INTRODUCTION
Franciscana dolphin, Pontoporia blainvillei (Gervais & d'Orbigny, 1844), is a small and endemic cetacean of the South-western Atlantic Ocean. The species is restricted to shallow marine and estuarine coastal waters from Golfo Nuevo (42°35′S), Chubut Province, Argentina (Crespo et al., Reference Crespo, Harris and González1998) to Itaúnas (18°25′S), Espírito Santo State, Brazil (Siciliano et al., Reference Siciliano, Di Beneditto and Ramos2002). Along its entire geographic distribution, this dolphin has been categorized as Vulnerable by the IUCN (Reeves et al., Reference Reeves, Dalebout, Jefferson, Karczmarski, Laidre, O'CorryCrowe, Rojas-Bracho, Secchi, Slooten, Smith, Wang, Zerbini and Zhou2012). Mortality in fishing gear is likely the most important threat to the conservation of this small cetacean (Reeves et al., Reference Reeves, Dalebout, Jefferson, Karczmarski, Laidre, O'CorryCrowe, Rojas-Bracho, Secchi, Slooten, Smith, Wang, Zerbini and Zhou2012); a maximum of 360–539 dolphins are by-caught in the entire Buenos Aires province (Negri et al., Reference Negri, Denuncio, Panebianco and Cappozzo2012). Moreover, recent studies have indicated that the abundance of franciscana dolphins from southern Brazil and Uruguay is declining due to unsustainable incidental mortality levels, suggesting that the species might be facing the highest risk of extinction of any cetacean of the South-western Atlantic Ocean (Kinas, Reference Kinas2002; Secchi et al., Reference Secchi, Danilewicz and Ott2003).
Since the 1990s, the population structure of franciscana dolphin has been divided in two different geographic populations based on osteological characters and mitochondrial DNA (Pinedo, Reference Pinedo1991; Secchi et al., Reference Secchi, Wang, Murray, Rocha-Campos and White1998). Recently, this division has been revised by Cunha et al. (Reference Cunha, Medeiros, Barbosa, Cremer, Marigo, Lailson-Brito, Azevedo and Solé-Cava2014), suggesting to split the franciscana dolphin population into two Evolutionarily Significant Units (ESUs). The North ESU is a small size population found from Espírito Santo (~18°S) to the centre of Rio de Janeiro and the South ESU is a larger size population ranged from somewhere in the centre of Rio de Janeiro to Argentina (42°S) (Pinedo, Reference Pinedo1991; Cunha et al., Reference Cunha, Medeiros, Barbosa, Cremer, Marigo, Lailson-Brito, Azevedo and Solé-Cava2014). Using differentiation among genetic, morphometric and population parameters, Secchi et al. (Reference Secchi, Danilewicz and Ott2003) proposed four Franciscana Management Areas (FMAs) along the coast of South America: two coastal zones (FMAs I and II) in Brazilian waters, one zone (FMA III) that includes coastal waters of Southern Brazil and Uruguay, and one zone (FMA IV) in Argentine waters.
Reproductive studies about female franciscana dolphins are limited to Brazilian and Uruguayan populations (Kasuya & Brownell, Reference Kasuya and Brownell1979; Harrison et al., Reference Harrison, Bryden, McBrearty and Brownell1981; Ramos et al., Reference Ramos, Di Benedito and Lima2000; Rosas & Monteiro-Filho, Reference Rosas and Monteiro-Filho2001; Danilewicz, Reference Danilewicz2003; Bertozzi, Reference Bertozzi2009; Freitas da Silva, Reference Freitas da Silva2011). This highlights the need to conduct studies on reproductive aspects of female dolphins in FMA IV off Argentina. This study provides essential information to design new conservation management plans that fit the features exhibited for each population of franciscana dolphins. In this sense, the objectives of this work are to estimate reproductive aspects of female dolphins such as reproductive stage, size and age at sexual maturity, ovulation rate, and estimate the annual reproductive rate of female franciscana dolphins from Argentina. Furthermore, characteristics of the ovaries, reproductive resting and senescence and morphometric studies are presented.
MATERIALS AND METHODS
Sample data
Sample size of the ovarian morphometric parameters measured varied because complete data of each dolphin were not always available. Ovaries and data collected from 31 franciscana dolphins by-caught in artisanal fishing nets along the coast of Buenos Aires and Río Negro Provinces were used in the analyses. Dolphins were sampled from 1998 through 2011. The study area included localities of Buenos Aires Province from Necochea (38°37′S 58°50′W) to Bahía Blanca (38°44′S 62°14′W) and one locality of Río Negro Province, El Cóndor next to the Río Negro Estuary (41°03′S 62°48′W) recently identified as the southernmost breeding area reported to date for this vulnerable species (Failla et al., Reference Failla, Seijas, Esposito and Iñíguez2012) (Figure 1). Carcasses of the dolphins collected during reproductive season (from November to March) and were kept frozen (at −21°C) until their postmortem analysis following standard procedures (Winchell, Reference Winchell1982). Mammary glands were examined to determine whether the female was lactating or not; the uterus was also examined to determine if a foetus was present. Biometric parameters measured were standard length (SL, N = 31) and total weight (TW, N = 29). SL was measured as a straight line from the tip of the rostrum to the fluke notch, and TW was obtained by a dynamo to the nearest 0.002 kg.

Fig 1. Geographic distribution of the study areas along the Southern coast of Argentina. References: N: Necochea; CLA: Claromecó; MH: Monte Hermoso; BB: Bahía Blanca; C: El Condor, Río Negro Estuary.
