INTRODUCTION
Juveniles of many marine fish and especially flatfish inhabit coastal areas (e.g. estuaries and shallow water bays) where they find generally more abundant food, lower predation risks and higher temperatures than in offshore areas (Gibson, Reference Gibson1994). However, in these essential habitats, fish juveniles have also to face highly variable environmental factors and superimposed anthropogenic activities that may jeopardize survival and influence recruitment into the adult population (Beck et al., Reference Beck, Heck, Able, Childers, Eggleston, Gillanders, Halpern, Hays, Hoshino, Minello, Orth, Sheridan and Weinstein2001). Fish habitat utilization reflects trade-offs between spatial distribution of essential resources, the internal state of the organism and tolerance to ambient conditions (Huntingford, Reference Huntingford1993). In order to achieve habitat conservation and sustainable fisheries management, it is therefore critical to understand fish juveniles behaviour in terms of spatio-temporal dynamics within these coastal habitats (Rice, Reference Rice2005).
Common sole Solea solea (L.) is a widely distributed and most economically important flatfish species of the north-east Atlantic region. Most common sole stocks are reported to be overexploited (ICES, 2005). Previously both field and laboratory studies have focused on the behavioural processes occurring in O-group juveniles during the short colonization phase (Champalbert & Koutsikopoulos, Reference Champalbert and Koutsikopoulos1995; Amara et al., Reference Amara, Lagardère, Désaunay and Marchand2000). Once settled in shallow coastal areas individuals have to face a variety of natural and anthropogenic stressors that may impact their survival. However, apart from field studies that have focused on their spatio-temporal distribution until autumn (Dorel et al., Reference Dorel, Koutsikopoulos, Désaunay and Marchand1991; Rogers, Reference Rogers1992; Jager et al., Reference Jager, Kleef and Tydeman1993) and some other rare studies that have inferred indirectly about their movement capacities using conventional (Coggan & Dando, Reference Coggan and Dando1988) or natural (Vinagre et al., Reference Vinagre, Salgado, Costa and Cabral2008) tags, little is known on O-group sole behavioural activities during the post-colonization period.
Therefore, the present study aimed at bringing new insights on O-group sole swimming behaviour during the sensitive post-colonization period in order to better understand dynamics of individuals in shallow coastal areas. A laboratory video-tracking system was developed to measure the spontaneous swimming and burying activity. Since behavioural patterns in fish generally vary on a diel cycle, experimental set up was designed to measure behavioural activities during two consecutive days according to light period of the day (day–twilight–night). O-group common sole were caught throughout four different months after colonization until the onset of winter (i.e. June to November) in an Atlantic nursery ground (Pertuis Charentais, Bay of Biscay, France). In this region, colonization is centred around May so that, it is likely that individuals belong to the same cohort. Therefore, it was hypothesized that sampling month may be used as proxy of age/ontogenetic status. The objectives were to establish the baseline levels of these natural activities in O-group sole (i.e. normal/spontaneous behaviour) and test the effect of the ontogenetic status (sampling month) on these activities according to light periods under constant experimental conditions throughout the post-colonization period.
MATERIALS AND METHODS
This study was conducted under the approval of the Animal Care Committee of France under the official licence of M.L. Bégout (17-010).
Fish origin and maintenance
Thirty-one wild O-group juveniles were caught in the Pertuis Charentais, a main sole nursery ground for the Bay of Biscay (Le Pape et al., Reference Le Pape, Chauvet, Mahevas, Lazure, Guérault and Désaunay2003), in June, July, September and November 2004 in a mussel pole-culture area (46°15′80″N 1°13′40″W) using a push net to avoid injuries to the fish (Durieux et al., Reference Durieux, Galois, Bégout, Sasal and Lagardère2007). At the video-tracking laboratory, fish were maintained in aerated tanks (45×30×35 cm) with filtered sea water (temperature: 20±1°C; salinity: 35±1; oxygen concentration: 100% air saturation) and a sand substratum (light colour). Both temperature and salinity were set to constant value as a mean of disconnecting the experiment from field conditions in a consistent way for all the fish. Fish were fed daily with frozen Tubifex. They were maintained under these conditions for at least 10 days' acclimatization prior experimentation in order to avoid tidal rhythm effects (Gibson, Reference Gibson1973, Burrows, Reference Burrows1994) that could eventually prevent comparisons between months. An artificial photoperiod was applied: daylight period (8:00–21:00), a twilight transition period (7:00–8:00 and 21:00–22:00) and a night period (22:00–07:00).
