Introduction
Seagrass beds typically comprise continuous swards of plants, but patchy distributions may also occur at higher current velocities (Fonseca et al., Reference Fonseca, Zieman, Thayer and Fisher1983; Fonseca & Kenworthy, Reference Fonseca and Kenworthy1987; Frederiksen et al., Reference Frederiksen, Krause-Jensen, Holmer and Laursen2004). Structures that have attracted much current interest are the so called ‘fairy circles’ or ‘fairy rings’ (Borum et al., Reference Borum, Raun, Hasler-Sheetal, Pedersen, Pedersen and Holmer2014; Ruiz-Reynés et al., Reference Ruiz-Reynés, Gomila, Sintes, Hernández-García, Marbà and Duarte2017). This appellation refers to the similarity to circles of terrestrial plants such as mushrooms and toadstools in terrestrial woodlands and lawns which are surrounded by a great deal of folklore, their name alluding to supernatural origins.
The structures take two forms. Firstly, there are circular patches bare of vegetation within otherwise continuous swards (Ruiz-Reynés et al., Reference Ruiz-Reynés, Gomila, Sintes, Hernández-García, Marbà and Duarte2017). Such bare patches may arise from obvious anthropogenic damage such as bomb impacts in Posidonia oceanica meadows (Meinesz & Lefèvre, Reference Meinesz, Lefèvre, Boudouresque, Jeudy de Grissac and Olivier1984; Pasqualini et al., Reference Pasqualini, Pergent-Martini and Pergent1999) or, as in the case of the Isles of Scilly, abrasion of the seabed by mooring chains as boats on fixed moorings move around with the tide (Jackson et al., Reference Jackson, Higgs, Allsop, Cawthray, Evans and Langmead2011; Warwick, Reference Warwick, Ray and McCormick-Ray2014), which is particularly prevalent on Scilly in St Mary's Harbour. These patterns may, however, arise naturally from self-organized patchiness in which facilitative and competitive interactions occur among plants with clonal growth such as seagrasses (Ruiz-Reynés et al., Reference Ruiz-Reynés, Gomila, Sintes, Hernández-García, Marbà and Duarte2017).
Secondly, there are circles of seagrass with lush fringes of rapidly growing ramets on the outer side of the circles with virtually bare centres, which are more akin to their terrestrial fungal counterparts and arguably more deserving of the fairy circle appellation. Circles of the eelgrass Zostera marina can be observed in shallow waters of highly exposed shores with sandy sediments (Fonseca & Kenworthy, Reference Fonseca and Kenworthy1987), in which case die-off in the patch centres primarily seems to be caused by sand accumulating and burying shoots within the patches during storm events with heavy sand movement. However, in 2008 pictures taken by a tourist showed such circles in the shallow waters off the chalk cliffs on the barren, chalky seabed on the island Møn in Denmark, where the environment is very different with low sediment loads and no burying of shoots (Borum et al., Reference Borum, Raun, Hasler-Sheetal, Pedersen, Pedersen and Holmer2014). This prompted much public speculation (bomb craters, landing marks for aliens, fairies!), but the more prosaic explanation is that the pools of acid volatile sulphides and chromium-reducible sulphur in the trapped mud, which were found to increase from the outer to the middle positions of the radially expanding Zostera marina clones, killed the oldest and weakest plants in the centre by sulphide poisoning (Borum et al., Reference Borum, Raun, Hasler-Sheetal, Pedersen, Pedersen and Holmer2014).
Worldwide, Zostera fairy circles of this second type occur quite rarely in small numbers. None have been reported on Scilly in recent seagrass surveys (Jackson et al., Reference Jackson, Higgs, Allsop, Cawthray, Evans and Langmead2011; Potouroglou et al., Reference Potouroglou, Kenyon, Gall, Cook and Bull2014; Marine Ecological Surveys Limited, 2017; Bull & Kenyon, Reference Bull and Kenyon2020), perhaps due to the nature of the survey techniques employed and to their objectives, which have been to determine any changes in the aerial extent of the beds rather than the fine structure within them, and the health of the stands in terms of the incidence of ‘wasting disease’.