Age determination
Teeth were collected at mid-length of the left lower jaw of each animal and preserved in 70% alcohol. Age determination was obtained by counting growth layers groups (GLGs) in histological sections of teeth decalcified with the commercial acid mix RDO® and sectioned by cryostat at −21°C (see Pinedo & Hohn, Reference Pinedo and Hohn2000; Panebianco et al., Reference Panebianco, Negri and Cappozzo2012 for further information on the technique used). On-centre histological sections were stained with Mayer's haematoxylin. Three different readers determined the age by countering the number of GLGs in the dentine and cementum. In this work, one GLG represents 1 year of age (Pinedo & Hohn, Reference Pinedo and Hohn2000).
Sexual maturity stage determination
The reproductive tract of each dolphin was removed and the ovaries were separated and fixed in 10% formalin solution. After fixation, ovaries were externally examined and then sliced at ~1 mm to assure that all corpora – corpus luteum (CL) and corpus albicans (CA) – resulting from an ovulation were counted (Danilewicz, Reference Danilewicz2003). Females were categorized as sexually immature when no corpora were present on the ovaries (Figure 2A). Females with at least one corpus in the ovaries were classified as sexually mature (Figure 2B). Females were defined as resting when they had at least one CA and showed no signs of gestation or lactation (no CL, no foetus, no milk in mammary glands, no enlarged uterine horn indicating recent parturition), as pregnant when both a CL and a foetus were found, and as lactating when milk was found in the mammary glands. Histological analyses were performed on the ovaries according to Harrison et al. (Reference Harrison, Bryden, McBrearty and Brownell1981): cross-section slices of the ovaries were embedded in Histoplast® (Biopack), sectioned with a digital microtome into 4–7 µm thick sections and stained with haematoxylin-eosin to ensure that all corpora were counted.
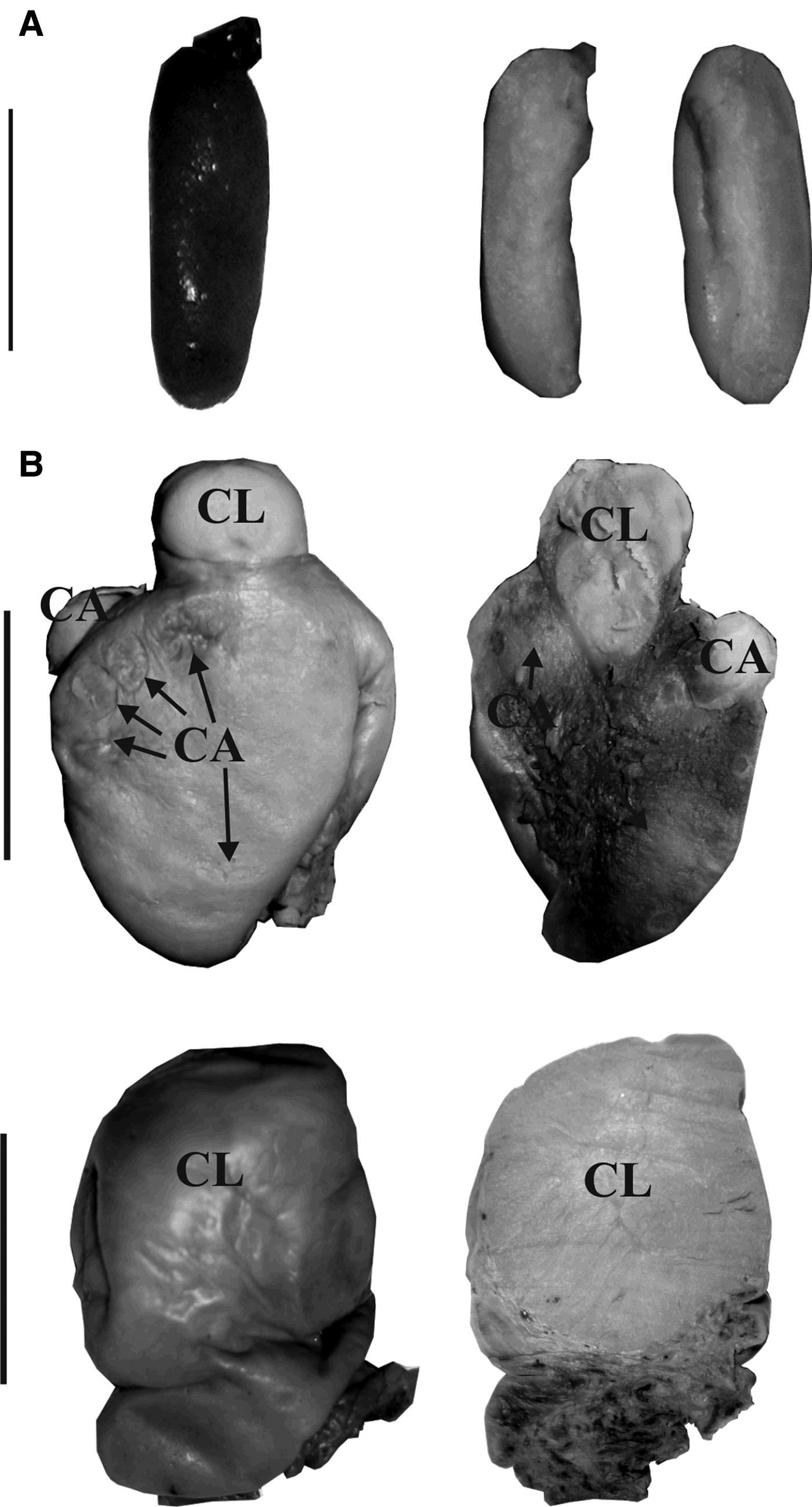
Fig. 2. External and internal macroscopic appearance of (A) immature and (B) mature ovaries of franciscana dolphins. The scars represent corpora lutea (CL) and corpora albicantia (CA). Scale bars: (A) 1 cm; (B) 2 cm.
