Introduction
Sea urchins from shallow waters are considered to be entirely tropical or temperate, and very limited overlap occurs in the subtropics. The genus Arbacia is represented by species that are distributed in shallow waters from temperate to the tropics, such as Arbacia lixula (Linneo, 1758), A. punctulata (Lamarck, 1816) and A. stellata (Blainville, 1825). Some species are restricted to temperate and sub-Antarctic zones in the southern hemisphere, such as A. spatuligera (Valenciennes, 1846), A. crassispina (Mortensen, 1910) and A. dufresnii (Blainville, 1825) (Lessios et al., Reference Lessios, Lockhart, Collin, Sotil, Sanchez-Jerez, Zigler, Perez, Garrido, Geyer, Bernardi, Vacquier, Haroun and Kessing2012). However, A. crassispina and A. dufresni are probably not separate species, and represent two morphs. They are not distinct in either the mitochondrial or the nuclear gene, but Lessios et al. (Reference Lessios, Lockhart, Collin, Sotil, Sanchez-Jerez, Zigler, Perez, Garrido, Geyer, Bernardi, Vacquier, Haroun and Kessing2012) does not formally synonymize the two species (Gianguzza & Bonaviri, Reference Gianguzza, Bonaviri and Lawrence2013).
Reproduction is one of the most important features of the life history. Species that are widely distributed such as A. stellata adjust their life-history patterns according to local conditions. Consequently, success of populations is directly related to settlement and recruitment rates, which in turn are related to the reproductive strategy (Stearns, Reference Stearns2000). Giese & Pearse (Reference Giese, Pearse, Giese and Pearse1974) established that species should synchronize reproduction with environmental conditions that will be most favourable for the survival of offspring, and often gametogenesis begins before these conditions occur. Gametogenesis in echinoids is modulated by several endogenous and environmental factors (Giese & Pearse, Reference Giese, Pearse, Giese and Pearse1974; Byrne, Reference Byrne1990; Mercier & Hamel, Reference Mercier and Hamel2009).
Reproduction has been studied for some populations of Arbacia (Tavares, Reference Tavares2004; Kino, Reference Kino2010; Wangensteen et al., Reference Wangensteen, Turon, Casso and Palacín2013; Epherra et al., Reference Epherra, Gil, Rubilar, Perez-Gallo, Reartes and Tolosano2015). Tavares (Reference Tavares2004) found that in A. lixula from Paraná state in Brazil, mature individuals were present during the whole sampling period, with female gonads in the resting state and male gonads in proliferation. The same species (A. lixula) in north-west Spain exhibits a seasonal cycle with spawning late spring to summer (from May to July) and during autumn (from October to November); the gonadosomatic index (GSI) followed the same trend as the photoperiod and has a strong relation with the average sea surface temperature (SST). Asynchrony was observed among individuals with several stages of development in the same gonad (Wangensteen et al., Reference Wangensteen, Turon, Casso and Palacín2013). Arbacia punctulata reproduces throughout the summer (June to August) in Florida and this pattern may vary with inter-annual temperature fluctuations (Harvey, Reference Harvey1956). Arbacia dufresni presents two spawning periods, the first during spring and the second and more intense during summer in Nuevo Golfo, Argentina (Brogger et al., Reference Brogger, Martinez and Penchaszadeh2010). Arbacia spatuligera reproduces during spring in Bahia Concepción in Chile (Bay-Schmith, Reference Bay-Schmith1981). Although it has been reported that the species of Arbacia are mainly gonochoric, Shapiro (Reference Shapiro1935) and Harvey (Reference Harvey1956) reported incidences of hermaphroditism. Finally, Epherra et al. (Reference Epherra, Gil, Rubilar, Perez-Gallo, Reartes and Tolosano2015) on the eastern coast of Patagonia looked at two sea urchin populations of A. dufrensii, with different oceanographic regimes, and found that one population presented a strong seasonal pattern of reproduction with gonads showing re-absorption and accumulation of reserves at different seasons, in contrast to the other where mature gametes were found throughout the year. Most reproductive aspects have been documented for several species of the genus Arbacia, but there are no data on the reproductive cycle of A. crassispina, as well as A. stellata (Gianguzza & Bonaviri, Reference Gianguzza, Bonaviri and Lawrence2013).
