Introduction
Limpets are common inhabitants and recognized keystone species on intertidal rocky shores worldwide, playing a crucial role in structuring local communities and ensuring ecosystem balance, integrity and stability (Jenkins et al., Reference Jenkins, Coleman, Santina, Hawkins, Burrows and Hartnoll2005; Coleman et al., Reference Coleman, Underwood, Benedetti-Cecchi, Åberg, Arenas, Arrontes, Castro, Hartnoll, Jenkins, Paula, Della Santina and Hawkins2006; Burgos-Rubio et al., Reference Burgos-Rubio, De la Rosa, Altamirano and Espinosa2015; Henriques et al., Reference Henriques, Delgado, Sousa and Ray2017). In addition, due to the easy access to intertidal areas, diverse limpet species have been harvested for human consumption since the establishment of ancestral populations along the coastline. For instance, in the Iberian Peninsula, true limpets (patellogastropods) have been traditional seafood since prehistoric times (Middle Palaeolithic) (Bicho & Haws, Reference Bicho and Haws2008; Fa et al., Reference Fa, Finlayson, Finlayson, Giles-Pacheco, Rodríguez-Vidal and Gutiérrez-López2016; Verdún-Castelló & Casabó i Bernad, Reference Verdún-Castelló and Casabó i Bernad2020). Nowadays, various limpet species are collected for seafood by both professional fishermen and recreational harvesters, under exploitation levels that depend on the geographic location, ease of access to intertidal areas and species abundance, and that reflect local/regional heritage, cultural legacy and gastronomic tradition (Sousa et al., Reference Sousa, Riera, Vasconcelos, Gouveia, Pinto, Delgado, Alves, González, Freitas, Henriques, Ray, Diarte-Plata and Escamilla-Montes2020a). In Portugal, limpets are professionally and/or recreationally harvested in some scattered locations sporadically exploited along the mainland (Vasconcelos et al., Reference Vasconcelos, Umapathy, Moura, Pereira, Carvalho and Gaspar2019), whereas these activities are ancestral, popular, widespread and intense in the archipelagos of Madeira (Henriques et al., Reference Henriques, Sousa, Pinto, Delgado, Faria, Alves and Khadem2012; Sousa et al., Reference Sousa, Vasconcelos, Henriques, Pinto, Delgado and Riera2019a, Reference Sousa, Vasconcelos, Riera, Pinto, Delgado and Henriques2019b, Reference Sousa, Riera, Vasconcelos, Gouveia, Pinto, Delgado, Alves, González, Freitas, Henriques, Ray, Diarte-Plata and Escamilla-Montes2020a, Reference Sousa, Henriques, Vasconcelos, Pinto, Delgado and Riera2020b, Reference Sousa, Pinto, Vasconcelos and Riera2020c; Cañizares et al., Reference Cañizares, Castejón, Haroun, Nogueira and Andrade2021) and Azores (Santos et al., Reference Santos, Hawkins, Monteiro, Alves and Isidro1995; Côrte-Real et al., Reference Côrte-Real, Hawkins and Thorpe1996; Martins et al., Reference Martins, Jenkins, Hawkins, Neto, Medeiros and Thompson2011, Reference Martins, Borges, Vale, Ribeiro, Ferraz, Martins, Santos and Hawkins2017; Diogo et al., Reference Diogo, Pereira and Schmiing2016).
Studies on the general morphology, morphometric relationships and relative growth of molluscs are performed with distinct purposes and provide useful information for diverse research fields, namely systematics and taxonomy, biology and ecology, fisheries assessment and management (Gaspar et al., Reference Gaspar, Santos and Vasconcelos2001, Reference Gaspar, Santos, Vasconcelos and Monteiro2002; Mauro et al., Reference Mauro, Arculeo and Parrinello2003; Vasconcelos et al., Reference Vasconcelos, Barroso and Gaspar2016, Reference Vasconcelos, Moura, Pereira, Pereira and Gaspar2018a, Reference Vasconcelos, Pereira, Carvalho and Gaspar2018b, Reference Vasconcelos, Santos, Pereira, Moura, Carvalho and Gaspar2022; Faria et al., Reference Faria, Martins, Pita, Ribeiro, Hawkins, Presa and Neto2017). The study of relative growth (isometry vs allometry, i.e. comparison of growth rates between body parts or measurements) is still the basis and founding principle for analysing morphometrics and investigating shape variation among closely related organisms (Huxley, Reference Huxley1932; Huxley & Teissier, Reference Huxley and Teissier1936). In the particular case of limpets, such studies aim mainly to assess the influence of diverse abiotic factors as drivers of changes in shell growth, shape and dimensions, mostly environmental variables such as shore topography, wave exposure and intensity, tidal height and desiccation stress (Baxter, Reference Baxter1983; Nolan, Reference Nolan1991; Côrte-Real et al., Reference Côrte-Real, Hawkins and Thorpe1996; Cabral & Silva, Reference Cabral and Silva2003; Lomovasky et al., Reference Lomovasky, de Aranzamendi and Abele2020; Vafidis et al., Reference Vafidis, Drosou, Dimitriou and Klaoudatos2020).