Analogous circles of Posidonia oceanica, a seagrass species endemic to the Mediterranean, occur in very shallow water, often in sheltered lagoons where conditions are at the extremes of the environmental tolerances of this species to salinity and temperature. Termed ‘atolls’, they are relatively rare, but have been found in a number of locations in Turkey, Marsala (western Sicily), Saint-Florent (Corsica) and Libya (Boudouresque et al., Reference Boudouresque, Ballesteros, Ben Maiz, Boisset, Bouladier, Cinelli, Cirik, Cormaci, Jeudy De Grissac, Laborel, Lanfranco, Lundberg, Mayhoub, Meinesz, Panayotidis, Semroud, Sinnassamy, Span and Vuignier1990, Reference Boudouresque, Bernard, Bonhomme, Charbonnel, Diviacco, Meinesz, Pergent, Pergent-Martini, Ruitton and Tunesi2006; Pergent et al., Reference Pergent, Djellouli, Hamza, Ettayeb, Alkekli, Talha, Alkunti, Pergent-Martini, El Asmi and Le Ravallec2007; Tomasello et al., Reference Tomasello, Di Maida, Calvo, Pirrotta, Borra and Procaccini2009 and references therein).
Materials and methods
A series of 37 high-resolution aerial vertical photographs taken in 1996 (exact date unknown), covering the entire archipelago of Scilly (49°54′N 6°18′W), were carefully examined for evidence of circular structures within the areas that Zostera marina beds have been recorded to occur. They were taken vertically at a time of low tide, high water clarity and little surface rippling by wind, so that the detailed features of the beds were particularly clear. Measurements of the diameters of circles were made from these photographs, which were scaled in relation to the size of fixed structures on land in the same photograph, as determined from Google Earth. These measurements are accurate to about the nearest metre. In addition, a high-resolution vertical photograph of the archipelago taken by the UK Environment Agency on 26 September 2007 was similarly examined, as was a high resolution (50 cm) satellite image of the archipelago (Pléiades – Isles of Scilly) taken on 11 March 2020, again at low tide with clear water, and any circles were measured and scaled in the same way. This scaling was confirmed by independent assessment generated from the geolocated Pléiades satellite image using QGIS software. These vertical images were supplemented by an oblique intertidal photograph taken at Old Grimsby Harbour, Tresco, on 11 September 2014 and an oblique drone sequence taken in Higher Town Bay, St Martin's, Isles of Scilly in early August 2021.
