INTRODUCTION
Humpback whales (Megaptera novaeangliae) are known for travelling long annual migration distances, from high-latitude areas used for feeding (feeding areas) to low-latitude areas used for breeding and calving (Evans, Reference Evans1987).
Fasting for long periods between feeding bouts and travelling great distances in the interim is common among the baleen whales. This creates the potential for complex patterns of population structure (Hoelzel, Reference Hoelzel1998). As a migratory whale, humpbacks fast during winter, on their breeding grounds, relying on their large fat reserves built up during the summer feeding season (Clapham, Reference Clapham1997). Successful winter migrations to low latitudes while fasting require that sufficient energy is stored as lipids before summer feeding ceases. Additionally, fasting migrators must be large enough to have sufficient body volume to accommodate these lipid stores. The seasonal variations of feeding intensity impose annual cycles of fattening and fasting on these animals that can be as extreme as those of small hibernating terrestrial mammals (Berta & Sumich, Reference Berta and Sumich1999).
Humpback whales are also one of the best examples of the seasonal contrasts experienced by the whales during the year. For them, as for many of the migratory whale species, the year is strongly divided both geographically and behaviourally (Clapham, Reference Clapham1997) and despite their nomadic tendencies, the movements of humpback whales are not unstructured (Clapham, Reference Clapham1996). During summer, they spend their time in high latitudes, feeding as much as they can to create a great blubber layer, but do not mate. In winter, they travel to low latitude areas, in tropical waters, where they mate and calve, fasting for long periods such as weeks or even months (Clapham, Reference Clapham1997).
At the end of each winter's breeding season, whales return to specific feeding grounds. Although there is some movement between these areas during the summer feeding season, by and large they represent discrete populations, at least until the following winter, when they will once again all mix on their breeding grounds. The area in which a particular humpback will feed during its life is determined by its mother, who will lead the calf back to her own feeding ground a few weeks after its birth in the tropics (Martin et al., Reference Martin, Katona, Mattila, Hembree and Waters1984; Clapham, Reference Clapham1996). Given the humpback's known wanderlust, we might expect a gradual mixing of populations (and genes) over long periods of time, particularly since the ocean has none of the natural barriers to movement that often separate animal populations on land (Clapham, Reference Clapham1996).
Although this species has been under worldwide protection from commercial whaling since 1966 (Martin et al., Reference Martin, Katona, Mattila, Hembree and Waters1984; Iwasaki & Kubo, Reference Iwasaki and Kubo2001), its population has seriously decreased in many stocks. Therefore, the full comprehension of the worldwide migratory patterns and gene flow between oceanic populations of this species is crucial for the conservation perspective and also for the understanding of the humpback's ecology and genetics.
MIGRATION STUDIES
The humpback whale is a truly cosmopolitan species, found in all oceans of the world (Clapham, Reference Clapham1997), and it is famous for its extensive migrations (Hoelzel, Reference Hoelzel1998). Humpback prey is relatively sparsely distributed in the tropics, so that individual cetaceans feeding in this habitat range over large areas in search for food. Prey of different types is abundant in boreal regions and polar regions where cold, nutrient rich waters are brought to the surface by upwellings (Hoelzel, Reference Hoelzel1998).
Several hypotheses have been proposed for these annual migrations: a vestigial behaviour from when the smaller ocean basins meant closer feeding and wintering areas; the optimization of energy budgets by breeding in warm water; increased calf (offspring) growth and survivorship in warm protected waters; and avoidance of killer whale (Orcinus orca) predation at low latitudes (Hoelzel, Reference Hoelzel1998; Rasmussen et al., Reference Rasmussen, Palacios, Calambokidis, Saborío, Rosa, Secchi, Steiger, Allen and Stone2007).
For humpback whales, breeding areas are at approximately 20° latitude in both hemispheres (Clapham & Mead, Reference Clapham and Mead1999), while the feeding areas are found in temperate to polar waters within the same hemisphere. Migratory timing varies with age, sex and reproductive status (Craig et al., Reference Craig, Herman, Gabriele and Pack2003). The first whales to leave the feeding areas and arrive to the breeding areas are lactating females with their calf from the previous year (yearling), followed by immature individuals, mature males, ‘resting’ females (those neither pregnant nor lactating) and lastly pregnant females. By the time they have to go back to their summer feeding grounds, and also as the winter breeding season progresses, the whales leave the breeding ground in reverse order: females with calves are the last to leave for summer feeding grounds (Dawbin, Reference Dawbin and Norris1966).
