INTRODUCTION
Chondrichthyes reproductive modes may be divided according to the embryonic development: oviparity when embryonic development is external to the mother's body and viviparity when embryonic development is internal (Musick & Ellis, Reference Musick, Ellis and Hamlett2005). In the viviparity behaviour, the living Chondrichthyes show a diversity of reproductive modes and these can be divided into two major categories based on fetal nutrition: lecithotrophy and matrotrophy. Lecithotrophy is a developmental pattern in which the yolk, derived from the maternal liver, provides for embryonic nutrition. This is the dominant mode of reproduction in Chondrichthyes, e.g. Scyliorhinus canicula (Linnaeus, 1758), Centrophorus granulosus (Boch & Schneider, 1801), Squalus acanthias Linnaeus, 1758, Leucoraja erinacea (Mitchill, 1825) and Raja eglanteria Bosc, 1800.
Lecitrotropic species show a weight loss of –20% from egg to final embryo development. On the contrary, any value higher than –20% (and naturally all weight gain) is considered matrotrophy (Hamlett et al., Reference Hamlett, Kormanik, Storrie, Stevens, Walker and Hamlett2005; Storrie et al., Reference Storrie, Walker, Laurenson and Hamlett2009). Therefore, matrotrophic species show a very wide range of categories from the minimal weight losses/minimal weight gain (limited matrotrophy) to extensive maternal supplements such as uterine secretions (histotrophy), ova (ovatrophy), siblings (intrauterine cannibalism or adelphotrophy) or placental transfer (placentatrophy). There are a few number of species in which matrotrophy has been described, e.g. Carcharias taurus Rafinesque, 1810 and Galeocerdo cuvier (Péron & Lesueur, 1822) (Hamlett et al., Reference Hamlett, Kormanik, Storrie, Stevens, Walker and Hamlett2005; Storrie et al., Reference Storrie, Walker, Laurenson and Hamlett2009).
Deania calcea (Lowe, 1839), the birdbeak dogfish (Chondrichthyes: Squalidae) occurs between 70 and 1470 m of depth and is distributed in the Atlantic Ocean from Iceland to South Africa. In the Pacific it occurs in Japan, Taiwan Islands, Australia, New Zealand, Peru and Chile (Clarke et al., Reference Clarke, Connolly and Bracken2002; Compagno et al., Reference Compagno, Dando and Fowler2005). These authors (Compagno et al., Reference Compagno, Dando and Fowler2005) defined this species as being ovoviviparous, but others (e.g. Carrier et al., Reference Carrier, Pratt, Castro, Carrier, Musick and Heithaus2004) suggested the replacement of the designation ovoviviparity by aplacental viviparity, a characterization that includes a wide range regarding the mode of nourishing their embryos. The ovarian fecundity ranged from 7 to 17 follicles while the uterine fecundity ranged from 3 to 5 embryos. The length at first maturity estimated for females was 96.6 cm (~10 years) (Paiva, Reference Paiva2009).
Fatty acids, mainly eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, are known to play a major role in several biochemical processes in vivo and in vitro (Økland et al., Reference Økland, Stoknes, Remme, Kjerstad and Synnes2005; Remme et al., Reference Remme, Larssen, Bruheim, Sæbø, Sæbø and Stokes2006) and in the case of human newborn infants it is well known that they are carried from maternal circulation across the placenta into the fetal venous blood (Connor et al., Reference Connor, Lowensohn and Hatcher1996). In deep-sea fish, particularly in the shark species, there is scarce information about this fact.
Moreover, it is well known that marine predators, particularly long-lived species such as sharks, accumulate high levels of mercury (Hg) via the food web (Endo et al., Reference Endo, Hisamichi, Haraguchi, Kato and Ohta2008). Their longevity together with their high trophic position contribute to their accumulation of mercury (Turoczy et al., Reference Turoczy, Laurenson, Allinson, Nishikawa, Lambert, Smith, Cottier, Irvine and Stagnitti2000), and the evidence of maternal transferences can be documented by the mercury levels found in embryos and juveniles (Adams & McMichael Jr, Reference Adams and McMichael1999; Pethybridge et al., Reference Pethybridge, Cossa and Butler2010). As a top predator, it is likely that it has high heavy metal contamination levels (high bioaccumulation potential), which might become a serious problem in terms of human health since the species was recently introduced (and accepted by the consumers) in the market, either fresh, salty or dry, and exported to the Asian market (particularly the fins).