Results
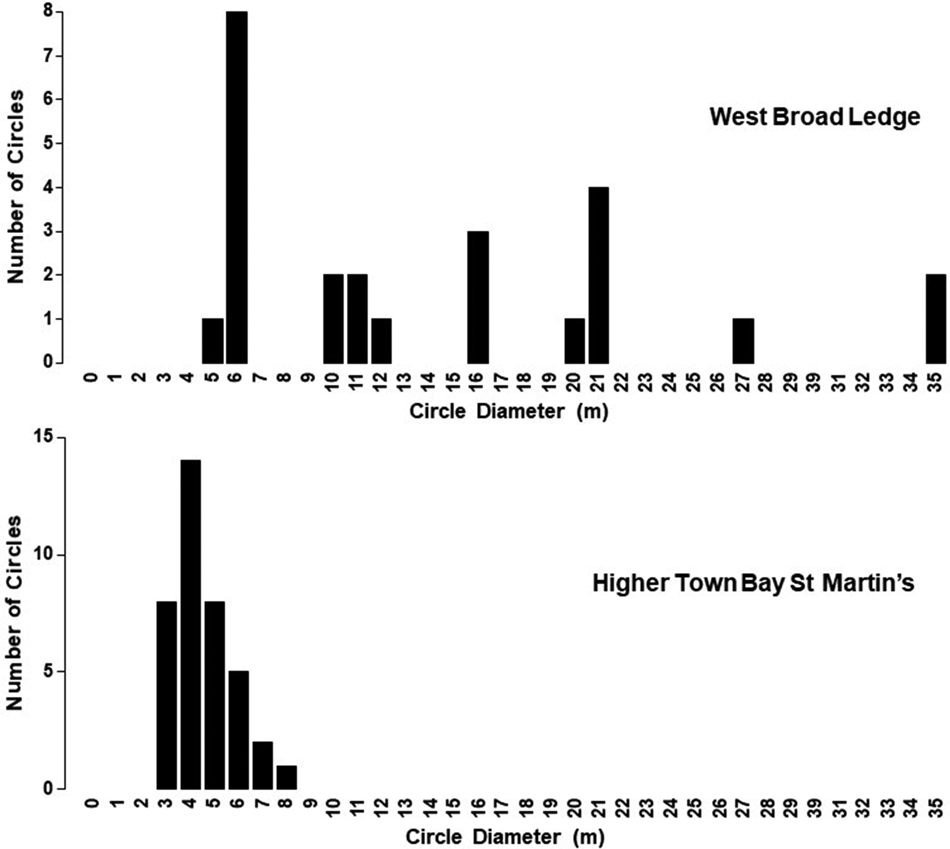
Although there was evidence in the 1996 photographs of isolated small circles in various areas, as was also the case in 2007 and 2020, the vast majority were found on the western fringes of the West Broad Ledge Zostera bed (49°57.34′N 6°18.23′W), the eastern edge of which borders the deep-water channel between Tean and St Martin's (Figures 1 and 2). Measurements of the diameters of the 25 recognizable circles in this group do not constitute a continuous series of increasing size but fall into discrete stepwise clusters with modal diameters of 6, 11, 16, 21, 27 and 35 m (Figure 3). In the 2007 photograph the circles that were so obvious in 1996 have started to break up or have disappeared and rather few of them can be matched between the two periods, but the one marked with an arrow in Figure 2 is a good example. They have all disappeared by 2020, leaving an entire striated bed (Figure 2). In the 2020 image there is a concentration of at least 38 small circles in the highly fragmented Z. marina bed in Higher Town Bay, St Martin's (Figures 4 and 5), with diameters of 3–8 m (Figure 3), equivalent to the smallest cohort at West Broad Ledge in 1996. Seventeen months later, in August 2021, many of these circles were clearly visible in the drone photograph (Figure 6) and could be matched with those in the 2020 satellite image by virtue of their configuration, but measurements were not possible due to the oblique nature of the image. In contrast, no circles were present at Higher Town Bay in 1996 when this bed comprised a more continuous, although striated, stand (Figure 4). However, small numbers of small circles were beginning to appear in 2007, although these were difficult to discern and enumerate within the fragmented bed (Figure 4).
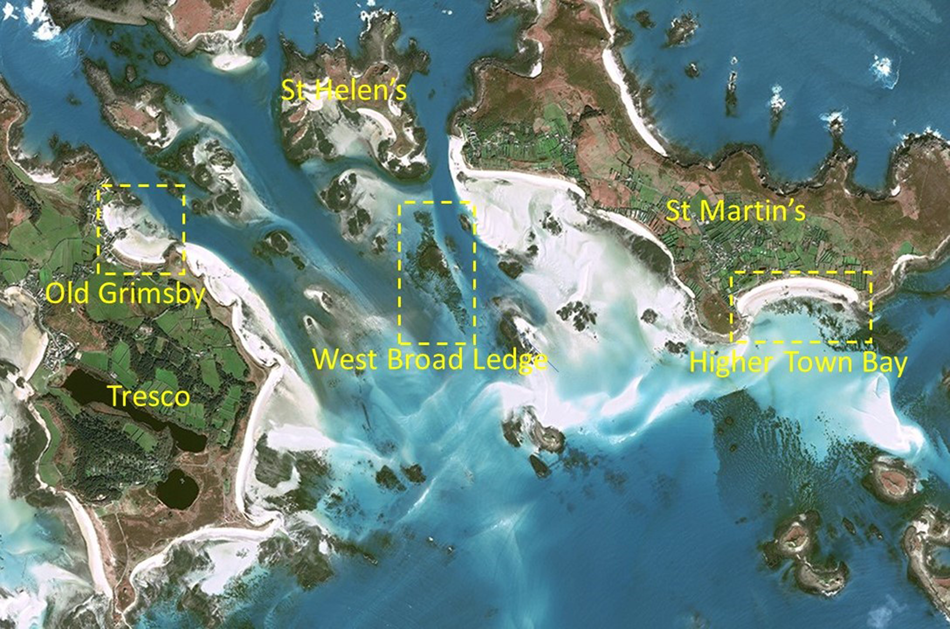
Fig. 1. 2020 satellite image showing the locations of the three seagrass areas exhibiting the fairy circles described in this article (dashed rectangles) in relation to the inhabited islands of Tresco and St Martin's and the uninhabited island of St Helen's. © CNES (2020), Distribution Airbus DS.
