INTRODUCTION
Polychaete worms of the family Sabellariidae are known for their ability to build cemented tubes of sand, which are typically attached to hard substrates. Some species have a gregarious lifestyle, forming extensive reefs in the shallow midlittoral and subtidal zones of beaches and estuaries, although colonies have been reported from depths of 100 m. These reefs are inhabited by a wide diversity of animals and plants (Gore et al., Reference Gore, Scotto and Becker1978; Porras et al., Reference Porras, Bataller, Murgui and Torregrosa1996) and may also function as sediment filters, altering local hydrodynamics and granulometry, and helping to reduce coastal erosion (Gram, Reference Gram1968). Sabellariids are considered important ecosystem engineers because of their ability to modify the environment and structure biological communities (Ataide et al., Reference Ataide, Venekey, Filho and dos Santos2014).
Several aspects of the reproductive biology of sabellariids are relatively well known, especially for species found in temperate waters. Laboratory studies have provided descriptions of fertilization and gamete development (Fauré-Frémiet, Reference Fauré-Frémiet1924; Waterman, Reference Waterman1934; Wilson, Reference Wilson1968b; Eckelbarger, Reference Eckelbarger1978b, Reference Eckelbarger1979; Peaucellier, Reference Peaucellier1984), as well as of the larval phase, metamorphosis and settlement (Wilson, Reference Wilson1968a, Reference Wilson1970a, Reference Wilsonb; Mauro, Reference Mauro1975; Eckelbarger, Reference Eckelbarger1977, Reference Eckelbarger, Rice and Chia1978a; Smith & Chia, Reference Smith and Chia1985; Pawlik, Reference Pawlik1988). Few population-level studies have been conducted (Wilson, Reference Wilson1971; Eckelbarger, Reference Eckelbarger1976; Curtis, Reference Curtis1978; Gruet & Lassus, Reference Gruet and Lassus1983; Culloty et al., Reference Culloty, Favier, Ni Riada, Ramsay and O'Riordan2010; Faroni-Perez, Reference Faroni-Perez2014).
The reef-building forms are relatively long-lived (average lifespans of 2–5 years), with high fecundity, larvae with a well-developed dispersal capacity, and gregarious settlement in response to chemical cues from the tubes of conspecifics (Gruet & Lassus, Reference Gruet and Lassus1983; Gruet, Reference Gruet1986; Pawlik, Reference Pawlik1988; Giangrande, Reference Giangrande1997). In temperate regions, reproductive patterns are associated with the seasonal variations in sunlight, temperature, and the availability of feeding resources (McCarthy et al., Reference McCarthy, Young and Emson2003; Dubois et al., Reference Dubois, Comtet, Retière and Thiébaut2007; Culloty et al., Reference Culloty, Favier, Ni Riada, Ramsay and O'Riordan2010), as well as extreme hydrodynamic disturbances characterized by high wave energy (McCarthy et al., Reference McCarthy, Young and Emson2003) and storms (Barry, Reference Barry1989). In tropical regions, however, few data are available on the biology of these organisms.
Sabellaria wilsoni Lana & Gruet, Reference Lana and Gruet1989 is endemic to the Atlantic coast of South America, from French Guiana to Argentina (Lana & Gruet, Reference Lana and Gruet1989; Lana & Bremec, Reference Lana, Bremec, Dauvin, Laubier and Reish1994). The species is found in the intertidal zone to depths of up to 25 m, inhabiting mixohaline and polyhaline environments in estuaries, beaches and shallow depths of the continental shelf (Lana & Bremec, Reference Lana, Bremec, Dauvin, Laubier and Reish1994; Lomônaco et al., Reference Lomônaco, Santos and Christoffersen2012; Ataide et al., Reference Ataide, Venekey, Filho and dos Santos2014). Sabellaria wilsoni typically forms small aggregations on rocky substrates (Lana & Gruet, Reference Lana and Gruet1989). On the Amazon coast, this species constructs extensive reefs on sandy beaches, which significantly modify local benthic assemblages (Ataide et al., Reference Ataide, Venekey, Filho and dos Santos2014). The reefs are subject to constant structural changes resulting from natural processes of erosion and reconstruction, a phenomenon also reported in other sabellariid species (Wilson, Reference Wilson1971; Gruet, Reference Gruet1986; Porras et al., Reference Porras, Bataller, Murgui and Torregrosa1996).
The present study focused on the reproductive biology of S. wilsoni, analysing the sex ratio and proposing a scale of gonadal development in males and females, and for the breeding season. Reproductive events were associated with the seasonal variations in climate (precipitation, wind conditions and hydrodynamics) and the structural conditions of the reefs built by these worms.