Humpback movement and migratory patterns can be studied by the comparison of photographs (photo-ID) of individually distinctive natural markings such as permanent scars or the pigment pattern on the ventral surface of the flukes, as well as comparison of their dorsal fin shape (Muñoz et al., Reference Muñoz, Félix, Flórez-González, Haase, Katona, Lodi, McOsker, Robertson, Stevick and Siciliano1998; Iwasaki & Kubo, Reference Iwasaki and Kubo2001; Stevick et al., Reference Stevick, De Godoy, Engel and Allen2006). The College of the Atlantic (Bar Harbor, Maine, USA) keeps the Antarctic Humpback Whale Catalogue and the North Atlantic Humpback Catalogue, which consists of a collection of identification photographs from throughout the southern hemisphere and North Atlantic, to identify re-sightings (Stevick et al., Reference Stevick, De Godoy, Engel and Allen2006). The great majority of humpbacks undertake the migration each year. However, a population of humpbacks in the Arabian Sea appears to remain in the region year-round, feeding and breeding in tropical waters but retaining a strongly seasonal breeding cycle (Clapham, Reference Clapham1998).
The individuals that are recorded in locations separated by hundreds or even thousands of miles give important information on patterns of movements, migration, and possible gene flow (Clapham, Reference Clapham1996). Studies based on photo-ID of tail pattern and coloration have identified individual humpbacks that have travelled approximately 5000 miles (about 8000 km) between their feeding and breeding grounds (see ‘Evidence of long movements’) (Clapham, Reference Clapham1997).
When migrating individuals are philopatric, returning to their natal breeding grounds, there is the potential for mixing of differentiated stocks in temporary assemblages on seasonal feeding grounds. This is one mechanism in which there can be genetically differentiated sympatric populations (Hoelzel, Reference Hoelzel1998).
For migratory cetacean stocks, there are a number of possible patterns of distribution and movement, depending on the species and the local environment. For example, primary movement may be between ‘private’ breeding and feeding grounds, with some possible movement across areas (Figure 1). In this case, stock structure may be expected to be apportioned between both feeding and breeding grounds (Hoelzel, Reference Hoelzel1998).

Fig. 1. Hypothetical migration from three populations. Complete arrows suggest primary and more probable movements between feeding and breeding areas. Dashed lines represent low-level secondary patterns of movement (Hoelzel, Reference Hoelzel1998, reproduced with permission).
The occurrence of multiple breeding stocks that converge on a single feeding area (Figure 2) is of great concern from the perspective of genetic conservation and management. In this case, differentiated breeding stocks mix in temporary assemblages, and the pattern of mixing can vary on both spatial and temporal scales. This could affect the differential impact of exploitation on the component stocks. For example, hunting in a given location for an extended period could result in takes from more than one stock as they move through the area (Hoelzel, Reference Hoelzel1998).

Fig. 2. Migrations from three hypothetical breeding grounds (A, B and C) to a unique feeding ground, represented by complete arrows. Return to non-natal breeding grounds is represented by dashed lines (only shown for population A) (Hoelzel, Reference Hoelzel1998, reproduced with permission).
GENETIC STUDIES
The observed seasonality in the whales' migration patterns results in a distribution called ‘antitropical’ (Davies, Reference Davies1962). This subject is crucial to the understanding of the genetic flow on whales, as when southern hemisphere humpbacks are in tropical waters (southern hemisphere winter), the northern hemisphere humpback whales are feeding at high latitudes (northern hemisphere summer). This situation reverses after 6 months, when the seasons invert. Consequently, there is always a vast geographical gap separating northern and southern whales (True, Reference True1904) and the effective interchange between northern and southern hemisphere populations is prevented, or limited, by the seasonal opposition of this migratory cycle (Baker et al., Reference Baker, Slade, Bannister, Abernethy, Weinrich, Lien, Urban, Corkeron, Calambokidis, Vasquez and Palumbi1994).
Also, in the northern hemisphere, where it is possible to find humpback whales in the Atlantic and in the Pacific Oceans, the movement between these populations is prevented by continental land masses (True, Reference True1904). In the southern hemisphere, humpback whales form a single circumpolar population distributed throughout the Southern Oceans (Mackintosh, Reference Mackintosh1965). Within each major oceanic population, humpback whales are thought to form relatively discrete subpopulations which are not separated by obvious geographical barriers (Chittleborough, Reference Chittleborough1965; Mackintosh, Reference Mackintosh1965; Katona & Beard, Reference Katona and Beard1990).