Ovarian morphometric characteristics
The ovaries were weighed (±0.1 g) and measured (±0.01 mm) in their three larger orthogonal dimensions (length, width and depth) with digital calipers before fixing them in 10% neutral buffered formalin solution, after which gonadal morphometric parameters were assessed (Table 1).
Table 1. Ovarian morphometric parameters calculated for female franciscana dolphins, Pontoporia blainvillei, from Argentina.

Length, weight and deepness of the right and left ovaries were statistically analysed by Wilcoxon matched-pairs-ranks test in order to verify the symmetry of the ovaries and the relationships between those variables were analysed by Spearman rank correlation. The number of corpora on each ovary was also recorded. The ovarian morphometric parameters (Table 1) correspond to each maturity group (Immature and Mature) were statistically compared by using Mann–Whitney U Test because parametrical statistical assumptions were not achieved (normality and homoscedasticity). Linear regressions between ovarian morphometric parameters: mean ovarian length (MOL), mean ovarian weight (MOW), mean ovarian deepness (MOD), relative ovarian weight (ROW) and index of maturity (IM) (Danilewicz et al., Reference Danilewicz, Claver, Perez-Carrera, Secchi and Fontoura2004; Bertozzi, Reference Bertozzi2009; Panebianco et al., Reference Panebianco, Negri and Cappozzo2012, Table 1) and SL were performed to investigate how development parameters vary according to sexual maturity stage and SL of the dolphins. Two different lineal regressions were fitted for mature and immature dolphins (the break points correspond to the mean length at sexual maturity (LSM) value of the 95% CI calculated by DeMaster method, see below), the regression slopes were compared with a Student t-test.
Attainment of sexual maturity
The age at sexual maturity (ASM) was estimated by using two different methods: the DeMaster method (1978) and the logistic regression method (Danilewicz, Reference Danilewicz2003).
According to DeMaster (Reference DeMaster1978), ASM was estimated as:
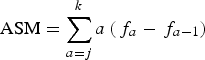 $${\rm ASM} = \sum\limits_{a = j}^k {a\left( {{\,f_a} - {\,f_{a - 1}}} \right)} $$
$${\rm ASM} = \sum\limits_{a = j}^k {a\left( {{\,f_a} - {\,f_{a - 1}}} \right)} $$Where f a is the fraction of animals sexually mature of the age class a, j is the age of the youngest sexually mature animal from the sample; and k is the eldest sexually immature dolphin from the sample.
The variance of ASM was expressed by the next equation:
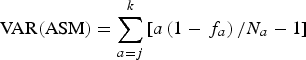 $${\rm VAR(ASM)} = \sum\limits_{a = j}^k {\left[ {a\left( {1 - {\,f_a}} \right)/{N_a} - 1} \right]} $$
$${\rm VAR(ASM)} = \sum\limits_{a = j}^k {\left[ {a\left( {1 - {\,f_a}} \right)/{N_a} - 1} \right]} $$Where N a is the total number of animals of age a.
The logistic regression method fits a sigmoid curve that represents the probability that a dolphin of age a was sexually mature. According to this approach the distribution of sexually mature and immature dolphins by age was:
Where x is the age of the dolphin, b is the slope of the regression and a is the intercept. To obtain the age when 50% of the animals are sexually mature (Y = 0.5), the last equation is simplified as ASM = −a/b by applying ln.
Length (LSM) and weight (WSM) at sexual maturity were also calculated using the equation of DeMaster (Reference DeMaster1978) modified by Ferrero & Walker (Reference Ferrero and Walker1995). The equation used for the calculation was the following:
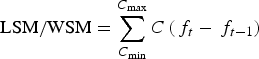 $${\rm LSM/WSM} = \sum\limits_{{C_{\min}}} ^{{C_{\max}}} {C\left( {{\,f_t} - {\,f_{t - 1}}} \right)} $$
$${\rm LSM/WSM} = \sum\limits_{{C_{\min}}} ^{{C_{\max}}} {C\left( {{\,f_t} - {\,f_{t - 1}}} \right)} $$Where C max represents the length/weight class of the sexually immature specimens with the highest length or weight, C min is the length/weight class of the sexually mature specimens with the lowest length or weight, C is the smallest value of the length/weight class t, and f t is the fraction of sexually mature animals from the length/weight class t. The calculation of the variance was expressed by the equation:
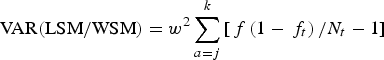 $${\rm VAR(LSM/WSM)} = {w^2}\sum\limits_{a = j}^k {\left[ {\,f\left( {1 - {\,f_t}} \right)/{N_t} - 1} \right]} $$
$${\rm VAR(LSM/WSM)} = {w^2}\sum\limits_{a = j}^k {\left[ {\,f\left( {1 - {\,f_t}} \right)/{N_t} - 1} \right]} $$Where N t is the total number of animals of the length/weight class t, and w 2 is the interval of classes, which in this case was 5 cm and 5 kg (Danilewicz, Reference Danilewicz2003).
