Introduction
Bioerosion is a natural process that converts calcareous substrate into sediments and dissolved carbonate and can thereby have a significant effect on the carbon cycle (Perry & Harborne, Reference Perry, Harborne, Hubbard, Rogers, Lipps and Stanley2016; Alvarado et al., Reference Alvarado, Grassian, Cantera-Kintz, Carballo, Londoño-Cruz, Glynn, Manzello and Enochs2017; Schönberg et al., Reference Schönberg, Fang, Carreiro-Silva, Tribollet and Wisshak2017a). Under favourable conditions for reef development, bioerosion does not exceed biological calcification (Scoffin et al., Reference Scoffin, Stearn, Boucher, Frydl, Hawkins, Hunter and MacGeachy1980; Perry et al., Reference Perry, Spencer and Kench2008; Enochs et al., Reference Enochs, Manzello, Carlton, Graham, Ruzicka and Colella2015). Nonetheless, several recent studies have suggested that bioerosion may intensify in future under conditions that can lead to coral reef demise, including environmental degradation, ocean acidification and El Niño impacts (Tribollet et al., Reference Tribollet, Godinot, Atkinson and Langdon2009; Perry & Harborne, Reference Perry, Harborne, Hubbard, Rogers, Lipps and Stanley2016; Schönberg et al., Reference Schönberg, Fang, Carreiro-Silva, Tribollet and Wisshak2017a). These predictions arose from previous observations of boring sponges functioning well under conditions stressful for corals (Cortés et al., Reference Cortés, Murillo, Guzmán and Acuña1984; Vicente, Reference Vicente1990; Schönberg & Suwa, Reference Schönberg, Suwa, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007; Schönberg et al., Reference Schönberg, Fang, Carballo, Carballo and Bell2017b) and by an apparent preference by boring sponges for dead coral substrate (Chaves-Fonnegra et al., Reference Chaves-Fonnegra, Zea and Gómez2007; Nava & Carballo, Reference Nava and Carballo2008; Schönberg & Ortiz, Reference Schönberg, Ortiz, Riegl and Dodge2009; Schönberg, Reference Schönberg2015). In the eastern and western Pacific, coral bleaching during El Niño events has occurred throughout the last three decades and is associated with a decrease of live coral cover at many coral reefs (Glynn & Leyte-Morales, Reference Glynn and Leyte-Morales1997; Glynn, Reference Glynn1998; Carriquiry et al., Reference Carriquiry, Cupul-Magaña, Rodríguez-Zaragoza and Medina-Rosas2001; Glynn et al., Reference Glynn, Maté, Baker and Calderón2001; Reyes-Bonilla, Reference Reyes-Bonilla2001; Sheppard et al., Reference Sheppard, Spalding, Bradshaw and Wilson2002; Glynn, Reference Glynn2011). The response of boring sponges related to the increase of dead corals after El Niño impacts has been reviewed by Cortés et al. (Reference Cortés, Murillo, Guzmán and Acuña1984) and Rützler (Reference Rützler2002), who documented the abundance increase of Cliona caribbaea at Caribbean coral reefs. Schönberg & Ortiz (Reference Schönberg, Ortiz, Riegl and Dodge2009) studied the whole assemblage of boring sponges, and they documented an escalation of sponge abundance after two bleaching events that occurred at the Great Barrier Reef in 1997/98 and 2003/04. On Mexican Pacific coral reefs, boring sponge assemblages have been intensively studied over the last two decades (e.g. Carballo et al., Reference Carballo, Cruz-Barraza and Gómez2004, Reference Carballo, Bautista-Guerrero, Nava, Cruz-Barraza and Chávez2013; Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014; Nava & Carballo, Reference Nava and Carballo2016). Such