Distribution of A. stellata extends along the Pacific coast of North, Central and South America, from southern California to Peru, including the Galapagos Islands and the peninsular coasts of the Gulf of California (Mortensen, Reference Mortensen1935; Clark, Reference Clark1948; Brusca, Reference Brusca1980; Metz et al., Reference Metz, Gómez-Gutiérrez and Vacquier1998; Hooker et al., Reference Hooker, Solís-Marín and Lleellish2005; Burcham & Caruso, Reference Burcham and Caruso2015). Arbacia stellata is not very abundant, living in small numbers and as solitary individuals (Morris et al., Reference Morris, Abbott and Haderlie1980; Lessios, Reference Lessios2005; Carlton, Reference Carlton2007), but is starting to appear as one of the most common species at its northern distribution, being the third most common in localities as Laguna Beach in southern California (Burcham & Caruso, Reference Burcham and Caruso2015). With its increase along de Baja California and California, A. stellate could have an impact on local sea urchin populations or other benthic invertebrates of commercial or ecological value. Its reproductive strategy may be critical to their success in further expansion to northern habitats. To fill in this information gap, the present work examines in detail the reproductive cycle of A. stellata in a one-year period in the northern extreme of its distribution. We also describe for the first time detailed aspects of its gametogenesis. We performed a detailed histological examination to quantify the progression of cellular events associated with gonad growth, gametogenesis, maturation and spawning.
Materials and methods
Sample collection and measurement
About 20 individuals of Arbacia stellata (mean test diameter of 47.1 ± 6.0 mm, mean high test of 26.0 ± 4.1 mm and mean wet weight of 62.7 ± 19.4 g) were haphazardly collected monthly by scuba from November 2013 to October 2014. Specimens were collected on rocky substratum at ~5 m depth in Punta Banda (31°44′39.92″N 116°42′13.69″W), located off the north-west coast of the Baja California peninsula, Mexico. Samples were transported to the Laboratorio de Ecología y Biología del Desarrollo of the Instituto de Investigaciones Oceanológicas at the Universidad Autónoma de Baja California (UABC), where they were placed in seawater at 4°C for 24 h (to prevent spawning) for their subsequent dissection, measurement and extraction of gonads.
Samples were dissected and macroscopic characteristics of gonads recorded such as colour, texture, position, shape and size. Gonads were extracted and the wet weight was estimated with a digital scale (±0.001 g). Volume of the gonad was measured by displacement of the fluid method until a hydrostatic equilibrium was reached, using a variation of the Mohr–Westphal balance (Scherle, Reference Scherle1970; Nagy & Pieri, Reference Nagy and Pieri1975).
Sea surface temperature (SST), net primary production (NPP) and chlorophyll, as well as photosynthetically active radiation (PAR) were obtained from satellite information through monthly images. The information was obtained from the Ocean Colour Web portal at 4 km in the HDF format and with a level of L3 Processing (in decimal degrees Long-Lst) from the Aqua Modis sensor. Photoperiod (Fp) was estimated using the model developed by the KCANE Project of the Department of Physical Oceanography at CICESE.
Histological processing and microscopic examination
Gonads were fixed in Bouin's solution for 72 h and preserved in 70% alcohol. Soon after they were dehydrated in graded ethanol series, cleared in Citrisolv®, infiltrated and embedded in paraffin wax using the routine inclusion technique for the Histokinette and finally sectioned at 7 µm and stained with haematoxylin and eosin as described by Humason (Reference Humason1979).
Stages of gonad development were identified on the basis of the cellular characteristics of the nucleus, cytoplasm, and follicular and germinal epithelial wall, according to the criteria proposed by Pérez et al. (Reference Pérez, Boy, Morriconi and Calvo2010). The reproductive cycle was defined based on the proportion of individuals found every month in each of the gonadal developmental stages. Six stages were considered for both sexes: (1) immature, (2) growth, (3) pre-maturity I, (4) pre-maturity II, (5) maturity and (6) spawned.
Oogenesis and spermatogenesis were described using the histological sections. At least 100 oocytes per gonad, per female and sectioned through the nucleus, were measured (Feret diameter) using the image analysis package Image J 1.41. Oocyte size frequencies were compared with GSI.