MATERIALS AND METHODS
Study area
Algodoal-Maiandeua Island is located on the Brazilian Amazon coast (00°36′S 047°34′W), and is surrounded on three sides by rivers and estuarine channels, with its northern portion facing the Atlantic Ocean (Figure 1). The climate is humid tropical with a mean annual temperature of 27.7 ± 1.1°C (Martorano et al., Reference Martorano, Pereira, Cézar and Pereira1993) and a mean annual precipitation (30-year record) from 2300 to 2800 mm (Moraes et al., Reference Moraes, Costa, Costa and Costa2005). Precipitation varies considerably over the course of the year, with a well-marked rainy season from January to July, with total rainfall of around 1657 mm; and a dry season from August to December, with total rainfall of 490 mm (Moraes et al., Reference Moraes, Costa, Costa and Costa2005). The region is dominated by semidiurnal macrotides with amplitude of 4–7 m (Silva et al., Reference Silva, Pereira, Gorayeb, Vila-Concejo, Sousa, Asp and Costa2011). The island's beaches are characterized by their fine sand and wide midlittoral zone of 200–400 m (Rosa Filho et al., Reference Rosa Filho, Gomes, Almeida and Silva2011), and the presence of rocky outcrops (lateritized sandstone) which are often colonized by S. wilsoni.

Fig. 1. (A) Location of the Sabellaria wilsoni reef on Algodoal-Maiandeua Island in Pará, northern Brazil. Reef conditions: (B) preserved; (C) eroded; (D) under reconstruction.
Sampling
Specimens were collected each month between May 2008 and April 2009 on a continuous platform-type reef (according to the classification of Gruet, Reference Gruet1971) with a total area of 900 m2, located on an estuarine beach on the island. Each month, fragments (two to three) of ~2 l were extracted from the reef and fixed in a 10% formalin-saline solution for 24 h and then transferred to 70% ethanol. The samples were extracted from the central portion of the reef, based on the assumption that it contained animals of a similar age, especially given the constant settlement of juveniles on the eroded margins of the reef (Gruet & Lassus, Reference Gruet and Lassus1983; Culloty et al., Reference Culloty, Favier, Ni Riada, Ramsay and O'Riordan2010).
In the laboratory, the animals were extracted from their tubes and the length of the thorax + abdomen (the caudal appendix was excluded because it is easily lost) was measured for about 100 individuals, using a stereoscopic microscope. Although for other sabellariids, the opercular crown is considered a good proxy of individuals’ size and age, we did not find a close relationship (R 2 = 0.27, F = 55.8, P < 0.05, N = 100) between the diameter of the opercular crown and the overall length (thorax + abdomen) for fixed specimens of Sabellaria wilsoni. This was the main reason for using body length, despite the possible effects of fixation on the contraction of organisms. The specimens were allocated to one of seven body (thorax + abdomen) length classes (0–3, 3–6, 6–9, 9–12, 12–15, 15–18 and 18–21 mm). Whenever possible, at least three specimens from each size class were selected for histological analysis. The specimens were dehydrated in ethanol, diaphanized in xylene, infiltrated and embedded in paraffin, and then sectioned longitudinally with a thickness of 5 µm for mounting on slides (three for each worm), stained with haematoxylin-eosin.
These slides were examined under a photomicroscope to determine the sex of each specimen and its stage of gonadal development, based on the classification of Culloty et al. (Reference Culloty, Favier, Ni Riada, Ramsay and O'Riordan2010) proposed for Sabellaria alveolata (Linnaeus, 1767). Three different segments of the abdomen of each specimen (anterior, middle and posterior) were observed. The individual was considered to be in reproductive condition when the majority of the segments were mature or spawning. For females, the numbers of oocytes in pre-vitellogenesis, early vitellogenesis and late vitellogenesis were quantified in three segments (anterior, middle and posterior). Thirty females were selected randomly from the total sample, to measure the diameter of the oocytes at different stages of development. These measurements were obtained using Image Tools 2.0, based on images obtained from the optical microscope. A stage micrometer slide was used to calibrate the equipment at magnifications of 40 and 100×.
Samples of surface water were collected each month during ebb tide to determine salinity levels, using a refractometer. Data on air temperatures, precipitation levels and wind speeds and direction were obtained from the meteorological station in Salinópolis (~30 km from the study site), provided by the Brazilian National Meteorological Institute (INMET). Information on significant wave heights (Hs) and the bottom shear stress of the waves was produced by numerical modelling in the Physical Oceanography Laboratory (LOF) at the Federal University of Pará (UFPA) in Belém, Brazil. These data were used to characterize the variations in local hydrodynamics. This modelling focused on the north-western portion of Algodoal-Maiandeua Island, where the reefs are located, and was based on bathymetric readings and in situ measurements of bottom roughness, in addition to the harmonic constants (provided by Sea Research Foundation, FERMAR – Brazil), and the wind and rainfall data from Salinópolis (provided by INMET). The simulation covered the 2-year period of 2008 and 2009, and the results were validated and filtered for the specific study period.
As the polychaete reefs on Algodoal-Maiandeua Island undergo structural modifications related to natural erosive and reconstructive processes, typical of those found for other species (Gruet, Reference Gruet1986; Porras et al., Reference Porras, Bataller, Murgui and Torregrosa1996), the condition of the reefs was recorded each month, and classified in one of three stages, based on the percentage cover of consolidated tubes within the overall spatial extent of reef, the height of aggregates and the visual appearance of erosion of patchiness (Silva, Reference Silva2015). Thus, the reefs were considered: (i) preserved, with a compact, continuous construction (percentage cover above 80%) (Figure 1B); (ii) eroded, with open spaces within the reef (percentage cover from 30 to 60%) and detached blocks (Figure 1C); or (iii) under reconstruction, for low, relatively small reefs (Figure 1D).