investigations revealed that in the Mexican Pacific boring sponges are the chief agents of erosion and substrate remodelling, especially at the reef edges (Carballo et al., Reference Carballo, Bautista-Guerrero and Leyte-Morales2008a). They are also important producers of sediments in the silt and fine sand fractions (Neumann, Reference Neumann1966; Fütterer, Reference Fütterer1974; Rützler, Reference Rützler1975; Nava & Carballo, Reference Nava and Carballo2016). In the Mexican Pacific, when the abundance of boring sponges inhabiting different substrate types is compared, attached dead coral substrate is more heavily invaded by these sponges than live corals and coral rubble. Nonetheless, coral rubble often harbours the entire local range of species (Carballo et al., Reference Carballo, Bautista-Guerrero, Nava, Cruz-Barraza and Chávez2013; Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). Throughout the Mexican Pacific, communities of boring sponges are uniformly composed of the same species assemblage, dominated by C. vermifera (Carballo et al., Reference Carballo, Bautista-Guerrero, Nava, Cruz-Barraza and Chávez2013; Nava & Carballo, Reference Nava and Carballo2013; Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). Recent studies documented that the reproduction and abundance of this species is not negatively affected by heat stress, suggesting resilience to global change conditions and a potential threat by this species for coral reefs recurrently impacted by thermal anomalies such those occurring during El Niño events (Carballo et al., Reference Carballo, Bautista-Guerrero, Nava, Cruz-Barraza and Chávez2013). The El Niño event of 2015–16 lasted from April 2015 to January 2016 (NOAA, 2017), reaching 2.87 units of the Oceanic Niño Index. Simultaneously, severe damage became visible on coral reefs along the Mexican Pacific coast, including significant coral bleaching and mortality. The aim of the present study was to evaluate the effects of El Niño events in the Mexican Pacific and the response of local boring sponge assemblages to the increase of unprotected reef area made up by dead corals.
Materials and methods
Study location
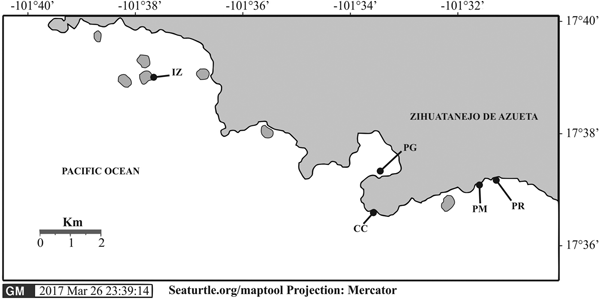
Five sites harbouring coral reefs were selected along the coast of Zihuatanejo, Guerrero, Mexico (Figure 1). All sites were predominantly colonized by pocilloporid corals. The first site, Playa Las Gatas (17°37′16.8″N 101°33′08.55″W) is located south of Zihuatanejo Bay. The local coral community forms mounds up to 1 m in diameter, being distributed between 3 and 6 m water depth, spreading over a rocky bottom, and being intersected by patches of sand and coral rubble. The coral reef of Islote Zacatoso (17°39′13.2″N 101°37′13.3″W) is located 1 km eastward from the Ixtapa Zihuatanejo Resort. This site harbours a fringing reef that extends down to 10.6 m depth, covering an area of about 1.9 ha. The third site at Caleta de Chon (17°36′52.8″N 101°33′16.3″W) is located 1.5 km south-east of Zihuatanejo Bay and inside a cove that is about 100 m long, 150 m wide and up to 13 m depth, but corals mostly occur between 6 and 7 m depth. Playa Manzanillo (17°37′11.4″N 101°31′27.6″ W) and Playa Riscalillo (17°37′0.7″N 101°31′03.5″W) are located 4 km south-east of Zihuatanejo Bay. Their fringing reefs extend to 6 m depth. The latter two reefs are considered the most conserved reefs in the area, mainly by their large area of 40 ha each, and their high cover of living corals.