The main goal of this paper is to evaluate the maternal–embryonic relationship in the birdbeak dogfish in order to evaluate if this species is lecithotrophic or matrotrophic considering chemical features (fatty acid composition and mercury level) and biological approaches.
MATERIALS AND METHODS
Sampling
Birdbeak dogfish were captured with deep-water longlines in the Portuguese continental slope, between September 2008 and May 2009, at depths around 1400–1450 m. Only females in stage 3 (mature: adult) and stage 6 (mature: extrusion), according to the maturity stage key suggested by Figueiredo et al. (Reference Figueiredo, Moura, Neves and Gordo2008), were considered for this study; in the former, mature eggs were analysed and in the latter, both the pregnant females and their almost full-term embryos were studied. Total length and total weight were recorded for each fish to the nearest millimetre and centigram, respectively, using an ichthyometer and a precision balance; total weight and diameter were recorded for each egg; total length, total weight and sex were recorded for each embryo. A single thick segment of the uteri of pregnant females was removed and stored in 10% formaldehyde solution buffered at pH = 7.0. Tissue samples were dehydrated in a graded series of ethanol (70–96%) and embedded in methacrylate resin. Two 3 µm sections were cut from each slice and stained with toluidine blue.
Muscle samples collected from the dorsal region and head of each pregnant female (six individuals labelled a to e in Table 1) and their respective embryos (10 individuals) as well as eggs (10 egg samples) from the last stage of ovarian phase were frozen and kept at 18°C until further analysis; the analyses were performed at least two times.
Table 1. Characterization of reproductive females in maturity stage 6 (labelled from a to e), and their respective embryos, and eggs of birdbeak dogfish used in this study.

Biological analysis
The presence of lecithotrophy or matrotrophy in this species was tested using the method and sample size described in previous studies—Guallart & Vicent (Reference Guallart and Vicent2001), Remme et al. (Reference Remme, Synnes and Stoknes2005) and Moura et al. (Reference Moura, Nunes, Bandarra, Gordo and Figueiredo2011). Ten homogenized eggs and ten embryos were dried at 60°C, over a period of 7 to 15 days, until a constant weight was reached; the samples were then incinerated at increasing temperatures until 500°C (samples were kept at this later temperature during 16 hours). The weight of wet, dry and ash, from both eggs and embryos samples were registered, so as to determine the content of water, organic and inorganic matter. Mean dry weight between eggs and embryos was then compared using the Mann–Whitney test using a significant level of 0.05 (Zar, 1999).
Furthermore, the uteri samples were histologically analysed to study the villi length, the type of vascularization and to detect the eventual presence of secretor cells.
Fatty acid composition
The determination of the fatty acid methyl esters (FAME) profile was prepared based on the experimental procedure described by Lepage & Roy (Reference Lepage and Roy1986), as well as the procedures referred by Bandarra et al. (Reference Bandarra, Batista and Nunes2009), using 300 mg of frozen dried sample. FAMEs were analysed in a CP 3800 Varian gas chromatograph, equipped with an auto-sampler and fitted with a flame ionization detector (FID). The separation was carried out with helium as carrier gas in a DBWax polyethylene glycol column (30 m × 0.25 mm id) programmed to start at 180°C for 5 minutes, heating at 4°C/minute for 10 minutes and kept at 220°C for 25 minutes, with a detector at 250°C. A split injector (100:1) at 250°C was used. FAMEs were identified by comparison of their retention time with those of chromatographic Sigma standards. The values were expressed as a relative percentage of the total fatty acids.
Mercury levels
Total mercury determinations were carried out on 2 aliquots ranging from 10–50 mg of frozen–dried material and were directly analysed in a flameless atomic absorption spectrophotometry (AAS) using an automatic mercury analyser (AMA–254, Leco). The equipment was regularly calibrated using two sets of standard solutions. Certified reference material TORT-2 (lobster hepatopancreas), from the National Research Council of Canada, was tested in quadruplicate in the same conditions as the samples, in order to assess analytical method accuracy; the value certified is 0.27 ± 0.06 mg kg−1 and the value obtained was 0.28 ± 0.00 mg kg−1 (mean ± standard deviation).