Statistical analysis
The sex ratio was analysed using Chi-square with Yates’ correction (when at least one class of the table has an expected count smaller than 5) for the study period as a whole, for each month, and each body-length class. The length of male and female specimens collected each month was compared using the t-test. An Analysis of Variance (ANOVA) was used to compare body length in males and females (entire study period) and the changes in the diameter of the oocytes among the different vitellogenic stages. Tukey's a posteriori test was used to compare pairs of means. A 5% significance level was used in all analyses.
RESULTS
Environmental characteristics
Mean monthly precipitation during the rainy season (January–July) was 403 mm, vs 30 mm in the dry season, when temperatures and salinity were highest. From January to June, the winds were predominantly north-easterly, and relatively weak (mean 2.1 m s−1) compared with the strong easterlies (mean 3.9 m s−1) that predominated from July to December (Table 1). The height and bottom shear stress of the waves were greater during the dry season, reaching maxima in October and November 2008 (Table 1). The reef was well-preserved between May and August 2008 (Figure 1B), but began to erode in September, and had almost completely disappeared by December (Figure 1C). Recovery began in January 2009, when most of the bottom of the reef was already covered by new constructions (Figure 1D) (Table 1).
Table 1. Environmental characteristics and condition of the Sabellaria wilsoni reefs monitored between May 2008 and April 2009, on Algodoal-Maiandeua Island, Brazil.

Temp. = mean monthly temperature; Rainfall = total monthly precipitation; Velocity = mean monthly wind velocity; Hs = mean monthly significant wave height; Shear stress = mean monthly wave-induced bottom shear stress.
Sex ratio and length
The species is dioecious, and no hermaphrodites were observed. The majority (62%) of the 255 specimens that could be sexed were female, with only 38% males. The smallest individual for which the sex could be determined was 1.6 mm in length. The population was female-dominated (1.6 females: 1 male) during most months, with significant deviations during the study period as a whole (χ 2 = 15.6, d.f. = 1, P < 0.001), as well as in May, August, October and November 2008, and February 2009 (Figure 2A).
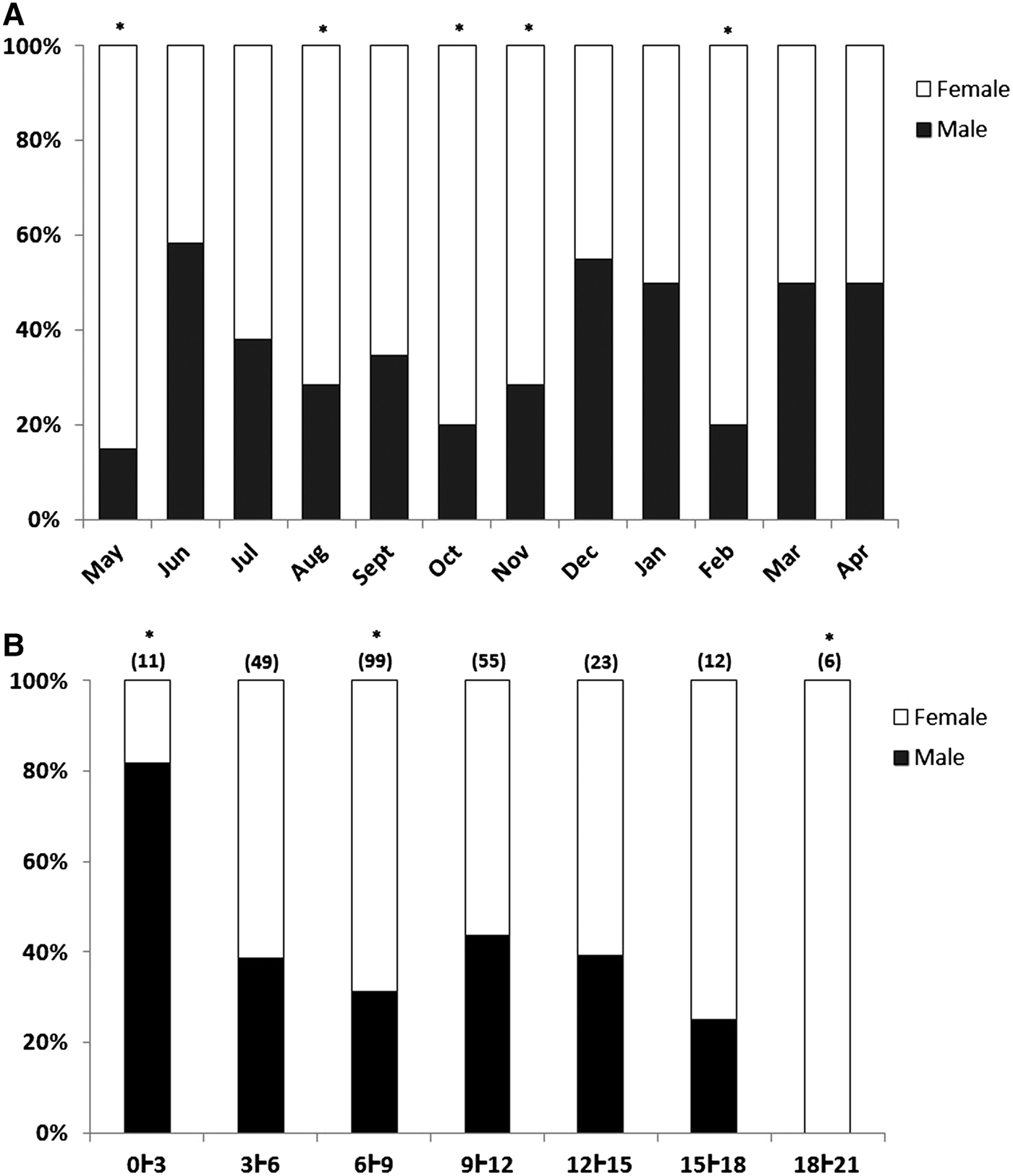
Fig. 2. (A) Proportion of male and female specimens of Sabellaria wilsoni collected between May 2008 and April 2009 on Algodoal-Maiandeua Island, Pará, Brazil. (B) Sex ratio by body-length class. The asterisks indicate a significant deviation from a ratio of 1:1 and the numbers on the bars represent the number of individuals for each class.
Males predominated significantly in the smallest size class (0–3 mm), whereas females predominated in all other size classes, with significant differences recorded in the 6–9 and 9–12 mm classes (Figure 2B). Females (![]() ${\bar x}$ = 8.8 ± 0.3 mm, ±SE – thorax + abdomen) were larger than males (
