Introduction
Hermit crabs are unique decapods as they need an external shelter to protect their soft abdomen; most of the hermit crab species use gastropod shells as shelters. The low shell availability in most tropical intertidal shores means that hermit crabs are continually looking for adequate shells (Vance, Reference Vance1972). Hermit crabs use different senses to find a new shell (Tricarico & Gherardi, Reference Tricarico and Gherardi2006). A chemical stimulus provides an important cue to find a potential new shell. Mesce (Reference Mesce1982) found that hermit crabs use calcium release from the gastropod shells to locate them. For instance, Clibanarius antillensis respond to different concentrations of calcium to orient to a potential shell (Chiussi et al., Reference Chiussi, Díaz, Rittschof and Forward2001). Several hermit crab species use the odours of decaying gastropods as cues of potentially available shells (e.g. Small & Thacker, Reference Small and Thacker1994; Rittschof & Hazlett, Reference Rittschof and Hazlett1997; Tran, Reference Tran2013). Hermit crabs can also rely on a combination of chemical and visual cues to adequately discriminate between different shell species. Clibanarius vittatus differentiate between different silhouettes (horizontal diamonds and rectangles; Diaz et al., Reference Diaz, Forward, Orihuela and Rittschof1994) and real shells when the visual cues are accompanied by gastropod odours (Diaz et al., Reference Diaz, Orihuela, Rittschof and Forward1995). This hermit crab species discriminates between shells through chemical cues from the flesh of dead gastropods; however, they ignore the chemical signals when they can visually perceive the preferred shell species (Diaz et al., Reference Diaz, Forward, Orihuela and Rittschof1994). Several crustaceans use bimodal cues (chemical plus visual) to enhance sensory acquisition (Hebets & Rundus, Reference Hebets, Rundus, Breithaupt and Thiel2010). The hermit crabs use a complex interaction of cues to find a shell. The chemical cues seem to inform about a potential new shell, while its recognition appears to be achieved by visual images (e.g. Chiussi et al., Reference Chiussi, Díaz, Rittschof and Forward2001; Gherardi & Tiedemann, Reference Gherardi and Tiedemann2004). However, the use of visual cues in the absence of odours to locate potential new shells has not been proved.
The rocky shores are environments exposed to back-and-forth water flow induced by the waves; where additionally, the complex topography (e.g. rocks and the emergent vegetation) modify the water motion (Lightbody & Nepf, Reference Lightbody and Nepf2006). In such regimes, the unpredictable direction and velocity of the water flow lead to irregular movement of chemical signals (Koehl, Reference Koehl2006; Hebets & Rundus, Reference Hebets, Rundus, Breithaupt and Thiel2010). The animals that track the environmental signals through odours are not equally affected by the turbulence. Swimming animals can follow chemical cues through the water column; however, a benthic organism can quickly lose the track if it moves over the ground (Mead et al., Reference Mead, Wiley and Koehl2003). Therefore, for benthic intertidal inhabitants, visual communication should be particularly important.
Calcinus californiensis is an aquatic hermit crab that inhabits the intertidal zone. This hermit crab prefers biconical shells (Stramonita biserialis) over a globular shell species (Nerita scabricosta) (Arce & Alcaraz, Reference Arce and Alcaraz2011). We tested if this hermit crab differentiates between these shell species (real shells) and their silhouettes, which represent the simplest visual version of a body. We hypothesized that if C. californiensis discriminate between shells by visual information, the individuals will attend more to the real shells and the silhouettes of S. biserialis over those of N. scabricosta.
Materials and methods
Collection of crabs and measurements
We collected hermit crabs, Calcinus californiensis, occupying shells of Nerita scabricosta in the intertidal zone at Troncones, Guerrero, Mexico during April 2018. Hermit crabs were collected by hand during low tides. The crabs were placed in individual containers (0.05 L) for transportation to our field laboratory. The crabs were kept in individual containers (0.20 L) submerged in the primary container with seawater (salinity of 35 PSU). The hermit crabs were housed at an ambient temperature of 27 ± 1°C in natural photoperiod for a maximum of 24 h. The experiments were conducted during the hours of high activity (0900 to 1400 h; Alcaraz & Kruesi, Reference Alcaraz and Kruesi2012).