The mean value and standard deviation for each component analysed were calculated based on analyses repeated twice. Data were also subjected to linear regressions and Pearson correlations to determine relationships between: (i) some fatty acids from the mother and her respective embryos; and (ii) the mercury levels in the mother, embryos, eggs, and the total length and total diameter.
To test the significant variation in the total of fatty acids among eggs, embryos and females, an analysis of variance was applied and normality and homogeneity assumptions of the data were confirmed; Tukey honestly significant difference test was applied when necessary. A principal component analysis (PCA) was performed to investigate differences in fatty acids among eggs, embryos and pregnant females. Ratios among EPA, DHA and arichidonic acid (ARA) were calculated for pregnant females and embryos. To evaluate the differences of Hg concentrations present in the embryos of each sex, the Mann–Whitney U-test was applied. PCAs were performed using CANOCO 4.5 and all other tests were executed with Statistica 8.3. A significance level of 0.05 was used (Zar, Reference Zar1999).
RESULTS
Biological analysis
The main characteristics of pregnant females, embryos and eggs are shown in Table 1. The total length and weight of the five pregnant females ranged between 98.40–106.60 cm and 3.80–6.55 kg, respectively (Table 1). Regarding the embryos, 6 females and 4 males were identified. The length and the weight were respectively within 19.3–25.8 cm and 23.61–51.79 g. Egg diameters ranged between 46 and 54 mm and the weight between 36.18 and 67.35 g (Table 1).
The comparison of the mean dry weight between eggs and embryos showed a significant difference (U = 6.00; P = 0.0481) revealed by a gain of 3.8% obtained in the embryos (Figure 1). Eggs showed a higher percentage of total organic and inorganic matter (47.74 ± 2.80% and 5.46 ± 2.20%, respectively) while the embryos showed a higher percentage of water content (63.52 ± 3.88%).

Fig. 1. Box plot of the dry weight variation between eggs and embryos.
Histological sections of the uteri of pregnant females showed a high level of both superficial and inner vascularization in the uterine villi as well as the presence of secretor cells (Figure 2).

Fig. 2. Uterine ville from pregnant females: (A) ville with blood vessel and secretory cell; (B) detail of a secretor cell. bv, blood vessel; sc, secretor cell.
Fatty acids composition
The fatty acids composition of the muscle of the reproductive female is shown in Table 2. Among the saturated fatty acids (SFA), the C16:0 (palmitic acid) was the dominant one. The C18:1 n-9 (oleic acid) was the prevailing monounsaturated fatty acid (MUFA), while C22:6 n-3 (DHA) was the main polyunsaturated fatty acid (PUFA). Among the n-6 fatty acids, C20:4 n-6 (ARA) was the dominant fatty acid in the pregnant females studied. As shown in Table 2, the n-3/n-6 ratio in muscle among the different reproductive females was found out to vary between 6.48 and 8.16.
Table 2. Mean and standard deviation of fatty acid composition of pregnant females (labelled from a to e) in maturity stage 6 (data are expressed as percentage of total fatty acids).

The embryos and eggs fatty acid composition are shown respectively in Table 3 and Table 4. Although the relative proportion of the fatty acid categories varies between egg and embryos, the C16:0, C18:1 n-9 and C22:6 n-3 were the dominant compounds. In the category of n-6 fatty acids the most abundant was the ARA. The n-3/n-6 ratios ranged between 4.10–5.72 and 5.47–6.79, respectively for embryos and eggs.
Table 3. Fatty acid composition of embryos of female birdbeak dogfish at maturity stage 6 (data are expressed as percentage of total fatty acids).

Table 4. Fatty acid composition of eggs of female birdbeak dogfish at maturity stage 3 (data are expressed as percentage of total fatty acids).

