INTRODUCTION
Heightened scientific attention to the fragility and susceptibility of cold-water coral ecosystems has demonstrated our poor understanding of basic ecological functions in the deeper-living species. Almost 50% of all scleractinian species known to date are azooxanthellate and over 41% of these live at depths greater than 50 m (Cairns, Reference Cairns2007), yet data on shallow-water hermatypic coral ecology far outweighs that of ahermatypic scleractinians. Limited collections at deeper depths, single time point collections and difficulties with international transport of samples can all be attributed to this paucity of information (Cairns, Reference Cairns2007).
Understanding the reproductive biology of any organism is fundamental to understanding its population dynamics. At present however, there are just a handful of scientific papers examining the reproductive processes of cold-water azooxanthellate reef-building corals (Brooke & Young, Reference Brooke and Young2003, Reference Brooke and Young2005; Burgess & Babcock, Reference Burgess, Babcock, Freiwald and Roberts2005; Waller & Tyler, Reference Waller and Tyler2005) and solitary scleractinians (Waller et al., Reference Waller, Tyler and Gage2002, Reference Waller, Tyler and Gage2005, Reference Waller, Tyler and Smith2008; Flint et al., Reference Flint, Waller and Tyler2007). The majority of data on reproduction in scleractinians is based on observations and experiments from tropical zooxanthellate species (Fadlallah, Reference Fadlallah1983; Richmond & Hunter, Reference Richmond and Hunter1990; Richmond, Reference Richmond and Birkeland1997; Goffredo et al., Reference Goffredo, Airi, Radetic and Zaccanti2006). The limited studies on cold-water corals have shown different patterns of reproductive ecology, such as the high proportion of gonochorism and larger oocyte sizes (Waller, Reference Waller, Freiwald and Roberts2005) compared with the predominance of hermaphroditism and smaller oocyte sizes in tropical species (Fadlallah, Reference Fadlallah1983).
Flabellum alabastrum Moseley 1873 and Flabellum angulare Moseley 1876 are solitary deep-water scleractinians found only in the Atlantic (Cairns, Reference Cairns1999). Flabellum alabastrum inhabits a depth-range from 401 to 2250 m in the north-east Atlantic (Zibrowius, Reference Zibrowius1980), whereas F. angulare has a much narrower distribution, 1647 to 2857 m (Zibrowius, Reference Zibrowius1980). Both of these solitary scleractinians belong to the suborder Caryophylliina, Vaughan & Wells 1943, and the family Flabellidae, Bourne 1905. Within this family, the genus Flabellum, Lesson 1831, has 42 species, all solitary azooxanthellate forms that are widely spread across all oceans (Cairns, Reference Cairns1999). The Flabellidae is a family with one of the highest numbers of species (Cairns, Reference Cairns1999), yet knowledge of any species ecology or physiology is sparse. Within the genus Flabellum, there are only two published ecological studies, one on the feeding ecology of F. alabastrum from the Newfoundland and Labrador continental slope (Sherwood et al., Reference Sherwood, Jamieson, Edinger and Wareham2008) and one on the reproductive ecology of Antarctic Flabellum spp. (Waller et al., Reference Waller, Tyler and Smith2008). This paper presents data on the reproductive ecology of these two azooxanthellate species.
MATERIALS AND METHODS
Samples for this study (Table 1) were used from the Discovery Collections (housed at the National Oceanography Centre, Southampton, UK), the SAMS Collections (housed at the Dunnstaffnage Marine Laboratory, Oban, UK) and directly from research cruises D249, D260 and D266 onboard the RSS ‘Discovery’. All samples were collected by Marinovitch semi-balloon otter trawl from either the Porcupine Seabight or the Rockall Trough in the north-east Atlantic. Samples were preserved in 4% formalin onboard and transferred prior to processing into 70% alcohol.
Table 1. Samples used for this study. Lat, latitude; Long, longitude; NR, non-reproductive.

Samples from each species from each season (see Table 1 for numbers) were decalcified using concentrated HCl until no carbonate skeleton remained. All individuals were then wet weighed and the number of mesenteries per polyp noted. Gametes were large enough to be identified using a dissecting microscope and so individuals of both species were examined to determine sex and number of fertile mesenteries. All females had three mesenteries dissected and mesenteries from three males per season were histologically processed. All specimens unable to be identified as male or female had three mesenteries dissected and histologically processed to examine for early stage gametes. Putative sizes of first reproduction are based on the smallest reproducing individuals found in samples. Sex-ratios were calculated from within months. Total sex-ratio was averaged from all samples from a species following a Chi-test between months.