${\bar x}$ = 8.8 ± 0.3 mm, ±SE – thorax + abdomen) were larger than males (![]() ${\bar x}$ = 7.8 ± 0.3 mm, ±SE – thorax + abdomen) in almost all months, with significant differences recorded for the period as a whole (F = 4.37, d.f. = 1, P = 0.04) and in July, December, February, March (Figure 3).
${\bar x}$ = 7.8 ± 0.3 mm, ±SE – thorax + abdomen) in almost all months, with significant differences recorded for the period as a whole (F = 4.37, d.f. = 1, P = 0.04) and in July, December, February, March (Figure 3).

Fig. 3. Mean body length (thorax + abdomen) of the male and female specimens of Sabellaria wilsoni collected between May 2008 and April 2009 on Algodoal-Maiandeua Island, Pará, Brazil. The vertical lines represent the SE.
The distribution of individuals in size classes (Figure 4), evaluating sexed and unsexed (individuals not subjected to histological analysis) specimens, indicates that S. wilsoni aggregates were formed by individuals of different age groups (youth and adults). From September to November (erosion phase), the recruitment of juveniles apparently slowed, and then increased markedly in December and January (Figure 4).

Fig. 4. Size-class frequency based on body length (thorax + abdomen) of specimens of Sabellaria wilsoni collected between May 2008 and April 2009 on Algodoal-Maiandeua Island, Pará, Brazil. N = 100 (in each month).
Gonadal development
The reproductive system is restricted to the abdominal region of the body in both sexes. The same stage of gametogenesis was present throughout an individual worm. The gonadal development of the specimens was classified in four stages in both males and females: (I) initial development, (II) maturing, (III) mature and (IV) spawning. The stages are shown in Figure 5 and described in Table 2.

Fig. 5. Stages of gonadal development in male and female specimens of Sabellaria wilsoni collected from Algodoal-Maiandeua Island, Pará, Brazil. (A and E) Initial development; (B and F) maturing; (C and G) mature; (D and H) spawning. ∗, Blood vessel; S, inter-segmental septum; T, gut wall; P, parapodium; PV, pre-vitellogenic oocyte; V, oocyte in early vitellogenesis; M, mature oocyte, late vitellogenic stage; sg, spermatogonia; sc, spermatocyte; sz, sperm; c, somatic cell, (thin arrow) oogonia; (thick arrow) inter-segmental septum.
Table 2. Description of the stages of gonadal development in female and male Sabellaria wilsoni, observed through histological examination.

Reproductive events in the females
The ovaries are associated with the blood vessels of the inter-segmental septa, which extend as far as the coelom (Figure 5A–D). Oocytes in different vitellogenic stages are present in the ovaries, although only oocytes in the final stage were released into the coelom. The oocytes increase progressively and significantly (F = 397.9, d.f. = 2, P < 0.01) in size over the course of vitellogenic development, with a mean diameter of 16.1 ± 2.9 µm in the previtellogenic stage, 34.4 ± 9.2 µm in the early, and 61.1 ± 1.1 µm in the late vitellogenic stages.