Experimental system
We used a Y-maze to test the hermit crabs' response to the visual stimuli. The Y-maze was 30 cm long, with a stem of 18 cm long and 9 cm width; each arm was 12 cm long and 7 cm width. The arms were separated by an angle of 60° (Y-split). A wall was hermetically glued at 5 cm from the end of each arm to isolate a compartment in which the stimuli were presented (Figure 1). We painted a line at 5 cm from the barrier wall of both arms to be used as the choice/decision criterion. The floor of the Y-maze was covered with sandpaper (No. 36) to simulate a natural substrate and to facilitate the walking of the crabs. The Y-maze was constructed following the recommendations of Jutfelt et al. (Reference Jutfelt, Sundin, Raby, Krång and Clark2017), considering the hermit crabs’ size and its walking speed (Alcaraz & García-Cabello, Reference Alcaraz and García-Cabello2017). The Y-maze received air from an external pump. The Y-mazes were lined with an opaque plastic to avoid reflexes and illumination effects to affect the perception of visual stimuli. We conducted pilot experiments to validate the design and construction of the Y-maze, as well as the hermit crabs’ responses.

Fig. 1. Schematic diagram of the Y-maze used for the experiments (A), photographs of the real shells of Nerita scabricosta and Stramonita biserialis presented in the Y-maze to the crabs (B), and silhouettes of the shells of S. biserialis and N. scabricosta (C).
The real shells and the silhouettes
The real shells presented to the crabs were undamaged and without epibionts, exhibiting their natural colour patterns. The silhouettes were made by taking photographs of real shells placed in the ground with the aperture down and forward-oriented (the real shells were presented in the same position). The photographs of the shells were digitally altered to look like silhouettes (Adobe Photoshop CC 2017). We printed the shell images the same size as the real shell from which the picture was taken. Therefore, we had a set of natural N. scabricosta and S. biserialis shells and their equivalent silhouettes of different sizes to present in each treatment. The size of the real shells and the silhouettes presented to the crabs were chosen according to the length of the shell occupied by the hermit crab. The size of the shell and the silhouette was calculated according to the equation that describes the relationship between the size of the chelae and the weight of the crab (Toledo, Reference Toledo2016).
Experimental design
The hermit crabs were presented to two binary sets of choices: (a) real gastropod shells of N. scabricosta and S. biserialis (Figure 1B); and (b) silhouettes of N. scabricosta vs S. biserialis (Figure 1C). We used silhouettes due to their constituting a visual scene constructed from the simplest visual element of a gastropod shell, representing the shape without revealing any other visual details (Franco et al., Reference Franco, Lapierre and Boyer2006).
The same individual was tested in both sets of stimuli (real shells and shell's silhouettes). Half of the hermit crabs (N = 20) were first presented to choose between the real shells of N. scabricosta and S. biserialis and then to their silhouettes; while, the other half (N = 20) were first presented to the shell's silhouettes and then to the real shells. The hermit crabs were randomly assigned to the order of the visual stimulus presentation (real shells and silhouettes). The crabs were tested the same day to both types of stimuli, within 25 min. The individuals were tested randomly. The hermit crabs were tested in the same shell type that they occupied in the field.
Testing procedure
The Y-maze was filled with 0.75 L of aerated seawater (27°C, 35 PSU). The visual stimuli were randomly assigned to the right or left arm of the maze. The hermit crab was placed at the edge of the stem of the Y-maze (Figure 1A), enclosed by a removable PVC cylinder (4 cm of diameter and 5 cm height). After 5 min, the crab was released by removing the cylinder. We registered the arm of the Y-maze chosen (stimulus) once the individual crossed the decision line (described above). At the end of the trial, we discarded the water and rinsed the Y-maze.
At the end of the experiments, we removed each hermit crab from its shell by heating its apex (Kellogg, Reference Kellogg1977). We measured the cephalothorax (shield length; digital calliper, ±0.1 mm) and weight (plate balance, OHAUS, ±0.01 g) of all hermit crabs. We weighed and measured the total length and width of shells and their aperture. The hermit crabs were sexed by the position of their gonopores. After taking all measurements, we returned hermit crabs with their original shells to the rocky shore from which we collected them.
