INTRODUCTION
The first studies on scleractinian corals on the Brazilian coast were done by Laborel (Reference Laborel1965), who identified a considerable number of taxa, as well as zonation patterns through profiles of different reefs along the coast (Laborel, Reference Laborel1969a, Reference Laborelb). Laborel also mentioned the impacts caused by the removal of numerous colonies of corals and hydrocorals, which were torn off and then burned to obtain lime (locally referred to as caieira) that was used to correct the soil pH for sugarcane plantations. This practice continued until the early 1970s. As a follow up to this research, the distributions of scleractinian and hydrocoral species on the Brazilian coast were characterized by Laborel (Reference Laborel1970), who included some reefs on the urban coast of Maceió.
The geographical distribution of coral species on reef ecosystems in Brazilian States was recorded by Belém et al. (Reference Belém, Rohlfs, Pires, Castro and Young1986). The north-east coast of Brazil has the largest concentration of reef ecosystems, starting from the Manuel Luiz Banks in the State of Maranhão (Amaral et al., Reference Amaral, Hudson, Steiner and Ramos2007) to the Abrolhos area on the south coast of the State of Bahia (Hetzel & Castro, Reference Hetzel and Castro1994). The Brazilian coast has great importance because one genus and six coral species are endemic (Wood, Reference Wood1983; Veron, Reference Veron1995). The endemism of three Brazilian species of the genus Mussismilia was described by Pires et al. (Reference Pires, Castro and Ratto1999) and also analysed by Nunes et al. (Reference Nunes, Fukami, Norris and Knowlton2008), who confirmed that this genus constitutes a monophyletic clade. The existing information about Brazilian reefs was considered sparse by Castro & Pires (Reference Castro and Pires2001), who looked at different areas of the north-east coast of Brazil, including an important area between the coast of the State of Rio Grande do Norte and extending south to the coast of Alagoas near the mouth of the San Francisco River. Shallow-water Scleractinia corals and Zoanthidea from the Coroa Grande reefs in the State of Pernambuco were characterized by Neves et al. (Reference Neves, Silveira, Johnsson and Longo2002). At Itacolomi reefs on the south coast of Bahia, coral species were studied by Castro et al. (Reference Castro, Amorim, Calderon and Segal2006).
On the coast of Alagoas, a general characterization of coral reefs including some information about faunal diversity was presented by Sovierzoski & Correia (Reference Sovierzoski, Correia and Salles1995), and the geomorphological and ecological aspects were described by Correia & Sovierzoski (Reference Correia and Sovierzoski2009). Knowledge of the benthic macrofauna on the Alagoas coast is limited to certain groups such as Porifera (Sarmento & Correia, Reference Sarmento and Correia2002; Cedro et al., Reference Cedro, Hajdu, Sovierzoski, Correia, Custódio, Lobo-Hajdu, Hajdu and Muricy2007) and Bryozoa, with a first record of a living catenicellid in the Atlantic Ocean (Vieira et al., Reference Vieira, Gordon and Correia2007).
This paper characterizes ecological and distributional aspects of scleractinian coral species found in the two different reef ecosystems located along the coast of Alagoas in north-east Brazil.
Study area
The study was carried out on the coast of Alagoas, which stretches for about 230 km, bordered in the north by the Persinunga River and in the south by the São Francisco River (8°55′S–36°10′W and 10°30′S–36°23′W). The coast of the State of Alagoas is divided into three areas with somewhat different environmental conditions (Figure 1).

Fig. 1. Map of the coast of the state of Alagoas, Brazil.
Geomorphological aspects of the coast of Alagoas were studied by Barbosa (Reference Barbosa1985), who characterized the different geological origins of coral reefs and sandstone reefs, and mapped the locations of these reefs. The north coast has more coral reefs, many of these near the coastline and others offshore, and only two areas with sandstone reefs (Figure 2). The central coast between Paripuiera until the city of Maceió is dominated by large and numerous coral reefs; before this area sandstone reefs are more common, and sometimes both types of reef occur together (Figure 3). On the south coast, coral reef areas are few, and the sandstone reefs are larger (Figure 4).