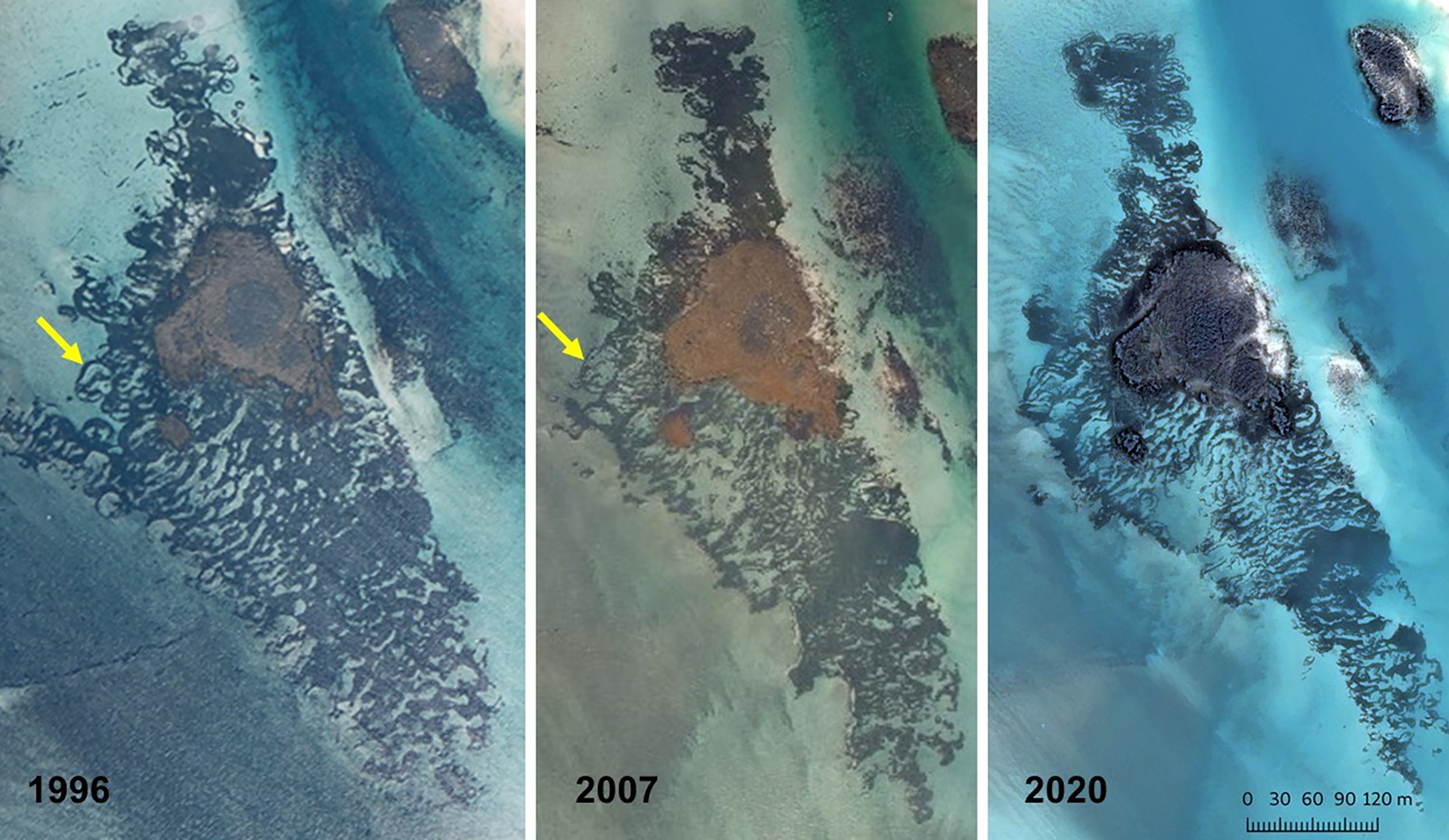
Fig. 2. West Broad Ledge seagrass bed in 1996, 2007 and 2020, the last image © CNES (2020), Distribution Airbus DS with a scale derived from those data.