Video-tracking set-up and experimental protocol
In the video tracking laboratory (isolated from external disturbances): three black circular arenas of 60 cm diameter, with walls drilled regularly with 5 mm diameter holes to allow water circulation, were placed individually in a 400 l tank with aeration and water filters that maintained a continuous water flow around the arena. A 5 cm deep layer of black sand (100 to 300 µm) was placed on the bottom of the arena in order to allow sole to bury and to provide an adequate colour contrast to allow fish detection by the image analysis system. Water was maintained at temperature around 20±1°C (by an air conditioning system in the laboratory); salinity at around 35±1 psu (by regularly changing seawater and when needed adjusting salinity with freshwater to compensate for evaporation); and oxygen concentration at around 100% air saturation (using air aerator systems). The arenas were illuminated laterally by artificial light with the same photoperiod used during maintenance of the fish: daylight period (8:00–21:00; white light: 0.3×1015 Q.cm−2.s−1), a twilight transition period (7:00–8:00 and 21:00–22:00; white light: 0.3×1014 Q.cm−2.s−1) and a night period (22:00–07:00; infrared light (PAR38 IR 175): 0.14×1014 Q.cm−2.s−1). Since fish are not sensitive to infrared light (Douglas & Hawryshyn, Reference Douglas, Hawryshyn, Douglas and Djamgoz1990), nocturnal behaviour was considered un-altered by the artificial lighting used to assess nocturnal swimming. Fish were not fed the day before the experiment nor during the subsequent video recordings.
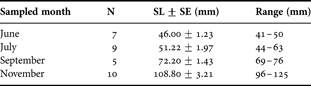
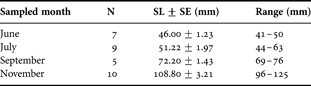
Above each arena, a digital black and white high sensitivity camera (Sony XCD-X700, XGA) linked to a computer equipped with Visilog 6.2 software (NOESIS, France) was mounted. Image acquisition proceeded with cyclic sub-sampling of each arena with 33 seconds duration sequences of 1 image s−1 and each image was saved in .jpeg format. Successive sequences relative to a same arena were separated by a time lag of 2 minutes because of a 7 seconds delay between each sequence acquisition. The resulting sub-sampling factor (SSF) was 1/3.6. For each month, at 11:00, the day of the beginning of recording, one fish was introduced per arena, so that three fish were recorded at a time. Videos were then recorded for 48 hours duration. After video recording, fish were individually measured (to 1 mm, standard length (SL)) (Table 1). To complete each month group, successive recordings took up to 8 days.
Table 1. Sample size (N), mean standard length (SL±SE (mm)), and size-range of O-group common sole Solea solea sampled in 2004 in the Pertuis Charentais nursery ground (France)
Fish detection was based on the colour contrast between the fish (appearing in white) and the black sandy bottom using image analysis (Visilog 6.2). The XY coordinates (mm) of the barycentre of the detected fish shape were extracted for each image/time. An integrated VBA module to Visilog 6.2 developed by NOESIS for this experiment, allowed automation of the acquisition and image treatment process.
Data analysis
Five to ten individuals per month (June, July, September and November) i.e. around 1500 hours of video-tracking were analysed (Table 1). Based on XY coordinates, four behavioural endpoints could be obtained: the distance travelled between consecutive images (Di) and the frequency of occurrence of three different activities: swimming (Si), burying (Bi), and immobility (Ii). When the fish was not detected, XY coordinates equalled 0, and the fish was considered buried. When fish was detected but distance travelled between two consecutive images was ≤5 mm s−1 (limit of motion detection of distance travelled between two consecutive images) fish was considered immobile. Alternatively when distance travelled was >5 mm s−1 fish was considered swimming. Distances travelled were transformed in body length (Bl) to avoid any effect of size on swimming speed (Peck et al., Reference Peck, Buckley and Bengtson2006). For each individual fish, hourly values of the four variables were calculated:

where SSF: sub-sampling factor; n: number of observations per hour; i: individual observation per second.
Dh is expressed in Bl h−1 and Sh, Bh and Ih are expressed in percentage.
Using all 48 hours' observations, individual data were averaged for specific light periods (day, twilight and night) prior to analysis. Given the nature of the data, i.e. non-independent light periods, one-way repeated measures ANOVAs were used to test differences for each behavioural variable (D, S, B and I) between month groups (between-subject factor: June, July, September and October) and light periods (within-subject factor: day, night and twilight) as well was interaction of both factors. Data were tested for normality with a Shapiro–Wilk test and for homoscedasticity with a Bartlett's test. Since data could not meet assumptions they were ranked prior to analysis (Kane et al., Reference Kane, Salierno, Gipson, Molteno and Hunter2004). Homogeneous groups were determined with the a posteriori multiple comparisons Newman and Keuls tests. Results are given as averages±SE (standard error). Statistical tests were performed using XLStat software and Systat. Tests were considered significant at P < 0.05.