Annual pregnancy rate (APR)
This parameter was estimated according to the Perrin & Reilly (Reference Perrin and Reilly1984) method as the proportion of sexually mature females that were pregnant adjusted by the gestation time in years (10.5 months–0.88 years; Kasuya & Brownell, Reference Kasuya and Brownell1979) and its variance was estimated (Perrin & Reilly, Reference Perrin and Reilly1984) by using the following equation:
Where N is the number of reproductive females in the sample. The estimation of this parameter was performed only for the mature dolphins.
Population reproductive parameters
LACTATION PERIOD (LP)
LP was estimated as follows (Perrin & Reilly, Reference Perrin and Reilly1984):
Where LP is lactation period in months, Gp is gestation period in months, L is the proportion of sexually mature females that are lactating and P is the proportion of sexually mature females that are pregnant. Gp was considered to be 0.88 months (Kasuya & Brownell, Reference Kasuya and Brownell1979).
LENGTH OF RESTING PERIOD (RP)
The resting period was calculated according to Perrin & Reilly (Reference Perrin and Reilly1984). The estimation included those females between ovulation cycles and those pregnant with embryos present.
Where RP is resting period in months, Gp is gestation period in months, R is the proportion of sexually resting mature females and P is the proportion of sexually mature females that are pregnant.
Statistical analyses
All the statistical analyses were performed by using the statistical software Statistica 7.0 (Statsoft, Inc.) and InfoStat (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzales, Tablada and Robledo2010). Data are given as mean ± SD and range. The level of statistical significance was set at P ≤ 0.05.
RESULTS
Ovarian morphometric characteristics
Sexual maturity stage determination and ovarian morphometric characteristics
The sampler set contained 19 immature dolphins and 12 mature, of which three were pregnant, two lactating, one lactating and pregnant and six resting. The age of the dolphins ranged from 0+ to 8 years old (Figure 3).

Fig. 3. Age distribution of female franciscana dolphins, Pontoporia blainvillei, incidentally captured from Southern Buenos Aires, Argentina.
The value of the morphometric variables of the right and left ovaries (length, weight and depth) did not vary significantly, thus indicating that the ovaries were symmetrical (Wilcoxon matched-pairs-ranks test, length (T = 139, N = 29), weight (T = 64.5, N = 26) and depth (T = 84.5, N = 24) P ≥ 0.1). Due to this fact, we decided to pool together the parameters of the right and left ovaries and work with the MOL, MOW and MOD (Tables 1 & 2) parameters.
Table 2. Summary of the ovarian morphometric parameters of female franciscana dolphins (Pontoporia blainvillei) from Argentina.
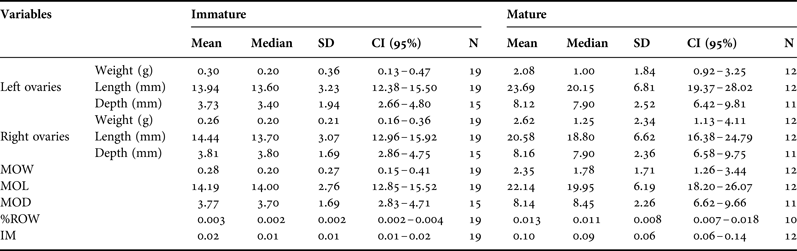
SD, standard deviation; CI, confidence interval; MOW, mean ovarian weight; MOL, mean ovarian length; MOD, mean ovarian depth; % ROW, percentage of relative ovarian weight; IM, index of maturity.
The total mass of the ovaries changed with both maturity stage (I: immature and M: Mature) and SL (Table 2, Figure 2). MOW (Range: I = 0.10–1.10 g, M = 0.55–5.25 g), %ROW (Range: I = 0.001–0.009, M = 0.004–0.027), MOL (Range: I = 8.45–19.50 mm; M = 14.40–34.50 mm), MOD (Range: I = 0.34–7.20 mm; M = 5.15–12.00 mm) and IM (Range: I = 0.001–0.009; M = 0.004–0.027) varied according to maturity groups (Mann–Whitney U test, MOL (U = 16, NI = 19, NM = 12), MOD (U = 8, NI = 15, NM = 12) and IM (U = 9, NI = 19, NM = 12) P ≤ 0.001). MOW and %ROW were the parameters that showed lower percentage of overlapping between maturity groups (10.7 and 15.1%), followed by MOD, IM and MOL (17.6, 19.2 and 19.6%). Resting female dolphins showed similar ovarian morphometric parameters values than mature dolphins (MOW, %ROW, MOL, MOD and IM) (Mann–Whitney U test, MOW: U = 7, NR = 4, NM = 8, P = 0.15; %ROW: U = 5, NR = 4, NM = 6, P = 0.17; MOL U = 5, NR = 4, NM = 8; MOT U = 5, NR = 4, NM = 7; IM U = 12, NR = 4, NM = 8; P ≥ 0.07). A significant difference in the location of the CL was observed (Mann–Whitney U test, P = 0.07, ~55% of the left ovaries showed at least one corpus and this number was around 82% for the right ovaries, Figure 2). Additionally, 33% of mature females showed corpora in both ovaries simultaneously.
The attainment of maturity, which corresponds to the dotted line in the Figure 4, coincided with an increase in total ovarian mass and length. Mature dolphins showed significantly higher slopes – of the linear regression analysis – than immature dolphins for all ovarian morphometric parameters excepting MOD for which the lineal regression for mature dolphins was not significant (Table 3).