While feeding in high-latitude waters, the humpback populations are spread among several relatively discrete feeding stocks. In contrast, it is possible that whales from different feeding grounds mix on the same breeding grounds, and probably interbreed (Mattila et al., Reference Mattila, Clapham, Katona and Stone1989; Katona & Beard, Reference Katona and Beard1990).
However, little is known about the extent of nuclear gene flow in humpback whale populations. Due to land masses, direct movement between the North Atlantic and North Pacific populations is not possible (True, Reference True1904), but migration between hemispheres is less clear. Considering the assumption that whales do not like to swim in warmer water, unless they are in their breeding season, Davies (Reference Davies1962) proposed the theory that cross-equatorial dispersal was more frequent in the Pleistocene, when it is thought that the equator had cold water projections. However, photo-ID records prove that the equator is not an absolute geographical barrier (Stone et al., Reference Stone, Flórez-Gonzalez and Katona1990), but it is still not known for sure whether these animals seen in both hemispheres are rare exceptions to the rule, occasional visitors or proper breeding migrants (Valsecchi et al., Reference Valsecchi, Pasboll, Hale, Glockner-Ferrari, Ferrari, Clapahm, Larsen, Mattila, Sears, Sigurjonsson, Brown, Corkeron and Amos1997).
Inter-oceanic exchange is also expected to happen to some degree in the absence of geographical barriers, yet its documentation remains an exceptional event. Since only a modest degree of migration is sufficient to confound structure between populations, dispersal events may be rare and therefore hard to identify (Pomilla & Rosenbaum, Reference Pomilla and Rosembaum2005). Previous documentations of migrations of this scale have centred on trans-equatorial movements (Stone et al., Reference Stone, Flórez-Gonzalez and Katona1990; Rasmussen et al., Reference Rasmussen, Palacios, Calambokidis, Saborío, Rosa, Secchi, Steiger, Allen and Stone2007) and just a couple of inter-oceanic migrants have been previously identified by Chittleborough (Reference Chittleborough1965).
Although several statistical approaches permit an indirect estimate of gene flow between populations, it is often very difficult to distinguish between current exchange and retention of common ancestral genetic states, as both scenarios produce a similar pattern of allele sharing (Pomilla & Rosenbaum, Reference Pomilla and Rosembaum2005).
For species with sex-biased dispersal or philopatry, the population structure of genetic variation can be different for the maternally inherited mitochondrial genome and the biparentally inherited nuclear genome. The contrasting structure of this genetic variation can, in turn, reveal the complexity of mating systems and one-way or return dispersal in species where demographic evidence is difficult to obtain (Baker et al., Reference Baker, Medrano-González, Calambokidis, Perry, Pilcher, Rosenbaum, Straley, Urban-Ramirez, Yamaguchi and Von Ziegesar1998). The following genetic studies on humpback whales are based mainly on mitochondrial and nuclear DNA.
Mitochondrial DNA
Genetic studies of humpback whales based on mitochondrial DNA (mtDNA) analysis have shown that humpback whale populations are distributed into three principal oceanic populations: the North Atlantic, the North Pacific and the Southern Oceans (Baker et al., Reference Baker, Palumbi, Lambertsen, Weinrich, Calambokidis and O'Brien1990, Reference Baker, Gilbert, Weinrich, Lambertsen, Calambokidis, Mcardle, Chambers and ÓBrien1993, Reference Baker, Slade, Bannister, Abernethy, Weinrich, Lien, Urban, Corkeron, Calambokidis, Vasquez and Palumbi1994). Mixing between those regions is not seen very often, and is considered slight, although occasional exchanges, especially between the North Pacific breeding grounds have been reported (Valsecchi et al., Reference Valsecchi, Pasboll, Hale, Glockner-Ferrari, Ferrari, Clapahm, Larsen, Mattila, Sears, Sigurjonsson, Brown, Corkeron and Amos1997).