Fig. 2. Coral and sandstone reefs on the north coast of Alagoas: (A) map of the area (adapted from Barbosa, Reference Barbosa1985); (B) Maragogi; (C) Porto da Rua.
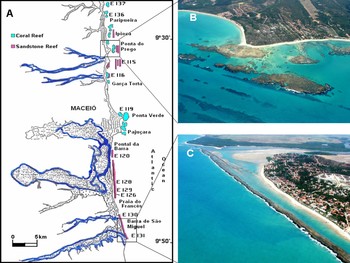
Fig. 3. Coral and sandstone reefs on the central coast of Alagoas: (A) map of the area (adapted from Barbosa, Reference Barbosa1985); (B) Ponta do Prego; (C) Barra de São Miguel.

Fig. 4. Coral and sandstone reefs on the south coast of Alagoas: (A) map of the area (adapted from Barbosa, Reference Barbosa1985); (B) Pontal do Coruripe; (C) Pontal do Peba.
Coral reefs are abundant on the northern and central coast. Their origin is based on calcareous sedimentary rock, made up of an aggregation of dead organisms, including skeletons of corals and hydrocorals associated with crusts of calcareous algae and the structures of other invertebrates including molluscs, bryozoans and echinoderms (Barbosa, Reference Barbosa1985). Many of these coral reefs form fringing reefs that are located near the beach line, where the top of the reef platform is exposed during low tides (Figure 5).

Fig. 5. Coral reefs at low tide on the Maceió city urban coast, Alagoas, Brazil.
Sandstone reefs are more common to the south of Maceió city and on the southern coast of Alagoas. Their geological origin derives from sand banks consolidated through the sedimentation process on calcium carbonate or iron oxide from the Quaternary period, starting with a chemical reaction with fresh water and the silica of seawater, which resulted in the sedimentary reefs (Barbosa, Reference Barbosa1985). These reefs form parallel lines along the coast and are generally located in front of river outlets, estuaries and coastal lagoons (Figure 6).

Fig. 6. Francês and Saco da Pedra sandstone reefs, Alagoas, Brazil.
In some areas along the coast of Alagoas, both reef formations occur together. Sometimes the sandstone reefs are positioned offshore and the coral reefs near the coastline (Figure 3B). In other areas, the sandstone reefs are near the coastline in the upper portion, and the coral reef platform extends toward the sea (Figure 7).

Fig. 7. On the coast of Paripueira (Sonho Verde), coral and sandstone reefs occur together.
MATERIALS AND METHODS
Field observations were made in the tide pools during low tide, by snorkelling and SCUBA dives at the reef edges along inlets and channels. Aerial and underwater photographs were taken during the summer months by the author. When necessary, specimens were collected and stored in individual plastic containers with seawater and magnesium chloride to anaesthetize them. All the biological material obtained was transported to the Setor de Comunidades Bentônicas/Universidade Federal de Alagoas for species identification. Specimens were then deposited in the Cnidaria collection of the Universidade Federal de Alagoas and the Museu Nacional/Universidade Federal do Rio de Janeiro.
RESULTS
The corals of the order Scleractinia recorded for reef ecosystems on the coast of Alagoas comprised 13 species in 9 families, all belonging to the subclass Hexacorallia, and included 5 endemic species.
Scleractinian corals