To quantify reproductive status, two indices were calculated for females and males, as well as for both sexes combined. (1) The traditional gonadosomatic index (GSI), calculated as GSI = [GW/(TBW − GW)] × 100, where GW = gonad wet weight and TBW = total body weight, and (2) the maturity index (MI); this index is also known as the microscopic maturity index because it provides a weighted mean of the microscopic stage of development of the gonads for the majority of individuals in the population at a certain time. Thereby, each MI value indicates its respective gonad development stage throughout the collecting period. It has been applied in several echinoderms (Sewell, Reference Sewell1992; Oyarzún et al., Reference Oyarzún, Marín, Valladares and Iriarte1999; Despalatović et al., Reference Despalatović, Grubelić, Šimunović, Antolić and Žuljević2004; Kazanidis et al., Reference Kazanidis, Lolas and Vafidis2014). The MI was calculated as MI = ∑ (ni × si)/N], where ni = the number of sea urchins at each microscopic gonadosomatic index stage (i.e. GDS), si = the numerical score attributed to each stage (i.e. GDS-I = 1, GDS-II = 2, GDS-III = 3, GDS-IV = 4, GDS-V = 5, GDS-V = 6), and N is the total number of sea urchins collected monthly. Thereby, each MI value indicates its respective gonad development stage throughout the collecting period. We tried to sample only one size of sea urchin for histological analysis. Table 1 summarizes the sizes and weights of the sea urchins sampled.
Table 1. Morphometric measurements of the specimens of Arbacia stellata analysed during the sampling period.

Monthly values of fecundity were quantified as the total number of vitellogenic oocytes (F vo) per female (actual fecundity gives an estimation of the number of eggs that would be released in the following spawning event, but not the precise eggs produced) from the mean volume of oocytes and the gonad volume (GV) in each female. Oocyte volume (OV) of at least 100 oocytes sectioned across the nucleus, per female, per month, was calculated assuming a spherical shape [OV = (4 × π × r3)/3] and averaged. Fecundity was estimated as follows:
where V g = volume of the gonad, V vo = mean volume of a vitellogenic oocyte, V nvo = mean volume of a non-vitellogenic oocyte, P = ratio between non-vitellogenic and vitellogenic oocytes: P = N nvo/N vo, N nvo = number of non-vitellogenic oocytes counted on a subsample of 100 oocytes per gonad, N vo = number of vitellogenic oocytes counted on a subsample of 100 oocytes per gonad (Ramírez-Llodra, Reference Ramírez-Llodra2002; Ramírez-Llodra et al., Reference Ramírez-Llodra, Tyler and Billett2002). It is important to point out that this is an estimate of fecundity based on converting non-vitellogenic oocytes into their equivalent mass to vitellogenic oocytes.
Statistical analysis
Sex ratio (male:female) was calculated monthly, and deviation from the expected ratio of 1:1 was tested with the Chi square goodness of fit test, using Yates' correction for continuity (v2c), due to degrees of freedom = 1 (Yates, Reference Yates1934; Zar, Reference Zar2010). The GSI and fecundity data did not fulfil the assumptions of normality and homoscedasticity (Shapiro–Wilk and Levene tests, respectively), for which non-parametric tests were applied. For GSI a Kruskal–Wallis one way analysis of variance on ranks was performed to identify significant differences among months, whereas a Mann–Whitney rank sum test was used to identify significant differences between sexes. To determine if there were significant differences among values of the MI regarding sexes, a Student's t-test was applied. To determine if there were significant differences among monthly values of fecundity a Kruskal–Wallis one way analysis of variance on ranks and Dunn's pairwise multiple comparison test were performed. The relationship of GSI and MI with the environmental variables, SST, photoperiod and net primary production was evaluated by a stepwise multiple linear regression (MLR). Statistical analyses were carried out using STATISTICA® 7. Statistical significance was accepted at P < 0.05 for all tests (Table 2 summarizes the statistical analysis used with the corresponding results obtained and shown in the following section).
Table 2. Summary of results obtained from the statistical analyses. GSI = Gonadosomatic Index, MI = Maturity Index. Statistical significance was accepted at P < 0.05 for all tests.

Results
Sex-ratio and gonad morphology
Arbacia stellata is confirmed to be a gonochoric species since no hermaphroditism was found from the total sample of 283 sea urchins, showing an annual sex ratio (m:f) of 0.96:1, which does not differ significantly from 1:1 (χ2 = 10.60, df = 11, α = 0.05), except in April 2014 (sex ratio 0.96:3). Gonads of A. stellata are irregular sacs positioned more skewed towards the aboral region. Colour of the female gonads varied from whitish-yellow in immature to dark purple in mature ones. In males, the colouration was usually milky yellow in all stages.
Gonad developmental stages
Females
The immature stage was characterized by the gonad lumen being occupied by a network of nutritive phagocytes of pale colour, with large vesicles of dark colour in the cytoplasm. Also, a few pre-vitellogenic oocytes with basophilic cytoplasm attached to the gonad walls were also observed (Figure 1A). The growth stage showed the beginning of vitellogenesis, characterized by the presence of primary vitellogenic oocytes, attached to the acini wall. The cytoplasm of these oocytes was stained pale purple showing a decrease in acidophilia. Distributed in the centre of the gonad lumen, nutritive phagocytes containing empty vacuoles or granular material were observed (Figure 1B).