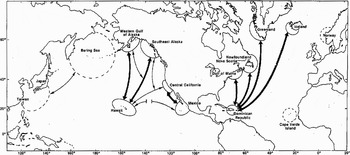
The Baker et al. (Reference Baker, Gilbert, Weinrich, Lambertsen, Calambokidis, Mcardle, Chambers and ÓBrien1993) study on mtDNA showed high levels of variation in humpback whales and substantial genetic differences between oceanic populations. High variation in extant populations would not be due to recent divergence or frequent oceanic interchange but to the persistence of the divergent humpback whale mtDNA lineages for millions of years. This study also calculated the minimum number of migration events necessary to explain the current geographical distribution of nucleotypes worldwide (further information and complete data in Baker et al., Reference Baker, Gilbert, Weinrich, Lambertsen, Calambokidis, Mcardle, Chambers and ÓBrien1993), which was found to be six. Of these, two have been from the Southern Oceans to the North Atlantic, two have been from the North Atlantic to the Southern Oceans, and two have been from the Southern Oceans to the North Pacific. The observations that humpback whales migrate from the Antarctic Peninsula to the equatorial coast of Colombia (Stone et al., Reference Stone, Flórez-Gonzalez and Katona1990) support the hypothesis that the Pacific coast of South America is a likely route for gene flow between oceans (Figure 3) (Baker et al., Reference Baker, Palumbi, Lambertsen, Weinrich, Calambokidis and O'Brien1990).

Fig. 3. Migratory destinations and population structure of humpback whales in the North Pacific and western North Atlantic Oceans, based on natural marked individuals recognized by photo-ID. Arrows connect seasonal habitats visited by individually identified whales but do not necessarily indicate migratory routes. Thick arrows connect regions with known strong migratory interchange and thin arrows connect regions with weak migratory interchange. The broken line connecting Hawaii and Mexico indicates the probable presence of an intervening seasonal migration to a feeding ground by individuals sighted on both winter grounds in alternate years (Baker et al., Reference Baker, Palumbi, Lambertsen, Weinrich, Calambokidis and O'Brien1990, reproduced with permission).
The Baker et al. (Reference Baker, Slade, Bannister, Abernethy, Weinrich, Lien, Urban, Corkeron, Calambokidis, Vasquez and Palumbi1994) study on the hierarchical structure of mtDNA gene flow among Megaptera novaeangliae worldwide demonstrated a high degree of geographical differentiation both within and between the three distinct oceanic populations of humpback whales, despite the nearly unlimited migratory potential of this species. Consequently, the analysis of mtDNA showed a striking degree of genetic structure within and among oceanic populations.
Humpback whales, as other cetacean species, have survived near-extinction after the massive hunting in the past without major loss of mtDNA variation. This apparent genetic diversity in humpback populations could be due to insufficient time since exploitation for the effects of drift to be measured (Baker et al., Reference Baker, Gilbert, Weinrich, Lambertsen, Calambokidis, Mcardle, Chambers and ÓBrien1993). Therefore, the long generation time and age structure of humpback whale populations may have allowed them to pass through a brief bottleneck without major loss of genetic variation (Nei et al., Reference Nei, Maruyama and Chakraborty1975).
Due to the tremendous mobility of humpback whales and the apparent absence of geographical barriers within ocean basins, the formation of significant genetic divisions between stocks indicates strong fidelity to migratory destinations, as a result of a calf's early maternally inherited experience (Baker et al., Reference Baker, Slade, Bannister, Abernethy, Weinrich, Lien, Urban, Corkeron, Calambokidis, Vasquez and Palumbi1994), especially in its first year of life (Baker et al., Reference Baker, Palumbi, Lambertsen, Weinrich, Calambokidis and O'Brien1990, Reference Baker, Medrano-González, Calambokidis, Perry, Pilcher, Rosenbaum, Straley, Urban-Ramirez, Yamaguchi and Von Ziegesar1998). The occasional ability of individual whales to visit alternative breeding grounds and, as sometimes seen, to move between feeding grounds argues against a strict behavioural imprinting or site-specific genetic programming underlying this fidelity. Instead, the life history of such whales suggests a likely mechanism for a ‘cultural’ transmission of migratory destinations (Baker et al., Reference Baker, Palumbi, Lambertsen, Weinrich, Calambokidis and O'Brien1990), as can be confirmed from the mtDNA analysis and its known maternal inheritance.
However, the results obtained from mtDNA analysis are only one genetic measure attributable to the segregation of maternal lineages. The observations of migratory interchange between breeding grounds suggest that the structure of nuclear DNA variation may be more complex (Baker et al., Reference Baker, Slade, Bannister, Abernethy, Weinrich, Lien, Urban, Corkeron, Calambokidis, Vasquez and Palumbi1994).
