INTRODUCTION
This study covers the scale worm species found during the MSM 20/4 cruise with RV ‘Maria S. Merian’ to explore the cold-water coral habitats in the eastern Gulf of Mexico and Straits of Florida as a contribution to the international TRACES initiative (Trans-Atlantic Coral Ecosystem Studies; see Hebbeln et al., Reference Hebbeln and Wienberg2012). During this expedition the coral habitat was visually inspected with a remotely operated vehicle (ROV) and selected megafauna was subsequently sampled using the ROV manipulator or with post-dive grab sampling along the ROV track. On board, species behaviour and interaction of species was documented by microscopic images and videos taken from live specimens immediately after sampling.
The scale worms found belong to the families Aphroditidae, Polynoidae and Sigalionidae. Most of the scale worm species are free-living, but among the Polynoidae about 23% are known to be commensal, with about 20% of the host species being cnidarians (Martín & Britayev, Reference Martín and Britayev1998; Eckelbarger et al., Reference Eckelbarger, Watling and Fournier2005). Altogether we found seven scale worm species, with four of them being new to science: one new aphroditid, five polynoids (among them three new species), and one sigalionid. Of the five polynoid species, three were found associated with a coral host. The other two and the aphroditid and sigalionid species occurred either on the sediment or rocks in the vicinity of the corals, or on dead coral fragments.
An extended taxonomic account is given for all species found, with detailed descriptions and figures for the new species. Moreover, the behaviour and interaction of the polynoid species found on a coral host are described and illustrated by live pictures and videos.
MATERIALS AND METHODS
The samples were collected during the MSM 20/4 cruise with RV ‘Maria S. Merian’ to the eastern Gulf of Mexico (Campeche Bank, West Florida Slope, south-west Florida Slope and Great Bahama Bank Mounds A and B) via grab, box corer or the manipulator arm of the MARUM-Cherokee ROV (see Hebbeln et al., Reference Hebbeln and Wienberg2012). The live organisms were sorted and stored in seawater (about 10°C) for subsequent macroscopic and microscopic documentation, which occurred for live coral host species within 1 h after collection due to fast degradation processes. Microscopic images and videos were acquired with a Keyence VHX-1000 digital camera system to characterize the species behaviour and potential host–symbiont interactions.
For in situ investigations and habitat characterizations of the scale worm host species we analysed the ROV video and image material. The main ROV camera was a Tritech Typhoon PAL colour zoom camera, mounted on a Pan & Tilt unit. One static DSPL Multisea Cam was overlooking the area directly in front of the ROV and still images were taken using a KONGSBERG OE-14 5 Megapixel camera, also mounted on the vehicle's Pan & Tilt unit. The Pan & Tilt unit was additionally equipped with a pair of red-coloured lasers for size measurements of objects on the sea-floor (distance between laser points: 16.5 cm). During bottom time, videos and minifilm framegrabs of the two main cameras were recorded and a still image camera acquired high resolution pictures (Hebbeln et al., Reference Hebbeln and Wienberg2012). Via timecode the video and image material was linked to the ROV navigation track and post-processed in the software ArcGIS.
The scale worm specimens are deposited at the Senckenberg Museum Frankfurt (SMF) (see collection database SeSam: http://sesam.senckenberg.de/). Comparative material has been loaned from the Natural History Museum, London, UK (BMNH).
In the ‘Material examined’ section, complete specimens are indicated by ‘cs’, while ‘af’, ‘mf’, and ‘pf’ refer to anterior, middle, and posterior fragments, respectively.
Specimens were studied using a stereo microscope and a compound microscope equipped with Nomarski interference contrast. Drawings were made using a camera lucida. In figures of anterior ends, the anteriormost elytra were either missing or removed.
Measurements: length (L) is measured from the anterior margin of the prostomium to the posterior border of the last segment (pharynx not included, if everted), width (W) is taken at the widest segment, including parapodia but excluding chaetae.
Information regarding distribution and habitat is based on the examined material and the references given in the respective list of synonymies.
SYSTEMATICS
Family aphroditidae Savigny in Lamarck, 1818
Genus Laetmonice Kinberg, 1856, emended
type species. Laetmonice filicornis Kinberg, 1856
DIAGNOSIS
Body dorsoventrally flattened; about 35 to 45 segments. Prostomium globular, with median antenna and one pair of stalked eyes; papillate facial tubercle present, situated below base of palps. Elytra 15 to 20 pairs on segments 2, 4, 5, 7 to 25, then on every third segment. Elytra not covered or covered by dorsal felt (formed by capillary notochaetae). Notochaetae of three kinds: (1) upper group: acicular tapering to fine tip; (2) middle group: slender, capillary; and (3) lower group: very stout, acicular with harpoon-shaped tip (sometimes hooded, missing in cirrigerous parapodia). Neurochaetae of two kinds: (1) stout, with lateral spine subdistally and inner recurved surface smooth, or with few denticles, or with row of numerous filamentous hairs; and (2) bipinnate, tapering to slender tips (only in segments 2 and 3). Pharynx without jaws.
REMARKS
So far the species of the genus Laetmonice Kinberg, 1856 were known to have no felt, e.g. L. hystrix (Savigny in Lamarck, 1818) or only a very thin layer covering the elytra, e.g. L. filicornis Kinberg, 1856 (see Pettibone, Reference Pettibone1966; Barnich & Fiege, Reference Barnich and Fiege2003). Since Laetmonice tunicata sp. nov. described below is characterized by a rather dense felt, we emend here the diagnosis for the genus.
Laetmonice tunicata sp. nov.
(Figures 2A–D & 3A,B)
TYPE MATERIAL
Holotype: (cs) SMF 22103, south-west Florida Slope, RV ‘Maria S. Merian’ MSM 20/4 Station 16353-1, grab, 24°58.428′N 84°17.916′W, 30 March 2012, 458 m, on rock fragments, leg. L. Beuck & A. Freiwald.
Paratype: (cs) SMF 22104, Great Bahama Bank Slope, RV ‘Maria S. Merian’ MSM 20/4 Station 16374-1, ROV14 Sample 2, 24°33.829′N 79°19.813′W, 4 April 2012, 632 m, on coral rubble, leg. L. Beuck & A. Freiwald.