Fig. 1. Micrographs of gonadal stages of Arbacia stellata females: (A) immature, (B) growth, (C) pre-maturity I, (D) pre-maturity II, (E) maturity and (F) spawning. NP, nutritive phagocyte; V, vesicles of dark colour; PO, pre-vitellogenic oocyte; L, lumen; VO, vitellogenic oocyte; RVO, residual vitellogenic oocyte.
In the pre-maturity I stage, the number and size of vitellogenic oocytes increased associated with a reduction in the abundance of nutritive phagocytes and glycoprotein material. The gonad contains oocytes in all stages of development but the number of mature oocytes is very low. The vitellogenic oocytes with acidophilic cytoplasm are pear-shaped and are projected into the lumen (Figure 1C). In the pre-maturity II stage, an increase in the number and size of the oocytes that retained the characteristic reddish colouration was observed, and a small number of mature eggs with a finely granular cytoplasm with no visible nucleus were found in the centre of the gonad lumen (Figure 1D).
At the maturity stage, the follicle contained numerous mature eggs, densely packed in the lumen, and a small number of nutritive phagocytes and primary oocytes were located along the follicle walls (Figure 1E). Finally the spawning stage was characterized by the presence of a small number of remaining eggs. At this stage, the wall of the follicle is thick and there is an increase in the number of nutritive phagocytes and a small number of primary oocytes (Figure 1F).
Males
The immature stage was characterized by the gonad lumen dominated by a light network of nutritive phagocytes of pale colour with small dark granules and vesicles; there were some spermatogonia attached to the gonad walls (Figure 2A). In the growth stage, a layer of spermatogonia and primary spermatocytes were located along the gonad wall and columns of almost regular spermatocytes are seen projected to the centre of each follicle. Remnant nutritive phagocytes dominate the centre of the lumen, although the amount of nutritive material had decreased (Figure 2B).

Fig. 2. Micrographs of gonadal stages of Arbacia stellata males: (A) immature, (B) growth, (C) pre-maturity I, (D) pre-maturity II, (E) maturity and (F) spawning. L, lumen; SP, spermatogonia; NP, nutritive phagocyte; PS, primary spermatocytes; S, spermatozoa; V, vesicles of dark colour; RS, residual spermatozoa.
In the pre-maturity I stage, conspicuous columns of spermatocytes were projected towards the lumen. The spermatozoa began to accumulate in the centre of the gonad. The number of nutritive phagocytes decreased and was displaced from the centre to the periphery (Figure 2C). The pre-maturity stage II had a similar structure to that of the pre-mature I, but the gonad contained free spermatozoa in the centre. The columns of spermatocytes were constant (Figure 2D).
In the maturity stage, the gonad was filled with densely packed spermatozoa. The spermatogenic layer became thin or disappeared. Nutritive phagocytes were located towards the periphery of the gonad, along the germinal epithelium (Figure 2E). In the spawning stage, the gonad lumen was almost empty although small clusters of spermatozoa and nutritive phagocytes were found. The gonad wall was very thin (Figure 2F).
Gonadosomatic and maturity indices
Oscillations of the GSI of females and males were similar during the studied period. The annual mean (±SD) for females was 5.6 ± 2.6, and 6.7 ± 2.2 for males, which were not significantly different (t = 21,014.000, P = 0.100). However, the monthly variations were significantly different (H = 143.406, df = 11, P < 0.001) (Figure 3). The month when the maximum value (10.03 ± 3.5) was observed was April 2014 and the lowest value (1.7 ± 0.8) occurred in December 2013. The interval of variation of GSI in each month was relatively broad (Figure 3).

Fig. 3. Monthly variation of the gonadosomatic index (GSI) of females, males and the total population of Arbacia stellata in Punta Banda, BC, Mexico. Every point represents the mean±Standard Deviation (SD) of the mean. On the x-axis, the first two months Nov. and Dec. correspond to the year 2013 and the following January–October to the year 2014.
The trend of the MI during the sampling months was similar in both females and males, although in December 2013 it was observed that females and males showed an inverse trend. Statistically no significant differences were found between sexes (T = −0.656, df = 22, P = 0.518) (Figure 4).

Fig. 4. Maturity index (MI) value of females, males, and the total average for the population of Arbacia stellata. On the x-axis, the first two months Nov. and Dec. correspond to the year 2013 and the following January–October to the year 2014.