Mature females showed body lengths of 2.3 to 21.7 mm, and occurred in all the months of the study period, with peaks in October–December 2008 and February–April 2009, when most females were mature or reproductive (Figure 6A). The quantitative analysis of the oocytes in different vitellogenic phases confirmed the occurrence of these two reproductive peaks (Figure 6B).

Fig. 6. Reproductive events observed in female specimens of Sabellaria wilsoni collected between May 2008 and April 2009 on Algodoal-Maiandeua Island, Pará, Brazil. (A) gonadal stages: (I) initial development; (II) maturing; (III) mature; (IV) spawning. (B) number of oocytes in: PV, previtellogenesis; VM, early vitellogenesis; VIT, late vitellogenesis; Total, mean total number of oocytes per female.
Reproductive events in the males
The testes are small structures arranged bilaterally, linked to the walls of the blood vessels that project from the inter-segmental septa within each abdominal segment (Figure 5E–H). The spermatogonia are stored in the testes and released into the coelomic cavity, together with coelomic support cells (Figure 5E), where spermatogenesis probably begins. The stages that precede the sperm were often observed aligned sequentially along the blood vessel, in a cordon arrangement (Figure 5F).
The smallest male had a body length of 1.6 mm, and the largest, 15.1 mm. As for the females, mature males occurred throughout the year, with peaks in October and November 2008 and March 2009, when more than 80% of the specimens were mature or reproducing (Figure 7).
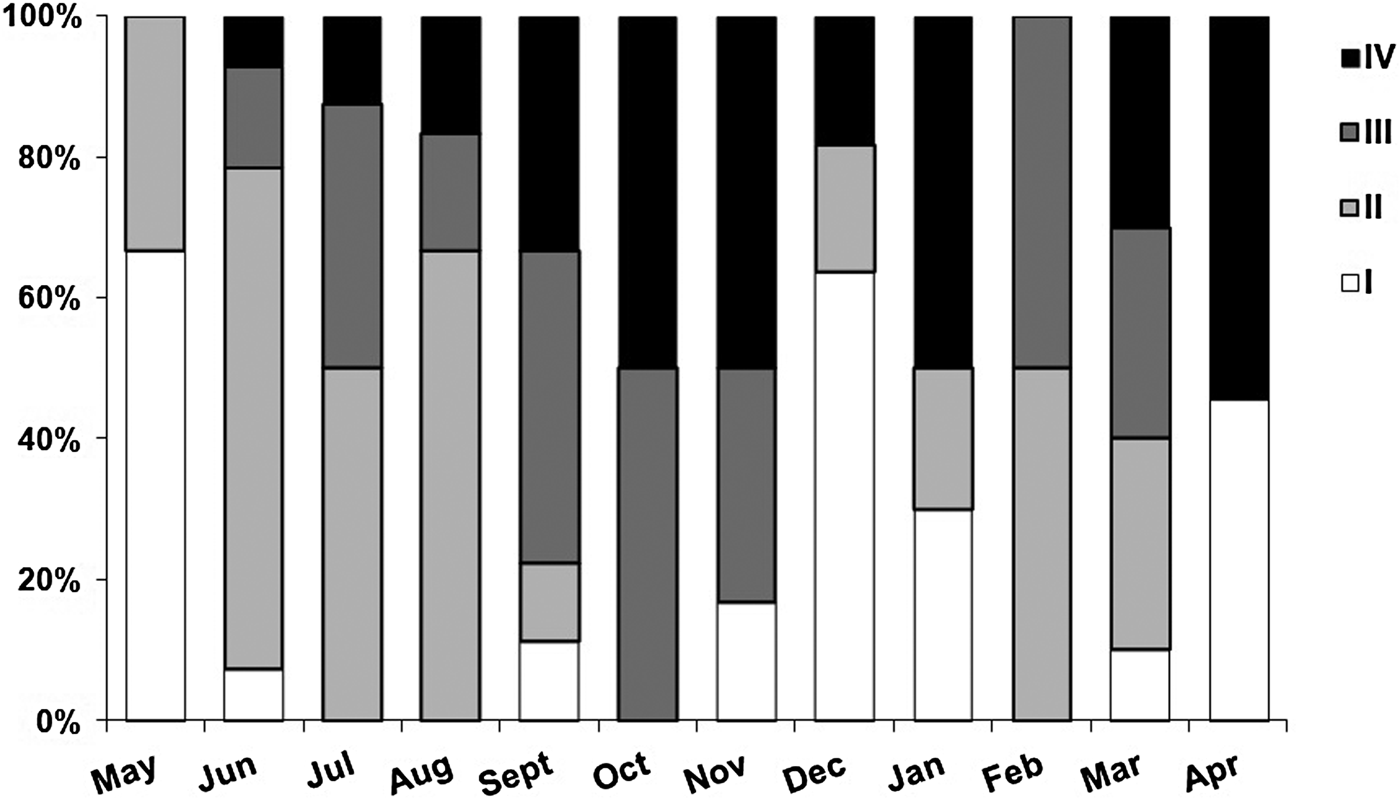
Fig. 7. The stages of gonadal development observed in male specimens of Sabellaria wilsoni collected between May 2008 and April 2009 on Algodoal-Maiandeua Island, Pará, Brazil. (I) initial development; (II) maturing; (III) mature; (IV) spawning.