Mesentery tissue was sequentially dehydrated to 100% propan-2-ol, followed by clearing with xylene for approximately 12 hours. Tissue was then embedded in molten paraffin wax (at 70°C) for 12 hours and poured into standard moulds. All female tissue was serially sectioned to give oocyte size–frequency and fecundity estimates, leaving 100 µm between slides. Five non-overlapping slides of male tissue were taken to stage spermatogenesis. All sections were stained using Masson's trichrome.
Sections were examined using an Olympus BH2 compound microscope with video camera attachment. Images were captured using Matrox Rainbow Runner and analysed using SigmaScan Pro V4 to calculate oocyte diameters. Feret diameter (the area if the oocyte was a perfect circle) was used as this normalizes the often irregular outline of oocytes.
Fecundity data were plotted with wet weight of polyps and a linear regression fitted to determine size-dependent fecundity information. Data were size corrected and mean monthly fecundities were plotted for all females. A Mann–Whitney U-test was used to compare between months.
RESULTS
Flabellum alabastrum
Flabellum alabastrum is a gonochoristic species, with gametes clearly visible when the tissue is decalcified. The total sex-ratio is 1:1 with little deviation within months (χ 2 = 0.615, P = 0.01). Putative size of first reproduction was 0.247g polyp wet weight.
OOGENESIS
Oogenesis (Figure 1A) can be divided into four stages:
Stage I—oogonia (unobserved, but <100 µm), expected to bud from the mesenterial lamellae;
Stage II—previtellogenic oocytes (<300 µm), containing a large nucleus;
Stage III—vitellogenic oocytes (<800 µm), larger yolk granules can be observed;
Stage IV—late vitellogenic oocytes (>800 µm), rarely observed, thick cortical granular layer present and a prominent nucleolus in the central nucleus observed.
Previtellogenic and vitellogenic oocytes were found within most females observed, late vitellogenic and oogonia were observed rarely. Females weighing less than 0.5g polyp wet weight, contained only previtellogenic oocytes and so were not included in percentage size–frequency plots, as these are considered juveniles. The maximum oocyte size observed was 925 µm diameter, indicative of lecithotrophic larval development.
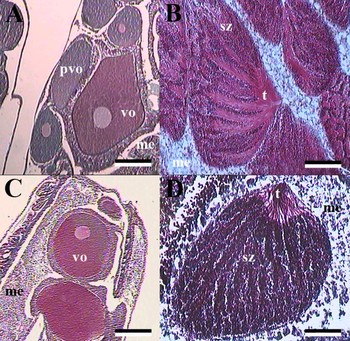
Fig. 1. (A) Flabellum alabastrum female mesentery showing previtellogenic and vitellogenic oocytes; (B) F. alabastrum male mesentery; (C) Flabellum angulare female mesentery; (D) F. angulare male spermatocyst. pvo, previtellogenic oocytes; vo, vitellogenic oocytes; me, mesogloeal envelope; sz, spermatozoa; t, spermatozoa tails. Scale bars: A, 500 µm; B, 250 µm; C, 600 µm; D, 350 µm; stained with Masson's trichrome.
SPERMATOGENESIS
In all months examined spermatocysts were at a late stage of development (Figure 1B), with spermatozoa tails clearly visible within the lumen. Spermatocysts appeared similar in morphology to that described for other deep-water scleractinians (Waller et al., Reference Waller, Tyler and Gage2002, Reference Waller, Tyler and Gage2005; Waller & Tyler, Reference Waller and Tyler2005).
PERIODICITY
Samples within a month sample were synchronous and so individual plots within a monthly sample were collated. Oocyte size–frequency diagrams (Figure 2), show little difference among monthly samples. Two cohorts appear to be developing and individuals are synchronous within populations. This suggests a quasi-continuous life history, with gametes being produced regularly. Male individuals were found only in the late stages of sperm development, this would also support a quasi-continuous pattern.

Fig. 2. Flabellum alabastrum sample mean oocyte size–frequency diagrams (error bars, ±SD; N = number of individuals; n = number of oocytes).
FECUNDITY
Fecundity is size-dependent and rises with polyp wet weight ((Figure 3A) R2 = 0.54; P = 0.01). There was no significant difference between the average fecundity for the months analysed (Figure 4A). Monthly average fecundity reaches a maximum of 2800 oocytes per polyp.

