples each) (Table 1). This middle size species is characterized for having reddish papillae concentrated at the base of the introvert, and an arrangement of tentacles at the distal end of the introvert (Figure 5E, F). It presents hooks in rings that are characterized for having a secondary tooth (Figure 6A). This species bore into coral skeletons (Cutler, Reference Cutler1994). Material examined: UNIANDES-IM 1692. South-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi.
PHASCOLOSOMA NIGRESCENS
This species was more abundant and frequent in skeletons of M. cavernosa > D. strigosa > M. annularis (Table 1). The trunk is usually brown with numerous papillae, and the introvert has pigmented bands (Cutler, Reference Cutler1994) (Figures 5J, K and 6B). This widespread species bore into coral skeletons (Cutler, Reference Cutler1994). Material examined: UNIANDES-IM 1693. South-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi.
ANTILLESOMA ANTILLARUM
This was the least abundant in the study, appearing in two samples. It was found, once each in skeletons of D. strigosa and M. annularis. The two organisms were about 25 mm each. The trunk is dark-brown with thick, dark papillae at the anterior and the posterior end of the animal. Antillesoma antillarum has been referenced in the literature as cosmopolitan, found in tropical and subtropical environments, boring into coral skeletons in shallow areas (Cutler, Reference Cutler1994).
PHASCOLION CF. GERARDI AND NEPHASOMA SP.
These were found in the three different coral skeletons, with the exception that Nephasoma sp. was not present in M. cavernosa. Phascolion cf. gerardi was more abundant and frequent in skeletons of M. cavernosa > D. strigosa > M. annularis (Table 1). Nephasoma sp. was more abundant and frequent in skeletons of D. strigosa than M. annularis. Although these two species are commonly found in coral skeletons or empty mollusc shells that they use as shelter, they are not considered borers like aspidosiphonids or phascolosomatids since they usually use pre-existing spaces (Cutler, Reference Cutler1994). Material examined Phascolion cf. gerardi: UNIANDES-IM 1691. South-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi. Material examined Nephasoma sp.: UNIANDES-IM 1690. South-western Caribbean Sea, Gaira Bay off Santa Marta, Magdalena, Colombia, 10 m depth. ID revised by G. Kawauchi.
DISCUSSION
The present study shows that the sipunculan fauna of Diploria strigosa, Montastraea cavernosa, and Montastraea annularis is relatively homogeneous in terms of abundance and number of species in the study site. The species found in the Santa Marta area are well known coral reef dwellers, and have been reported as typical organisms from dead coral substrate from the Caribbean and many other regions of the world (Murina, Reference Murina, Rice and Todorovic1975; Rice & Macintyre, Reference Rice, MacIntyre, Rutzler and MacIntyre1982; Peyrot-Clausade et al., Reference Peyrot-Clausade, Hutchings and Richard1992; Hutchings & Peyrot-Clausade, Reference Hutchings and Peyrot-Clausade2002; Schulze, Reference Schulze2005; Fonseca et al., Reference Fonseca, Dean and Cortés2006). Although only few studies have been carried out on sipunculans in detail, different authors have reviewed the ecological importance of this group in coral reef environments (Hutchings, Reference Hutchings1986; Glynn, Reference Glynn and Birkeland1997).