Statistical analyses
The shell choice of hermit crabs of C. californiensis was analysed with Generalized linear mixed models (GLMM) with a binomial distribution of the error term. The crab identity was used as the random factor; the sex (males and females), the weight of the crab and the treatment (a: silhouettes, b: real shells) were the fixed variables. The models we used included additive and interactive effects between variables. The Akaike information criterion (AIC) adjusted for small sample sizes (AICc) was used to select the best-fitting model. The AICc selects the most parsimonious model that best explains the variation in the data (Burnham & Anderson, Reference Burnham and Anderson2002). The model with the lowest AICc score provides the best fit to the data. A difference between any model and the best-fitting model larger than two units in their AICc scores (ΔAICc >2) indicates support for a real difference in the fit to the data (Johnson & Omland, Reference Johnson and Omland2004). We computed model-specific Akaike weights (w), which measure the relative support or weight of evidence in the data for each fitted model (Burnham & Anderson, Reference Burnham and Anderson2002). We calculated model-averaged regression coefficients that represent an ‘average model’ across our entire set of individual competing models based on the Akaike weights. The model averaging is a robust mathematical representation of the probability of success in the identification of the preferred shell as a function of treatment, size and sex. The model-weighted regression coefficients incorporate the uncertainty in the process of model selection by considering the relative support for each competing model, and are thus more robust than those derived from any single model alone (Burnham & Anderson, Reference Burnham and Anderson2002; Johnson & Omland, Reference Johnson and Omland2004). We performed all our statistical analyses in R (R Core Team 2016).
We tested within treatments for potential differences between males and females in the choice for S. biserialis or N. scabricosta using a χ 2 test. Finally, we tested if crab sizes were homogenously distributed in their shell choice using a Welch two sample t-test (used for unbalanced sample sizes; silhouettes: S. biserialis, N = 22, and N. scabricosta, N = 18; real shells: S. biserialis, N = 21, and N. scabricosta, N = 19).
Results
Our sample size consisted of 40 hermit crabs(18 females and 22 males). The preference of the stimulus (biconical vs globular shells) was best described by two models according to the model selection analysis (ΔAICc <2; Table 1). The sex and the body size were the two main variables that explained the preference of the crabs. The sex of the individuals and an additive effect of the size of the crabs were present in the most supported model (AICc = 103.14 and w = 0.43; Table 1). The next model with the best AICc included only sex of crabs (AICc = 104.49, ΔAICc = 1.36, and w = 0.22; Table 1). The size of the crab (measured in weight) and the probability of choosing the preferred shell species (S. biserialis) in both males and females were positively associated (Figure 2A, B). The choice of shells of N. scabricosta and S. biserialis was similar for real shells and the shell's silhouettes (AICc = 105.55, ΔAICc = 2.41, and w = 0.13; Table 1).

Fig. 2. Probability of choosing the real shell of Stramonita biserialis (A), and the silhouette of this species (B) as a function of the males and females’ crab size. Lines represent the estimated values from model averaging, the triangles show the observed data. Triangles of females are slightly displaced from their real values (0 and 1) to improve visualization.
Table 1. Model selection results that examine variation in the choice of preferred shell by hermit crabs as a function of experiments (silhouettes or real shells), sex and crab weight

According to the model averaging (treatment effect, sex and size), the preference of shells of N. scabricosta and S. biserialis was similar between silhouettes and real shells (Figure 2A, B). The larger individuals were more likely to choose S. biserialis than the smaller ones. The males chose with lower probability the biconical shell than females (Figure 2). The intercept for females was 0.61, whereas the intercept for males was 0.21 (Figure 2). The average slope (in logit scale) that represents the effect of crab weight on the probability of choosing S. biseralis was 2.54. Males and females differed in the shell they chose (silhouettes: χ 21 = 5.29, P = 0.02; real shells: χ 21 = 3.77, P = 0.05, Figure 3). The males preferred the globular shell N. scabricosta and females preferred the biconical shell S. biserialis in both experiments (Figure 3). Finally, the size of the hermit crabs was not biased between individuals that chose biconical shells S. biserialis and those that chose globular shells N. scabricosta in either experiment (silhouettes: t 236.58 = −1.06, P = 0.3; real shells: t 231.348 = −0.15, P = 0.88).