The distribution of SFA, MUFA and PUFA in the eggs, embryos and pregnant females is shown in Figure 3. The results of the eggs demonstrated that a significant part of the fatty acids were MUFA (varying from 37.88% to 42.47%) followed by PUFA, and SFA had the lowest proportion of total fatty acids. The results of embryos demonstrated that a significant part of the fatty acids were PUFA (varying from 41.51% to 45.73%) followed by the MUFA as observed in the fatty acid profile of eggs, the SFA of the embryos representing the lowest proportion. The results of pregnant females demonstrated that a significant part of the fatty acids were PUFA (varying from 40.60% to 54.01%) while the two other fatty acids categories showed similar proportions (SFA ≈ MUFA). Significant differences were found among eggs, embryos and pregnant females for all fatty acid groups (F >11; P < 0.05) with the exception of SFA for embryos and pregnant females (Tukey test P = 0.87).

Fig. 3. Distribution of saturated, monounsaturated and polyunsaturated fatty acids in eggs, embryos (Emb.) and pregnant female (PF) birdbeak dogfish at the maturity stage 6, expressed in percentage (mean ± SD; minimum–maximum).
A significant direct correlation was found between some fatty acids present in pregnant females and their respective embryos: C14:0 (r = 0.8025; P = 0.0005), 16:0 (r = 0.5753; P = 0.0110) and 20:5 n-3 (EPA) (r = 0.859; P = 0.0001).
The ratios EPA/ARA, DHA/ARA and DHA/EPA were calculated and the last two ratios were higher in pregnant females than in their respective embryos. The ratio EPA/ARA was higher in embryos than in pregnant females. The smaller embryos (6 and 7) stood out from the group of embryos in the ratios EPA/ARA and DHA/EPA since they showed a higher EPA/ARA ratio and a lower DHA/EPA ratio when compared with larger embryos.
Applying the Pearson correlation to the three most important fatty acids present in pregnant females (EPA versus ARA, DHA versus ARA and DHA versus EPA) showed that the only significant correlation was found between DHA versus EPA (r = –0.9663; P = 0.007). The Pearson correlation was also applied to the three most important fatty acids present in embryos and significant correlations were obtained for all the pairs: EPA versus ARA (r = –0.64; P = 0.045); DHA versus ARA (r = 0.73; P = 0.016); DHA versus EPA (r = –0.79; P = 0.006). The maternal ratio for essential fatty acids (EFA)/long-chain polyunsaturated fatty acids (LCPUFA) of pregnant females was also analysed. The mean value of this ratio, using as EFA the 18:2 n-6 linoleic acid (LA) and 18:3 n-3 α-linolenic acid (ALA), and as LCPUFA, ARA, EPA, and DHA was <0.02.
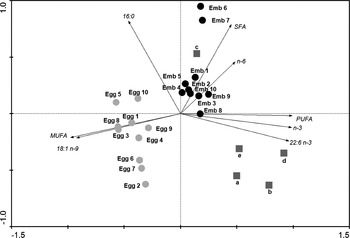
The results of PCAs of the total fatty acids and the most important fatty acid within each group (SFA, MUFA and PUFA) present in pregnant females and their respective embryos and eggs are shown in Figure 4. The first two axes explained 97.2% of the total variable. These analyses showed a clear separation among eggs, embryos and pregnant females. Eggs were mostly related to a high content of MUFA and particularly with the oleic acid. Embryos were particularly related to n-6 PUFA and pregnant females to n-3 PUFA and mostly DHA. It is worthwhile to mention that the smallest embryos analysed (6 and 7) and their mother showed the highest values of SFA and therefore constitute a specific group in the figure since they are isolated from the other females and embryos.

Fig. 4. Principal component analysis: total fatty acids and the most important ones within each group of fatty acids among pregnant females, their respective embryos and eggs. ![]() , pregnant females; •, respective embryos;
, pregnant females; •, respective embryos; ![]() , eggs.
, eggs.
Mercury levels
Total mercury level of pregnant females and their respective embryos are shown in Table 5. The total Hg level ranged from 0.02 to 0.22 mg kg−1 in the embryos while in the females the total Hg levels were near or greater than 1.00 mg kg−1, ranging between 0.9 ± 0.07 and 1.89 ± 0.18 mg kg −1.
Table 5. Total mercury levels of pregnant female birdbeak dogfish (labelled from a to e) and its embryos.