Fig. 8. Scleractinian corals: (A) Agaricia agaricites; (B) Agaricia fragillis agaricites; (C) Siderastrea stellata in the intertidal zone; (D) Siderastrea stellata in the subtidal zone; (E) Porites astreoides; (F) Porites branneri.
REMARKS
Observed in the subtidal zone of coral reefs and exposed in extreme low tides, which commonly form during summer on the coast of Alagoas. However, this species is more commonly found in the subtidal zone in protected areas of the reef platform. The colonies can be very dense, with small slides on their edges, and are coloured an intense yellow. The associated organism most frequently recorded was Echinometra lucunter Linnaeus, 1758 (Echinoidea). These echinoids appear to be positively associated with this coral species, because they facilitate the attachment of new coral larvae when they feed on macroalgae, which have faster growth rates and compete spatially with this coral species.
REMARKS
This species was found on the edge of coral reefs, where the seawater is clearer throughout the year, from the northern coast of Alagoas to the Maceió coast. In general the colonies were dark green to brown, and encrusted in areas with indirect light. In all reef areas, few colonies occurred, probably because they were situated on the edge of the coral reef where the substratum has countless irregularities such as caves and small channels with high hydrodynamics.
REMARKS
This is the most abundant coral species in all the reef ecosystems, including both coral and sandstone reefs. It occurs on the surface of the platform and at the edge of the reefs. Many new colonies of this coral were recorded on reefs close to the coastline, occupying available spaces in the reef substratum due to the impact caused in the past by the caieiras. The majority of these colonies in the intertidal zone were small, with low growth rates. On the coral reefs, colonies more than 2 m in diameter were observed in the permanently submerged areas within the reef tidal pools at the reef edge, in areas that were little impacted in the past. On the sandstone reefs, this coral occurred in areas that contained some water during low tide, such as small tide pools, mostly close to the top of the reef platform. Colour varied from beige to dark yellow to maroon, being darker in the permanently submerged colonies. It is an endemic species in Brazil, occurring on the north-eastern coast in the Manuel Luiz Banks on the coast of the State of Maranhão (Amaral et al., Reference Amaral, Hudson, Steiner and Ramos2007) to Cabo Frio on the coast of the State of Rio de Janeiro (Castro & Pires, Reference Castro and Pires2001).
REMARKS
This species occurs on coral reefs along margins and edges, mostly in the subtidal zone. These edges are exposed only in extreme low tides, which are very common on this coast in summer. The colonies are dark yellow, and range from a few centimetres to almost a metre in diameter in permanently submerged areas. In some reef areas, this coral species was associated with high densities of the echinoid Echinometra lucunter. Their habit of feeding on algae opens free space for coral recruitment and development.
REMARKS
This species is present in the subtidal zone on coral reefs, along the edge of the reef substratum and the colonies are brown. This species lives mostly in reef areas similar to that of Porites astreoides, although it is also sometimes found associated with the echinoid E. lucunter, probably because this sea urchin facilitates its colonization on the reef substratum.
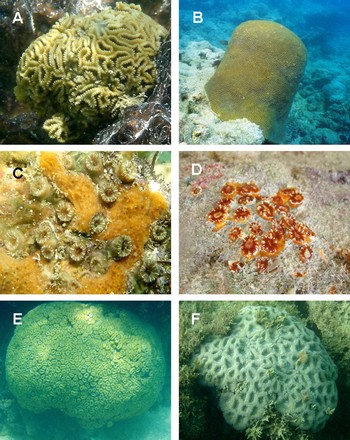
Fig. 9. Scleractinian corals: (A) Favia gravida; (B) Montastrea cavernosa; (C) Astrangia brasiliensis; (D) Phyllangia americana; (E) Mussismilia hartii; (F) Mussismilia hispida.
REMARKS
This coral is very common on coral reefs, from the intertidal zone on the top of the reef platform to the subtidal. Specimens can be frequently found in small pools or exposed directly to the air during large tidal variations. Generally, the colonies are different shades of yellow, with small sizes of up to only 10 cm in diameter. This species was among the most impacted by the caeiras coral harvesting. In some sandstone reefs, it can be observed in the intertidal zone, although it is very scarce, probably because of the influence of fresh water from the estuarine areas. It is an endemic species in Brazil, with a wide occurrence from the Manuel Luiz Banks in Maranhão (Amaral et al., Reference Amaral, Hudson, Steiner and Ramos2007) to the coast of Espírito Santo, also including Rocas Atoll and the Fernando de Noronha Archipelago (Castro & Pires, Reference Castro and Pires2001).
REMARKS
This coral typically occurs on the edges of the reef platforms in permanently submerged areas at depths of 3 to 20 m, more on the coral reefs than on the sandstone reefs. It appears to need clear water, and had a small number of specimens in shallow water and more colonies where the water is clear. Some colonies reached remarkable sizes of more than 2 m in diameter and 3 m in height, with colour varying from dark yellow to dark green or brown. In general, this coral attaches to the base of the reef edge, often touching the sand, and in areas of channels or inlets with considerable tidal flow. For this reason, many of these colonies had physical damage caused by boat anchors and chains, principally where there is intense tourist activity.