Fig. 4. Higher Town Bay seagrass bed in 1996, 2007 and 2020, the last image © CNES (2020), Distribution Airbus DS with a scale derived from that data. An enlargement of the section in the rectangle of the 2020 image is shown in Figure 5.
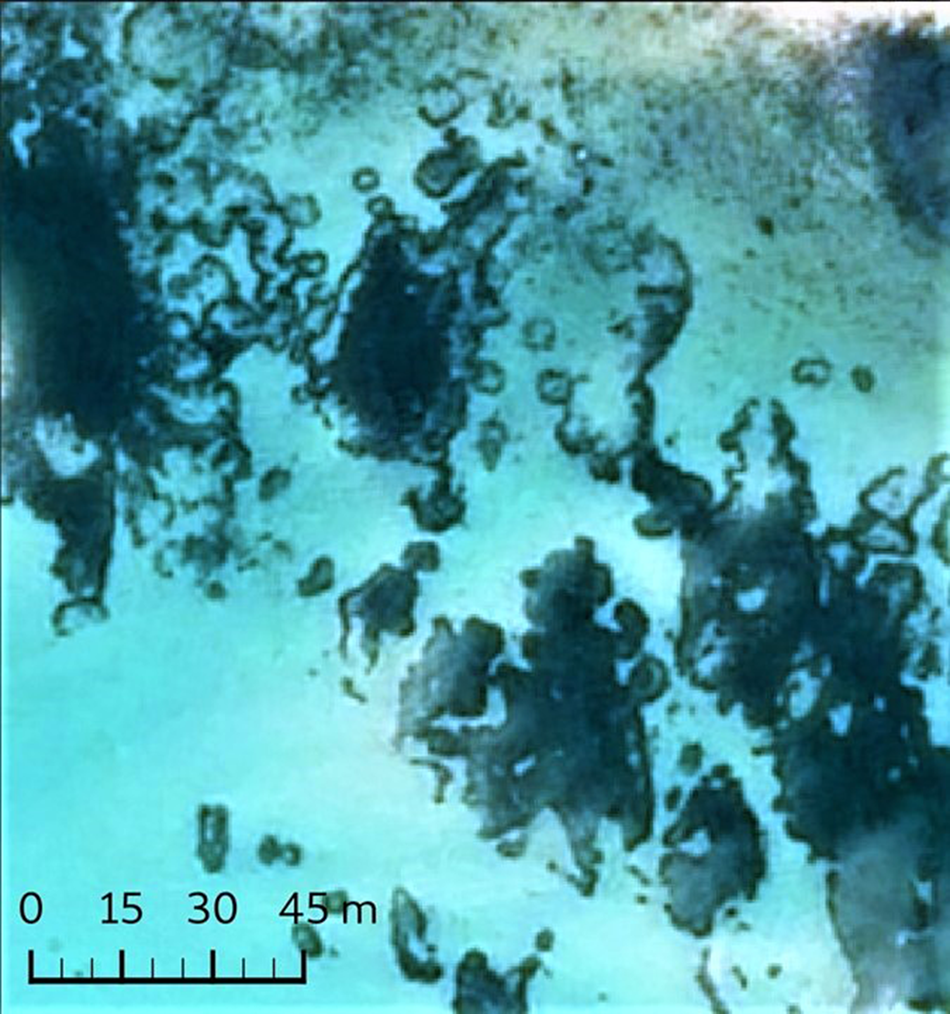
Fig. 5. Enlargement of the section in the rectangle of the 2020 Higher Town Bay image with the same resolution as Figure 4 but showing the circles more clearly.