Fig. 3. (A) Flabellum alabastrum potential fecundity plotted against decalcified polyp wet weight with a fitted regression line (95% confidence limits, f = y0 + a*x, size corrected to 3.690g polyp wet weight); (B) Flabellum angulare potential fecundity plotted against polyp wet weight, with a fitted regression line (95% confidence limits, f = y0 + a*x, size corrected to 2.538g polyp wet weight).

Fig. 4. (A) Flabellum alabastrum average potential fecundity per month analysed (error bars, ±SD, size corrected to 3.690g polyp wet weight); (B) Flabellum angulare mean potential fecundity for each month analysed (error bars, ±1SD, size corrected to 2.538g polyp wet weight).
Flabellum angulare
Flabellum angulare is also a gonochoristic species with a 1:1 sex-ratio (χ 2 = 0.719, P = 0.01), deviating little within monthly samples. Gametes were also clearly visible post-decalcification within and throughout mesenteries. Size of first reproduction is putatively 1.379g polyp wet weight.
OOGENESIS
Oogenesis can be divided into four stages (Figure 1C), similar to F. alabastrum. Oogonia (<100 µm), as in F. alabastrum, were rarely observed. Previtellogenic (<350 µm) and vitellogenic (<900 µm) were present in all female individuals examined. Late vitellogenic oocytes were also rarely observed (>900 µm). The maximum oocyte diameter observed was 1015 µm, indicative of lecithotrophic larval development.
SPERMATOGENESIS
In contrast to F. alabastrum there were four stages of spermatogenesis observed in F. angulare samples (Figure 1D):
Stage I, Early—loosely packed aggregations of spermatocytes within spermatocyst. Empty lumen;
Stage II, Maturing—some spermatozoa present, still largely empty lumen;
Stage III, Late—lumen packed with spermatozoa;
Stage IV, Spent—relict spermatozoa can be seen.
Mainly maturing and late stages of spermatogenesis could be observed in single individuals, though all males in September were at stages I and II.
PERIODICITY
Individual oocyte size–frequency diagrams were collated monthly, as individuals were synchronous. Oocyte size–frequency diagrams show a similar bimodal tendency in March and September, but less of a second peak in October (Figure 5). These data, together with the fecundity data, suggest a release of oocytes before or during September. Although the data are limited we interpret this as either seasonality or periodic synchrony of gamete release.

Fig. 5. Flabellum angulare sample mean oocyte size–frequency diagrams (error bars, ±SD; N = number of individuals; n = number of oocytes).
FECUNDITY
Fecundity is size-dependent in F. angulare, increasing numbers of oocytes are produced as wet weight increases ((Figure 3B) R2 = 0.528, P = 0.01). Figure 4B shows a marked variation in fecundity, with significant numbers of oocytes in October and March (U = 39.0, P = 0.329) and a significant decrease in September (U = 30, P = 0.036). Monthly average fecundity reaches a maximum of 550 oocytes per polyp for March samples.
DISCUSSION
Both species in this study are gonochoric, with no hermaphroditic individuals being found. The random selection of males and females within size-classes militates against the likelihood of sequential hermaphroditism. No brooded planulae were observed in any specimen examined (Table 1), and thus we suggest both species broadcast-spawn gametes. This is a strategy found widely in cold-water scleractinians examined to date (Waller, Reference Waller, Freiwald and Roberts2005).
Two oocyte cohorts were present in all samples of F. alabastrum. There was no marked difference in fecundity among samples. In males, spermatogenesis is nearly always in the late stages of development. These factors all contribute to the pattern of quasi-continuous release of gametes into the water column. Vitellogenic oocytes in F. angulare, however, were present in larger numbers in March and September, than in October, and fecundity was significantly lower in September than in March or October, suggesting vitellogenic oocytes were spawned before or throughout late September. Previtellogenic oocytes are then produced to increase the fecundity found in October. One important factor that is unable to be assessed in this study is interannual variability, which may have caused an offset in these results.