In this general context, the present work analysed and compared morphometric relationships (between shell length, width, height, total weight, area and volume), morphometric indices (ellipticity, conicity, density, surface area and volumetry) and relative growth (isometry vs allometry) among four sympatric limpet species from the Algarve coast (southern Portugal). The study comprised three true limpet species, namely the black-footed limpet (Patella depressa Pennant, 1777), the rough limpet (Patella ulyssiponensis Gmelin, 1791) and the common limpet (Patella vulgata Linnaeus, 1758), as well as the striped false limpet (Siphonaria pectinata Linnaeus, 1758). Overall, the study was based on the following working hypotheses regarding potential ecological implications of diverse morphological and morphometric features of these limpet species: (a) different limpet species display dissimilar morphometric relationships, indices and relative growth; (b) different morphometrics and relative growth reflect intra- and inter-specific variation in the limpet species’ main ecological traits, habitat features, distribution and position in the intertidal, and prevailing environmental conditions.
Materials and methods
Studied limpet species
Due to their broad distributional range along the NE Atlantic, limpets are quite common along European rocky shores and some species have their meridional and septentrional biogeographic limits in Portugal (Guerra & Gaudêncio, Reference Guerra and Gaudêncio1986; Boaventura, Reference Boaventura2000; Cabral, Reference Cabral2007; Borges et al., Reference Borges, Doncaster, MacLean and Hawkins2015; Simone & Seabra, Reference Simone and Seabra2017). Accordingly, diverse limpets co-occur in southern Portugal and the following four species inhabit rocky shores in the lower eulittoral zone along the Algarve coast: the true limpets P. depressa, P. ulyssiponensis and P. vulgata and the striped false limpet S. pectinata.
Both P. depressa and P. vulgata have their meridional biogeographic limit in southern Portugal (Fretter & Graham, Reference Fretter and Graham1976; Guerra & Gaudêncio, Reference Guerra and Gaudêncio1986; Southward et al., Reference Southward, Hawkins and Burrows1995; Boaventura, Reference Boaventura2000; Borges et al., Reference Borges, Doncaster, MacLean and Hawkins2015). In general, P. depressa (more wave-exposed areas) and P. vulgata (more sheltered areas) predominate in the mid-shore, whereas P. ulyssiponensis is a low-shore species that prevails in the lower algal zone or in shallow tidal pools, although their distribution might vary depending on local features of the rocky shore (Ballantine, Reference Ballantine1961; Fretter & Graham, Reference Fretter and Graham1976; Boaventura, Reference Boaventura2000; Cabral, Reference Cabral2007; Antit et al., Reference Antit, Gofas and Azzouna2008; Casal et al., Reference Casal, Aceña-Matarranz, Fernández-Márquez and Fernández2018). In contrast, the false limpet S. pectinata is an upper-shore species that occurs mainly on rocky surfaces subjected to moderate wave-action, which reduce the risk of dislodgement and provide suitable conditions for prolonged foraging activity (Ocaña, Reference Ocaña2003; Crocetta, Reference Crocetta2016).
Study area and sample collection
Field sampling was performed monthly during two consecutive years (January 2017–December 2018), at Praia da Luz (37°05ʹ06.5″N 08°43ʹ45.1″W) in Lagos (Algarve coast – southern Portugal) (Figure 1). The study area is moderately sheltered by near headlands and capes that protect this rocky shore against the North-west Atlantic Ocean swell, softening the local hydrodynamics due to lower wave energy, wind exposure and coast steepness (Boaventura et al., Reference Boaventura, Ré, Cancela da Fonseca and Hawkins2002; Vasconcelos et al., Reference Vasconcelos, Umapathy, Moura, Pereira, Carvalho and Gaspar2019). In the sampling zone, the rocky shoreline eroded by the wave action and seawater runoff creates small intertidal pools that preserve seawater for long periods.