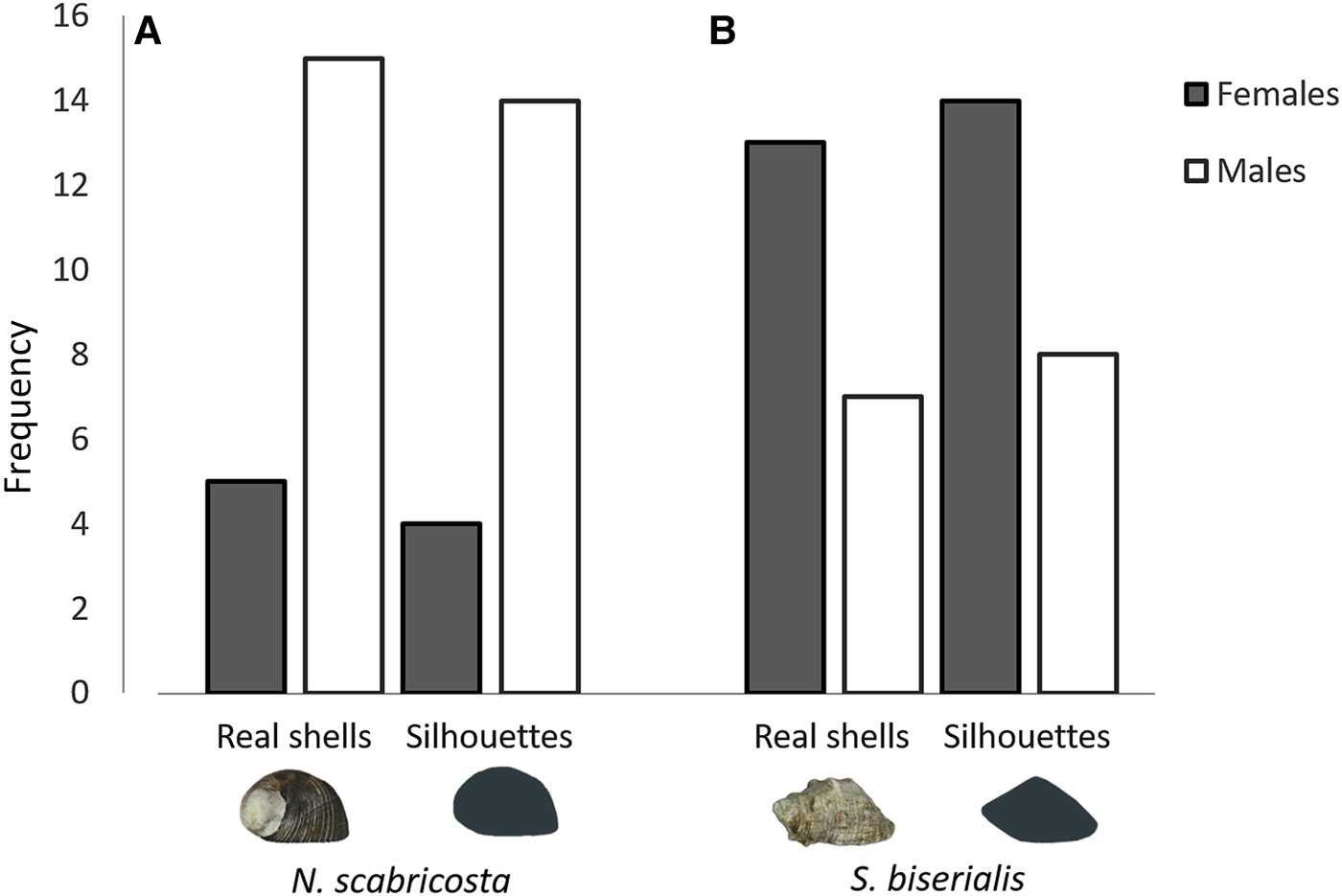
Fig. 3. Frequency of attendance to real shells and silhouettes of Nerita scabricosta (A) and real shells and silhouettes and of Stramonita biserialis (B) by male and female hermit crabs.
Discussion
Intertidal benthic animals, including hermit crabs, are exposed to dynamic water motion (Scully, Reference Scully1979; Hahn, Reference Hahn1998). The breaking waves disperse olfactory signals into the water column making visual ability critical for benthic inhabitants. The hermit crabs discriminated visually between the biconical and globular shells (Stramonita biserialis and Nerita scabricosta, respectively) in the absence of chemical information. Vision is one of the least understood sensory channels in aquatic invertebrates (Gherardi et al., Reference Gherardi, Cenni, Parisi and Aquiloni2010; Chiandetti & Caputi, Reference Chiandetti and Caputi2017); however, vision has shown to be valuable as a source of information for many species of crustaceans, such as in individual recognition (e.g. lobsters and crayfish; Van der Velden et al., Reference Van der Velden, Zheng, Patullo and Macmillan2008; Gherardi et al., Reference Gherardi, Cenni, Parisi and Aquiloni2010) and predator identification (Layne, Reference Layne1998). The hermit crabs of C. californiensis visually discriminated between the different shells in the absence of chemical information. In contrast to C. californiensis, the intertidal hermit crab Pagurus bernhardus do not react to silhouettes, and although these crabs respond to a block and predator's model approaching, they do not discriminate between them by its shape (Dalesman & Inchley, Reference Dalesman and Inchley2008).