ADDITIONAL MATERIAL
Laetmonice hystrix (Savigny in Lamarck, 1818): 1 specimen, SMF 10409, March 1996, Banyuls-sur-Mer, on Posidonia, leg. D. Cappellato. 1 specimen, SMF 10414, 21 July 1964, Rovinj (see Figure 3B).
DIAGNOSIS
Eyltra covered by dense layer of felt, formed by the capillary notochaetae. Stout neurochaetae with lateral spine subdistally and inner recurved surface usually with one to three denticles or smooth.
DESCRIPTION
(Based on holotype SMF 22103, Figure 2A–D, and paratype SMF 22104, Figure 3A.)
Prostomium globular, with long, smooth median antenna consisting of ceratophore and two-articled style (long basal and short distal article); eyes stalked, unpigmented; palps long slightly papillate. First or tentacular segment with a pair of tentaculophores, each bearing many capillary chaetae and a smooth dorsal and ventral tentacular cirrus consisting of a cirrophore and a two-articled style (Figure 2A).
Elytra 15 pairs on segments 2, 4, 5, 7, … , 23, 25, 28, 31, covered by rather dense layer of felt with entangled detritus (Figure 3A); elytra more or less oval, surface and margin smooth; first pair attached more or less centrally by elytrophore, the following attached eccentrically. On cirrigerous segments styles of dorsal cirri smooth, consisting of two articles, long, reaching beyond tips of neurochaetae. Styles of ventral cirri smooth, short, not reaching beyond neuropodia.
Beginning with segment 2, parapodia biramous; notopodia irregularly shaped with three groups of chaetae, neuropodia smaller with two to three chaetae (Figure 2B). Notochaetae of three kinds: (1) upper group: acicular tapering to fine tips; (2) middle group: slender, capillary; and (3) lower group: very stout, acicular with harpoon-shaped tips, sometimes hooded (missing in cirrigerous parapodia) (Figure 2C). Neurochaetae of two kinds: (1) stout, with lateral spine subdistally and inner recurved surface usually with one to three denticles (Figure 2D) or smooth; and (2) bipinnate, tapering to slender tips (only in segments 2 and 3).
Dorsum more or less smooth, ventrum with few papillae. Colour in life: greyish (Figure 3A).
MEASUREMENTS
Holotype SMF 22103, cs (Figure 2A–D): L 20 mm, W 7 mm for 32 segments. Paratype SMF 22104, cs: L 10 mm, W 3 mm for about 30 segments (Figure 3A).
ETYMOLOGY
The species is named for its dense felt (Latin tunica = coat).
REMARKS
Laetmonice tunicata sp. nov. is remarkable with regard to its rather dense felt (Figure 3A) and its stout neurochaetae with recurved surface showing one to three denticles or being smooth (Figure 2D).
Among the Laetmonice species occurring in the Atlantic, only L. hystrix (Savigny in Lamarck, 1818) present in the north-east Atlantic and Mediterranean has stout neurochaetae with denticles, but in this species the capillary notochaetae never form a felt covering the elytra (Figure 3B; see also Barnich & Fiege, Reference Barnich and Fiege2003).
We checked the original descriptions of the other Laetmonice species to be considered in this context (see Amaral & Nonato, Reference Amaral and Nonato1982; Augener, Reference Augener1906; Kinberg, 1856; Jirkov, Reference Jirkov1989; McIntosh, Reference McIntosh1885, 1900), i.e.:
-
L. assimilis McIntosh, Reference McIntosh1885 (north-east Atlantic, Arctic);
-
L. benthaliana McIntosh, Reference McIntosh1885 (South Atlantic, Southern Ocean);
-
L. britannica McIntosh, Reference McIntosh1900 (North and South Atlantic, see also Kongsrud et al., Reference Kongsrud, Budaeva, Barnich, Oug and Bakken2013);
-
L. filicornis Kinberg, 1856 (North Atlantic, Mediterranean);
-
L. parva Amaral & Nonato, Reference Amaral and Nonato1982 (south-west Atlantic);
-
L. nuchipapillata Augener, Reference Augener1906 (north-west Atlantic, West Indies); and
-
L. uschakovi Jirkov, Reference Jirkov1989 (North Atlantic).
They are all characterized by stout neurochaetae with filamentous hairs on the recurved inner surface and only a very thin (pseudo-) felt or no felt at all, and are thus easily differentiated from our species.
DISTRIBUTION AND HABITAT
North-west Atlantic, Gulf of Mexico. Found in 458–652 m depth. In MSM 20/4 Station. 16353-1 the substrate consisted of carbonate sandstone rock fragments with some coarse carbonate sand (foraminifera ooze) and shell debris and in MSM 20/4 Station 16374-1 of dead coral rubble of about 10 cm length, heavily colonized by diverse epibionts and the stalked crinoid Endoxocrinus (Diplocrinus) maclearanus minimus (Isselicrinidae) (cf. David et al., Reference David, Roux, Messing and Améziane2006).
Family polynoidae Kinberg, 1856
Genus Eunoe Malmgren, 1866
type species. Polynoe nodosa M. Sars, 1861.
DIAGNOSIS
Body dorsoventrally flattened, short, with up to 50 segments; dorsum more or less covered by elytra or short posterior region uncovered. Fifteen pairs of elytra on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, and 32. Prostomium with or without distinct cephalic peaks and three antennae; lateral antennae inserted ventrally to median antenna. Anterior pair of eyes dorsolateral at widest part of prostomium, posterior pair dorsal near hind margin. Parapodia with elongate acicular lobes with both acicula penetrating epidermis; tip of neuropodia extended to supra-acicular process. Notochaetae stout with blunt tip. Neurochaetae more numerous and more slender, with exclusively unidentate tips.
Eunoe purpurea Treadwell, Reference Treadwell1936
(Figure 4A–C)
Eunoe purpurea Treadwell, Reference Treadwell1936: 51, figures 1–6; Hartman, Reference Hartman1956: 273.

Fig. 1. Map of MSM 20/4 stations in the eastern Gulf of Mexico with presence of scale worm specimens. The bathymetry derives from Ocean Data View 2012.