The GSI and MI did not follow the same trend. During the months when the GSI showed the highest values (March to May 2014), the lowest MI values were recorded. Likewise, the months when the highest values of MI were observed (November, December and January of 2013 and from July to October of 2014) were also the months in which the GSI exhibited the lowest values (compare Figures 3 and 4).
Gametogenic cycle
The gametogenic cycle of Arbacia stellata is semi-continuous since a high asynchrony was observed among individuals and within the gonad in any of the months studied, for both sexes. Several gonadal stages were represented in the sampled individuals and even in the same gonad of each individual.
In females, the month in which a higher proportion of spawned urchins were observed (88.9%) was December 2013. Soon after, stages of immature and growth began to appear, with February 2014 being the month with the highest representation of these stages (25 and 41.7%, respectively). March and May 2014 were mainly represented by the stage of pre-maturity I (62 and 41%, respectively). As of June 2014, the stages of pre-maturity II and maturity began to be more representative; with August being mostly represented by the maturity stage (85.7%), and in September 2014 the spawning stage began to appear (Figure 5).

Fig. 5. Gametogenic cycle of females and males of Arbacia stellate in Punta Banda, BC, Mexico. On each x-axis, the first two months Nov. and Dec. correspond to the year 2013 and the following January–October to the year 2014.
In males, the maturity stage was present in most sampling months (June to October 2014), with the exception of April and May 2014, with August having the highest incidence (82%). The spawning stage was observed in different months including November 2013, January, February, June, September and October 2014. The pre-maturity I stage was also present in most sampling months, except for January 2013 and August 2014. The pre-maturity II stage was observed during all sampling periods. The immature stage was present from December 2013 to February 2014, with a higher incidence in December (33%). The growth stage started to be evident from December 2013 to May 2014, mostly represented in April (44%) (Figure 5).
Size structure of oocytes
Females showed a continuous production of pre-vitellogenic and vitellogenic oocytes. The most frequent size was the 80 µm interval and in all months this size coincided with the mean diameter of vitellogenic oocytes (85.9 ± 9.3 µm). The second most frequent size was 60 µm, which corresponds to the mean diameter of pre-vitellogenic oocytes (54.0 ± 8.5 µm). This was also observed in December 2013 and January 2014, when the number of gametes in the gonads was low. The maximum size of vitellogenic oocytes in the sampling area was 102.6 ± 9.8 µm (Figure 6).