DISCUSSION
The reefs of Sabellaria wilsoni found on Algodoal-Maiandeua Island underwent extreme changes in their structure during the course of the study. Hydrodynamics appears to be the principal factor determining the construction-reconstruction cycle of the local reefs. The reefs are destroyed during the dry season, when the easterly trade winds are more intense, generating higher and more frequent waves that resuspend more bottom sediments (Pereira et al., Reference Pereira, Mendes, Monteiro and Asp2009). The beginning of the reef erosion process also coincides with the equinoctial spring tides (September), which have a height of up to 5.3 m (DHN-Brasil, 2008), contributing to unusually strong tidal currents during this period (Pereira et al., Reference Pereira, Sozinho da Silva, da Costa, Asp, da Costa and Vila-Concejo2012). During the rainy season, when the winds are weaker and the waves less dynamic, the reefs were growing or being reconstructed (Silva, Reference Silva2015).
The destruction of sabellariid reefs may be related to the maturity of the construction, which breaks and erodes when the reef reaches its maximum possible height and breadth. A complete cycle of colonization (growth to destruction) may take 10 years in Sabellaria alveolata (Gruet, Reference Gruet1986), but in regions with strong hydrodynamics, as on Algodoal-Maiandeua Island, the reefs may be relatively short-lived, disappearing and being rebuilt in a matter of months (Porras et al., Reference Porras, Bataller, Murgui and Torregrosa1996; Dias & Paula, Reference Dias and Paula2001; Desroy et al., Reference Desroy, Dubois, Fournier, Ricquiers, Le Mao, Guerin, Gerla, Rougerie and Legendre2011). Extreme hydrodynamic conditions may negatively affect the reefs by increasing erosion rates, causing siltation (Gruet, Reference Gruet1971; Wilson, Reference Wilson1971), and possibly reducing the worms’ efficiency in filtering sediments (Vovelle, Reference Vovelle1965; Wilson, Reference Wilson1971; Pawlik & Butman, Reference Pawlik and Butman1993) and their recruitment (Wilson, Reference Wilson1974).
Reproduction in S. wilsoni was exclusively sexual. All individuals were dioecious and showed no clear sexual dimorphism, which is typical of sabellariids (Giangrande, Reference Giangrande1997; Capa et al., Reference Capa, Hutchings and Peart2012). The reproductive biology of S. wilsoni is similar to that of other sabellariid species (Fauré-Frémiet, Reference Fauré-Frémiet1924; Waterman, Reference Waterman1934; Wilson, Reference Wilson1968b; Eckelbarger, Reference Eckelbarger1978b, Reference Eckelbarger1984; Culloty et al., Reference Culloty, Favier, Ni Riada, Ramsay and O'Riordan2010; Faroni-Perez & Zara, Reference Faroni-Perez and Zara2014), in both morphological features (e.g. the shape and location of the sex organs and gametes) and physiological characteristics, as might be expected for one of the most specialized polychaete groups (Capa et al., Reference Capa, Hutchings and Peart2012). However, some of the features observed during the present study had not previously been recorded in the Sabellariidae.
Females of S. wilsoni were significantly larger than males, a pattern not found previously in sabellariids, but not unusual in either errant (Van Dover et al., Reference Van Dover, Trask, Gross and Knowlton1999; Britayev & Fauchald, Reference Britayev and Fauchald2005) or sedentary polychaetes (Zajac, Reference Zajac1991; Cotter et al., Reference Cotter, O'Riordan and Myers2003). Females are larger than males in a wide range of egg-laying marine species, especially since a larger body may confer greater reproductive success due to the potential for the production of larger numbers of eggs (Berglund, Reference Berglund1986; Premoli & Sella, Reference Premoli and Sella1995). In addition to being larger, female S. wilsoni were also more abundant than males during most months of the study period. Based only on external colouration, similar numbers of males and females have been recorded in populations of Phragmatopoma lapidosa Kinberg, 1866 (Eckelbarger, Reference Eckelbarger1976). Gruet & Lassus (Reference Gruet and Lassus1983) also recorded a 1:1 sex ratio for S. alveolata in France, based on the presence of gametes in the coelomic liquid. In contrast, Culloty et al. (Reference Culloty, Favier, Ni Riada, Ramsay and O'Riordan2010) recorded a male-biased sex ratio (1.4:1) in Irish S. alveolata, based on histological data.