REMARKS
The colonies of these corals were made up of several chalice-shaped polyps reaching up to 7 mm. The colonies were formed by several polyps, coloured yellow to brown. This coral was formed on the edges of both reef ecosystems, but was more common on sandstone reefs with little influence from estuarine water. Encrustations always formed in areas where the substratum showed countless irregularities, holes and small channels. It is an endemic species in Brazil, with a narrow distribution from Pernambuco to southern Bahia (Hetzel & Castro, Reference Hetzel and Castro1994).
REMARKS
This coral showed small colony sizes and numbers of polyps, with small chalices about 10 mm in size. The coloration varied from orange to dark red. In general, this coral was found on the edges of the reefs, but was more common in the substratum on sandstone reefs, mostly inside rifts and holes. Sometimes A. braziliensis can be found attached in the same areas.
REMARKS
This coral forms mostly on offshore reefs, on permanently submerged reef edges. It shows a preference for areas with low wave action, clear water and absence of turbidity. However, a larger number of these were also seen in sand areas on the reef edge. The colonies have a yellowish-brown coloration and a long shape between 10 and 15 cm.
REMARKS
This species mostly colonizes areas close to the edges of the reef platforms, in channels that are permanently submerged. It is abundant on coral reefs, occurring only occasionally on sandstone reefs. The colonies reach considerable size, in excess of 3 m in diameter, with greyish-green coloration. They have a branching form, as polyps only form at the base of the colony structure, which is also where fouling organisms, mostly sponges, were observed. In several reef regions far from the coast, this coral is abundant. However, because its polyp structure is easily broken, in areas with intense tourism activity this coral was more severely damaged than other species. It is an endemic species in Brazil, ranging from Rio Grande do Norte to Espírito Santo, including Rocas Atoll and the Fernando de Noronha Archipelago (Castro & Pires, Reference Castro and Pires2001).
REMARKS
This species showed different ecological characteristics than the previously cited species. These colonies were observed in shaded areas where there are rifts and burrows, on the reef platforms and along the reef edges. The colonies occur more frequently on coral reefs, although they are present on sandstone reefs, in locations where fresh water influence is minimal. Colonies reach at most a metre in diameter, and range in colour from greyish-brown to dark green. This coral has a more compact polyp structure than M. hartti, and therefore mechanical impact affects relatively few of its colonies. However, in areas of intense tourist activity, colonies were severely damaged. It is an endemic species in Brazil, with a wide occurrence from the Manuel Luiz Banks on the coast of Maranhão (Amaral et al., Reference Amaral, Hudson, Steiner and Ramos2007) to southern Brazil in the State of São Paulo, including Rocas Atoll and the Fernando de Noronha Archipelago (Castro & Pires, Reference Castro and Pires2001).
REMARKS
This coral appears as a solitary polyp, ranging in colour from dark green to brown and grey. Specimens were recorded only on the coral reefs off northern Alagoas. They occur far from the coast where the water is clear most of the year, with little turbidity during the rainy season. The polyps grow up to 7 cm in size, and are fixed to shed areas on the reef substratum. They were always found fastened to the rifts and whole walls, distributed along the reef pools and the edges of the reef platforms, and in protected areas with low water movement.
Spatial distribution
The scleractinian coral species on the coast of Alagoas showed a spatial distribution based on the geomorphological origin of the substratum and environmental factors, principally the influence of seawater turbidity and hydrodynamics along the Alagoas coast.
The coral reefs have features such as fringing reefs that form banks near the beach line, and other reef banks in irregular patches distributed along the offshore continental shelf. The coastal reefs have an intertidal zone on the platform surface with approximately 1 km2 emerging during low tides, when they are near the beach line, where Favia gravida and Siderastrea stellata are more common in small sizes in the tidal pools. In the subtidal zone where there is little hydrodynamism, Agaricia agaricites, Porites astreoides and Porites branneri are common. In the reef pools and the bore reef zone there are many areas with Mussismilia hartii and Mussismilia hispida and some associated Montastrea cavernosa. On the offshore coral reefs that remain submerged at depths up to 15 or 20 m, the colonization is similar to other reef areas; however, in some caves Scolymia wellsi can be found (Figure 10).