Fig. 2. Laetmonice tunicata sp. nov., holotype (SMF 22103): (A) anterior end, frontal view, left dorsal tentacular cirrus broken; (B) left, elytrigerous parapodium from segment 23, anterior view, ventral cirrus missing; (C) tip of harpoon-shaped notochaeta; (D) tips of neurochaetae. Scale bars: A, B, 2 mm; C, D, 250 µm.
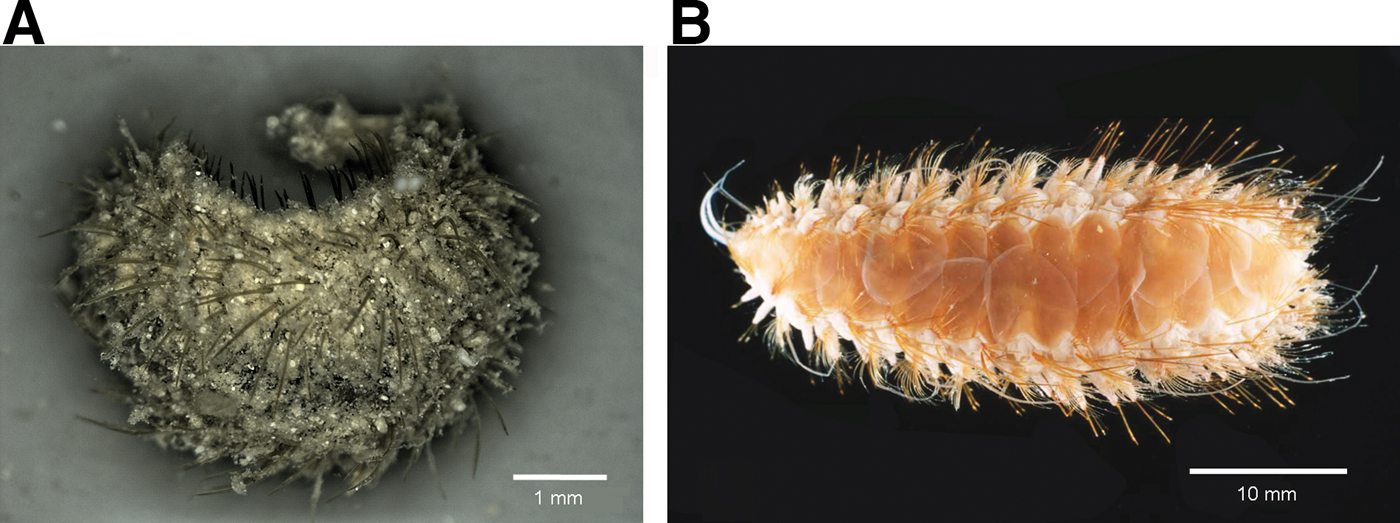
Fig. 3. (A) Laetmonice tunicata sp. nov., paratype (SMF 22104), living animal, with felt covering dorsum; (B) Laetmonice hystrix (SMF 10414), preserved animal, without felt on dorsum.

Fig. 4. (A) Bathypathes cf. alternata colony (length ~40 cm) in situ on patchily distributed Lophelia pertusa rubble; (B) alive Eunoe purpurea crawling on B. cf. alternata; (C) detail showing name giving purple pigmentation on pharynx and prostomium; (D) Stylaster erubescens colony (length ~8.5 cm) in situ on boulder together with large anemone; (E) detail of stylasterid showing tunnel with specimen of Harmothoe dannyi sp. nov. crawling out (arrow); (F) anterior end of alive H. dannyi sp. nov. Scale bars: B, 1 mm; C, F, 500 µm; E, 1 cm.

Fig. 5. (A) Fan-shaped Acanthogorgia armata colony (length ~16 cm) in situ on boulder from mass-flow deposit; (B) tunnel induced by polynoid Gorgoniapolynoe caeciliae on A. armata (arrow); (C) detail of tunnel formed by expanded bases of polyps; (D) G. caeciliae crawling out of tunnel; (E) detail of prostomium showing characteristic modified first elytra; (F) G. caeciliae hunting between coral polyps with everted pharnyx. Scale bars: B, 2 mm; C, D, 1 mm; E, F, 500 µm.

Fig. 6. (A) Several Candidella imbricata colonies in situ on boulders (colony in centre of image about 17 cm in length); (B) three tunnels with polynoid Gorgniapolynoe caeciliae on C. imbricata; (C, D) G. caeciliae crawling in tunnel; (E) detail of tunnel formed by modified coral scales (note difference of diameter of coral branch without tunnel); (F) detail of prostomium of G. caeciliae with characteristic modified first elytra. Scale bars: B, 2 mm; C, E, 1 mm; D, F, 500 µm.
Acanthicolepis longicirrata Treadwell, Reference Treadwell1941: 26, figures 1–4.
MATERIAL EXAMINED
1 specimen (af), SMF 22105, west Florida Slope, RV ‘Maria S. Merian’ MSM 20/4 Station 16334-1, ROV 6 Sample 6, 26°20.195′N 84°45.492′W, 27 March 2012, 508 m, on antipatharian coral Bathypathes cf. alternata Brook, 1898, leg. L. Beuck & A. Freiwald.
DIAGNOSIS
Elytra with few scattered papillae at outer lateral and posterior margin and on posterior half of elytron; surface covered by many rounded microtubercles. Neurochaetae tapering to long bare, unidentate tip. Characteristic purple pigmentation at the pharynx and prostomium (in life and in ethanol; Figure 4B, C).
REMARKS
Eunoe purpurea was originally described by Treadwell (Reference Treadwell1936) from off Bermuda and later by the same author as Acanthicolepis longicirrata Treadwell, Reference Treadwell1941 also from off Bermuda. Both species were synonymized by Hartman (Reference Hartman1956) in her revision of species erected by Treadwell. Eunoe purpurea is easily recognized due to its rounded microtubercles on the elytra and its characteristic purple pigmentation on the pharnyx and prostomium in life and in ethanol (Figure 4B, C).