In theory, a 1:1 sex ratio would be expected where male and female investment in the offspring is equal, and mortality rates are similar in the two sexes over the different stages of the life cycle (Charnov & Bull, Reference Charnov and Bull1989). A biased sex ratio may be the result of environmental or genetic factors, or some combination of the two (Shuker et al., Reference Shuker, Moynihan and Ross2009). In sedentary tubicolous polychaetes, which release their gametes into the water for external fertilization and without sequential hermaphroditism, a female-biased sex ratio may be related to age-specific differences in survivorship determined by variable recruitment or life-history patterns (Werren & Charnov, Reference Werren and Charnov1978; Werren & Taylor, Reference Werren and Taylor1984), polygenic systems (Premoli et al., Reference Premoli, Sella and Berra1996) or differential production of females due to the lower cost of producing daughters and/or the possibility of enhancing reproductive success (Premoli & Sella, Reference Premoli and Sella1995).
The larger size and greater abundance of the female S. wilsoni recorded in the present study could indicate the allocation of a considerable amount of energy to the reproductive process (Zajac, Reference Zajac1991). However, the high proportion of males in smaller size classes (0–3 mm), with a subsequent reduction in the larger classes (Figure 2), may originate from differential longevity of males and females. Little is known about the physiological longevity of polychaetes, although female-biased sex ratios and size dimorphism, males being smaller than females, have been interpreted as the result of a longer lifespan for females (Britayev & Zamishliak, Reference Britayev and Zamishliak1996; Jollivet et al., Reference Jollivet, Empis, Baker, Hourdez, Comtet, Jouin-Toulmond, Desbruyeres and Tyler2000).
The reproductive structures of S. wilsoni are similar to other sabellariids. The ovaries and testes are simple structures associated with the blood vessels of the inter-segmental septa found in the abdominal segments. Oogenesis is intra-ovarian, with the oocytes linked to the ovaries until the final stages of the vitellogenic process. All the different developmental stages were found in the ovary, while the spermatogonia were released into the coelom to mature together with the support cells.
Four stages of gonadal maturity were identified for S. wilsoni (initial development, maturation, mature and spawning). Unlike S. alveolata, an inactive stage (worms with no signs of sexuality) and resting or recuperating (spent worms, recently spawned) were not identified in S. wilsoni. The absence of inactive individuals may have been the result of sampling. The smallest individual collected had a body length of 1.6 mm, while observations in the field and unpublished data confirmed the existence of recently settled worms smaller than 1 mm.
Sampling fragments from the centre of the reef, as done here, cannot encompass all the spatial heterogeneity and physiological states of reef-builders. The sabellariid reef areas most exposed to the waves and constantly eroded are referred to as high mortality, with shorter stays of worms and high densities of juveniles (Gruet & Lassus, Reference Gruet and Lassus1983; Culloty et al., Reference Culloty, Favier, Ni Riada, Ramsay and O'Riordan2010; Lomônaco et al., Reference Lomônaco, Santos and Christoffersen2012). Survival in areas with higher environmental energy and instability may demand significant additional energy for tube construction and maintenance of the reefs, reducing the energy available for conversion to body mass (Lomônaco et al., Reference Lomônaco, Santos and Christoffersen2012) and reproductive effort (Giangrande, Reference Giangrande1997). Polychaete species can form populations or subpopulations with different patterns of maturation and spawning, according to physical environmental fluctuations (Bhaud, Reference Bhaud1972; Giangrande, Reference Giangrande1990, Reference Giangrande1997).
The evidence indicates that individuals of S. wilsoni reach sexual maturity very rapidly. Eckelbarger (Reference Eckelbarger1976) noted that the first signs of sexual traits are observed in P. lapidosa between 6 and 8 weeks after settling. In Phragmatopoma californica (Fewkes, 1889), sexual maturation occurs 1 to 3 months after settling (Barry, Reference Barry1989). In south-eastern Brazil, Faroni-Perez (Reference Faroni-Perez2014) observed that individuals of P. caudata measuring ~4 mm (length of thorax + abdomen) contained gametes, while recently settled juveniles measured around 3 mm. No precise data are available on sexual maturation in other Sabellaria species, although the worms are known to mature during their first year (Wilson, Reference Wilson1971; Gruet & Lassus, Reference Gruet and Lassus1983).
The presence of mature S. wilsoni throughout the year, and the lack of evidence of a resting gonadal stage, indicate that at this location, the species breeds continuously. This is a typical pattern in tropical organisms, given the relatively high and stable temperatures throughout the year, which support relatively constant levels of productivity (Longhurst & Pauly, Reference Longhurst and Pauly1987). In the northern hemisphere, mature sabellariid individuals have also been observed throughout the year, such as S. alveolata in France (Gruet & Lassus, Reference Gruet and Lassus1983), P. lapidosa in Florida (Eckelbarger, Reference Eckelbarger1976; McCarthy et al., Reference McCarthy, Young and Emson2003) and P. californica in California (Barry, Reference Barry1989). Despite these findings, marked seasonal patterns have been observed in spawning (Culloty et al., Reference Culloty, Favier, Ni Riada, Ramsay and O'Riordan2010), the abundance of larvae (Curtis, Reference Curtis1978; Dubois et al., Reference Dubois, Comtet, Retière and Thiébaut2007), and recruitment and settling (Wilson, Reference Wilson1971; Eckelbarger, Reference Eckelbarger1976; Dubois et al., Reference Dubois, Comtet, Retière and Thiébaut2007). Wilson (Reference Wilson1971) monitored populations of S. alveolata over a 9-year period in Cornwall (UK), and found that spawning occurred over a period of only a few days in mid-July, in the boreal summer. Geographic variation in spawning patterns and sexual maturation appears to be common in polychaetes, related primarily to environmental factors such as temperature, photoperiod, and the availability of food resources (Bhaud, Reference Bhaud1972).