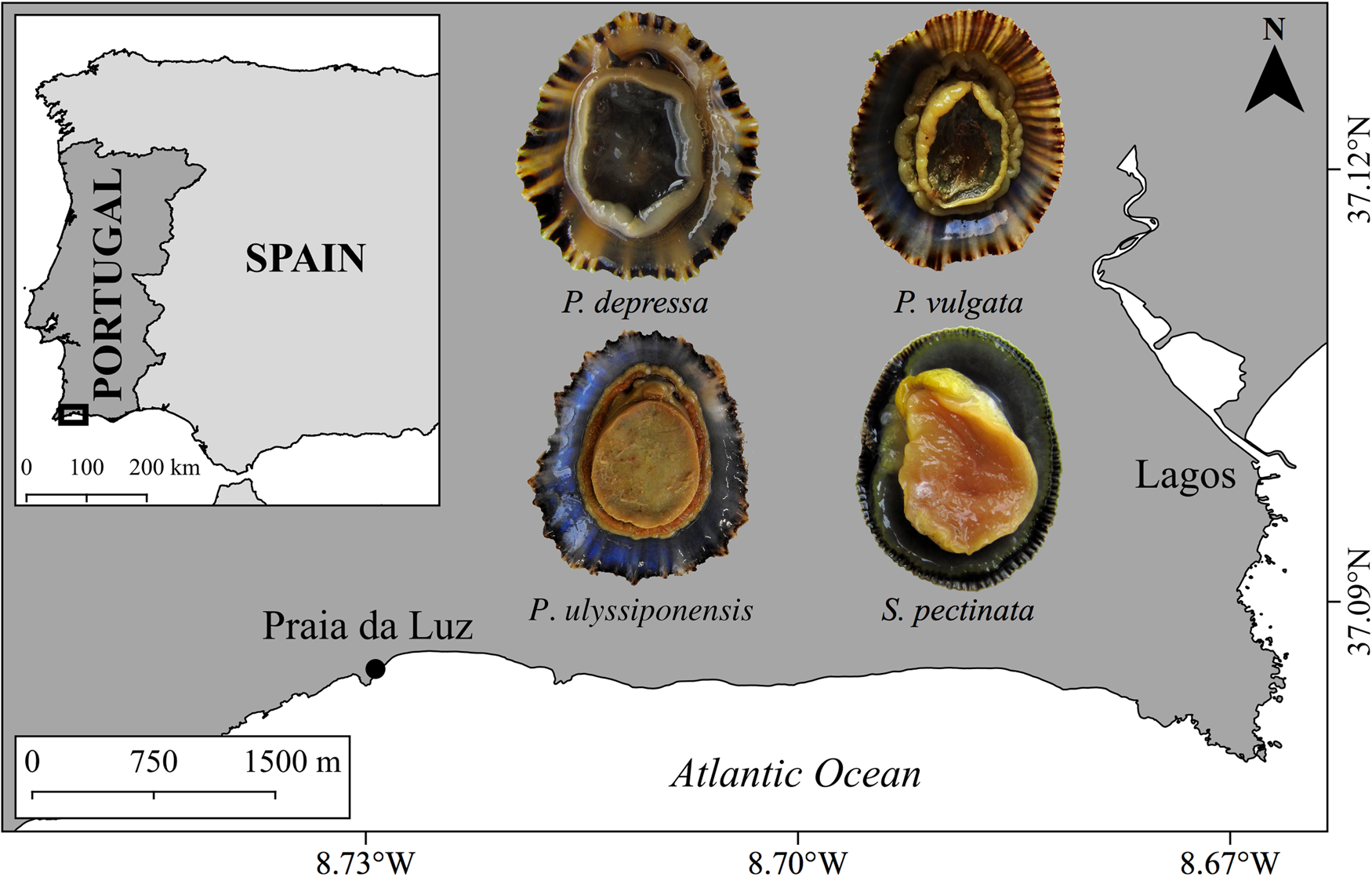
Fig. 1. Map highlighting the geographic location of the sampling site at Praia da Luz (Algarve coast – southern Portugal), together with photographs (ventral view) of the studied species: Patella depressa, Patella ulyssiponensis, Patella vulgata and Siphonaria pectinata.
The four limpet species (P. depressa, P. ulyssiponensis, P. vulgata and S. pectinata) were caught manually using a harvesting knife along an intertidal area of ~3000 m2 (~100 m long × 30 m wide). Specimens (~15–20 individuals/month) were collected randomly during low tide, roughly at the same tidal level. Immediately after field sampling, individuals were kept in identified plastic bags and preserved in ice until further laboratory procedures.
Biological sampling and shell morphometrics
In the laboratory, species identification was confirmed based on the main shell features described and illustrated in specialized literature (Christiaens, Reference Christiaens1973; Fretter & Graham, Reference Fretter and Graham1976; Titselaar, Reference Titselaar1998) and species names followed the most recent taxonomic nomenclature available at the World Register of Marine Species – WoRMS (WoRMS Editorial Board, 2021; http://www.marinespecies.org). Damaged specimens (eroded or broken shell border) were discarded and epibionts (seaweeds and encrusting invertebrates) were removed from the shell surface with a scalpel and/or hard brush to avoid biased measurements and weighing.
Individuals were separated per species, counted, measured using a digital calliper (precision of 0.01 mm) and weighed on a top loading digital balance (precision of 0.01 g). The morphometric variables included the measurement of the three shell axes, namely shell length (SL), shell width (SW) and shell height (SH) (Figure 2), complemented by the determination of total weight (TW) after blotting dry on absorbent paper to drain seawater from the shell surface and mantle cavity to avoid biased weighing.

Fig. 2. Schematic illustration of the three shell axes measured in the four limpet species (Patella depressa, Patella ulyssiponensis, Patella vulgata and Siphonaria pectinata): shell length (SL), shell width (SW) and shell height (SH).
In addition, based on these measurements and weights, the following shell areas and volumes were calculated using specific equations for parabolic cones, previously employed in other studies involving diverse limpet species (Jones et al., Reference Jones, Jones, Baxter, Naylor and Hartnoll1979; Baxter, Reference Baxter1983; Lowell, Reference Lowell1984; Khouw, Reference Khouw2006; Cabral, Reference Cabral2007; Cabral & Natal Jorge, Reference Cabral and Natal Jorge2007):
• Shell base area: SbA = π × [(SL + SW)/4)]2
• Shell surface area: SsA = 3.6 × [(SL + SW)/4] × √{[(SL + SW)/4)]2 + [(4/3) × SH)]}
• Shell internal volume: SiV = π × SL × SW × SH/12
• Shell total volume: StV = {π × [(SL + SW)/4]2 × SH}/2
Data treatment and statistical analyses
Initially, all data were checked for the occurrence of outliers, which were corrected or eliminated before further treatment and analyses. Morphometric relationships were established through regression analysis (least squares method on raw data) by fitting the power function (Y = aXb) and the degree of association between variables was assessed through the correlation coefficient (r).