Fig. 6. Screenshot from a video filmed in Higher Town Bay in early August 2021 by Dominic Heydon, reproduced with permission.
Discussion
These are not the first records of these circles on Scilly. Early observations attribute them to the sites of ancient hut-circles, submerged for centuries as a result of sea-level rise (Thomas, Reference Thomas1985). Thomas observed from a boat that ‘On 11 September 1956 [we] noticed a complex of circular features about 5 m each in diameter. They were indicated by rings of large seaweed-festooned rocks against a sandy bottom, just west of Tean Sound channel and north of Spence's Broad Ledge – now called West Broad Ledge.’ He also recounts that ‘During a photographic flight (22 September 1979, mostly at 700 feet, during LST) Dr Peter Fowler noted various ‘seaweed circles’ outlined below the water against light sand. He photographed them because, as he points out, if real hut-circles do exist now below LST they will look like these.’ The photographs, figs 21 and 22 in Thomas (Reference Thomas1985), show several circles of varying size and completeness ‘in relation to the deep-water channel between Tean and St Martin's’, i.e. West Broad Ledge. Thomas admits that ‘These were cases where closer examination, without diving, was not possible’ and it seems likely that the ‘rings of large seaweed-festooned rocks’ were wishfully imagined rather than actually seen. Thomas does report that, at a close-by location, divers examined an unquestionable submerged ancient village comprising six square and one circular hut, a more realistic conurbation than a city of many perfectly circular buildings up to 35 m in diameter! Note that such circles occur on Scilly in greater abundance than has been recorded anywhere else in the world.
The circular nature of these structures is strongly suggestive of the unrestricted clonal growth of genets arising from individual seedlings, expanding equally in two dimensions. In established Z. marina beds reproduction is vegetative, i.e. by growth of the rhizome, although seedling recruitment may exceed vegetative reproduction in areas of sediment disturbance (Phillips & Menez, Reference Phillips and Menez1988; Reusch et al., Reference Reusch, Stam and Olsen1998). Potouroglou et al. (Reference Potouroglou, Kenyon, Gall, Cook and Bull2014) quantified Z. marina flowering and non-flowering shoot density annually from 1996 to 2012 around the Isles of Scilly, finding that patchiness was positively associated with flowering in the previous year and that, although local populations were maintained largely through vegetative reproduction, sexual reproduction may contribute to colonization of vacant habitat. Zostera marina can achieve high colonization potential by having high reproductive effort (Verhagen & Nienhuis, Reference Verhagen and Nienhuis1983).