Nuclear DNA
Studies done in four microsatellite loci by Valsecchi et al. (Reference Valsecchi, Pasboll, Hale, Glockner-Ferrari, Ferrari, Clapahm, Larsen, Mattila, Sears, Sigurjonsson, Brown, Corkeron and Amos1997), in an attempt to calculate the genetic distance between humpback populations in four major regions (the North Atlantic, the North Pacific, and two widely separated Antarctic regions, East Australia and the Antarctic Peninsula), located in three oceans (North Atlantic, North Pacific and Southern Oceans) provide some results relating to this issue.
Significant heterogeneity between the oceanic populations is seen, including between mitochondrially distinct populations within a single ocean. By implication, and as expected, the world's humpback whale populations are not panmictic. Among those comparisons where significant heterogeneity exists, the greatest values are between Northern Atlantic and Northern Pacific humpback populations. The next greatest are the transequatorial comparisons: East Australia–North Pacific, Iceland–Antarctic Peninsula and North Atlantic–Antarctic Peninsula. Finally, there is even a weak but significant heterogeneity within a single ocean (Valsecchi et al., Reference Valsecchi, Pasboll, Hale, Glockner-Ferrari, Ferrari, Clapahm, Larsen, Mattila, Sears, Sigurjonsson, Brown, Corkeron and Amos1997).
Also, the two Antarctic populations show higher levels of allelic diversity when measured by allele number, although the difference is not significant. This result is consistent with the Antarctic populations being historically larger, especially prior to human exploitation. Nuclear genetic differentiation occurs between the North Atlantic populations, even though photo-ID studies indicate that mixing occurs on a common breeding ground. In the Pacific Ocean, studies using photo-ID also confirm occasional movements across the equator. However, the efforts to study trans-equatorial migration are still little, and these results suggest that inter-hemisphere migrations might exist (see ‘Evidence of long movements’), but the genetic differentiation between the humpback populations is still present (Valsecchi et al., Reference Valsecchi, Pasboll, Hale, Glockner-Ferrari, Ferrari, Clapahm, Larsen, Mattila, Sears, Sigurjonsson, Brown, Corkeron and Amos1997).
The nuclear studies indicate that gene flow between oceanic populations of humpback whales is low, and may even be restricted between different populations within the same ocean basin. However, it is important to point out that Baker et al. (Reference Baker, Medrano-González, Calambokidis, Perry, Pilcher, Rosenbaum, Straley, Urban-Ramirez, Yamaguchi and Von Ziegesar1998) suggests that on a global scale, microsatellite variation may provide inconsistent estimates of genetic distances within and between oceanic populations, perhaps as a result of inappropriate assumptions about models and rates of mutations at these loci.
The parallel analysis of nuclear DNA and mtDNA variation allows the partitioning of maternal and biparental components of dispersal and population structure. The complex nature of the nuclear genome makes using these primers more difficult than using comparable mtDNA primers, but the potential for examining many different independent loci makes these primers attractive choices in population genetics surveys (Palumbi & Baker, Reference Palumbi and Baker1994).
Comparisons of the results obtained from mtDNA analysis to those gained by analysing nuclear actin intron sequences, as carried out by Palumbi & Baker (Reference Palumbi and Baker1994), show that on a worldwide scale, nuclear and mitochondrial data lead to similar estimates of gene flow. In both analyses, the North Atlantic and South Pacific populations are genetically distinct from the Northern Pacific population (Baker et al., Reference Baker, Gilbert, Weinrich, Lambertsen, Calambokidis, Mcardle, Chambers and ÓBrien1993), with both analyses from nuclear and mtDNA giving similar estimates of gene flow.
However, over smaller spatial scales comparisons of the two analyses show contrasting patterns. Within two of the North Pacific breeding regions (Hawaii and California) nuclear markers revealed significantly less heterogeneity relative to that revealed by mtDNA sequences (Baker et al., Reference Baker, Palumbi, Lambertsen, Weinrich, Calambokidis and O'Brien1990, Reference Baker, Gilbert, Weinrich, Lambertsen, Calambokidis, Mcardle, Chambers and ÓBrien1993; Palumbi & Baker, Reference Palumbi and Baker1994). Such a discrepancy suggests that males disperse more than females and that nuclear gene flow may occur where mitochondrial exchange does not. In other words, this discrepancy may be the result of gender-biased migration between breeding grounds. If males tend to move between breeding grounds more often and breed more successfully than do females, then nuclear alleles may be mixed more thoroughly than are the maternally inherited mitochondrial sequences. The comparison of mtDNA haplotypes and intron allele frequencies confirmed previous observations that population differentiation in humpback whales is less marked for nuclear loci than for maternal lineages (Palumbi & Baker, Reference Palumbi and Baker1994).