DISTRIBUTION AND HABITAT
North-west Atlantic: off Bermuda in 900–1640 m (Treadwell, Reference Treadwell1936, Reference Treadwell1941) and in the Gulf of Mexico in 508 m on Bathypathes cf. alternata (Figure 4A, B; this study).
North-east Atlantic: in the northern Bay of Biscay, Saint Nazaire Terrace (VITAL Expedition 2002) in 1250–1470 m on Madrepora oculata (R.B., unpublished observation, see below).
According to our observations the specimen of E. purpurea was found crawling on the antipatharian and probably does not stimulate the coral to produce any galls or tunnels as described for Gorgoniapolynoe caeciliae or Harmothoe dannyi sp. nov. below and reported for other polynoids associated to antipatharian corals (Pettibone, Reference Pettibone1991a).
Genus Gorgoniapolynoe Pettibone, 1991
type species. Gorgoniapolynoe bayeri Pettibone, 1991.
DIAGNOSIS
Body dorsoventrally flattened, with up to about 60 segments; mid-dorsum uncovered by elytra, except in anterior segments. Fifteen pairs of elytra on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, and 32. Anterior one to three pairs of elytra modified, with translucent, chitinous central area. Prostomium with or without cephalic peaks and three antennae; lateral antennae lateroventral to median antenna (i.e. ventral and removed from median antenna). Two pairs of eyes. Parapodia with elongate acicular lobes with noto- and neuroacicula penetrating epidermis; tip of neuropodia extended to supra-acicular process. Notochaetae few, stout with blunt tip. Neurochaetae more numerous, of same width as notochaetae; usually with bidentate tip. Prominent glandular area on bases of ventral cirri beginning with segments 11–18.
Gorgoniapolynoe caeciliae (Fauvel, Reference Fauvel1913)
(Figures 5A–F, 6A–F & 7A, B)
Polynoe caeciliae Fauvel, Reference Fauvel1913: 24, figure 7A–D; Fauvel, Reference Fauvel1914: 69, pl. 4 figures 1–6, 18–19.

Fig. 7. Tunnels with polynoid Gorgoniapolynoe caeciliae specimens found on (A) undamaged Acanthogorgia armata colony (length ~16 cm) and on (B) a branch of a fan-shaped Candidella imbricata colony (length ~30 cm) collected from the upper current-exposed part of a very large boulder (colony dimensions: ~30 cm high and ~65 cm wide).
Gorgoniapolynoe caeciliae: Pettibone, Reference Pettibone1991b: 704, figures 12–14.
MATERIAL EXAMINED
1 specimen (cs), SMF 22106, south-west Florida Slope, RV ‘Maria S. Merian’ MSM 20/4 Station 16347-1, ROV 3 sample 2, 25°16.221′N 84°26.864′W, 29 March 2012, 539 m, on octocoral Acanthogorgia armata Verrill, 1878, leg. L. Beuck & A. Freiwald (Figures 5A–F, 7A).
1 specimen (cs), SMF 22107, south-west Florida Slope, RV ‘Maria S. Merian’ MSM 20/4 Station 16347-1, ROV 8 sample 4, 25°16.256′N 84°26.805′W, 29 March 2012, 539 m, on octocoral Candidella imbricata (Johnson, 1862) together with Munidopsis sp. (Crustacea), l leg. L. Beuck & A. Freiwald (Figure 7B).
7 specimens (cs), SMF 22108, south-west Florida Slope, RV ‘Maria S. Merian’ MSM 20/4 Station 16350-1, ROV 9 sample 6, 24°58.476′N 84°17.933′W, 30 March 2012, 464 m, on octocoral Candidella imbricata (Johnson, 1862), leg. L. Beuck & A. Freiwald (Figure 6A–F).
DIAGNOSIS
First pair of elytra modified, chitinous area small, leaving wide, clear medial border on elytra. Prostomial lobes rounded, without cephalic peaks. (Figures 5E & 6F).
DISTRIBUTION AND HABITAT
Widely distributed in the north-west and north-east Atlantic, in 400–1500 m depth, associated with octocorals of the families Acanthogorgiidae, Coralliidae and Primnoidae (Bayer, Reference Bayer1964; Pettibone, Reference Pettibone1991b; Eckelbarger et al., Reference Eckelbarger, Watling and Fournier2005).
The specimens examined in this study were found on Acanthogorgia armata Verrill, 1878 (Acanthogorgiidae) (Figure 5A–F) and on Candidella imbricata (Johnson, 1862) (Primnoidae) (Figure 6A–F). Our pictures of live A. armata corals confirm the description by Pettibone (Reference Pettibone1991b) for A. aspera Pourtalès, 1867. In both cases the polynoid G. caeciliae induces modifications in the growth of the corals, i.e. the bases of the polyps are expanded to form protective tunnels for the worms (see detail in Figure 5C). The investigated host colony of A. armata was collected entirely and undamaged (length ~16 cm). In total, this coral harboured 12 individuals of G. caeciliae, with each polynoid associated with a specific tunnel (Figure 7A). The tunnels are arranged paralleling the axes of the host branches and without specific side preference around the axis; they are formed by a thin layer of sclerites located between the inner axis and the surface of the coral. The tunnels enlarge the diameter of the branch to about double size (~3–4 mm) and measure between 2–4.5 cm in length. Gorgoniapolynoe caeciliae specimens occurred either inside their individual tunnel or partly outside, preferentially on the oral opening, rarely on the cloacal opening and in one case also with the posterior end partly outside a fracture along a tunnel. Additionally, we could observe the polynoid leaving the tunnel and hunting for prey between the branches of the coral with its proboscis everted (Figure 5D–E; see Supplementary materials and methods section—before References). When trying to take a worm, which was partly outside its tunnel with forceps, its chaetae interfered with the sclerites, making the attempt unsuccessful.