Fig. 6. Frequency distribution of the oocyte diameter of Arbacia stellata (November 2013–October 2015) in Punta Banda, BC, Mexico.
Fecundity
Mean fecundity (±SD) was estimated at 1.7 × 109 ± 9.4 × 109 vitellogenic oocytes per female. Statistically significant differences were observed among months (H = 45.445, df 11, P ≤ 0.001). Months that showed significant differences were April 2014 with November 2013; December 2013 with September and October 2014; and January 2014 with December 2013 and September 2014. The ranges of fecundity values within months were relatively broad (Figure 7).
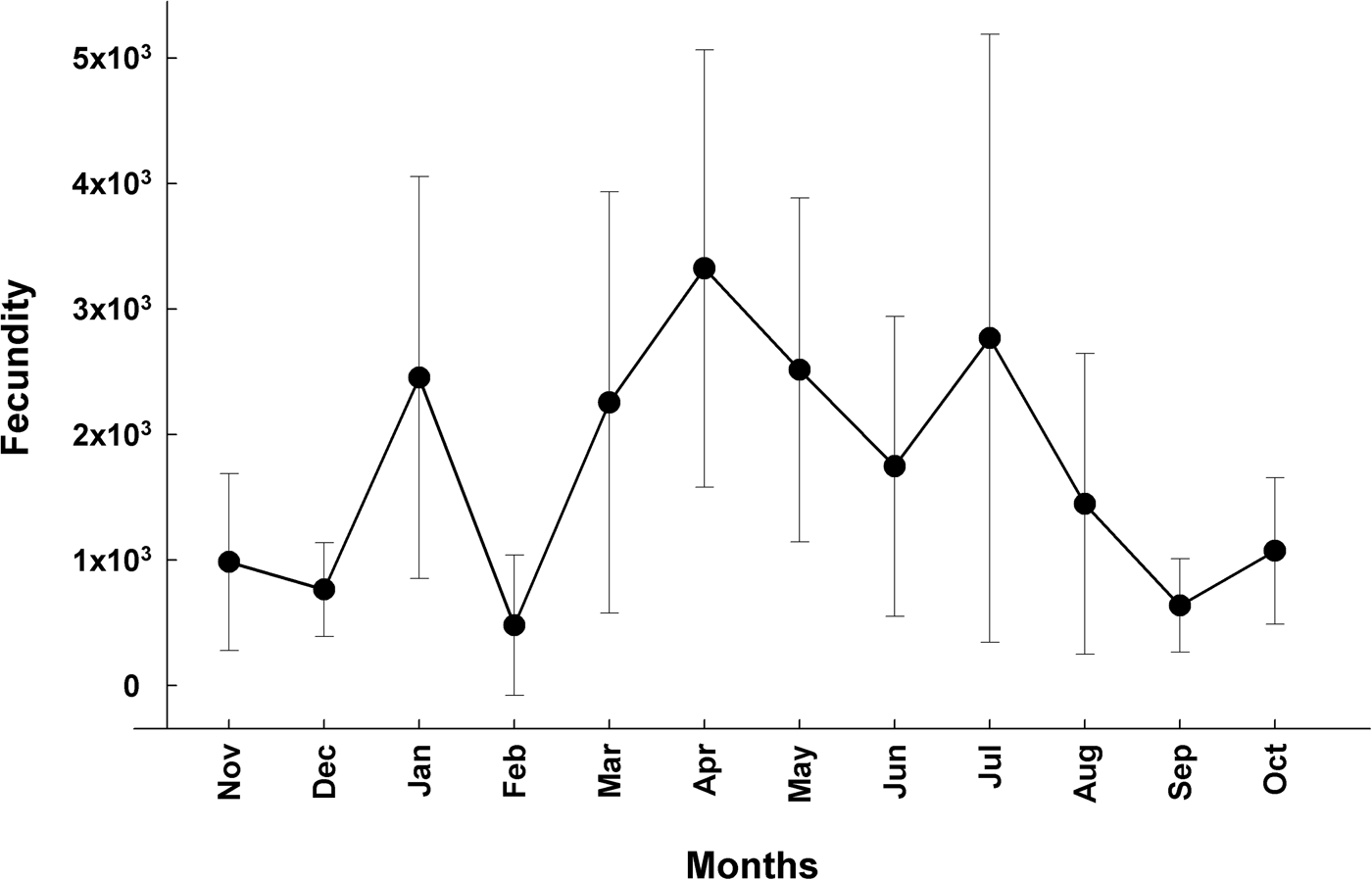
Fig. 7. Monthly variation (Mean±SD) of fecundity values of Arbacia stellata in Punta Banda BC, Mexico. On the x-axis, the first two months Nov. and Dec. correspond to the year 2013 and the following January–October to the year 2014.
Environmental variables
Minimum values of SSW occurred from November 2013 to February 2104, with the lowest value in December 2013 (15°C). From March 2014, the temperature began to increase to a maximum value in July 2014 (23°C). From August 2014, temperature began to descend again. Maximum values of light hours were reached from May to June 2014, and from August values started to decrease. The minimum values were recorded from November 2013 to January 2014, with the lowest value in December (Figure 8A). Mean monthly values of chlorophyll a started to increase from March 2013 and showed the maximum value in April 2014, then decayed progressively until reaching the minimum value in September (Figure 8B). Net primary production started to increase from May 2014 reaching its maximum value in June and started to decline in August to its minimum value recorded in February 2014 (Figure 8B).

Fig. 8. Annual variation of the mean values: (A) photoperiod and temperature; (B) chlorophyll a and net primary production in Punta Banda, Baja California, Mexico. On each x-axis, the first two months Nov. and Dec. correspond to the year 2013 and the following January–October to the year 2014.
The multiple linear regression (MLR) analysis of the GSI with the environmental parameters was solved in one step (GSI = 3.363 + (1.833 × chlorophyll)) and indicated that the total variation explained for the GSI in terms of the selected variable (chlorophyll) was 50%, with a multiple correlation value of 0.70 and a P level <0.05. In the case of MI, the MLR model was solved in two steps (MI = 1.574 + (0.154 × temperature) – (0.367 × chlorophyll)). The total variation explained by the selected variables was 53%, with a multiple correlation value of 73% and a P level <0.05. For fecundity the MLR analysis was also solved in two steps: Fecundity = −1,422,366.292 + (777,496.760 × chlorophyll) + (102,199.394 × temperature). The total variation explained with the selected variables was 55%, with a multiple correlation value of 0.74 and a P level <0.05.