For derived instantaneous swimming speeds, only values >5 mm s−1 (limit of motion detection of distance travelled between two consecutive images) were considered. Swimming speed frequencies were calculated for the different month groups with individual speed-classes of 2 cm s−1 and a range of 0.5 to 20 cm s−1.
RESULTS
Significant differences were detected for both factors (i.e. sampled months and light periods) for all measured behavioural variables D, B, S and I (Table 2; Figure 1). Independently of the light periods, D and S decreased significantly between June and July, while B increased significantly. Between July and September, D, S and I decreased significantly, while B increased significantly. The pattern observed for these four variables was independent from the light periods. Between September and November, B decreased significantly and I increased significantly independently of the light periods for both variables whereas no difference was detected for D and S. D and S were significantly higher at night and twilight than during the day, B was significantly lowest at night, intermediate at twilight and highest during the day independently of sampled months. I was significantly highest at night independently of sampled months.

Fig. 1. Average distance travelled (Bl h−1) (A) and frequency occurrence (%) for the three different types of activity swimming (B), burying (C) and immobility (D) per sampled month and light period for O-group common sole Solea solea captured in 2004 in the Pertuis Charentais area (France). Artificial light time period was set as: day, 8:00–21:00; twilight, 7:00–8:00 and 21:00–22:00; night, 22:00–7:00. Values were calculated on the total 48 hours' observation period.
Table 2. Results of one-way repeated measures ANOVAs testing differences in mean behavioural variables (distance travelled and swimming, burying and immobility occurrences; ranked transformed data) between months (between-subject factor), light periods (within-subject factor) and interaction of both factors for O-group common sole Solea sole a sampled in the Pertuis Charentais nursery ground (France). Multiple comparisons Newman and Keuls tests post hoc. Jn, June; Jl, July; Sept, September; Nov, November; Twi, twilight period.

For fish sampled in June, swimming speed frequencies displayed a bimodal distribution (Figure 2). The first mode for speed comprised between 0.5 and 2 cm s−1 (0.4 Bl s−1) and a second for speed of 8–10 cm s−1 (2.2 Bl s−1), each representing 25% of the overall swimming speeds. For fish sampled in July, September and November, swimming speed frequencies displayed a unimodal distribution with 50–60% of swimming speeds between 0.5 and 2 cm s−1.

Fig. 2. Swimming speed (cm s−1) frequency distribution calculated on the total 48 hours' observation period for each sampled month of common sole Solea solea captured in the Pertuis Charentais area (France). 10 cm s−1 corresponds to 2.2, 2.0, 1.4 and 0.9 Bl s−1 in June, July, September and November respectively.
DISCUSSION
Our data were obtained under experimental conditions using a video tracking system at a scale of observation enabling the assessment of daily spontaneous swimming activities (levels and rhythms) of O-group common sole for the first time. This method appears to be one of the only non-harmful methods of quantifying free activity of O-group sole. However, caution should be applied in extrapolating these data to field observations. Swimming activity over two days showed relatively high inter-individual variability as often observed for fish (Bourke et al., Reference Bourke, Magnan and Rodriguez1997; Salvanes & Hart, Reference Salvanes and Hart1998; Mehner, Reference Mehner2006). Body size is well known to influence fish swimming activity so that in our study we used relative distance travelled in proportion of body size in order to minimize size effect within and between sampled months. Alternatively it is important to consider that the low sample size routinely employed in behavioural studies (here five to ten replicates per sampled month) and often due to technical constraints might also have the effect of raising the variability. Nevertheless, some clear temporal trends in the behavioural activities from the post-colonization period untill autumn were observed.
O-group sole showed a relatively clear circadian activity in line with the artificial light conditions of the experiment, with higher swimming activity at night and twilight than during the day and alternatively more burying activity during the day than during the night. This nocturnal activity pattern is consistent with previous studies on young O-group sole (Macquart-Moulin et al., Reference Macquart-Moulin, Champalbert, Howell, Patriti and Ravaivoson1991; Champalbert & Marchand, Reference Champalbert and Marchand1994; Champalbert & Koutsikopoulos, Reference Champalbert and Koutsikopoulos1995), older 2-group (Lagardère et al., Reference Lagardère, Ducamp, Frikha and Sperandio1988; Laffargue et al., Reference Laffargue, M-L and Lagardère2006) and in general in other flatfish (Gibson, Reference Gibson1973; Burrows, Reference Burrows1994; Hurst & Duffy, Reference Hurst and Duffy2005). Champalbert et al. (Reference Champalbert, Macquart-Moulin, Patriti and Chiki1991) demonstrated that sole > 30 mm were photonegative at most light intensities except at twilight. In the field, in addition to circadian rhythms, flatfish display tidal rhythms in swimming activity which has been shown for juveniles to correspond to a trade-off between foraging, predator avoidance and the selection of suitable environmental conditions (Burrows, Reference Burrows1994; Gibson et al., Reference Gibson, Phil, Burrows, Modin, Wennhage and Nickell1998). In our study, fish were acclimatized for ten days prior to the experiment in order to prevent any confounding effect of tidal rhythms between sampled months.