In the case of Candidella imbricata, which is characterized by scales at the bases of the polyps, these scales are enlarged to form tunnels for the polynoids as shown in Figure 6C–E (cf. also Pettibone, Reference Pettibone1991b). At Station 16347-1 (ROV dive 8) C. imbricata occurred very frequently between 544 and 427 m water depth (see Figure 6A), in contrast to Station 16350-1 (dive 9) where just two colonies were documented in 464 m. The fan-shaped, current oriented C. imbricata colonies occur, like those of A. armata, on boulders, blocks or outcropping bedrocks and individual C. imbricata colonies observed are up to 45 cm in length. The commensal G. caeciliae was very abundant on the C. imbricata host samples collected. One long branch (~30 cm in length) of a C. imbricata colony collected from the upper current-exposed part of a very large boulder (colony dimensions: ~30 cm high and ~65 cm wide) at Station 16347-1 (ROV dive 8) revealed in total 36 polynoids (Figure 7B). The individual tunnels measure 1–4.5 cm in length. The majority of the tunnels open at least with one side to a ramification point of the coral.
Bayer (Reference Bayer1964) observed G. caeciliae also on several species of the octocoral genus Corallium. In this case, the worm induces an abnormal growth of the branchlets which form covered tunnels for the worms (cf. also Pettibone, Reference Pettibone1991b).
Genus Harmothoe Kinberg, 1856
type species. Harmothoe spinosa Kinberg, 1856 (revised by Barnich et al., Reference Barnich, Fiege, Micaletto and Gambi2006).
DIAGNOSIS
Body dorsoventrally flattened, short, with up to about 50 segments; dorsum more or less covered by elytra or short tail region uncovered. Fifteen pairs of elytra on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, and 32. Prostomium with distinct cephalic peaks and three antennae; lateral antennae inserted ventrally to median antenna. Position of anterior pair of eyes variable, posterior pair situated dorsally near hind margin. Parapodia with elongate acicular lobes with noto- and neuroacicula penetrating epidermis; tip of neuropodia extended to supra-acicular process. Notochaetae stout with blunt tip. Neurochaetae more numerous and usually more slender; with tips falcate or straight, either all bidentate with a subdistal secondary tooth or some bi- and some unidentate.
Harmothoe dannyi sp. nov.
(Figures 4D–F & 8A–I)
TYPE MATERIAL
Holotype: (cs), SMF 22109, west Florida Slope, RV ‘Maria S. Merian’ MSM 20/4 Station 16326-1, ROV 5 sample 2, 26°24.550′N 84°46.679′W, 26 March 2012, 499 m, on hydrocoral Stylaster erubescens Pourtalès, 1868, leg. L. Beuck & A. Freiwald.
ADDITIONAL MATERIAL
Harmothoe evei Kirkegaard, 1980: holotype, BMNH ZB 1983.1767, RRS ‘Shackleton’ Station 1903/7, off Brittany, 47°29.94′N 9°33.40′W, April 1977, SMBA sledge, 4250 m.
Harmothoe bellani Barnich & Fiege, 2000: holotype, SMF 9087, western Mediterranean, Banyuls-sur-Mer, near Laboratoire Arago, in Posidonia, 10 m, 10 September 1997, leg. D. Fiege & R. Barnich.
DIAGNOSIS
Anterior eyes dorsolateral at widest part of prostomium. Elytral margin with few, scattered papillae; microtubercles globular to conical in anterior half of elytra, getting larger, spine-shaped towards posterior margin. Neuropodial supra-acicular process long, digitiform. Neurochaetae all with falcate, bidentate tip, secondary tooth distinctly set off.
DESCRIPTION (BASED ON HOLOTYPE)
Body with 39 segments. At anterior end (Figures 4F & 8A), prostomium bilobed, with distinct cephalic peaks; ceratophore of median antenna in anterior notch, lateral antennae inserted ventrally, styles of antennae papillate, tapering; anterior pair of eyes situated dorsolaterally at widest part of prostomium, posterior pair dorsally near hind margin of prostomium; palps papillate, tapering.
Tentaculophores inserted laterally to prostomium, without notochaetae, each with a dorsal and ventral tentacular cirrus; styles of cirri papillate, tapering. Second segment with first pair of elytra, biramous parapodia, and long buccal cirri. Following segments with tapering, short, smooth ventral cirri.
Fifteen pairs of elytra, covering dorsum, on segments 2, 4, 5, and 7, then on every second segment to 23, 26, 29, and 32, last seven segments cirrigerous; elytral margin with few, scattered papillae; microtubercles globular to conical in anterior half of elytra, getting larger, spine-shaped towards posterior margin (Figure 8B, C). Cirrigerous segments with distinct dorsal tubercles; dorsal cirri with cylindrical cirrophore, style papillate, tapering.

Fig. 8. Harmothoe dannyi sp. nov., holotype (SMF 22109): (A) anterior end; styles of median antenna, right tentacular and dorsal cirri, and right palp missing; (B) left, middle elytron from unknown segment; (C) detail of posterior margin of same; (D) left, cirrigerous parapodium from segment 8, posterior view, chaetae omitted; (E) distal half of long notochaeta; (F) distal part of middle neurochaeta; (G) tip of same; (H) tip of upper neurochaeta; (I) tip of lower neurochaeta. Scale bars: A, B, 1 mm; C, E, F, 100 µm; D, 500 µm; G–I, 50 µm.
Parapodia biramous; noto- and neuropodia with elongate acicular lobe, tips of noto- and neuroacicula penetrating epidermis; tip of neuropodial acicular lobe extended to long, digitiform supra-acicular process (Figure 8D). Notochaetae stouter than neurochaetae, with distinct rows of spines and blunt tip (Figure 8E); neurochaetae with distinct rows of spines, all with falcate, bidentate tip, secondary tooth distinctly set off (Figure 8F–I).
Colour in life: yellow-greenish (Figure 4F).
MEASUREMENTS
Holotype: (SMF 22109) cs in two fragments: L 15 mm, W 3.5 mm for 39 segments.
ETYMOLOGY
The species is named after Danny Eibye-Jacobsen, polychaetologist at the Danish Natural History Museum Copenhagen, for his continuous help and support during the last years.
REMARKS
Due to the presence of spine-shaped microtubercles on the elytra, H. dannyi sp. nov. might be confused with H. evei Kirkegaard, 1980 and H. bellani Barnich & Fiege, 2000 (see Barnich & Fiege, Reference Barnich and Fiege2009). Harmothoe evei, however, clearly differs by the following characters: (1) neurochaetae with rather straight tip and secondary tooth in bidentate neurochaetae slender, fragile while neurochaetae in H. dannyi sp. nov. show a falcate tip and the secondary tooth is distinctly set off; and (2) the neuropodial supra-acicular process is much shorter than in H. dannyi sp. nov.