This discrepancy between mtDNA and nuclear DNA results may be due either to differences in genetic drift in mitochondrial and nuclear genes or to preferential movement of males, which do not transmit mtDNA to their offspring, between separate breeding grounds. Opposing mtDNA and nuclear DNA results can help clarify otherwise hidden patterns of structure in natural populations. The use of several different loci will also allow comparisons of estimates of gene flow that are not confounded by the inherent differences between maternally and biparentally inherited genes (Palumbi & Baker, Reference Palumbi and Baker1994), providing additional evidence for the complexity of male and female gene flow and the significance of genetic management units within oceanic populations of humpback whales (Baker et al., Reference Baker, Medrano-González, Calambokidis, Perry, Pilcher, Rosenbaum, Straley, Urban-Ramirez, Yamaguchi and Von Ziegesar1998).
EVIDENCE OF LONG MOVEMENTS
There are reports of humpback whales migrating long distances, from their feeding to their breeding grounds, and also in the opposite direction (Rasmussen et al., Reference Rasmussen, Palacios, Calambokidis, Saborío, Rosa, Secchi, Steiger, Allen and Stone2007). Evidence on these migratory patterns is based on movements of individuals identified by natural markings as part of a large-scale international collaboration, with participation of several organizations (Stevick et al., Reference Stevick, Aguayo, Allen, Avila, Capella, Castro, Chater, Rosa, Engel, Félix, Flórex-González, Freitas, Haase, Llano, Lodi, Muñoz, Olavarría, Secchi, Sheidat and Siciliano2004). Trans-oceanic migration events appear to be very rare in humpback whales, but both Matthews (Reference Matthews1937) and Mackintosh (Reference Mackintosh1942) reported humpback whale catches near the equator during the austral winter off the western coasts of South America and Africa, and suggested that some southern hemisphere whales winter in areas north of the equator (Rasmussen et al., Reference Rasmussen, Palacios, Calambokidis, Saborío, Rosa, Secchi, Steiger, Allen and Stone2007). Chittleborough (Reference Chittleborough1965) also demonstrated that extensive movement across stocks occurs, as shown by the movement of marked whales from Western Australia into the South Pacific.
Considering the North Atlantic, animals which feed at sites off the United States, Canada and west Greenland migrate southward to Caribbean waters during the winter where mating probably takes place (Perkins et al., Reference Perkins, Bryant, Nichols and Patten1982; Whitehead et al., Reference Whitehead, Chu, Perkins, Bryant and Nichols1983). Photo-ID matches provide evidence that humpback whales from the Icelandic feeding grounds and those from feeding areas in the north-western Atlantic utilize a common breeding area and may thus interbreed. The Icelandic photographs that proved to match with Caribbean-photographed humpbacks suggest that there is a significant interchange of animals between these areas (number of recognized humpbacks = 3, mean migration distance = 5958 km) (Figure 3) (Martin et al., Reference Martin, Katona, Mattila, Hembree and Waters1984).
The migratory destinations of humpback whales from the Antarctic Peninsula feeding region and the breeding grounds off the coasts of South America are still uncertain. The number of individuals re-sighted in the Antarctic Peninsula differed dramatically between eastern and western South America. From the western South American humpback population, 43 individuals were identified (through photo-ID) off the Antarctic Peninsula. On the other hand, no animals from Brazil were re-sighted in either the Antarctic Peninsula or off western South America (Figure 4). This evidence suggests that the north-western coast of South America represents an important breeding ground destination for those whales that feed off the Antarctic Peninsula. However, no support concerning the movements of humpback whales between the Antarctic Peninsula and the east coast of South America is provided (Stevick et al., Reference Stevick, Aguayo, Allen, Avila, Capella, Castro, Chater, Rosa, Engel, Félix, Flórex-González, Freitas, Haase, Llano, Lodi, Muñoz, Olavarría, Secchi, Sheidat and Siciliano2004), suggesting that humpback whales from the north-eastern coast of South America probably feed in other regions of the Antarctic (Muñoz et al., Reference Muñoz, Félix, Flórez-González, Haase, Katona, Lodi, McOsker, Robertson, Stevick and Siciliano1998).