Both aerial photographs and satellite imagery show that the majority of seagrass beds on Scilly are of the striated type associated with high current velocities. Patchy seagrass vegetation often reflects processes of recovery from disturbances as well as the particular hydrodynamic conditions of the seagrass habitats (Bell et al., Reference Bell, Fonseca, Stafford, Larkum, Orth and Duarte2006; Duarte et al., Reference Duarte, Fourqurean, Krause-Jensen, Olesen, Larkum, Orth and Duarte2006), and patch formation through seedling establishment has been well documented (e.g. Duarte & Sand-Jensen, Reference Duarte and Sand-Jensen1990; Olesen & Sand-Jensen, Reference Olesen and Sand-Jensen1994; Vidondo et al., Reference Vidondo, Duarte, Middelboe, Stefansen, Lützen and Nielsen1997). However, in an experiment in which shoots and rhizomes of Z. marina were experimentally removed, Boese et al. (Reference Boese, Kaldy, Clinton, Eldridge and Folger2009) concluded that natural seedling production played no part in recovery, but this was due exclusively to rhizome growth from adjacent perennial beds. On the other hand, Kendrick et al. (Reference Kendrick, Waycott, Carruthers, Cambridge, Hovey, Krauss, Lavery, Les, Lowe, Vidal, Ooi, Orth, Rivers, Ruiz-Montoya, Sinclair, Statton, van Dijk and Verduin2012, Reference Kendrick, Orth, Statton, Hovey, Ruiz-Montoya, Lowe, Krauss and Sinclair2017) have argued from the evidence of genetic analysis that sexual reproduction and seed are more important for recruitment and the persistence of seagrass beds than previously thought.
The unvegetated or partially vegetated centres of the circles have been attributed to burial during storm events with heavy sand movement (Fonseca & Kenworthy, Reference Fonseca and Kenworthy1987) or to sulphide poisoning (Borum et al., Reference Borum, Raun, Hasler-Sheetal, Pedersen, Pedersen and Holmer2014), but a stress factor need not necessarily be invoked to account for shoot mortality (Duarte et al., Reference Duarte, Fourqurean, Krause-Jensen, Olesen, Larkum, Orth and Duarte2006). As the genet expands radially, older shoots may eventually senesce and die, but younger shoots may continue to thrive and extend away from the point where a seedling originally produced the genet. Senescent shoots may also be more susceptible to mortality resulting from the physical disturbance effects of storms. The average rhizome elongation rate for Z. marina is 26 cm year−1 (Marbà & Duarte, Reference Marbà and Duarte1998), corresponding to an increase in circle diameter of 52 cm year−1. If this rate is realized on Scilly, circle diameters would correspond to ages of 11.5, 21.1, 30.8, 40.4, 51.9 and 67.3 years. Although this is of similar magnitude to similar calculations of Z. marina genet longevity at sheltered sites in the Baltic (Olesen & Sand-Jensen, Reference Olesen and Sand-Jensen1994; Reusch et al., Reference Reusch, Stam and Olsen1998), it has to be admitted that, in a dynamic environment subjected to frequent disturbance by storms and where colonization of bare sediment is occurring, longevity of this magnitude seems unrealistic! The three size increments between the diameters of the first four cohorts of circles at West Broad Ledge (Figure 3) are exactly the same, i.e. 5 m, which would represent 9.6 years. (Little significance can be attached to the increments between the two larger circles, represented by only one and two individuals respectively; Figure 3). Furthermore, calm and relatively storm-free years which might permit the establishment of seedlings are unlikely to occur with such regularity. Such a regular pattern might arise, however, if the growth increments represented a similar rate of seasonal growth in consecutive years, but this would then imply a linear rhizome growth rate of 2.5 m year−1, which would be exceptional for Zostera marina, although several other seagrass species can even exceed this (Marbà & Duarte, Reference Marbà and Duarte1998). The arrowed circle in Figure 2 was estimated on the above assumption to be 5 years old in 1996 (Figure 3) and was still present in 2007, so they can have a lifespan of around 16 years, which seems more realistic than 67! The occurrence of the circles is certainly sporadic, with their gradual disappearance over the period of this study at one site (West Broad Ledge) and appearance at another (Higher Town Bay).
The suggestion that sexual reproduction contributes to the colonization of bare sediment in the vicinity of established meadows (Potouroglou et al., Reference Potouroglou, Kenyon, Gall, Cook and Bull2014) is supported by a personal observation of two identical circles of Z. marina ~5–6 m in diameter exposed during a low spring tide on bare sediment just up-shore of the Old Grimsby Harbour seagrass bed, Tresco, on 11 September 2014 (Figure 7). None had been observed in the vicinity in previous or subsequent years. This implies that the circles had achieved this size in a single growing season, but had subsequently been destroyed by natural or anthropogenic disturbance in an area subject to quite extensive boating activity including the presence of numerous mooring chains. The balance of evidence thus supports the contention that the rhizomes arising initially from seedlings have an exceptionally high growth rate for this species, which would be an evolutionary adaptation for rapid recolonization in such a physically disturbed environment, and that the proliferation of fairy circles both at West Broad Ledge and Higher Town Bay represent later and earlier stages in this colonization process respectively, perhaps after severe sediment disturbance caused by storms.