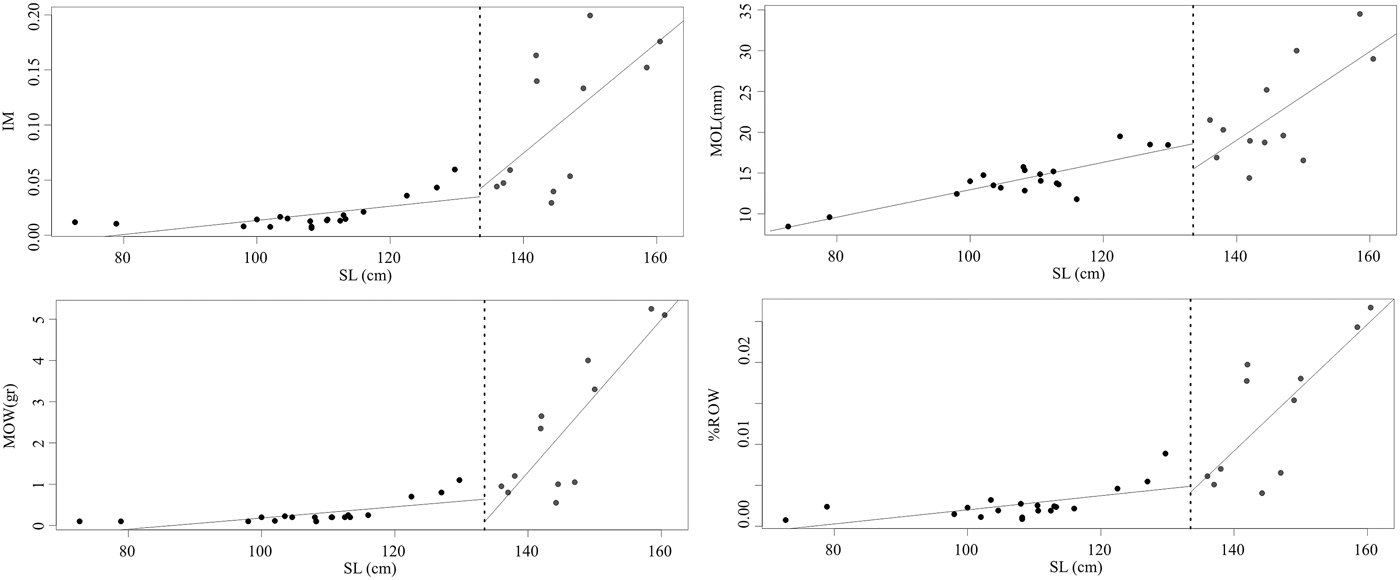
Fig. 4. Ovarian morphometric parameters as a function of standard length of female franciscana dolphins from Argentina. Dotted line corresponds to the mean Length at Sexual Maturity value. Black circles correspond to immature dolphins and grey circles to mature dolphins.
Table 3. Linear regression of the ovarian morphometric parameters as a function of standard length, and the statistical significance of linear regression slope comparison according to sexual maturity stage.

MOL, mean ovarian length; MOW, mean ovarian weight; MOD, mean ovarian depth; %ROW, percentage of relative ovarian weight; IM, index of maturity. Regression model: y = bx + a; SE, standard error; ST, Student t-test significance: *P < 0.05; ** P < 0.001; *** P < 0.0005. Statistical parameters: MOL: t 27 = 2.02, P = 0.02; MOW: t 27 = 4.23, P < 0.01; MOD: t 22 = 0.17, P = 0.43; IM: t 26 = 2.17, P = 0.02; ROW: t 26 = 3.23, P < 0.01.
Attainment of sexual maturity
The oldest immature female was 4 years old and the youngest mature female was 3 years old, thus suggesting that the age at attainment of sexual maturity is between 3 and 4 years old. In fact, the ASM was estimated at 3.92 ± 0.09 years (95% CI = 3.33–4.51) by using the DeMaster method and 3.42 years by the logistic regression method. Those ASM values were close to the average age of the three females (3.67 years) collected in the year when they attained sexual maturity, i.e. with only one CL.
The length of the immature and mature dolphins ranged from 72.70 to 129.70 cm and from 136.00 to 160.50 cm respectively; LSM was estimated at 133.47 ± 11.11 cm (95% CI = 126.93–140 cm). The immature and mature dolphins weighed from 3.58 to 30.50 kg and from 26.50 to 52.00 kg respectively, WSM was calculated at 32.68 ± 2.72 kg (95% CI = 29.41–35.95 kg).
Annual pregnancy rate (APR)
APR for female franciscana dolphins from FMA IV was 0.36 ± 0.02 (95% CI = 0.10–0.65). The proportion of mature, lactating, pregnant and resting females was 0.39, 0.25, 0.33 and 0.33 respectively, and one female was simultaneously pregnant and lactating.
No evidence of senescent females was found since the oldest females (8 years old) were pregnant or lactating and both showed CL in their ovaries. Based on the ASM, the number of corpora in the ovaries and the relationship between the number of corpora and the age of the dolphins (Linear regression, y = 1.13x−2.72, r2 = 0.66, F (1,10) = 17,11, P = 0.003, N = 11), the ovulation rate is 0.39 for sampled population. Neither the gestation period nor length at birth were estimated because of the low number of neonates collected – two, in December and February (SL = 75.8 ± 4.4 cm, TW = 5.0 ± 3.4 g).