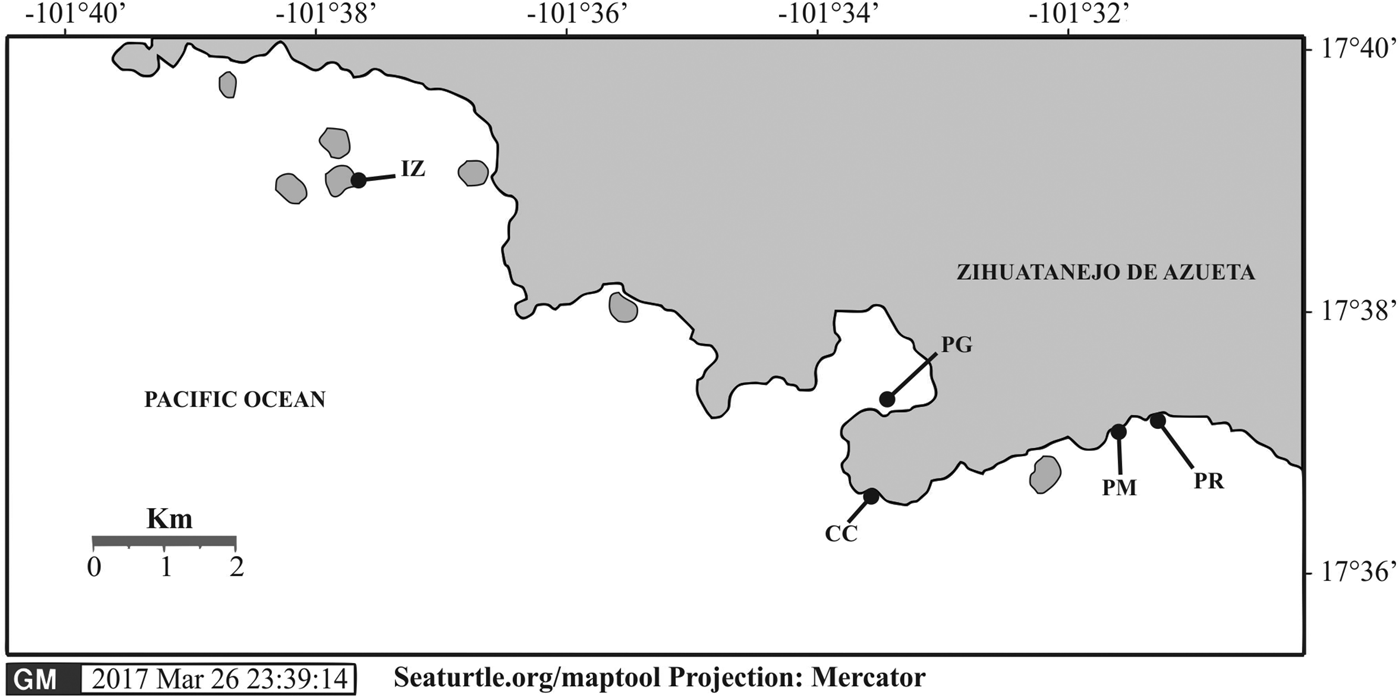
Fig. 1. Location of coral reefs surveyed at Zihuatanejo Guerrero, México. IZ, Islote Zacatoso; PG, Playa Las Gatas; CC, Caleta de Chon; PM, Playa Manzanillo; and PR, Playa Riscalillo.
Environmental and habitat characterization
During September 2015 we recorded environmental indicators of water quality and substrate characteristics at each site. Water transparency was assessed using standard Secchi disk extinction depth measurements (Preisendorfer, Reference Preisendorfer1986). The Secchi disk was tied to a nylon string and submerged to the maximum depth at which the disk was visible. The depth (m) was then recorded. This procedure was repeated at least nine times over 3 days on every survey. Hierarchical means were calculated, first by each day and then across the days. The sedimentation rate (kg m−2 day−1) was recorded using six sediment collection bottles attached to three vertical PVC pipes anchored at 1 m above the reef. Sediments were collected over a period of 4 days per site and occasion and were rinsed with distilled water at the laboratory prior to oven-drying and weighing (Cortés & Risk, Reference Cortés and Risk1985).