Independently of the type of variables (linear, ponderal, area or volume), relative growth was analysed through the regression slope (allometry coefficient – b). In relationships between linear variables (SW and SH vs SL) isometry occurs for b = 1, between linear and area variables (SbA and SsA vs SL) isometry occurs for b = 2, and between linear and ponderal or volume variables (TW, SiV and StV vs SL) isometry occurs for b = 3, in practice reflecting a similar growth rate of both variables throughout ontogeny (Huxley & Teissier, Reference Huxley and Teissier1936; Mayrat, Reference Mayrat1970). Accordingly, a t-test (H0: b = 1 or 2 or 3; HA: b ≠ 1 or 2 or 3) was applied to confirm whether the regression slope denotes isometric growth (b = 1 or 2 or 3) or allometric growth (negative allometry: b < 1 or 2 or 3; positive allometry: b > 1 or 2 or 3).
In addition, aiming to further analyse the shell shape and better describe the general morphology of limpet species, five morphometric indices (ellipticity, conicity, density, surface area and volumetry) based on coupled ratios between the linear, ponderal, area or volume variables were calculated through the following equations (some concepts and terminologies adapted from Cabral, Reference Cabral2003, Reference Cabral2007; Cabral & Silva, Reference Cabral and Silva2003; Reference Cabral and Natal JorgeCabral & Natal Jorge, 2007; Battelli, Reference Battelli2016):
• Ellipticity index (elongation ratio): EI = SW/SL
• Conicity index (convexity ratio): CI = SH/SL
• Density index (weight ratio): DI = TW/SL
• Surface area index (area ratio): SI = SsA/SL
• Volumetry index (volume ratio): VI = StV/SL
These morphometric indices were compared between species through analysis of variance (ANOVA). Whenever ANOVA assumptions (normality of data and homogeneity of variances) were not achieved, the non-parametric Kruskal–Wallis test (ANOVA on ranks) was performed. In addition, variation in the morphometric indices during growth was analysed by plotting EI, CI, DI, SI and VI against individual size (SL). For each species, the main trends and eventual size-dependency (variation throughout ontogeny) in the morphometric indices was assessed through a t-test (H0: b = 0; HA: b ≠ 0) applied to the respective regression slope. All statistical analyses were performed following Sokal & Rohlf (Reference Sokal and Rohlf1987) with significance level considered at P < 0.05.
Results
The descriptive statistics, morphometric relationships and relative growth of the four limpet species are compiled in Table 1. A total of 1482 individuals were analysed (P. depressa = 354; P. ulyssiponensis = 306; P. vulgata = 408; S. pectinata = 414). These fairly representative samples presented a broad range in individual size and weight for all species analysed, namely P. depressa (17.2–41.6 mm SL/0.4–9.1 g TW), P. ulyssiponensis (24.6–52.5 mm SL/2.2–34.8 g TW), P. vulgata (19.5–42.7 mm SL/1.6–16.0 g TW) and S. pectinata (9.7–31.3 mm SL/0.1–5.1 g TW) (Table 1).
Table 1. Descriptive statistics, morphometric relationships and relative growth in four limpet species from the Algarve coast (southern Portugal)

N, number of individuals; SL, shell length (mm); SW, shell width (mm); SH, shell height (mm); TW, total weight (g); SbA, shell base area (cm2); SsA, shell surface area (cm2); SiV, shell internal volume (cm3); StV, shell total volume (cm3); Size and weight data presented as mean ± SD and respective range (minimum–maximum); r, correlation coefficient; b, allometry coefficient; SE, standard error; 95% CI, 95% confidence interval. Asterisks denote statistical level (P-value): n.s.not significant, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; A−, negative allometry; I = , isometry; A+, positive allometry.
Independently of the species and variables (linear, area or ponderal), all morphometric relationships were highly significant (P < 0.001) and characterized by high correlation coefficients (r = 0.761–0.994) (Table 1). The correlations were always higher in relationships involving shell areas (r = 0.988–0.994) and volumes (r = 0.959–0.981), compared with those involving total weight (r = 0.916–0.977), shell width (r = 0.947–0.968) and shell height (r = 0.761–0.910). The regression slopes (allometry coefficients) ranged in the intervals b = 0.904–1.248 (linear variables), b = 1.980–2.105 (area variables) and b = 2.934–3.365 (ponderal and volume variables). Among the 28 morphometric relationships, isometries (14 = 50.0%) and positive allometries (13 = 46.4%) clearly prevailed over negative allometries (1 = 3.6%). In P. depressa, all morphometric relationships were hyperallometric (7 A+), being mostly isometric in P vulgata (6 I = ), P. ulyssiponensis (4 I = ) and S. pectinata (4 I = ). The only hypoallometry (1 A−) was recorded in the relationship between SH and SL of P. vulgata (Table 1).