Calcinus californiensis also recognized the shell by its silhouette. Our experimental design did not allow identification of the visual features used by the hermit crabs to discriminate the different shell types. Though, C. californiensis can distinguish between the two shells using the simplest elements of a visual scene (silhouette). The consistent individual choice, of the real shells and the silhouettes, suggest that hermit crabs do not need to use colour and three-dimensional structure to discriminate between different shells. Discrimination between different shells by their silhouettes can be advantageous since colour can be an inaccurate signal in intertidal environments, as most of the surfaces are covered with algae (i.e. encrusted) and other epibionts that modify the colour pattern.
Hermit crabs of C. californiensis had been shown to prefer shells of S. biserialis over the globular shells of N. scabricosta (Arce & Alcaraz, Reference Arce and Alcaraz2012). Therefore, we expected that C. californiensis would choose the real shells and the silhouettes of Stramonita biserialis. In contrast, males and females chose different shell species. The females were more attracted to real shells and silhouettes of S. biserialis, while males were more interested in shells and silhouettes of N. scabricosta. The intersexual difference in shell choice is interesting. The differences in shell choice between males and females resemble the pattern of occupancy of shells in the wild, where males occupy shells of N. scabricosta more frequently than females, and females occupy biconical shells of S. biserialis (and Cantharus sanguinolentus) more than males (Arce & Alcaraz, Reference Arce and Alcaraz2011). Males and females could be dealing with different challenges and thus diverge in their preference. The shells of S. biserialis are relatively heavier and have thicker walls, that protect hermit crabs from shell-breaking predators, in comparison with the shells of N. scabricosta (Alcaraz & Arce, Reference Alcaraz and Arce2017). However, the males occupying the relatively lighter shells of N. scabricosta exhibit higher foraging rates (Alcaraz & García-Cabello, Reference Alcaraz and García-Cabello2017), body growth (Alcaraz et al., Reference Alcaraz, Chávez-Solís and Kruesi2015) and rate of metabolism than males using shells of S. biserialis (Alcaraz and Kruesi, Reference Alcaraz and Kruesi2009). The relatively low weight of N. scabricosta can make these shells more advantageous for males (usually more active than females) during searching for mates and copulation (Turra, Reference Turra2005). Thus, it is not surprising that the males preferred shells of N. scabricosta over the ones of S. biserialis. The likelihood to attend to shells of S. biserialis increased positively with body size, independently of the sex and the visual presentation of the shell (real and silhouettes). Turra & Leite (Reference Turra and Leite2004) found a similar tendency where the choice for a particular type of shell depended on the body condition of the crab (size/weight). The snails of N. scabricosta reach a smaller size than the ones of S. biserialis in Troncones (and thus their shells); therefore, males that reach a relatively large size could modify their preference toward a shell species that reaches an adequate size for them.
The intertidal hermit crab C. californiensis discriminated between two different shells using just their visual sensory system; additionally, the crabs discerned between shells using the minimal visual feature of the resource. Our study suggests that the visual system is more important than previously supposed for animals inhabiting intertidal shores. As far as we are aware, this is the first study that emphasizes the ecological relevance of the visual abilities as sufficient to obtain a vital resource in animals living on the rocky shores.
Author ORCIDs
Guillermina Alcaraz, 0000-0002-5485-0671
Acknowledgements
We thank field and laboratory assistance from Álvarez-Galicia A. and Burciaga L M. We also thank Dr Zuñiga-Vega J for his review of an early version of this manuscript. Also, we thank F. Arreola, A. Ramírez and the people of the Mechanical Workshop of the Facultad de Ciencias, UNAM for the manufacture and design of all Y-mazes.
Financial support
This study was supported by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, Universidad Nacional Autónoma de México (PAPIIT- UNAM) IN-216418, and the Dirección General de Asuntos del Personal Académico (DGAPA-UNAM) for a postdoctoral fellowship to MSR.