Two additional population parameters were estimated, Lactation Period (LP) was estimated at 7.95 months and Length of Resting Period (RP) at 10.5 months (Figure 5).

Fig. 5. Reproductive chronology of female franciscana dolphins. The seasonality of the reproductive events was modelled on the data presented in this work (Pregnancy, Resting and Lactating periods) and from previous work (Mating and Birth period according to Danilewicz, Reference Danilewicz2003). Bars represent the mean time of each reproductive event and the gradient pattern the time lag that may occur at the beginning and end of each event shown.
DISCUSSION
No ovulation asymmetry was found in female franciscana dolphins from FMA IV and the evidence supports that both right and left ovaries are functional. This outcome is consistent with that observed by Rosas & Monteiro-Filho (Reference Rosas and Monteiro-Filho2001) for franciscana dolphins belonging to FMA II, although ovarian asymmetry was described in dolphins from FMA III (Brownell, Reference Brownell1984; Danilewicz, Reference Danilewicz2003), where only the left ovary was active. The lack of asymmetry was also evident as both ovaries were able to ovulate from the attainment of sexual maturity onwards – the by-caught dolphins analysed in this work accumulated similar amounts of corpora in both ovaries (Table 4). This fact has been previously described as a general trend in cetacean species (Dabin et al., Reference Dabin, Cossais, Pierce and Ridoux2008).
Table 4. Distribution of corpora lutea and albicantia in the ovaries of franciscana dolphins from Argentina.
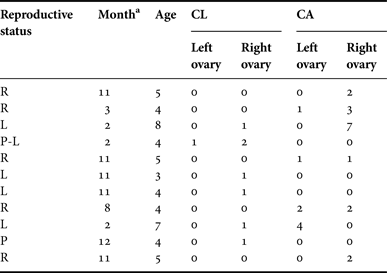
a The month when the dolphins were by-caught. Age in GLGs. R, resting; L, lactating; P, pregnant and P-L, pregnant and lactating females; CL, number of corpora lutea; CA, number of corpora albicantia.
The analyses on ovarian morphometric parameters revealed that ovaries clearly increase in size as a function of SL. Similar trends have been reported by Ramos et al. (Reference Ramos, Di Benedito and Lima2000), Danilewicz (Reference Danilewicz2003) and Rosas & Monteiro-Filho (Reference Rosas and Monteiro-Filho2001), for FMAs Ib, IIIa and IIb respectively (Cunha et al., Reference Cunha, Medeiros, Barbosa, Cremer, Marigo, Lailson-Brito, Azevedo and Solé-Cava2014), thus suggesting that this is a common feature among all populations of Pontoporia blainvillei. This feature has also been observed in several cetacean species (Marsh & Kasuya, Reference Marsh and Kasuya1984; Hohn et al., Reference Hohn, Read, Fernandez, Vidal and Findley1996).
Annual ovulation rates can be calculated when the sample is large enough according to age classes (Marsh & Kasuya, Reference Marsh and Kasuya1984, Reference Marsh and Kasuya1986; Perrin & Donovan, Reference Perrin and Donovan1984), although observations on smaller samples – as in this work – can only indicate a trend in the number of ovulations in relation to age. In this context, we found that the number of CL and CA increased with the age from puberty (3–4 years old) to at least 8 years old for the dolphins, indicating that CL and CA are detectable in the ovaries for at least 4 years in this species. Read & Hohn (Reference Read and Hohn1995) also found a similar positive trend between the number of corpora and age for harbour porpoise, Phocoena phocoena, from the Gulf of Maine. Harrison et al. (Reference Harrison, Bryden, McBrearty and Brownell1981) published similar results for franciscana dolphins from Uruguay, although and according to his findings, the CAs do not persist throughout life in this species, as occurs in certain large whales (Perrin & Donovan, Reference Perrin and Donovan1984). The former authors have demonstrated that older females from Uruguay (161–175 cm SL) did not accumulate a growing number of CA with increasing age. Based on the accumulation pattern of the corpora scars in the ovaries, the ovulation rate and the presence of a simultaneously pregnant and lactating female (Table 4), we suggest that franciscana dolphins might be monoestrous and breed annually (Ovulation rate: 0.39). We found that some females might also ovulate twice a year (Table 4), but only one of these ovulations might result in pregnancy. Several studies have estimated fecundity rates from CA counts and discussed these in relation to the age of the female (Marsh & Kasuya, Reference Marsh and Kasuya1984; Berta & Sumich, Reference Berta and Sumich1999; Chivers, Reference Chivers, Perrin, Würsig and Thewissen2002). Harrison et al. (Reference Harrison, Bryden, McBrearty and Brownell1981) reached a similar conclusion (i.e. annual breeding and monoestrous) based on ovarian characteristics of Pontoporia blainvillei from Uruguay by using similar methodology. Later, Danilewicz (Reference Danilewicz2003) confirmed the same outcome for specimens from Rio Grande do Sul, Brazil. Despite this valuable information, it is worth pointing out that multiple ovulations have been reported, particularly in young adult females, as well as the existence of accessory CL; consequently, CA count may differ from the actual number of ovulatory events (Harrison et al., Reference Harrison, Brownell, Boice and Harrison1972; Dabin et al., 2008). In addition, a recent study on postmortem observations on the ovaries of a captive bottlenose dolphin, Tursiops truncatus, has proposed that only the CAs resulting from a gestation would be persistent, which would imply that the others had completely healed out – or become completely resorbed (Brook et al., Reference Brook, Kinoshita and Benirschke2002).