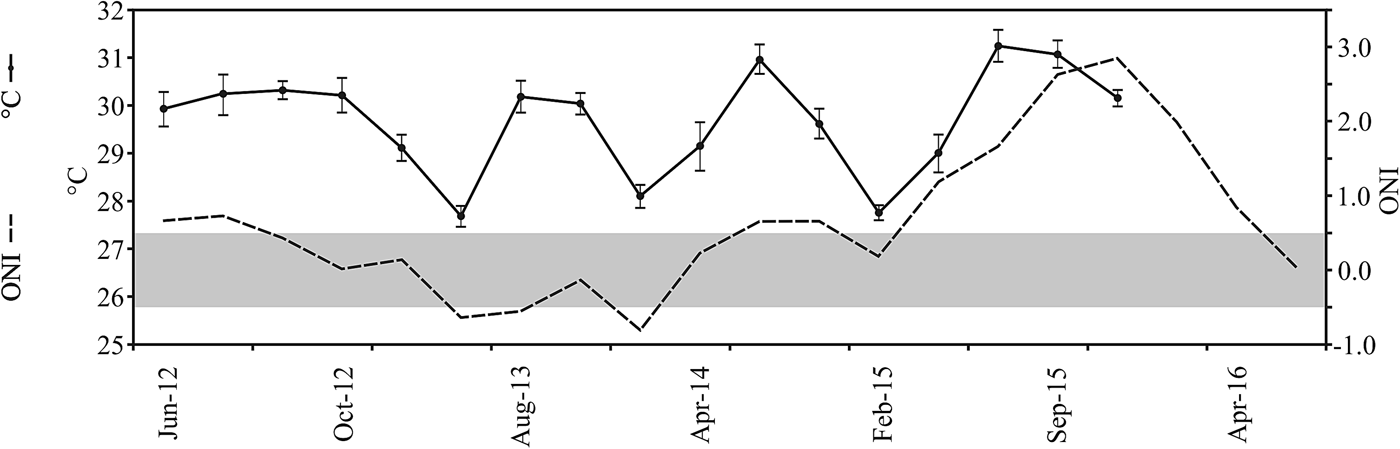
Water temperature (°C) and illumination (lux) above the bottom were recorded at 5 m depth using a waterproof Hobo pendant temperature/light data logger (Onset, Bourne, USA). This device was programmed to measure both variables every 5 min for 72 h. At the end of each survey the data logger was collected and its temperature/illumination data were downloaded to a computer. Additional records of water temperature made at Islote Zacatoso between June 2012 and December 2015 were also considered. They were also sampled at 5 m depth using Hobo data loggers and they were averaged per quarterly surveys. Records of sea surface temperature (SST) anomalies in the Niño 3 region (5N–5S, 150W–90W) during the same period were obtained from the National Centers for Environmental Information (NOAA, 2017). A standard thermal threshold of 0.5°C for temperature anomalies during the Oceanic Niño Index (ONI) was considered to detect the beginning and the end of an El Niño event (NOAA, 2017).
Cover of major substrate components was assessed during June 2015 using five 20 m line transects laid at 5 m depth at each reef, oriented parallel to the shoreline (Loya, Reference Loya1972; Gattuso et al., Reference Gattuso, Pichon, Delesalle, Canon and Frankignoulle1996; Chaves-Fonnegra et al., Reference Chaves-Fonnegra, Zea and Gómez2007). Along each transect, the length of the line covering each substratum category was recorded to the nearest centimetre and their proportion (%) of the length of transect (20 m = 100%) was considered. Substrate categories were the following:
(a) Healthy corals: These were all living coral colonies with natural and bright tissue colouration, either greenish or brownish. Pocilloporid corals with white branch tips were considered as normal.
(b) Pale corals: The tissue of respective coral was noticeably pale, but still not entirely bleached. During the study this condition was common at several sites where pale corals were scattered among healthy and bleached corals.
(c) Bleached corals: These corals were still covered with tissue, but had either almost or totally lost their colouration, caused by the expulsion of their symbiotic zooxanthellae. The coral tissue became transparent, and the coral skeleton was visible, creating the impression that the respective coral was white.
(d) Dead corals: The skeletons of freshly dead coral colonies had a white or greyish colouration. Although their surface remained without erosion at the time of the surveys, it was occasionally colonized by filamentous algae. All eroded coral substrate covered by crustose or articulated calcareous algae were considered, as old dead corals.
(e) Coral rubble: This represented unattached fragments of dead corals that could potentially be relocated by strong water movement. Such fragments were usually covered by calcareous algae, and their size ranged between 1 and 5 cm in diameter. When they were larger, their own weight stabilized them, and we no longer considered them as ‘unattached’.