Fig. 10. Scleractinian species distribution on coral reef: (A) typical three-dimensional aspect; (B) cross-section in relation to the tidal variation and the water depth.
The sandstone reefs, when located near the beach line, have a small intertidal zone on the surface of the reef platform that emerges during low tides and remain exposed for some time. In some of these areas there were many Siderastrea stellata in the tidal pools, and some Favia gravida. These reefs are more influenced by fresh and estuarine water, which causes reduced salinity and increased turbidity in this coastal zone, principally during the winter rainy season, when many algae colonize the reef substratum. The sandstone reefs that are little influenced by fresh water or are located offshore, generally remain submerged at depths of up to 5 to 10 m. In the breaker zone on the reef crest it is possible to find Astrangia braziliensis and Phyllangia americana in protected areas such as caves. Colonies of Mussismilia hartii, Mussismilia hispida and Montastrea cavernosa can be very common from 2 to 20 m deep, depending on the water turbidity (Figure 11).

Fig. 11. Scleractinian species distribution on sandstone reef: (A) typical three-dimensional aspect; (B) cross-section in relation to the tide variation and water depth.
The coastal reef ecosystems of Alagoas show differences in the coral species distribution. The north coast has the largest areas of reefs, formed mostly by coral reefs located near the coastline and offshore, and also has higher concentrations of corals, mostly in the subtidal zone where the water is clear most of the year and less turbid in the winter during the rainy season. On the central coast, the largest coral reef areas occur near the beach line until Maceió city, where the more resistant corals on the top of the reef platform are exposed during low tide. The south coast has fewer coral species, because few coral reefs and the sandstone reef occur, due to the greater influence of larger discharges of fresh and estuarine water originating from the rivers and lagoons, which causes reduced salinity and increased water turbidity, mainly during the winter period when rains occur (Table 1).
Table 1. Scleractinian coral species distribution on the coast of Alagoas, Brazil.

XXXX, <75%; XXX, <50%; XX, <25%; X, <1%; –, 0%.
DISCUSSION
The species of scleractinian corals on the coast of the State of Alagoas differed in their qualitative and quantitative cover distribution on coral and sandstone reefs. Qualitative aspects were characterized by Laborel (Reference Laborel1969b), who reported a similar distribution of the species Agaricia agaricites, Favia gravida and Montastrea cavernosa from coral reefs on the Maceió coast, but actually Siderastrea stellata was more common. However, Laborel (Reference Laborel1969a) found qualitative differences, recording four additional species from Alagoas such as Agaricia fragilis, Astrangia brasiliensis, Phyllangia americana and Mussismilia hispida.
The influence of fresh and estuarine water, which reduce the salinity during the dry season, determine the spatial distribution of corals and explain the concentrations of these species on the northern and central coasts and in areas where coral reefs are more common. The environmental aspects that influence the coral distribution along the Alagoas coast also influence all the benthic fauna on both reef ecosystems, as described by Correia & Sovierzoski (Reference Correia and Sovierzoski2009), and specific fauna groups such as Porifera (Sarmento & Corrreia, 2002; Cedro et al., Reference Cedro, Hajdu, Sovierzoski, Correia, Custódio, Lobo-Hajdu, Hajdu and Muricy2007) and Bryozoa (Vieira et al., Reference Vieira, Gordon and Correia2007).
The present study demonstrated the importance and the distribution of scleractinian corals for reef ecosystems in Alagoas, and added new information about the corals from the Brazilian coast, for which more information is needed, as mentioned by Castro & Pires (Reference Castro and Pires2001). The diversity of corals of Alagoas is higher than in other coastal reefs, because 13 coral species with 5 endemic taxa were found, and this genetic aspect was confirmed by Nunes et al. (Reference Nunes, Fukami, Norris and Knowlton2008).
The Alagoas coastal reefs differ somewhat in coral cover composition and abundance in shallow water, compared to other coastal reefs in north-east Brazil, such as the Coroa Grande reefs in Pernambuco (Neves et al., Reference Neves, Silveira, Johnsson and Longo2002) and the Itacolomis reefs on the south coast of Bahia (Castro et al., Reference Castro, Amorim, Calderon and Segal2006).
ACKNOWLEDGEMENTS
Special thanks to Hilda H. Sovierzoski for suggestions and assistance during the fieldwork and to Janet W. Reid for revising the English version.