A linear relationship between Hg concentrations and total length of pregnant females was obtained (r = 0.8242; P = 0.000002). There is a close relationship between the Hg level of a pregnant female and the Hg level of its embryos (r = 0.7655; P = 0.0009). Moreover, no statistical relationship was found between the Hg level in embryos and the embryos total length (r = 0.0315; P = 0.647791). Also, there were no statistical differences concerning Hg levels between female and male embryos (U = 12.00; P = 1). The total mercury level in ten eggs of female birdbeak dogfish was quantified and ranged between 0.02 and 0.06 mg kg−1, with a mean value of 0.035 ± 0.016 mg kg−1. A close relationship between the Hg egg concentration and the egg total diameter (r = 0.6061; P = 0.0080) was observed.
DISCUSSION
This is the first study to assess the maternal–embryonic nutritional relationship in elasmobranchs considering the pregnant females associated with their embryos. Until now, this species has been considered as not having a maternal contribution (Compagno et al., Reference Compagno, Dando and Fowler2005); however, the biological and chemical results observed in the present work prove that there is a matrotrophic strategy for the birdbeak dogfish.
Biological analysis
In the lecithotrophic strategy, the embryonic development occurs without any maternal supplement. According to Hamlett et al. (Reference Hamlett, Kormanik, Storrie, Stevens, Walker and Hamlett2005), the presence of matrotrophy implies: (a) a dry weight gain during development from egg to embryo and that gain can be minimal to extensive but any weight gain greater than –20% is considered to be matrotrophy; and (b) there is a transfer of substances between the pregnant female and its respective embryos. The gain of 3.8% in dry weight from egg to embryo obtained in our results supports the matrotrophy strategy. Furthermore, the presence of secretor cells in the uterine villi also contributes to the possibility of transference of nutritional substances between the pregnant females and their embryos. This high metabolic activity might be due to the high vascularization present in the long uterine villi which may contribute to an effective exchange of respiratory and excretory products between the females and their embryos.
Fatty acids composition
In both pregnant females and embryos, the PUFA are the dominant fatty acids, followed by MUFA and SFA. This high content of PUFA in the embryos suggests a matrotrophic contribution since the PUFA content in the egg is lower. The higher content of MUFA in the embryos when compared with that of the pregnant females is probably a result of the absorption of the MUFA from the eggs.
While analysing the PUFA in both embryos and pregnant females it could be observed that the former had a lower percentage of n-3 and a higher n-6 than the latter. However, it must be pointed out that two embryos (those with the smallest size) presented the highest n-3 percentage (and their mother the smallest n-3 percentage among the other females). One possible explanation can be the transference of a larger amount of a n-3 compound at the beginning of the development of the embryos since, as it happens in mammals, these fatty acids are very important for the development of certain organs and for several biochemical and physiological responses of the organism (Remme et al., Reference Remme, Larssen, Bruheim, Sæbø, Sæbø and Stokes2006). In human newborn infants it is well known that these fatty acids are carried from maternal circulation across the placenta into fetal venous blood, but in deep-sea fish species nothing is known about such occurrence (Connor et al., Reference Connor, Lowensohn and Hatcher1996; Innis, Reference Innis2007). It is also well known that for humans the intake of n-3 LCPUFA or of their precursor is necessary for the correct development of the foetus, because the n-3 fatty acids are essential in reproduction processes, membrane development, brain and visual functions (Matorras et al., Reference Matorras, Perteagudo, Sanjurjo and Ruiz1999).
Eicosanoid precursors, especially ARA and EPA, are implicated in many physiological processes including reproductive functions, and as precursors of prostaglandins (Tocher et al., Reference Tocher, Bendiksen, Campbell and Bell2008; Torres et al., Reference Torres, Penha-Lopes, Narciso, Macia and Paula2008). The values of the EPA/ARA ratio obtained in this study were among the range of values presented by Økland et al. (Reference Økland, Stoknes, Remme, Kjerstad and Synnes2005) for other deep-sea species, indicating that these fatty acids may be actively used by both pregnant females and their respective embryos.