Individual variation of the accumulation rate of corpora was observed in the present study; this is a common feature in cetaceans and is a reflection of differences in the annual ovulation rate between individuals as well as variation in the attainment of sexual maturity (Kasuya & Marsh, Reference Kasuya and Marsh1984; Read & Hohn, Reference Read and Hohn1995; Brook et al., Reference Brook, Kinoshita and Benirschke2002; Murphy et al., Reference Murphy, Pierce, Law, Bersuder, Jepson, Learmonth, Addink, Dabin, Santos, Deaville, Zegers, Mets, Rogan, Ridoux, Reid, Smeenk, Jauniaux, López, Alonso Farré, González, Guerra, García-Hartmann, Lockyer and Boon2010). Many other factors might affect ovulation rates by affecting hormonal production, such as climate, e.g. living in temperate vs tropical waters through variation in light intensity and day-length as perceived by the hypothalamus, free-living vs captive condition, stress, and species-specific differences in mating systems (Dabin et al., Reference Dabin, Cossais, Pierce and Ridoux2008). It is also important to note that our analyses only included dolphins that were by-caught, resulting in a bias sample – older franciscana dolphins (larger than 160 cm) and dolphins with calves are very rarely entangled or found stranded (Negri et al., in press) – due to this further analysis is needed in order to obtain more accurate outcomes about ovulation pattern.
Cetaceans’ species that exhibit annual reproduction included small odontocetes like the Dall's porpoise, Phocoenoides dalli, and harbour porpoise, and baleen whales, such as the large baleen minke whale, Balaenoptera acutorostrata (Lockyer, Reference Lockyer1984; Hohn & Brownell, Reference Hohn and Brownell1990; Clapham, Reference Clapham1992; Ferrero & Walker, Reference Ferrero and Walker1999). Many small cetacean species have relatively young ages at sexual maturity as well as short lifespans. Species that do not have a strictly annual reproduction, such as the dwarf sperm whale, Kogia sima, and the large humpback whale, Megaptera novaeangliae, show longer gestation periods than those species with a strictly annual reproduction, as do franciscana dolphins from Argentina. References to the presence of senescent females’ dolphins are scarce. Marsh & Kasuya (Reference Marsh and Kasuya1984) found an association between follicle abundance and the incidence of follicular atresia with senescence in pilot whales, Globicephala sp., over 50 years old. Later, Myrick et al. (Reference Myrick, Hohn, Barlow and Sloan1986) also found evidence of senescence in old spotted dolphins.
The ASM found in this work was lower than the one found by Corcuera & Monzón (Reference Corcuera and Monzón1990) (ASM = 5–7, N = 12) and Corcuera (Reference Corcuera1996) (ASM = 4.3–4.4, N = 27) for franciscana dolphins from FMA IV. LSM was also lower in this work than the value found by Corcuera in 1996 (133.47 cm vs 140 cm). Nevertheless, ASM and LSM values were close to those found by Danilewicz (Reference Danilewicz2003) in franciscana dolphins from Rio Grande do Sul (FMA III, 3.5–3.7 years old and 138.9 cm, N = 72) and São Paulo and Paraná (FMA II, 3.6–4 years old and 128–130 cm, Freitas da Silva, Reference Freitas da Silva2011, N = 35), whereas the same parameters were higher than those found in dolphins from Rio de Janeiro (3 years old and 130 cm; Ramos et al., Reference Ramos, Di Benedito and Lima2000). An earlier study on dolphins collected in Uruguay showed lower ASM (2.7 years old), but a higher LSM (140 cm) (Kasuya & Brownell, Reference Kasuya and Brownell1979). These variations in LSM and ASM have been noticed in previous studies where it has also been demonstrated that those parameters could vary with decreasing population abundance caused by incidental mortality in small odontocetes (Kasuya, Reference Kasuya1985; Read & Gaskin, Reference Read and Gaskin1990). Nevertheless, we cannot exclude differences in the methodologies used and the low number of samples as possible sources of variation in the estimated values of ASM and LSM. Comparison of age at attainment of sexual maturity within a population over time is useful in identifying fluctuations in reproductive patterns; mainly in populations under human pressure such as franciscana dolphins, the most threatened small cetacean in the South-western Atlantic Ocean due to incidental mortality (Reeves et al., Reference Reeves, Dalebout, Jefferson, Karczmarski, Laidre, O'CorryCrowe, Rojas-Bracho, Secchi, Slooten, Smith, Wang, Zerbini and Zhou2012). Even though previous studies have demonstrated that ASM and LSM vary – i.e. diminish with decreasing population abundance caused by incidental mortality – in small odontocetes (Kasuya, Reference Kasuya1985; Read & Gaskin, Reference Read and Gaskin1990), we cannot exclude differences in the methodologies used and the low number of samples as possible sources of variation in the estimated values of ASM and LSM. Based on the variation of ASM and LSM and ovarian weight between FMA II, III, IV and FMA I (Ramos et al., Reference Ramos, Di Benedito and Lima2000; Rosas & Monteiro-Filho, Reference Rosas and Monteiro-Filho2001; Danilewicz, Reference Danilewicz2003; Freitas da Silva, Reference Freitas da Silva2011; this study) we suggest that those differences supported the existence of the two distinctive ESU described by Cunha et al. (Reference Cunha, Medeiros, Barbosa, Cremer, Marigo, Lailson-Brito, Azevedo and Solé-Cava2014). Note that the existing information about reproductive biology of franciscana dolphins is relatively old and scarce which enhanced the value of the information presented in this work.