Burying behaviour represented 80% of the activity in autumn months (September and November). This behaviour is linked to a strategy for predation avoidance (Gibson, Reference Gibson1997) but also energy saving with lower basal metabolism while buried (Howell & Canario, Reference Howell and Canario1987). Sole from June and July buried clearly less than fish from September and November. The latter were often immobile at the surface of the substratum, especially at night. As burying capacity of juvenile flatfish is a strong logistic function of body size relative to the sediment grain size (Gibson & Robb, Reference Gibson and Robb1992), June and July fish, due to their relatively small size, are likely to have been limited by the size of the sand grains used in the experiment (100–300 µm).
The O-group sole showed a strong decline in swimming activity from June to July (around 8 fold in distance travelled and 4 fold in % swimming), with very low activity remaining until November. This drastic change was also observed in term of swimming speed frequency distributions. Individuals sampled in June displayed a high proportion of fast swimming speeds (around 10 cm s−1 or 2 Bl s−1) that typically corresponds to off bottom swimming with large scales movements (Champalbert & Koutsikopoulos, Reference Champalbert and Koutsikopoulos1995; Hurst & Duffy, Reference Hurst and Duffy2005) whereas from July onwards the majority of swimming speeds were lower than 2 cm s−1 which is characteristic of the foraging behaviour of juvenile flatfish searching for benthic prey (Lagardère et al., Reference Lagardère, Bégout, Lafaye and Villotte1994; Hill et al., Reference Hill, Burrows and Hughes2000, Reference Hill, Burrows and Hughes2002). Both environmental (i.e. current, salinity, light, and food availability) and endogenous factors are considered to play a determinant role in nursery colonization (Champalbert & Koutsikopoulos, Reference Champalbert and Koutsikopoulos1995). Here, experimental conditions were the same for all sampled months so that we assume that our observations reflect an ontogenetic change in swimming activity. O-group sole juveniles in the Bay of Biscay colonize nursery grounds around May (Amara et al., Reference Amara, Lagardère, Désaunay and Marchand2000). Around one month post-colonization, O-group sole juveniles still demonstrate a relatively high swimming activity. This behaviour may be a relic of an exploratory behaviour associated with colonization of new habitat (Hurst & Duffy, Reference Hurst and Duffy2005). Low activity from July onwards may reflect the transition to a well established sedentary behaviour. From an ecological point of view, this behaviour may be interpreted as giving a relatively high resiliency of O-group sole in confined areas of the shallow coastal habitat. This is consistent with field observations of potential O-group sole movements in different nursery grounds based on density distributions (Dorel et al., Reference Dorel, Koutsikopoulos, Désaunay and Marchand1991; Rogers, Reference Rogers1992; Jager et al., Reference Jager, Kleef and Tydeman1993), recapture of tagged individuals (Coggan & Dando, Reference Coggan and Dando1988) and use of natural tags (Vinagre et al., Reference Vinagre, Salgado, Costa and Cabral2008).
In the field it has been reported that O-group sole operate migration to deeper waters prior to winter in order to avoid very low water temperature occurring in shallow part of the habitat at this time (Dorel et al., Reference Dorel, Koutsikopoulos, Désaunay and Marchand1991; van der Veer et al., Reference van der Veer, Dapper and Witte2001). Since we did not observe any increase in swimming activity at this time under constant experimental conditions, this suggests that such migratory behaviour prior to winter is mainly influenced by hydroclimatic factors.
In conclusion, using a laboratory video-tracking system this study provides quantified spontaneous swimming activity baseline levels of O-group common sole through their first period of life in coastal shallow areas. O-group sole showed a clear ontogenetic change in swimming activity between June and July, demonstrating the transition between the exploratory behaviour of late colonization and the well established sedentary behaviour until the onset of winter. These results highlight the potential movement limitations of O-group sole in coastal areas during this highly critical period.
ACKNOWLEDGEMENTS
IFREMER and the Conseil Régional Poitou Charentes funded the study. The VBA module for image acquisition and treatment automation was developed in collaboration with D. Péral (NOESIS). J. Grizon was the boat pilot for fish sampling in the field. P. Pineau, M. Prineau and N. Lachaussée helped in the set-up and maintenance of the experimental infrastructures. We are grateful to E. Hunter from the CEFAS Lowestoft laboratory and to anonymous referees for valuable comments and corrections on the manuscript.