Distinction from H. bellani is based on the numerous, long papillae at the elytral margin and surface and the longer papillae on the appendages such as dorsal and tentacular cirri, while in H. dannyi sp. nov. there are only few, scattered and short papillae at the elytral margin and papillae on the appendages are much shorter.
DISTRIBUTION AND HABITAT
So far only known from the type locality, the west Florida slope in the Gulf of Mexico, north-west Atlantic. Found in 499 m depth on Stylaster erubescens Pourtalès, 1868 (Stylasteridae, Hydrozoa) colonizing a downslope transported boulder (diameter ~17 cm) together with a large anemone and various epilithic sponges (Figure 4D, E).
As described above for G. caeciliae, specimens of H. dannyi sp. nov. seem also to stimulate their coral host to produce protective tunnels (see Figure 4E, arrow). Although most Harmothoe species are free-living, those reported to live associated with corals seem to have a preference for hydrocorals and were all found in the Pacific so far: e.g. Harmothoe irritans (Marenzeller, Reference Marenzeller1904) found on Stenohelia profunda Moseley, 1881 and Errina macrogastra Marenzeller, Reference Marenzeller1904 off Galapagos; Harmothoe melanicornis Britayev, Reference Britayev1981 found on Allopora sp. in the Central Pacific; Harmothoe vinogradovae Averincev, 1978 found on an unidentified hydrocoral off Tasmania and Harmothoe zibrowii (Hartmann-Schröder, Reference Hartmann-Schröder1992) found on Pseudosolanderia sp. off New Caledonia (cf. Britayev, Reference Britayev1981; Hartmann-Schröder, Reference Hartmann-Schröder1992; Marenzeller, Reference Marenzeller1904).
Harmothoe cedrici sp. nov.
(Figures 9A–J & 10A)

Fig. 9. Harmothoe cedrici sp. nov., holotype (SMF 22110): (A) anterior end; styles of median antenna tentacular and dorsal cirri, and palps missing; (B) left, anterior elytron from unknown segment; (C) detail of posterior margin of same; (D) dorsal cirrus from unknown segment (free in vial); (E) left, elytrigerous parapodium from segment 15, posterior view, chaetae omitted; (F) distal half of long notochaeta; (G) distal part of middle neurochaeta; (H) tip of same; (I) tip of upper neurochaeta; (J) tip of lower neurochaeta. Scale bars: A, B, D, 1 mm; C, F, G, 100 µm; E, 500 µm; H–J, 50 µm.

Fig. 10. (A) Harmothoe cedrici sp. nov., holotype (SMF 22110), living animal, anterior end; (B) Subadyte campechensis sp. nov. holotype (SMF 22111), living animal, anterior end. Scale bars: A, 1 mm; B, 500 µm.
TYPE MATERIAL
Holotype: (cs), SMF 22110, Great Bahama Bank Slope (Mound A), RV ‘Maria S. Merian’ MSM 20/4 Station 16373-1, ROV 13 sample 1, 26°33.554′N 79°21.262′W, 4 April 2012, 682 m, on coral rubble (dead Lophelia pertusa fragments), leg. L. Beuck & A. Freiwald.
ADDITIONAL MATERIAL
Harmothoe aequespina (Langerhans, 1884): syntypes (fragments), NHMW 235; Madeira, ded. 1884.
Harmothoe anoculata Hartmann-Schröder, Reference Hartmann-Schröder1975: holotype ZMH P13601, 7 paratypes ZMH P13602, ‘Meteor’ 3 Station 30, off Portugal, 45°55.4′N 14°07.9′W, 14 March 1966, 5260 m.
DIAGNOSIS
Anterior eyes dorsolateral at widest part of prostomium. Elytral margin with few, short, scattered papillae; microtubercles globular to conical, smaller in anterior half of elytra, getting larger towards posterior margin. Neuropodial supra-acicular process short, digitiform. Noto- and neurochaetae woth prominent rows of spines, neurochaetae mostly with falcate, bidentate tip, secondary tooth slender, fragile.
DESCRIPTION (BASED ON HOLOTYPE)
Body with 36 segments. At anterior end (Figure 9A), prostomium bilobed, with distinct cephalic peaks; ceratophore of median antenna in anterior notch, lateral antennae inserted ventrally, styles of antennae papillate, tapering; eyes small, only slightly pigmented, anterior pair of eyes situated dorsolaterally at widest part of prostomium, posterior pair dorsally near hind margin of prostomium; palps papillate, tapering.
Tentaculophores inserted laterally to prostomium, each with notochaetae and a dorsal and ventral tentacular cirrus; styles of cirri papillate, tapering. Second segment with first pair of elytra, biramous parapodia, and long buccal cirri. Following segments with tapering, short, smooth ventral cirri.
Fifteen pairs of elytra, covering dorsum, on segments 2, 4, 5, and 7, then on every second segment to 23, 26, 29, and 32, last four segments cirrigerous; elytral margin with few, short, scattered papillae; microtubercles globular to conical, smaller in anterior half of elytra, getting larger towards posterior margin (Figure 9B, C). Cirrigerous segments with distinct dorsal tubercles; dorsal cirri with cylindrical cirrophore, style papillate, tapering (Figure 9D).
Parapodia biramous; noto- and neuropodia with elongate acicular lobe, tips of noto- and neuroacicula penetrating epidermis; tip of neuropodial acicular lobe extended to short, digitiform supra-acicular process (Figure 9E). Notochaetae stouter than neurochaetae, with prominent rows of spines and blunt tip (Figure 9F); neurochaetae with prominent rows of spines reaching up to secondary tooth, mostly falcate with bidentate tip, secondary tooth slender, fragile (might be abraded) (Figure 9G–J).
Colour in life: greyish-yellow (Figure 10A).
MEASUREMENTS
Holotype: (SMF 22110) cs, L 15 mm, W 4 mm for 36 segments.