The morphometric indices (ellipticity, conicity, density, surface area and volumetry) of the four limpet species are compiled in Table 2 and illustrated in Figure 3. These five indices revealed a remarkable variation in shell shape and clear morphological diversity between species: EI ranged from 0.737 in S. pectinata to 0.844 in P. vulgata, CI varied between 0.223 in S. pectinata and 0.382 in P. vulgata, DI ranged from 0.061 in S. pectinata to 0.275 in P. ulyssiponensis, SI varied between 0.142 in S. pectinata and 0.274 in P. ulyssiponensis, and VI ranged from 0.029 in S. pectinata to 0.141 in P. ulyssiponensis (Table 2). The true limpets (P. depressa, P. ulyssiponensis and P. vulgata) displayed invariably higher morphometric indices than the false limpet (S. pectinata). All morphometric indices presented highly significant differences (Kruskal–Wallis: P < 0.001) among limpet species, namely EI (H = 866.935), CI (H = 1123.419), DI (H = 1053.906), SI (H = 900.314) and VI (H = 1019.370), excepting the ellipticity (Dunn's test: Q = 1.287; P > 0.05) and surface area (Dunn's test: Q = 1.653; P > 0.05) of P. depressa and P. vulgata (Table 2). Overall, these indices highlight diverse morphological features of the species, namely the wider and rounder shells of P. vulgata and P. depressa, compared with the longer and more elliptic shells of P. ulyssiponensis and S. pectinata. All species displayed shells with significantly different conicity, which was highest in the more conical P. vulgata and lowest in the more flattened S. pectinata. Similarly, all species had diverse density indices, ranging from the clearly lighter S. pectinata to the gradually heavier P. depressa, P. vulgata and P. ulyssiponensis. Accordingly, both the surface area and volumetry indices were invariably lowest in S. pectinata and highest in P. ulyssiponensis compared with the remaining limpet species (Table 2).

Fig. 3. Variation in the morphometric indices (ellipticity, conicity, density, surface area and volumetry) as a function of specimen size in four limpet species (Patella depressa, Patella ulyssiponensis, Patella vulgata and Siphonaria pectinata) from the Algarve coast (southern Portugal). r, correlation coefficient; b, regression slope. Asterisks denote statistical level (P-value): n.s.not significant, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Table 2. Morphometric indices (ellipticity, conicity, density, surface area and volumetry) in four limpet species from the Algarve coast (southern Portugal)

SL, shell length (mm); SW, shell width (mm); SH, shell height (mm); TW, total weight (g); SsA, shell surface area (cm2); StV, shell total volume (cm3); Data presented as mean ± SD and respective range (minimum–maximum). In each morphometric index, different superscript letters (a, b, c or d) denote statistically significant differences between species (K–W: P < 0.05).
Most morphometric indices were size-dependent in the four limpet species, except for the ellipticity of P. vulgata and S. pectinata and the conicity of P. ulyssiponensis, whose linear regressions against specimen size were not statistically significant (r = 0.014–0.093; P > 0.05) (Figure 3). However, all the remaining relationships displayed slopes significantly different from zero reflecting gradual changes in shell shape during growth, almost invariably positive slopes (b = 0.0007–0.0084) indicative of increasing trends in those morphometric indices during growth and only one negative slope (b = −0.0012) denoting the declining shell conicity throughout the ontogeny of P. vulgata. Overall, although with variable levels of statistical significance (P < 0.05–0.001), the density, surface area and volumetry indices of all species were clearly size-dependent and increased markedly during the limpets’ growth and lifespan (Figure 3).
Discussion
Limpets are keystone and foundation species in intertidal rocky shores, playing a crucial role in shaping and structuring the local communities. However, despite being extensively studied worldwide, for diverse purposes mostly related to the species’ general biology and ecology, the information available on limpets’ morphometric relationships, indices and relative growth is still relatively scarce and dispersed. In fact, to the authors’ best knowledge concerning the present limpet species (P. depressa, P. ulyssiponensis, P. vulgata and S. pectinata), comparisons with previous studies on those subjects are limited to populations of P. ulyssiponensis from mainland Portugal (Cabral, Reference Cabral2003, Reference Cabral2007; Cabral & Silva, Reference Cabral and Silva2003) and Tunisia (Boukhicha et al., Reference Boukhicha, Ben Hassine and Tlig-Zouari2013) and to populations of P. vulgata from Scotland (Jones et al., Reference Jones, Jones, Baxter, Naylor and Hartnoll1979; Baxter, Reference Baxter1983) and mainland Portugal (Cabral, Reference Cabral2003, Reference Cabral2007; Cabral & Silva, Reference Cabral and Silva2003) (Table 3). In this context, the present study provided valuable information to further analyse and compare limpets’ shape, morphometrics and relative growth, as well as to discuss their main trends and connections with species’ ecological traits, distribution, habitat and environment, within this highly dynamic and complex scenario of intertidal ecosystems.
Table 3. Comparison of morphometric relationships and relative growth in Patella ulyssiponensis and Patella vulgata populations from the Algarve coast (southern Portugal) and from other geographic locations throughout their distributional range in the North-east Atlantic Ocean

SL, shell length (mm); SW, shell width (mm); SH, shell height (mm); TW, total weight (g); SiV, shell internal volume (cm3); N, number of individuals; r, correlation coefficient; b, allometry coefficient; SE, standard error; 95% CI, 95% confidence interval. Asterisks denote statistical level (P-value): n.s.not significant, P > 0.05; ***, P < 0.001; A−, negative allometry; I = , isometry; A+, positive allometry. Some r values were square-root transformed and some b values were anti-log transformed from original data.