Discussion
In the sampling area Arbacia stellata was gonochoric, with a semi-continuous reproductive cycle for both sexes and partial spawning throughout the year. The minimum GSI value recorded in December 2013 recovered significantly faster in subsequent months, when the highest proportion of urchins at immature (Stage I) and growth (Stage II) stages was observed.
GSI has been widely used to evaluate reproduction, however, it is not sufficient to describe the cycle in echinoids because the increase of gonads not only corresponds to size and number of gametes, but also to somatic cells, phagocytes or storage of nutrients (Walker et al., Reference Walker, McGinn, Harrington and Lesser1998, Reference Walker, Unuma, Lesser and Lawrence2007; Ebert et al., Reference Ebert, Hernandez and Russell2011). For A. stellata, high GSI values not only reflect gamete abundance, but also the increase of nutrients in immature urchins and germ cells in portions of the gonad at the growth stage (Stage II) of development. According to the MLR analysis, GSI has a positive relationship with chlorophyll, so the increase in its value at the beginning of the year and its maximum value in April (spring) could indicate a nutrient assimilation during spring. During this period, the maximum values of chlorophyll were recorded and correspond to the time when upwelling occurs near the sampling site at Punta Banda (Durazo, Reference Durazo2015; Oliva-Méndez et al., Reference Oliva-Méndez, Delgadillo-Hinojosa, Pérez-Brunius, Valencia-Gasti, Huerta-Diaz, Palacios-Coria and Martín Hernández-Ayón2018), therefore this high availability of nutrients was reflected in the thickening of the gonad rather than in the proliferation of gametes.
The broad monthly variations of the GSI may indicate intra-population variability in the development of the gonads. This feature was observed in both females and males, so we suggest a synchrony between sexes.
The MI had a positive relation with temperature and a negative relation with chlorophyll a. Its values started to increase at the beginning of the summer when higher temperatures prevail, chlorophyll a starts declining, and the peak of NPP is reached. On the other hand, maximum values are reached in autumn (when temperature starts descending) and are maintained until the beginning of winter. However, MI in females showed its maximum value in December 2013, with a large percentage of individuals in the spawning stage, this being the month with the highest reproductive activity for females, despite being the coldest month of the study period (15°C). This condition was also reported for the sand dollar Dendraster excentricus, in which the main gamete maturation apparently occurs during the winter (December–February) at temperatures close to the lowest recorded in the Punta Banda estuary (14–18°C) (Olivares-Bañuelos et al., Reference Olivares-Bañuelos, Figueroa-Flores and Carpizo-Ituarte2012).
Although the sequence of oogenesis in A. stellata was similar to that described for other echinoids (oogenesis started with primary germ cells that developed through several vitellogenic stages and produced eggs) (Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991), no synchrony was observed in the development of the gonad, where from two to four stages of development within the same gonad were observed. Likewise, pre-vitellogenic and vitellogenic oocytes were present in almost all sampling months, with vitellogenic ones being the most frequent, as reported by Wangensteen et al. (Reference Wangensteen, Turon, Casso and Palacín2013) for A. lixula in the Mediterranean. Other authors who studied reproduction of Arbacia also reported this characteristic (Kino, Reference Kino2010; Epherra et al., Reference Epherra, Gil, Rubilar, Perez-Gallo, Reartes and Tolosano2015).
Changes in the frequency of diameter distribution of oocytes and eggs indicate the presence of an annual cycle in the production of gametes. However, in A. stellata no oocyte growth sequence could be detected throughout the year and a predominance of mature gametes was observed. This type of reproduction has been observed in other species of echinoids, such as Holopneustes purpurascens (Williamson & Steinberg, Reference Williamson and Steinberg2002), Stylocidaris affinis (Holland, Reference Holland1967), Paracentrotus lividus (Byrne, Reference Byrne1990), Arbacia lixula (Wangensteen et al., Reference Wangensteen, Turon, Casso and Palacín2013) and Dendraster excentricus (Olivares-Bañuelos et al., Reference Olivares-Bañuelos, Figueroa-Flores and Carpizo-Ituarte2012).
Our results are in contrast with most of the echinoids that have well-defined annual reproductive cycles, with predictable discrete stages of development (Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991). On the other hand, although mature individuals were observed throughout the year, a stage of repose could be inconspicuous, indicating that production of gametes requires a brief period of resting or does not occur in some individuals. Pearse & Phillips (Reference Pearse and Phillips1968) observed something similar for Echinometra mathaei on the west coast of Australia, suggesting that this stage is likely to be short or perhaps does not normally occur.