ETYMOLOGY
The species is named after the carcinologist Cédric d'Udekem d'Acoz, research associate at the Royal Institute of Natural Sciences Belgium, for his continuous donations of scale worms in excellent condition during the past years.
REMARKS
Elytral characters of Harmothoe cedrici sp. nov. with its globular microtubercles remember those of H. aequespina (Langerhans, 1884), H. anoculata Hartmann-Schröder, Reference Hartmann-Schröder1975 and H. vagabunda Pettibone, Reference Pettibone1985. But Harmothoe cedrici sp. nov. clearly differs from these three species by its noto- and neurochaetae with prominent rows of rather long spines. Additionally H. aequespina shows many long papillae at the outer elytral margin while in H. cedrici sp. nov. there are only few, short scattered papillae at the elytral margin. Both, H. anoculata and H. vagabunda, have non-pigmented eyes while in H. cedrici sp. nov. eyes are small, but pigmented. Harmothoe vagabunda differs also by the absence of a neuropodial supra-acicular and in H. anoculata the microtubercles are smaller and more conical (see Hartmann-Schröder, Reference Hartmann-Schröder1975; Pettibone, Reference Pettibone1985; Barnich & Fiege, Reference Barnich and Fiege2009).
DISTRIBUTION AND HABITAT
So far only known from the type locality, the Great Bahama Bank Slope in the Gulf of Mexico, north-west Atlantic. Found in 682 m depth on coral rubble.
Genus Subadyte Pettibone, Reference Pettibone1969
type species. Polynoe pellucidus Ehlers, 1864.
DIAGNOSIS
Body dorsoventrally flattened, short, with about 40 segments covered by elytra. Fifteen or sixteen pairs of elytra on segments 2, 4, 5, 7, 9, 11, 13, 15, 17, 19, 21, 23, 26, 29, 32 (15 pairs) and 34 (16 pairs). Prostomium without distinct cephalic peaks, with three antennae; lateral antennae inserted ventrally to median antenna. Anterior pair of eyes situated dorsolaterally at widest part of prostomium, posterior pair dorsally near hind margin. Parapodia with elongate acicular lobes with noto- and neuroacicula penetrating epidermis; tip of neuropodia not extended to supra-acicular process. Notochaetae stout with blunt or notched tip. Neurochaetae more numerous and more slender, with basal semi-lunar pockets and bidentate tip.
Subadyte campechensis sp. nov.
(Figures 10B & 11A–L)

Fig. 11. Subadyte campechensis sp. nov, holotype (SMF 22111): (A) anterior end; style of right dorsal tentacular cirrus and right palp missing; (B) right elytron from segment 4; (C) detail of posterior margin of same; (D) left, cirrigerous parapodium from segment 12, posterior view, chaetae omitted; (E) distal half of short notochaeta; (F) tip of same; (G) distal half of long notochaeta; (H) tip of same; (I) distal part of middle neurochaeta; (J) tip of same; (K) tip of upper neurochaeta; (L) tip of lower neurochaeta. Scale bars: A, B, 1 mm; C, 250 µm; D, 500 µm; E, G, I, 100 µm; F, H, J–L, 50 µm.
TYPE MATERIAL
Holotype: SMF 22111, Campeche Bank, RV ‘Maria S. Merian’ MSM 20/4 Station 16321-1, box corer, 23°50′N 87°08′W, 25 March 2012, 640 m, on fine mud, leg. L. Beuck & A. Freiwald.
ADDITIONAL MATERIAL
Subadyte pellucida: SMF 10467, Banyuls-sur-Mer, 10 September 1997, between Posidonia, 10 m, near to the Laboratory, leg. D. Fiege & R. Barnich.
DIAGNOSIS
Elytra with scattered flask-shaped papillae and some large, triangular papillae with terminal constriction present in a row near posterior margin. Long notochaetae with distinctly bidentate tip, similar to tip in neurochaetae.
DESCRIPTION (BASED ON HOLOTYPE)
Body with 18 segments, posterior four segments regenerating. At anterior end (Figure 11A), prostomium bilobed, without cephalic peaks; ceratophore of median antenna in anterior notch, lateral antennae inserted ventrally, styles of antennae papillate, tapering; eyes large, anterior pair dorsolateral at widest part of prostomium, posterior pair dorsal near hind margin of prostomium; palps papillate, tapering.
Tentaculophores inserted laterally to prostomium, without notochaetae, each with a dorsal and ventral tentacular cirrus; styles of cirri papillate, tapering. Second segment with first pair of elytra, biramous parapodia, and long buccal cirri. Following segments with tapering, short, smooth ventral cirri.
Number of elytra unknown, those present covering dorsum, on segments 2, 4, 5, and 7, then on every second segment to 17; outer lateral and posterior elytral margin with few, scattered papillae, mostly flask-shaped, some large, triangular macropapillae with terminal constriction present in a row near posterior margin; due to detritus sticking to elytral surface, presence of papillae on surface difficult to evaluate (Figure 11B, C). Cirrigerous segments without dorsal tubercles; dorsal cirri with cylindrical cirrophore, style papillate, tapering.
Parapodia biramous; noto- and neuropodia with very elongate, tapering acicular lobe, tips of noto- and neuroacicula penetrating epidermis; tip of neuropodial acicular lobe not extended to supra-acicular process (Figure 11D). Notochaetae much stouter than neurochaetae, with prominent rows of spines, short notochaetae with blunt, entire tip, long notochaetae with bidentate tip with distinctly set off secondary tooth, similar to tip in neurochaetae (Figure 10E–H); neurochaetae with rows of spines less distinct, all with bidentate tip (Figure 11I–L).
Colour in life: rather transparent, except for some brownish pigment on ceratophores of antennae, yellow and brownish pigmentation on dorsum and dark pharynx (Figure 10B).
MEASUREMENTS
Holotype: (SMF 22111) af with posterior four segments regenerating, L 5.5 mm, W 2.5 mm for 18 segments.
ETYMOLOGY
The species is named after the type locality, the Campeche Bank in the Gulf of Mexico.