We found a lower APR in comparison with that found by Danilewicz (Reference Danilewicz2003) for franciscana dolphins from Brazil (0.66) and harbour porpoise (0.95; Read & Hohn, Reference Read and Hohn1995) whereas it was similar to the one found for spotted dolphins, Stenella attenuata (0.33; Myrick et al., Reference Myrick, Hohn, Barlow and Sloan1986). We also found a low proportion of lactating females and high proportion of resting females (0.33), indicating that pregnancy is likely to fail or not occur, so females must wait until the next mating season (Figure 5). This low pregnancy rate could be explained by sampling bias due to lower vulnerability of larger females and females with calves to by-catch (Danilewicz et al., Reference Danilewicz, Claver, Perez-Carrera, Secchi and Fontoura2004; Negri et al., in press). A different reason that may explain the difference between the results found here with those previously reported is due to sampling period that coincided with the breeding season of the species, the resting females recovered may have not ovulated yet during that specific mating period. This may account for the lower pregnancy rate estimated in the current study compared with more northern management areas for this species. Further studies are likely to provide new insight into habitat use of franciscana dolphins from Argentina.
Harrison et al. (Reference Harrison, Bryden, McBrearty and Brownell1981) and Kasuya & Brownell (Reference Kasuya and Brownell1979) estimated lactation period duration for franciscana in Uruguay as at least 8 months, based on lactating females and their ovarian characteristics respectively. The lactation period was also estimated between 7.5 and 8.5 months in franciscana dolphins from South-eastern Brazil, included in the North ESU (FMA II; Ramos et al., Reference Ramos, Di Benedito and Lima2000), and at least 7 months in dolphins from northern Buenos Aires from South ESU (FMA IVa; Rodríguez et al., Reference Rodríguez, Rivero and Bastida2002; Denuncio et al., Reference Denuncio, Bastida, Danilewicz, Morón, Rodríguez-Heredia and Rodríguez2013). We achieved a similar outcome (7.95 months, estimated according Perrin & Reilly, Reference Perrin and Reilly1984) to the ones found in other small coastal odontocetes with a short lifespan, such as the harbour porpoise and vaquita, Phocoena sinus (Read & Hohn, Reference Read and Hohn1995; Hohn et al., Reference Hohn, Read, Fernandez, Vidal and Findley1996). Therefore, franciscana dolphins have a lactation period duration similar to other small, coastal dolphins with a short lifespan.
Knowledge of the natural history of a species is vital for understanding its biology, conservation status and for ensuring its continued survival. Framed within the evolutionary theory of life history of mammals the concept of ‘slow-fast continuum’ arose (Stearns, Reference Stearns1992). This slow-fast continuum concept, driven by body size and ecology of a species (Harvey & Purvis, Reference Harvey, Purvis and McGlade1999), explains the great variation in life history strategies observed throughout the mammalian orders, including cetaceans. Small odontocetes, such as the harbour porpoise, are located closer to the fast end of the slow-fast continuum as they reach sexual maturity relatively early, have a low life expectancy (around 20 years) and very high reproductive rates (~0.9) (Read & Hohn, Reference Read and Hohn1995; Hohn et al., Reference Hohn, Read, Fernandez, Vidal and Findley1996). Those small cetaceans represent one extreme of life history strategies compared with larger cetaceans (Read & Hohn, Reference Read and Hohn1995). Species with a ‘fast’ life history strategy are expected to show a short calving interval and a large offspring size per lifetime, although it was not the case for harbour porpoise and franciscana dolphin, when only a few offspring are produced per lifetime (Read, Reference Read1990; Danilewicz, Reference Danilewicz2003).
Estimation of reproductive parameters of females and understanding their life history strategy (Perrin et al., Reference Perrin, Holts and Miller1977; Kasuya, Reference Kasuya1985; Myrick et al., Reference Myrick, Hohn, Barlow and Sloan1986) are important for monitoring the possible impacts that exploitation, habitat degradation, pollution and other factors may have on a population. This study provides novel data about reproductive aspects of female franciscana dolphins, including the first information obtained on its southern limit distribution, which can aid conservation management of this species in Argentina.
ACKNOWLEDGEMENTS
This work is part of the PhD thesis of M.V. Panebianco presented at the University of Buenos Aires (UBA), Argentina. Our sincere gratitude to all artisanal fishermen whom made this project possible. Many people collaborated with the fieldwork and laboratory procedures and we want to thank D. Rodríguez, M.N. Paso-Viola (CADIC/CONICET), F.H. Pérez (Ecocentro), M. Sotelo and M.V. Massola (RUM Bahía Blanca, Bahía Falsa y Bahía Verde); the staff of Fundación Cethus, V.A. Seijas, L. Russo Lacerna y R. Espósito (Río Negro Estuary). This study was carried out with permission of the Dirección de Fauna de la Provincia de Río Negro, Argentina (Exp. No. 132264-DF-2010).
FINANCIAL SUPPORT
This work was carried out with the financial support of the Félix Azára Foundation, the Society of Marine Mammalogy, the Cetacean Society International, University of Valencia, Fundación Cethus, Organization of American States, Western Hemispheric Migratory Species Initiative, the Rufford Foundation and Whale and Dolphin Conservation.