The significant Pearson correlation obtained between DHA and EPA confirmed the importance of DHA in reproductive processes comparatively to EPA, a pattern which was also observed by Rodríguez et al. (Reference Rodríguez, Pérez, Díaz, Izquierdo, Fernández-Palacios and Lorenzo1997) and Duttaroy (Reference Duttaroy2009). In fact, the presence of a higher content of DHA is justified by its importance for the ontogenic development of fish because it maintains membrane fluidity, impulse propagation, synaptic transmission, and it functions as a cytosolic signal transducing factor for various gene expressions during the critical period of brain development (Duttaroy, Reference Duttaroy2009). EFA and LCPUFA are very important for fetal growth and development (Duttaroy, Reference Duttaroy2009). The maternal ratio EFA/LCPUFA of pregnant females analysed in the present study revealed that LA and ALA were actively used at this female stage, being in accordance with Duttaroy (Reference Duttaroy2009) who found that maternal EFA metabolism is crucial for fetal growth and development.
The egg fatty acid composition follows the same trend found in other deep-water shark species studied by Remme et al. (Reference Remme, Synnes and Stoknes2005): Centrophorus squamosus (Bonnaterre, 1788), Etmopterus princeps Collett, 1904, Centroscymnus crepidater (Barbosa du Bocage & Brito Capello, 1864), Centrocysmus coelolepis (Barbosa du Bocage & Brito Capello, 1864) and Centroscymnus fabricii (Reinhardt, 1825). A significant part of the fatty acid composition was MUFA, followed by PUFA and SFA. This pattern (with large percentages of C16:0, C18:1 n-9 and C22:6 n-3) is relatively close to other marine organisms (Remme et al., Reference Remme, Synnes and Stoknes2005). SFA provide an adequate level needed for the physiological and structural functions of future embryos (DRI, 2005).
The presence of rich PUFA, particularly DHA, in birdbeak dogfish eggs may contribute to the fluidity of the membranes, which may also be a prerequisite for maintaining normal cell functions at low temperatures (Pickova et al., Reference Pickova, Kiessling, Pettersson and Dutta1999); the high DHA content is essential in the development of the nervous system, a fundamental part of all the animals (Pickova et al., Reference Pickova, Kiessling, Pettersson and Dutta1999; Remme et al., Reference Remme, Synnes and Stoknes2005; Kuratko & Salem Jr, Reference Kuratko and Salem2009).
Mercury levels
The pregnant females, whose mercury levels were <1.5 mg kg−1, had embryos with a total mercury level varying from 1 to 5% of their mothers. On the contrary, in the pregnant females, whose mercury levels were >1.5 mg kg−1, the mercury level of the embryos ranged from 7 to 12%. According to Adams & McMichael Jr (Reference Adams and McMichael1999) and Pethybridge et al. (Reference Pethybridge, Cossa and Butler2010) the mercury levels in embryos and juveniles can be viewed as the base concentration for the overall population, and indicate the transfer of mercury from maternal sources. The transmission of mercury from maternal sources may be an important factor contributing to total mercury concentrations in shark muscle and through bioaccumulation adults have a bigger potential to contain excessive mercury levels (Adams & McMichael Jr, Reference Adams and McMichael1999).
Our results showed a close relationship between the Hg level of pregnant females and the Hg level of their respective embryos and this fact corroborates the possible existence of a maternal contribution. Eggs showed very low mercury levels (≤0.06 mg kg−1) although mature females showed the highest levels (2.24 ± 0.10 mg kg−1) among all the maturity stages analysed for female birdbeak dogfish (Paiva, Reference Paiva2009). This agrees with results described by Wiener & Spry (Reference Wiener, Spry, Beyer, Heinz and Redmon-Norwood1996) who reported that the amount of mercury transferred from the adult female to the eggs during oogenesis is small, since the analyses of eggs showed low levels of mercury. The same results were obtained by Hammerschmidt et al. (Reference Hammerschmidt, Wiener, Frazier and Rada1999) who reported that the concentration of mercury in the ovaries and developing eggs are lower than those found in most other tissues and organs of female fish exposed to mercury either in the laboratory or in natural waters.
ACKNOWLEDGEMENT
We would like to thank ‘ArtesanalPesca’ (Sesimbra, Portugal) for their support in providing the birdbeak dogfish specimens used in this work.