Semi-continuous gamete production of A. stellata with high percentage of mature organisms from summer to winter, coinciding with low seawater temperatures and short days, is in contrast with what would be expected of populations in temperate regions of the northern hemisphere. Reproductive cycles tend to be more synchronized with increasing distance from the equator, and seasonality becomes more pronounced (Pearse, Reference Pearse1968, Reference Pearse1970). Seasonality of reproduction is also synchronized to maximize the survival of spawning, exposing the larvae to more favourable conditions for development (Giese & Pearse, Reference Giese, Pearse, Giese and Pearse1974; Benítez-Villalobos & Martínez-García, Reference Benítez-Villalobos and Martínez-García2012; Benítez-Villalobos et al., Reference Benítez-Villalobos, Hernando Avila-Poveda, Díaz-Martínez and Ruiz Bravo-Ruiz2015).
Most intense spawning of A. stellata during summer, autumn and early winter may have evolved to avoid the spring upwelling season, which brings the rising of carbon-enriched water (DIC) and low pH (~7.8) (Feely et al., Reference Feely, Christopher, Sabine, Hernández-Ayón, Ianson and Hales2008; Cervantes-Díaz et al., Reference Cervantes-Díaz, Hernández-Ayón, Durazo-Arvizu, Linacre-Rojas, Camacho-Ibar, Lara-Lara, Siqueiros-Valencia, Bazán-Guzmán, Paz, Wong, Bazan and Saynes2013). This could negatively affect calcification of pluteus larvae of sea urchins (Stumpp et al., Reference Stumpp, Wren, Melzner, Thorndyke and Dupont2011; Dorey et al., Reference Dorey, Lançon, Thorndyke and Dupont2013). Additionally, the correlation of GSI with chlorophyll a, and the opposite in the MI, may indicate the accumulation of nutrients in the gonad during the spring ensuring that gametogenesis and spawning coincide with non-upwelling periods, when low salinity and high pH (~8) prevail in the area (Cervantes-Díaz et al., Reference Cervantes-Díaz, Hernández-Ayón, Durazo-Arvizu, Linacre-Rojas, Camacho-Ibar, Lara-Lara, Siqueiros-Valencia, Bazán-Guzmán, Paz, Wong, Bazan and Saynes2013). These water conditions could greatly favour larval development, ensuring maintenance of A. stellata populations.
A semi-continuous reproduction pattern allows individuals to reproduce when they find a suitable signal, considering also, that they can mature different cohorts of gametes in the same gonad. Then, new recruits can enter the population during the most intense period and/or through small breeding events during the year (Williamson & Steinberg, Reference Williamson and Steinberg2002). This reproductive activity throughout the year, with other ecological adaptations of recruits and juveniles, could explain the increasing abundance of A. stellata in the study area.
Arbacia stellata is moving northward in the California region, increasing its abundance. Engle & Richards (Reference Engle and Richards2001) reported the occurrence of A. stellata in Channel Islands, California, and concluded warmer waters probably brought the larvae during El Niño 1997–1998. They proposed that the absence of juveniles and presence of larger individuals in Laguna Beach populations suggest that the animals are older and possibly come from previous recruitment events. We suggest this species reproduces continuously during most of the year in Baja California, with the highest intensity during the coldest period. This observation supports the hypothesis that larvae of A. stellata in southern California were able to recruit during warmer periods. Species with wide distributions could adapt aspects of their life history to the local conditions. Reproduction and optimal development of larvae are important aspects of these important life history features (Stearns, Reference Stearns1992, Reference Stearns2000). Byrne et al. (Reference Byrne, Andrew, Worthington and Brett1998) found that the pattern of spawning may vary among localities and years. Because A. stellata has a wide distribution in the Pacific, reproduction of particular populations could vary with conditions in each locality. For Arbacia stellata in its northern distribution, the possibility of an extended reproductive season could have strong implications for its dispersal, permanent presence and possible impacts in this region.
Author ORCID
Eugenio De Jesús Carpizo-Ituarte, 0000-0002-3253-2357
Financial support
This work was supported by CONACYT, project Genes de respuesta al estrés como bioindicadores para evaluar el efecto del cambio global en organismos estructuradores del bentos en el Pacífico Mexicano (EJC-I, Grant No. 181597) and IIO-UABC project Biología del desarrollo para el aprovechamiento sostenible y conservación de los recursos costeros (EJC-I, Grant No. 403/1030). JPD-M received a CONACYT scholarship for doctoral studies and the results presented here are part of her dissertation research.