Fig. 7. Two identical circles of Zostera marina ~5–6 m in diameter exposed during a low spring tide on bare sediment just up-shore of the Old Grimsby Harbour seagrass bed, Tresco, on 11 September 2014. Photograph by R.M. Warwick.
This suggested mechanism for the formation of the circles is clearly not applicable to the ‘atolls’ of Posidinia oceanica, which occur in very sheltered areas of the Mediterranean. These may be only a few metres across (micro-atolls) and, like the circles of Zostera, may also be derived from nearly circular patches where plagiotropic (horizontal) shoots only grow outwards, with the shoots in the central area dying (Pergent & Pergent-Martini, Reference Pergent and Pergent-Martini1995; Boudouresque et al., Reference Boudouresque, Bernard, Bonhomme, Charbonnel, Diviacco, Meinesz, Pergent, Pergent-Martini, Ruitton and Tunesi2006). However, this is where the similarity ends. Where genetic studies have been undertaken, the individual atolls have been shown to be composed of multiple genotypes (Tomasello et al., Reference Tomasello, Di Maida, Calvo, Pirrotta, Borra and Procaccini2009). They may also reach relatively large sizes (macro-atolls). In the Ain Al-Ghazala lagoon on the eastern coast of Libya, for example, the diameter of these atolls is on average 20–30 m and they can approach 70 m in several areas, with their rims comprising a series of micro-atolls (Pergent et al., Reference Pergent, Djellouli, Hamza, Ettayeb, Alkekli, Talha, Alkunti, Pergent-Martini, El Asmi and Le Ravallec2007). The very slow rhizome growth of this species, averaging 2 cm year−1 (Marbà & Duarte, Reference Marbà and Duarte1998), has prompted the suggestion that ‘their construction in all likelihood dates back over several centuries, even thousands of years’ (Pergent et al., Reference Pergent, Djellouli, Hamza, Ettayeb, Alkekli, Talha, Alkunti, Pergent-Martini, El Asmi and Le Ravallec2007).
The observations and conclusions in this paper are based on very scant evidence and certainly need to be supported, or refuted, by a more frequent series of images to determine the growth rates of the circles, and genetic studies of their clonal nature. Hopefully, however, this note will promote some interest in pursuing such studies.
Data
I confirm that all the data supporting the findings of this study are available within the article.
Acknowledgements
My curiosity regarding the seagrass circles was sparked by the many people who responded to my post on the Isles of Scilly Notice Board Facebook page asking for suggestions as to their possible causes, and in particular to Pamela Broad and Glynis Greenman who pointed me in the direction of the archaeological literature and to Julia Day for lending me her copy of the scarce book by Charles Thomas (Thomas, Reference Thomas1985). Nike Bianchi alerted me to the work on Posidonia atolls. James Bull (Swansea University) provided encouragement and advice on seagrass ecology, but the conclusions expressed in this paper are my own. Roger Covey (Natural England) provided the 1996 aerial photographs. The 2007 UK Environment Agency data contain public sector information licensed under the Open Government Licence v3.0. The 2020 Pléiades – Isles of Scilly satellite image was obtained by the NERC Earth Observation Data Acquisition and Analysis Service (NEODAAS) at Plymouth Marine Laboratory, with additional image processing by Emma Sullivan. In retirement, I acknowledge the facilities provided to me by my Honorary Research Fellowship at the Plymouth Marine Laboratory and my Sir Walter Murdoch Distinguished Collaborator award and Adjunct Professorship at Murdoch University.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
I declare none.