More than 90% of the specimens found living inside the coral skeletons of D. strigosa, M. cavernosa and M. annularis were bioeroders with the exception of Phascolion cf. gerardi and Nephasoma sp., which usually use pre-existing spaces (Cutler, Reference Cutler1994). The most important sipunculans reported as dead coral skeletons dwellers are the species of Phascolosoma Leuckart, 1828 Aspidosiphon Diesing, 1851 and Lithacrosiphon Shipley, 1902 (Rice, Reference Rice1976; Cutler, Reference Cutler1994). Aspidosiphon fischeri and Phascolosoma perlucens were the most abundant and frequent species in all three skeletal substrates analysed, comprising 73% of the total fauna found in the present study. Rice (Reference Rice, Rice and Todorovic1975) reported Aspidosiphon fischeri as especially abundant in the Southern Caribbean, and this species has also been reported on the Pacific coast of Panama and the Galapagos Islands (Cutler, Reference Cutler1994). Likewise, P. perlucens has been considered one the most abundant rock boring species in the great Caribbean, and its dominance may be related to the prolonged breeding season (Rice, Reference Rice, Rice and Todorovic1975). It has been reported to have circumtropical distribution (Cutler, Reference Cutler1994); however, a recent study has suggested that it could be a complex of cryptic species (Kawauchi & Giribet, Reference Kawauchi and Giribet2010). Aspidosiphon parvulus and Aspidosiphon steenstrupii were found in small proportions in the three skeletal substrates and have a low frequency of occurrence. Aspidosiphon parvulus has only been reported in the Western Atlantic and Caribbean region in reefs from Belize (Schulze & Rice, Reference Schulze and Rice2004), and Panama (Collin et al., Reference Collin, Díaz, Norenburg, Rocha, Sánchez, Schulze, Schwartz and Váldes2005; Schulze, Reference Schulze2005) where it is very common. On the other hand, A. steenstrupii is a worldwide-distributed species that has been reported as one of the dominant species in reefs from the Great Barrier Reef (Hutchings & Peyrot-Clausade, Reference Hutchings, Peyrot-Clausade, Choat, Barnes, Borowitzka, Joll, Davies, Flood, Hatcher, Hopley, Hutchings, Kinsey, Orme, Pichon, Sale, Sammarco, Wallace, Wilkinson, Wolanski and Bellwood1988), the Tropical Eastern Pacific (Cantera et al., Reference Cantera, Orozco, Londoño-cruz and Toro-Farmer2003; Fonseca et al., Reference Fonseca, Dean and Cortés2006), and the Caribbean (Rice & Macintyre, Reference Rice, MacIntyre, Rutzler and MacIntyre1982). Hutchings & Peyrot-Clausade (Reference Hutchings, Peyrot-Clausade, Choat, Barnes, Borowitzka, Joll, Davies, Flood, Hatcher, Hopley, Hutchings, Kinsey, Orme, Pichon, Sale, Sammarco, Wallace, Wilkinson, Wolanski and Bellwood1988) found that this species was one of the most abundant sipunculans at Lizard Island (Great Barrier Reef), reaching higher densities in shallow areas. Aspidosiphon elegans made up only a small percentage of the total abundance in this study; it was absent from D. strigosa and rare in samples of M. cavernosa and M. annularis. Notwithstanding, it is another widespread species, also found in reefs from the Western Pacific and the Indian Ocean. In Moorea, French Polynesia, studies have found that this species is the most abundant sipunculan in samples of dead Porites Link, 1807 (Hutchings & Peyrot-Clausade, Reference Hutchings, Peyrot-Clausade, Choat, Barnes, Borowitzka, Joll, Davies, Flood, Hatcher, Hopley, Hutchings, Kinsey, Orme, Pichon, Sale, Sammarco, Wallace, Wilkinson, Wolanski and Bellwood1988, Reference Hutchings and Peyrot-Clausade2002) and Rice & Macintyre (Reference Rice, MacIntyre, Rutzler and MacIntyre1982) have reported very high densities from the barrier reef of Belize. Other species like Phascolosoma nigrescens, Nephasoma sp., Phascolion cf. gerardi, Antillesoma antillarum and Lithacrosiphon cristatus appeared only in a small number of samples and at low densities. The later was distributed in the three different coral skeletons analysed, and has been reported in the Caribbean as synonymous of L. alticonus or L. gurjanovae, also reaching high densities in reefs from Belize (Rice & Macintyre, Reference Rice, MacIntyre, Rutzler and MacIntyre1982).