The present limpet species survive and thrive in very harsh and unstable environments, therefore some characteristics of the coast (exposed or sheltered), shore level (upper, middle or lower limits), wave exposure, hydrodynamic fluxes and tidal cycle, with consequent physical, chemical and biological abrupt changes (Boaventura et al., Reference Boaventura, Ré, Cancela da Fonseca and Hawkins2002, Reference Boaventura, Cancela da Fonseca and Hawkins2003), are reflected and correlated with particular features of their shell shape (Cabral, Reference Cabral2007). Accordingly, the morphometric relationships established in this study revealed diverse trends and patterns in the relative growth of these four limpet species. The relationship SW vs SL revealed diverse trends in the relative growth of P. depressa (b = 1.117) and P. ulyssiponensis (b = 1.033) with positive allometries indicative of progressive widening of the shell, compared with P. vulgata (b = 1.033) and S. pectinata (b = 1.006) with isometries suggestive of balanced growth rates in shell length and width throughout ontogeny.
For comparison purposes, and certainly reflecting contrasting environmental conditions between study areas, especially in terms of higher exposure and stronger hydrodynamics, a previous study registered isometric growth in P. ulyssiponensis (b = 1.022) from western Portugal (Cabral, Reference Cabral2007), whereas for P. vulgata hypoallometry (b = 0.924) was reported in Scotland (Jones et al., Reference Jones, Jones, Baxter, Naylor and Hartnoll1979) and there are reports of hyperallometries in Scotland (b = 1.062–1.080) (Baxter, Reference Baxter1983) and western Portugal (b = 1.042) (Cabral, Reference Cabral2007) (Table 3). Limpets’ shell shape is strongly influenced by their latitudinal distribution and vertical position on the shore (Bouzaza & Mezali, Reference Bouzaza and Mezali2018). In fact, other studies that recorded hyperallometric growth between SW and SL suggested that limpets at higher tidal levels (compared with those lower down the shore) and limpets inhabiting sheltered sites (compared with those in more exposed areas), both tended to have slightly broader shells (Balaparameswara Rao & Ganapati, Reference Balaparameswara Rao and Ganapati1971; Bannister, Reference Bannister1975; Jones et al., Reference Jones, Jones, Baxter, Naylor and Hartnoll1979; Baxter, Reference Baxter1983). Apparently, narrower shells are helpful and advantageous to reduce drag and avoid dislodgement under stronger wave action (Branch & Marsh, Reference Branch and Marsh1978; Baxter, Reference Baxter1983).
In the present study, the relationship SH vs SL also displayed clear inter-specific differences, ranging from positive allometries in P. depressa (b = 1.248) and S. pectinata (b = 1.092), isometric growth in P. ulyssiponensis (b = 1.026) and a negative allometry in P. vulgata (b = 0.904), reflecting differential and divergent trends in the expression of shell height compared with shell length throughout the species ontogeny. In the case of S. pectinata, hyperallometric growth in shell height is probably related to its quite harsh intertidal habitat, subject to long periods of aerial exposure and prone to desiccation (Vermeij, Reference Vermeij1973), where higher and heavily ridged shells (strong radial ribs) help to dissipate heat and keep soft body temperature (Vermeij, Reference Vermeij1973; Cabral, Reference Cabral2007; Boukhicha et al., Reference Boukhicha, Ben Hassine and Tlig-Zouari2013). The same principle applies to the positive allometry in P. depressa that occurs mainly in the middle shore level in both exposed and sheltered coasts along mainland Portugal, strongly influenced by the tidal cycle and repeatedly emerged for considerable periods, thus more susceptible to desiccation than species inhabiting lower shore levels (Guerra & Gaudêncio, Reference Guerra and Gaudêncio1986; Boaventura et al., Reference Boaventura, Ré, Cancela da Fonseca and Hawkins2002, Reference Boaventura, Cancela da Fonseca and Hawkins2003). However, although being advantageous under thermally stressful conditions, taller shells are energetically more expensive in highly hydrodynamic environments (Baxter, Reference Baxter1983), compared with flattened shells better adapted for handling stronger wave impact and current flow (Khouw, Reference Khouw2006). In the particular case of P. ulyssiponensis this isometric growth, indicative of equivalent growth rates between shell length and height, probably reflects the fact that this species inhabits lower shore levels and is submerged most of the time (Cabral, Reference Cabral2007).