Fig. 4. Potential migratory destinations between the Antarctic Peninsula feeding grounds to the breeding grounds off South America. Arrows serve to connect potential migratory endpoints and are not intended to indicate routes of travel (Stevick et al., Reference Stevick, Aguayo, Allen, Avila, Capella, Castro, Chater, Rosa, Engel, Félix, Flórex-González, Freitas, Haase, Llano, Lodi, Muñoz, Olavarría, Secchi, Sheidat and Siciliano2004, reproduced with permission).
These findings were confirmed when humpback whales from the southern Pacific were sighted off Panama and Costa Rica in the austral winter as far north as 11°N using this area for calving and mating. Evidence of this long movement is a report of seven individuals, including a mother and a calf, who have travelled approximately 8300 km, from a wintering area off the Pacific coast of Central America to feeding areas off Antarctica. This movement is the longest ever taken by any mammal. These whales were observed as far north as 11°N off Costa Rica, in an area also used by a boreal population during the opposite winter season, resulting in unique spatial overlap between northern and southern hemisphere populations (Rasmussen et al., Reference Rasmussen, Palacios, Calambokidis, Saborío, Rosa, Secchi, Steiger, Allen and Stone2007).
Stone et al. (Reference Stone, Flórez-Gonzalez and Katona1990) also reported a humpback whale that has migrated from the Antarctic Peninsula to Colombia (8334 km according to Stone et al. (Reference Stone, Flórez-Gonzalez and Katona1990) and 7878 km according to Rasmussen et al. (Reference Rasmussen, Palacios, Calambokidis, Saborío, Rosa, Secchi, Steiger, Allen and Stone2007); the discrepancy is due to the methods that are utilized by each of the authors). Again, it is suggested that this whale probably belongs to a population that feeds in Antarctica and breeds off the coast of Colombia, giving interesting implications for the possibility of migration between hemispheres (Stone et al., Reference Stone, Flórez-Gonzalez and Katona1990). Félix et al. (Reference Félix, Caballero and Olavarría2007) presents examples of studies conducted in recent years in different locations in the south-eastern Pacific, which have provided an overview of the genetic diversity, which seems to be the lowest among humpback whale stocks in the southern hemisphere, and have shown a migratory relationship between the surveyed feeding areas in the Antarctic and the Colombian and Ecuadorian breeding grounds.
These trans-equatorial migrations between Antarctica and Central America are common for at least part of the humpback whale population in the eastern South Pacific. This area in Central America is considered an overlap area for whales from the northern and the southern hemispheres. Central America is a breeding ground for eastern North Pacific humpback whales migrating from areas off California and also, as reported, a wintering area for whales feeding off Antarctica as well (Félix et al., Reference Félix, Caballero and Olavarría2007). Medrano-González et al. (Reference Medrano-González, Baker, Robles-Saavedra, Murreli, Vásquez-Cuevas, Congdon, Straley, Calambokidis, Urbán-Ramirez, Flórez-González, Olavarría-Barrera, Aguayo-Lobo, Nolasco-Soto, Juárez-Salas and Villavinceio-Llamosas2001) suggests that eastern north and south Pacific populations share genetic traits indicating a trans-equatorial exchange, probably off Central America. When it is the boreal winter season, the austral hemisphere is having its summer season, so that the whales in the southern hemisphere are starting to head south, for their feeding grounds (Rasmussen et al., Reference Rasmussen, Palacios, Calambokidis, Saborío, Rosa, Secchi, Steiger, Allen and Stone2007). Therefore, despite opposite migration schedules, it might be possible for an occasional individual from the southern hemisphere to breed with members of the northern hemisphere population (Stone et al., Reference Stone, Flórez-Gonzalez and Katona1990).
Movements of individuals between feeding and breeding migratory destinations within ocean basins have been extensively demonstrated through the hunting of marked whales, photographic capture–recapture, satellite tagging and telemetry and genetic identification (Mackintosh, Reference Mackintosh1942; Chittleborough, Reference Chittleborough1965). As with trans-oceanic migrations, inter-oceanic migrations appear to be very uncommon among the humpback whales. However, Pomilla & Rosenbaum (Reference Pomilla and Rosembaum2005) reported a match indicating an inter-oceanic migration from an individual from Madagascar and Gabon (Figure 5). The samples belonged to a male individual and shared a locally rare mtDNA lineage. A comparison of dorsal fin characteristics confirmed the genetic match.