REMARKS
Although the holotype of Subadyte campechensis sp. nov. is incomplete and, thus, the number of elytra is unknown, the specimen can be attributed to the genus Subadyte, due to the following characters: neurochaetae with basal semi-lunar pockets; presence of distinct rows of spines in noto-and neurochaetae; noto-and neuropodia with elongate acicular lobes; prostomium with indistinct cephalic peaks and ventrally inserted lateral antennae (see also Pettibone, Reference Pettibone1969; Barnich & Fiege, Reference Barnich and Fiege2003).
Subadyte campechensis sp. nov. is easily distinguished from the eight other species of Subadyte known worldwide by its very characteristic triangular macropapillae with terminal constriction on the elytra and its long notochaetae with distinctly bidentate tip.
Table 1 summarizes the main characters of all considered species. As visualized in this table, the nine species fall into three groups: (1) S. albanyensis, S. chesterfieldensis and S. gracilis with tip of long notochaetae entire (as it is in the short notochaetae of all considered species); (2) S. micropapillata, S. mjoebergi and S. pellucida with tip of long notochaetae notched (i.e. presenting a slit or notch, but not a set-off secondary tooth); (3) S. mexicana, S. papillifera and S. campechensis sp. nov. with tip of long notochaetae distinctly bidentate with subdistal secondary tooth (similar to the bidentate neurochaetae in many polynoid species). However, the presence of the large triangular macropapillae on the elytra in S. campechensis sp. nov. allows the distinction from S. mexicana with digitiform macropapillae and from S. papillifera with club-shaped, nodular macropapillae. For further details on the species considered above see references in Table 1.
Table 1. Comparative table of all Subadyte species known worldwide.

DISTRIBUTION AND HABITAT
So far only known from the type locality, the Campeche Bank in the Gulf of Mexico, north-west Atlantic. Found in 640 m depth on sediment surface consisting of fine mud with some live astrorhizid foraminiferans, large soft worm tubes, pteropod shells, and coral fragments (Hebbeln et al., Reference Hebbeln and Wienberg2012).
Family sigalionidae Kinberg, 1856
Genus Leanira Kinberg, 1856
type species. Leanira quatrefagesi Kinberg, 1856.
DIAGNOSIS
Body elongate, with numerous segments. Elytra numerous, on segments 2, 4, 5, 7, continuing on alternate segments to 25 or 27, then on every segment to end of body. Prostomium oval, fused to first segment, with three antennae; ceratophore of median antenna without auricles or ctenidia; lateral antennae fused to inner dorsal sides of tentaculophores; paired palps long, emerging ventrally to tentaculophores, with long inner and shorter outer palpal sheaths. Bulbous facial tubercle medial to inner palpal sheaths. Tentaculophores with single aciculum, a pair of tentacular cirri, and two bundles of capillary chaetae. Segment 2 with first pair of elytra; biramous parapodia with stylodes; buccal cirri longer than following ventral cirri. Segment 3 without dorsal cirri or tubercles. Cirriform branchiae present and row of three ctenidia dorsal to notopodia present from about segment 30. Parapodia biramous. Notopodia with well-developed bracts with stylodes; notochaetae capillary, spinous. Neuropodia with bilobed postchaetal lobe with stylodes; neurochaetae compound spinigers, with blades relatively short, canaliculate; with or without additional simple spinous neurochaetae. Style of ventral cirri with basal knob. Pharynx with eleven pairs of border papillae, two pairs of jaws.
Leanira robusta Verrill, Reference Verrill1885
Leanira robusta Verrill, Reference Verrill1885: 682, pl. 40 figure 175; Pettibone, Reference Pettibone1970: 10, figure 5.
MATERIAL EXAMINED
1 specimen (af), SMF 22112, Campeche Bank, RV ‘Maria S. Merian’ MSM 20/4 Station 16308-1, box corer, 23°50.121′N 87°10.484′W, 22 March 2012, 565 m, on muddy pteropod–foraminiferan ooze with coral fragments, leg. L. Beuck & A. Freiwald.
DIAGNOSIS
Prostomium ventrally without labial lobes. Tentaculophores with few stylodes. Neurochaetae mostly compound spinigers, some additional simple neurochaetae present. Base of neuropodia ventrally without tubular segmental papillae.
REMARKS
The examined specimen is an anterior fragment in rather bad condition, without elytra.
DISTRIBUTION AND HABITAT
So far only known form off Massachusetts, north-west Atlantic, in 183–230 m (Pettibone, Reference Pettibone1970). Our specimen from the eastern Gulf of Mexico represents a new record for the region. It was found in 565 m water depth on muddy pteropod-foraminiferan ooze with living crinoids, sponges, bryozoans, serpulids, barnacles and brachiopods attached to coral fragments.
ACKNOWLEDGEMENTS
We want to express our sincere thanks to the captain, crew and shipboard staff of RV ‘Maria S. Merian’ during cruise MSM20/4. We are grateful to chief scientist Dierk Hebbeln and thank the scientific participants. Special thanks to the team of the QUEST ROV from MARUM, Bremen University, led by Nicolas Nowald, for their skilful operations and great support during MSM20/4. For identification of the coral hosts we would like to thank Manfred Grasshoff (Octocorallia) from the Senckenberg in Frankfurt, Germany, and Stephen Cairns (Stylasteridae) and Dennis Opresco (Antipatharia) from the Smithsonian in Washington, USA. Charles Messing from Nova in Florida, USA, is also thanked for identifying the crinoid found together with the new Laetmonice. Comparative material was kindly sent on loan by Emma Sherlock (Natural History Museum, London, UK). Helmut Zibrowius (Marseille) is thanked for making polynoid specimens from Ifremer cruise VITAL to the Bay of Biscay in 2002 available to R.B.
FINANCIAL SUPPORT
This study was supported by the EU F.P. VII Projects HERMIONE (contract no. 226354) and CoralFISH (contract no. 213144), and by the Hessian initiative for the development of scientific and economic excellence (LOEWE) at the Biodiversity and Climate Research Centre (BiK-F), Frankfurt, Germany.
Supplementary materials and methods
The supplementary material referred to in this article can be found online at journals.cambridge.org/mbi.