Reproductive patterns in sabellariids may also be affected by extreme hydrodynamic disturbances, which destroy their colonies, eliminating adults and providing new opportunities for the settlement of juveniles (Barry, Reference Barry1989; McCarthy et al., Reference McCarthy, Young and Emson2003). In Florida (USA), observations of recruitment patterns in P. californica (Barry, Reference Barry1989) and of spawning in P. lapidosa (McCarthy et al., Reference McCarthy, Young and Emson2003) found facultative breeding peaks following major storms and wave-related disturbances, apparently an adaptive response to severe damage caused by the waves. This response would maximize reproductive output in situations of an increased probability of mortality, and potential recruitment success resulting from the reduced competition and greater available space.
The findings of Barry (Reference Barry1989) and McCarthy et al. (Reference McCarthy, Young and Emson2003) appear to be consistent with the life history of S. wilsoni which, while reproducing continuously throughout the year, showed two primary reproductive peaks. The first occurred during the dry season, from October to December 2008, when the reefs were undergoing maximum levels of erosion. The second peak occurred during the rainy season, from January to March 2009, when the reefs were recuperating and being recolonized. This appears to indicate that on the Amazon coast of Pará, the S. wilsoni reefs pass through periods of erosion and reconstruction associated with seasonal fluctuations in the local climate, which appear to modulate the reproductive strategy of the species.
Hydrodynamic disturbances may affect the reproductive cycle, advancing or delaying the breeding period (Giese & Kanatani, Reference Giese, Kanatani, Giese, Pearse and Pearse1987). Sabellariids such as P. lapidosa can undergo vitellogenesis and produce mature eggs within only a few days (Eckelbarger, Reference Eckelbarger1979). Both P. californica and P. lapidosa release their gametes immediately when disturbed or removed from their tubes (Eckelbarger, Reference Eckelbarger1976; McCarthy et al., Reference McCarthy, Young and Emson2003). Hydrodynamic disturbances may also permit colonization by new recruits in habitats that were fully occupied prior to the disturbance (Sousa, Reference Sousa, Bertness, Gaines and Hay2001). In this case, surviving individuals may increase their reproductive effort in an attempt to occupy the available space (Pawlik & Chia, Reference Pawlik and Chia1991; Sousa, Reference Sousa, Bertness, Gaines and Hay2001).
The morphometric results (Figure 1.4) indicate that in this population of S. wilsoni, recruitment continued throughout the study period, peaking in June through August 2008. In the following months and until November 2008, recruitment was reduced. However, it increased from December 2008 to January 2009 (early recuperation). Although the duration of the planktonic larval stage in S. wilsoni is not known, this period lasts between 2 weeks and 3 months in most other sabellariids (Eckelbarger, Reference Eckelbarger, Rice and Chia1978a). Therefore, the December–January recruits may have been produced during the preceding October–November spawning peak; however, confirmation of this would require more reliable data on the duration of larval development, the possible connectivity among local populations, and the regional oceanographic processes involved in the retention and dispersal of the larvae.
Conditions in the rainy season may favour the development of reefs in this region, when the gentler hydrodynamics facilitates the persistence of aggregates of individuals. While salinity is low (7–18) during this period, the species is euryhaline (Lana & Gruet, Reference Lana and Gruet1989; Lana & Bremec, Reference Lana, Bremec, Dauvin, Laubier and Reish1994). As S. wilsoni feeds on suspended particles, resources may also be more abundant during this period, when the seasonal increase in runoff and fluvial discharge increases the input of nutrients, suspended organic matter and chlorophyll-a (da Costa et al., Reference da Costa, de Sousa, Pinheiro, Pereira and da Costa2011). In south-eastern Brazil, Faroni-Perez (Reference Faroni-Perez2014) also recorded higher densities of juvenile P. caudata during the rainy season, associated with the increased input of nutrients from the adjacent estuarine system.
The results of the present study are consistent with the known capacity of sabellariids to tolerate unstable environmental conditions. Their life history is typical of opportunistic r-strategists, adapted for survival in unpredictable, short-lived habitats.
ACKNOWLEDGEMENTS
We are grateful to the late Dr André Souza dos Santos for his assistance with the identification of species. We thank Dr Stephen Ferrari for his valuable comments and Dra Janet W. Reid for language revision of the manuscript. Thanks also to two anonymous reviewers for their comments, which helped us to improve the manuscript.
FINANCIAL SUPPORT
Financial support was provided by the National Council for Scientific and Technological Development (CNPq-Brazil) through Universal project no. 486204/2007. The first and second authors were also awarded scholarships by CNPq-Brazil.