Proportionally flatter shells are more common in limpets highly exposed to strong wave action at higher shore levels, where this morphometric/morphological feature helps them to adhere firmly and remain attached to the substratum, avoiding being swept away by hydrodynamic forces and alleviating desiccation stress (Orton, Reference Orton1928; Bannister, Reference Bannister1975; Branch & Marsh, Reference Branch and Marsh1978; Baxter, Reference Baxter1983; Khouw, Reference Khouw2006; Cabral, Reference Cabral2007; Harley et al., Reference Harley, Denny, Mach and Miller2009). In addition, since predators tend to select smaller prey that are easier to handle, flattened shells (i.e. proportionally greater investment in increasing shell length and/or width compared with shell height) is probably an advantageous strategy of limpets to avoid predation (Silva et al., Reference Silva, Hawkins, Boaventura and Thompson2008). Similarly, previous studies also reported isometric growth in the relationship SH vs SL of P. ulyssiponensis from mainland Portugal (b = 1.035 and b = 1.059) (Cabral & Silva, Reference Cabral and Silva2003; Cabral, Reference Cabral2007), whereas possibly due to specific shell shape adaptations to particular environmental conditions, P. vulgata population displayed positive allometries (b = 1.216–1.322) in Scotland (Baxter, Reference Baxter1983) and mainland Portugal (b = 1.045 and b = 1.216) (Cabral & Silva, Reference Cabral and Silva2003; Cabral, Reference Cabral2007) (Table 3). In addition, some differences might also be due to the population geographic positioning within the species distributional range (i.e. weaker adaptation and lower fitness towards the northward and southward limits of each species), eventually reinforced by local features and stressors such as wave exposure and hydrodynamics, shore level and tidal height, availability of intertidal pools and shadow, predation and harvesting. For instance, the occurrence and abundance of the boreal P. vulgata decreases southwards (Borges et al., Reference Borges, Doncaster, MacLean and Hawkins2015), being scarcer in southern Portugal where it reaches its meridional biogeographic limit, thus becoming more sensitive to water temperature, suffering stronger thermal stress and higher risk of desiccation in this southern edge of its distributional range (Guerra & Gaudêncio, Reference Guerra and Gaudêncio1986). Consequently, in this area P. vulgata tends to inhabit microhabitats, namely tidal pools and shade zones, especially on vertical and humid surfaces (Guerra & Gaudêncio, Reference Guerra and Gaudêncio1986), displaying a behavioural adaptation to adverse environments that also influences and modulates limpet morphology and morphometrics (Harley et al., Reference Harley, Denny, Mach and Miller2009).
In the present study, limpets’ total weight was composed of both shell weight (deposition of shell material) and tissues weight (somatic growth). The morphometric relationship TW vs SL presented distinct trends between species, with hyperallometries in P. depressa (b = 3.362) and P. ulyssiponensis (b = 3.298) against isometric growth in P. vulgata (b = 2.967) and S. pectinata (b = 2.981). These positive allometries in both P. depressa and P. ulyssiponensis reflect a proportionally higher growth rate in total weight compared with shell length, probably associated to increased shell deposition that improved shell thickness and weight throughout ontogeny (Jones et al., Reference Jones, Jones, Baxter, Naylor and Hartnoll1979), which indicates suitable feeding conditions for those species in the study area because starvation reduces calcium deposition and decreases the growth rate in shell weight (Zischke et al., Reference Zischke, Watabe and Wilbur1970). In general, limpets inhabiting higher shore levels develop heavier shells to improve protection against solar radiation (Balaparameswara Rao & Ganapati, Reference Balaparameswara Rao and Ganapati1971), but the continuous deposition of material and increased shell thickness during growth also helps to maintain the limpets’ resistance against compression forces (Cabral & Natal Jorge, Reference Cabral and Natal Jorge2007). For example, boreal populations of P. vulgata from the Orkney Islands (Scotland), subjected to severe environments and intense hydrodynamics, presented hyperallometries (b = 3.341–3.610) in their weight–length relationships (Baxter, Reference Baxter1983) (Table 3). In addition, by also including tissues weight (somatic growth), weight–length relationships constitute a simple and practical condition index (Anderson & Gutreuter, Reference Anderson, Gutreuter, Nielsen and Johnson1983; Richter et al., Reference Richter, Luckstadt, Focken and Becker2000). Similarly to the presently non-harvested populations from southern Portugal (isometries in P. vulgata and S. pectinata and hyperallometries in P. depressa and P. ulyssiponensis), isometric and positive allometric growth in the relationship TW vs SL were considered indicators of ecosystem health and population fitness in P. aspera populations from Marine Protected Areas (MPAs) in the archipelago of Madeira (Sousa et al., Reference Sousa, Pinto, Vasconcelos and Riera2020c).