Most zooxanthellate and shallow water azooxanthellate scleractinians have some form of reproductive periodicity, usually either lunar or temperature-dependent (Fadlallah, Reference Fadlallah1983; Richmond & Hunter, Reference Richmond and Hunter1990; Goffredo et al., Reference Goffredo, Arnone and Zaccanti2002, Reference Goffredo, Airi, Radetic and Zaccanti2006). Within this study, F. alabastrum, the shallower species, appears quasi-continuous, and F. angulare appears to reproduce periodically. Though these species are found below the permanent thermocline, there have been many instances of reproductive cues in the deep-sea (Tyler et al., Reference Tyler, Pain and Gage1982, Reference Tyler, Harvey, Giles and Gage1992, Reference Tyler, Gage, Paterson and Rice1993; Billet et al., Reference Billett, Lampitt, Rice and Mantoura1983) and have even been related to deep-water scleractinians (Waller & Tyler, Reference Waller and Tyler2005). Eckelbarger & Watling (Reference Eckelbarger and Watling1995) proposed that increased food in the environment could affect reproduction in three ways: (1) it can initiate gametogenesis followed soon after by spawning; (2) it can initiate spawning for planktotrophic larvae; or (3) it can initiate and synchronize gametogenesis, with a spawning event occurring after a time period. Seasonal phytoplankton blooms reaching the benthos in the Porcupine Seabight occur around July (Lampitt et al., Reference Lampitt, Bett, Kiriakoulis, Popova, Ragueneau, Vangriesheim and Wolff2001), and so in F. angulare, which spawns in late August/September, this food fall could be the seasonal cue to induce vitellogenesis (Fadlallah, Reference Fadlallah1983; Richmond & Hunter, Reference Richmond and Hunter1990). Indeed in Lophelia pertusa, a reef building cold-water scleractinian, this same bloom is thought to initiate gametogenesis (Waller & Tyler, Reference Waller and Tyler2005). Sherwood et al. (Reference Sherwood, Jamieson, Edinger and Wareham2008) have shown F. alabastrum to have a mixed to carnivorous diet, and so is unlikely to be limited to feeding primarily within phytodetrital food falls. With this feeding strategy, F. alabastrum is likely to have food available year around, and this could explain its non-seasonal reproductive habit. There is unfortunately no data on the feeding ecology of F. angulare, but with its seasonal reproductive habit, there is the potential that this species has a more selective diet (perhaps to fresher phytodetritus) that is only supplied to the deep sea seasonally.
Corals, including scleractinians, are also renowned for their reproductive plasticity. Within a genus there are often differing reproductive patterns (Hartnoll, Reference Hartnoll, Keegan, Ceidigh and Boaden1977; Fadlallah, Reference Fadlallah1983; Szmant-Froelich, Reference Szmant-Froelich1984; Szmant, Reference Szmant1986; Richmond & Hunter, Reference Richmond and Hunter1990), and even the same species having differing patterns (poecilogeny) in different locations (Richmond & Jokiel, Reference Richmond and Jokiel1984; Kruger et al., Reference Kruger, Schleyer and Benyahu1998). So it is not unusual within the Cnidaria for F. alabastrum to be quasi-continuous and F. angulare to be seasonal, and this is likely to be a consequence of environmental conditions being different at these different depths (Eckelbarger & Watling, Reference Eckelbarger and Watling1995). Indeed the only other published reproductive study within the genus Flabellum (or the family Flabellidae) showed Antarctic species of Flabellum to be quasi-continuous brooders, a pattern frequently observed in invertebrates from Antarctic waters (Waller et al., Reference Waller, Tyler and Smith2008).
During trawls from RRS ‘Discovery’ between 2000 and 2002 it was noted that the two species were mutually exclusive in each trawl (personal observation). Although Zibrowius (Reference Zibrowius1980) has shown their depth-ranges to be very similar, the different depth-range in a specific area may have led to allopatric speciation, emphasized by the slight differences in reproductive pattern. Such speciation has been observed in shallow sympatric populations of the echinoid Echinometra (Palumbi & Metz, Reference Palumbi and Metz1991). More detailed surveys of the distribution of the two species of Flabellum in the north-east Atlantic would provide interesting insights into their population ecology.
ACKNOWLEDGEMENTS
This research was supported by the European Union ‘Atlantic Coral Ecosystem Study’ contract No. EVK3-CT1999-00008. Dave Billett, I.G. (Monty) Priede and the captains and crew of the RSS ‘Discovery’ were all instrumental in obtaining specimens for this study. We also thank John Gage†, Peter Lamont and Murray Roberts for samples obtained from DML, Oban. R. Waller is currently supported by a SOEST Young Investigator fellowship from the University of Hawaii at Manoa, and this paper is SOEST contribution number 7893.