The relatively homogeneous structure of sipunculans found among coral skeletons in this study confirms that the type of coral played a minor role in conditioning the assemblage, and other factors are more important in structuring the community. Physical conditions of the skeleton at the time of collection directly affect the density of different macroborers including sipunculans. The dynamics of sipunculan colonization of dead coral skeletons is spatially and temporally variable (Davies & Hutchings, Reference Davies and Hutchings1983; Kiene, Reference Kiene, Choat, Barnes, Borowitzka, Joll, Davies, Flood, Hatcher, Hopley, Hutchings, Kinsey, Orme, Pichon, Sale, Sammarco, Wallace, Wilkinson, Wolanski and Bellwood1988). Davies & Hutchings (Reference Davies and Hutchings1983) have indicated that sipunculans are not a pioneer community, and two or three years after the death of the colony the substrate could become suitable for colonization by this group. Generally, this infestation follows a succession pattern, in which the time when the skeleton became available is important. Our dead coral samples were taken in situ, directly from the reef matrix that at the moment of the collection looked superficially similar (covered by filamentous algae). There is no information about the time when the coral died and the skeleton began a succession process. Despite the data being homogeneous across the different coral species, between samples it was highly variable and exhibited marked differences in abundance and composition. It can be suggested that this variability is due to collection of coral skeletons of different ages and degrees of succession. Marked variation, within and between samples of dead coral substrates collected in situ, has also been mentioned (Hutchings & Peyrot-Clausade, Reference Hutchings, Peyrot-Clausade, Choat, Barnes, Borowitzka, Joll, Davies, Flood, Hatcher, Hopley, Hutchings, Kinsey, Orme, Pichon, Sale, Sammarco, Wallace, Wilkinson, Wolanski and Bellwood1988; Peyrot-Clausade & Brunel, Reference Peyrot-Clausade and Brunel1990), and it has been attributed to samples at different stages of development (succession pattern) that are determined by the time since the coral colony died. Generally, the macroboring community develops over time increasing in abundance and richness (Hutchings & Peyrot-Clausade, Reference Hutchings and Peyrot-Clausade2002). In reefs from Madagascar, Peyrot-Clausade & Brunel (Reference Peyrot-Clausade and Brunel1990) have found that the level of infestation was more correlated with the degree of coral degradation than the type of coral, since sipunculans were less abundant in new dead heads (undamaged) than in old ones.
The values of the present study are low compared to reefs in other places of the Caribbean. The only study on the Caribbean coast of Colombia was done by Moreno-Forero et al. (Reference Moreno-Forero, Navas and Solano1998), in skeletons of Acropora palmata (Lamarck, 1816) around Islas del Rosario (Cartagena, Colombia), and although they did not report the composition at the species level, they found densities up to 213 organisms dm−3, which are much higher than the values found in the present study. It appears that the sipunculan community is highly influenced by the reef zone they inhabit. Higher densities are found on or near the reef crest in shallow areas, than in other parts of the reef, where light regime and agitation of water can play an important role in influencing the distribution of sipunculans species (Rice & Macintyre, Reference Rice, MacIntyre, Rutzler and MacIntyre1982). This study was conducted in a patchy fringing reef, at 10 m depth, where there is a lack of a reef crest zone. This is in contrast, to the samples from Moreno-Forero et al. (Reference Moreno-Forero, Navas and Solano1998), which came from dead coral samples taken on the reef crest, off Isla Grande (Islas del Rosario). The type of coral morphology (branching vs massive), which influence the abundance and composition of cryptobiota in dead coral skeletons (Highsmith, Reference Highsmith1981a), could be also a factor related to the low densities found in our study. Smaller coral chunks (i.e. branching species) have higher surface to volume ratio than bigger coral chunks having relatively solid centre (i.e. massive species), and branching species tend to have higher percentage of dead surface, thus supporting more organisms (Highsmith, Reference Highsmith1981b). This statement is in accordance with the densities found by Moreno-Forero et al. (Reference Moreno-Forero, Navas and Solano1998), which represents dead coral chunks from branching Acropora palmata.
Sipunculans found in dead coral skeletons of the massive species D. strigosa, M. cavernosa and M. annularis from the Santa Marta area have been reported as typical coral reef dwellers elsewhere. Phascolosoma perlucens and Aspidosiphon fischeri, the two most abundant species in this study are two of the most common species in the Caribbean. The three skeletal substrates analysed shared similar species distribution; however, the marked variability between samples may be the result of skeletal blocks of different ages and succession stages. This study contributes to the knowledge about the cryptobiota and coral reef diversity for the region.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the Marine Biology Program of the Universidad Jorge Tadeo Lozano at Santa Marta, especially to A. Franco and H. Valencia for support in the field trips and for the laboratory facilities. We are indebted to Tatiana Rico who helped in the collection and identification of the specimens, and to P. Lecompte and M. Bolaños who helped improving earlier versions of this manuscript. We also thank Anja Schulze for preliminary identification of some species and G. Kawauchi who confirmed the morphotypes. We greatly appreciate the comments from two anonymous referees that helped to clarify and improve this manuscript.