Fig. 5. Possible migration routes for humpback whales around Africa. Stars indicate the locations where the two matching samples were collected, in Madagascar and in Gabon. Shading indicates humpback whale distribution in the breeding destinations (white) and in the feeding grounds (dark grey). Dark grey arrows indicate population seasonal migratory routes; white dotted arrows indicate three plausible alternative deviations from the main routes that the sampled individual might have undertaken during a migration from the Indian to the South Atlantic Ocean passing through the feeding grounds (Pomilla & Rosenbaum, Reference Pomilla and Rosembaum2005 and US National Geophysical Data Center; reproduced with permission).
Recent observations of matches (from individual migrators) from different oceans reinforced the idea that humpback whales everywhere are great wanderers both in their regular migrations within the same ocean and in the less frequent trans-oceanic and trans-equatorial migrations (Clapham, Reference Clapham1996).
CONCLUSION
Although the humpback whales have a cosmopolitan distribution through the world's oceans and very long migratory potential, trans-equatorial and inter-oceanic migrations are rare among individuals of this species. Therefore and, as expected, the humpback populations are not panmictic on a worldwide scale, despite the absence of obvious geographical barriers. The inversion of the seasons in the northern and southern hemispheres plays an important role in explaining why those migration patterns do not happen very often among the humpback populations. This pattern called ‘antitropical’ creates a vast geographical gap separating austral and boreal whales, due to the inversion of the seasons between both hemispheres.
Although previous research demonstrates that most humpback whales do not cross the equator or move between oceans, the evidence that individuals occasionally do so, has important implications in gene flow, population differentiation and population management. The apparent low rate of this type of migration among humpback populations could be an underestimate due to the difficulty of comparisons between photo-ID of different oceanic catalogues, photograph quality between catalogues and also to the low incidence of studies in worldwide migration routes and genetic studies. Further studies in this subject are highly recommended, by a combination of both demographic approaches and genetic studies.
The estimate of gene flow among the humpback whale population worldwide should include both nuclear and mtDNA analyses to facilitate the discrimination of male and female dispersal patterns and to maximize resolution and rates. Besides, the use of both genetic analyses gives complementary information on the complexity of the problem and dynamics of the system, helping clarify questions concerning the mating systems of humpback whales and the possibility of reproductive isolation between stocks within the same ocean and among oceanic populations. The photo-ID comparisons between different populations also provide evidence of naturally marked individuals that have taken these routes, possibly responsible for gene flow among humpback oceanic populations worldwide.
The trans-equatorial migrations are mainly described for individuals from feeding areas off the Antarctic Peninsula to breeding areas in Central America, especially Costa Rica, Panama and Colombia. The breeding grounds in low latitude areas off Central America could be an overlap of breeding grounds for different populations, as suggested by both migratory and genetic analysis, and where the gene flow among oceanic populations would be more likely to happen, despite the opposite seasons in the northern and southern hemispheres. The combination of a demographic and comparative genetic approach, using both nuclear and mtDNA confirm the power of these analyses for the understanding of humpback mating systems, migratory routes and worldwide gene flow.
As previously suggested by many researchers and reiterated in this paper, understanding the worldwide migration patterns and the gene flow among humpback oceanic populations is crucial for the conservation perspective, especially considering the development of international agreements and protection areas by the competent institutions responsible for this matter, ensuring that natural levels of genetic diversity can be conserved and marine biodiversity preserved.
ACKNOWLEDGEMENTS
The authors would like to thank Milton Marcondes, veterinary from the Instituto Baleia Jubarte (Brazil), who provided essential assistance in revising this article; David Janiger, from the Natural History Museum of Los Angeles County, who provided many of the references; Sérgio Furtado dos Reis, professor at University of Campinas, who helped with many of the ecology concepts; Caio Eduardo Dias Bonafé Jr. and Enrico Aoyama, for formatting the figures. The figures used in this article were reproduced with permission, so that we would like to thank Oxford University Press, for permission to use Figure 1 and 2; C. Scott Baker and Nature Publishing Group for permission to use Figure 3; Peter Stevick and JCRM for permission to use Figure 4; and Cristina Pomilla, Howard C. Rosenbaum and US National Geophysical Data Center, for permission to use Figure 5.