As expected, resulting from calculations based on mathematical equations involving the three linear measurements already discussed above (SL, SW and SH), limpets’ shell areas (SbA and SsA) and volumes (SiV and StV) also displayed some similar trends in terms of relative growth. For instance, the positive allometries in shell base area presented by P. depressa and P. ulyssiponensis (denoting increased growth rate in SbA during growth) are usually influenced by the species’ vertical distribution on the shore and prevailing conditions (Khouw, Reference Khouw2006). On the one hand, a smaller shell base area helps to reduce water loss and avoid desiccation under exposed conditions (Lowell, Reference Lowell1984; Khouw, Reference Khouw2006; Cabral, Reference Cabral2007), while on the other hand a larger contact area improves a limpet's tenacity, because the adhesion force to hard substrate (attachment strength to the home scar) is proportional to the foot surface area (Branch & Marsh, Reference Branch and Marsh1978; Jones et al., Reference Jones, Jones, Baxter, Naylor and Hartnoll1979; Grenon & Walker, Reference Grenon and Walker1981; Baxter, Reference Baxter1983; Cabral, Reference Cabral2007). Regarding the shell surface area, this morphometric feature is essentially related with functional trade-off between limpets and the surrounding temperature, i.e. heat loss and cooling using seawater retained inside the shell to decrease body temperature, alleviate thermal stress and avoid desiccation (Vermeij, Reference Vermeij1973; Cabral, Reference Cabral2007; Boukhicha et al., Reference Boukhicha, Ben Hassine and Tlig-Zouari2013). Accordingly, one positive allometry (P. depressa) and three isometries (P. ulyssiponensis, P. vulgata and S. pectinata) recorded in the relationships SsA vs SL of these limpet species in this study area, further confirms their adaptation and ability to cope with the aerial exposure and thermal stress during low tide. Following some general trends recorded in shell dimensions and areas, enhanced shell volumes (SiV and StV) throughout ontogeny also constitute a useful morphological adaptation against heating stress and desiccation risk (Cabral, Reference Cabral2007). In practice, more voluminous limpets have a larger reservoir of seawater (i.e. store more inner water because a higher portion of the available shell volume is void of tissue), which allows losing a smaller fraction of body water, lowering body temperature and alleviating desiccation during periods of environmental stress (Vermeij, Reference Vermeij1973; Branch & Marsh, Reference Branch and Marsh1978; Lowell, Reference Lowell1984; Nolan, Reference Nolan1991; Cabral, Reference Cabral2007). In the present study, the relationships SL vs SiV and SL vs StV revealed the same type of relative growth in P. ulyssiponensis and P. vulgata (isometries) as well as in P. depressa and S. pectinata (hyperallometries), suggesting a differential adaptation of these limpet species to thermal stress induced by the aerial exposition during emersion periods (variable depending on the shore level) in intertidal areas along southern Portugal. Just for comparison purposes, positive allometric growth was recorded in the relationship SiV vs SL in two Scottish populations of P. vulgata from Easthaven (Jones et al., Reference Jones, Jones, Baxter, Naylor and Hartnoll1979) and from the Orkney Islands (Baxter, Reference Baxter1983) (Table 3).
Subsequently, the calculation of morphometric indices (ellipticity, conicity, density, surface area and volumetry) clearly confirmed their practicality and usefulness for further describing and interpreting limpets’ shell shape. In fact, despite consisting of simple ratios between some variables, these morphometric indices corroborated data on shell morphometrics and relative growth, providing valuable insights of inter- and/or intra-specific variation in some shell features of these limpet species. Confirming high diversity in shell shape, the conicity (CI: P. vulgata > P. ulyssiponensis > P. depressa > S. pectinata), density and volumetry (DI and VI: P. ulyssiponensis > P. vulgata > P. depressa > S. pectinata) were significantly different among all limpet species. The ellipticity (EI: P. depressa = P. vulgata > P. ulyssiponensis > S. pectinata) and surface area indices (SI: P. ulyssiponensis > P. depressa = P. vulgata > S. pectinata) also displayed considerable inter-specific variation among most limpet species. Unfortunately, intra- and inter-specific comparisons of these morphometric indices with analogous data available from previous studies with these limpet species are quite scarce and limited to the ellipticity and conicity indices of P. ulyssiponensis and P. vulgata from Portugal and Tunisia (Cabral, Reference Cabral2003, Reference Cabral2007; Cabral & Silva, Reference Cabral and Silva2003; Boukhicha et al., Reference Boukhicha, Ben Hassine and Tlig-Zouari2013). Following some general trends in shell shape and morphometrics already discussed above, the present population of P. ulyssiponensis scored an ellipticity index of 0.765, quite similar to those previously recorded along mainland Portugal, namely 0.764 (Cabral, Reference Cabral2003) to 0.769 (Cabral, Reference Cabral2007), and all higher than that reported in Tunisia (0.707) (Boukhicha et al., Reference Boukhicha, Ben Hassine and Tlig-Zouari2013), whereas P. vulgata from this study area presented a higher EI (0.844) compared with other populations from the Portuguese coast, which ranged from 0.793 (Cabral, Reference Cabral2003) to 0.799 (Cabral, Reference Cabral2007). The conicity index of P. ulyssiponensis from the Algarve coast (CI = 0.305), fell within the range previously registered along mainland Portugal, namely 0.303 (Cabral, Reference Cabral2007), 0.318 (Cabral, Reference Cabral2003) and 0.322 (Cabral & Silva, Reference Cabral and Silva2003), being slightly higher than in Tunisia (CI = 0.296) (Boukhicha et al., Reference Boukhicha, Ben Hassine and Tlig-Zouari2013), while P. vulgata recorded a CI of 0.382 in this study area, also comparatively higher than in other populations from mainland Portugal, with reported CIs of 0.345 (Cabral & Silva, Reference Cabral and Silva2003), 0.346 (Cabral, Reference Cabral2003) and 0.356 (Cabral, Reference Cabral2007).
Acknowledgements
The present study was supported by the research project ‘Contributo para a Gestão Sustentada da Pequena Pesca e da Apanha (PESCAPANHA)’, funded by the Fisheries Operational Programme (MAR 2020) and cofinanced by the European Maritime and Fisheries Fund (EMFF 2014–2020). The author Flávio Janeiro received a research grant (Ref: IPMA-2019-054-BI) awarded by IPMA within the framework of the project PESCAPANHA. Authors greatly acknowledge the Associate Editor (Professor Jan G. Hiddink) and two anonymous reviewers for providing constructive comments that improved the overall quality of this article.