(f) Filamentous algae: They were represented by filamentous blue-green, red and green algae growing above rocks and dead corals.
(g) Metamorphic rocks and sand: These substrata were unavailable for settlement by boring sponges. They were abundant at sites such as Playa Las Gatas, were terrigenous rocks and constituted a stable substrate consisting of boulders.
The coral mortality index was used to assess the conservation status of the surveyed coral reefs, resulting from the ratio of live and dead corals (Gómez et al., Reference Gómez, Aliño, Yap and Licuanan1994; Holmes et al., Reference Holmes, Edinger, Limmon and Risk2000):
where: CMI = coral mortality index, DC = cover of dead corals + cover of coral rubble (%), LC = cover of living corals, including cover of healthy + pale + bleached coral categories (%). This index ranges between 0 (where all substrata are living corals) and 1 (where all substrata are dead corals).
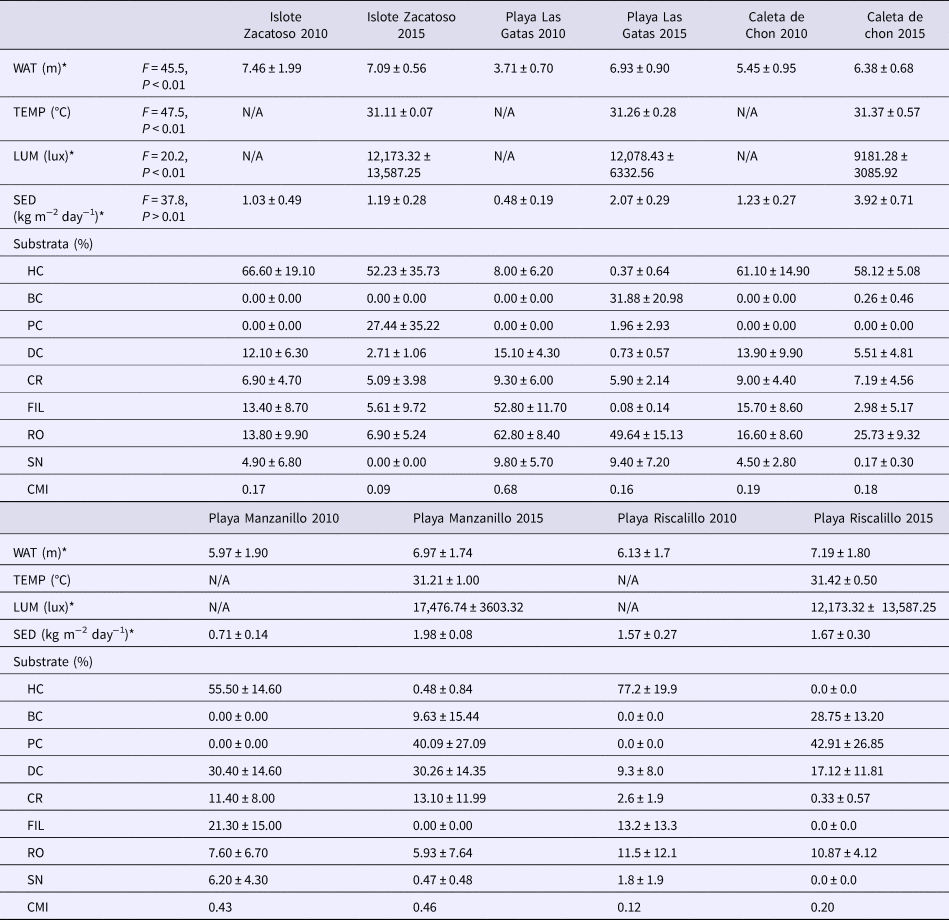
Earlier records of the environmental parameters used in the present study, coverage of live and dead corals, and the coral mortality index were available for the summer 2010 (Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). These data were considered for comparison purpose and are summarized in Table 1.
Table 1. Environmental parameters and composition of coral reefs in the study area (mean ± SD) recorded during summer 2010 (Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014) and 2015 (present study)
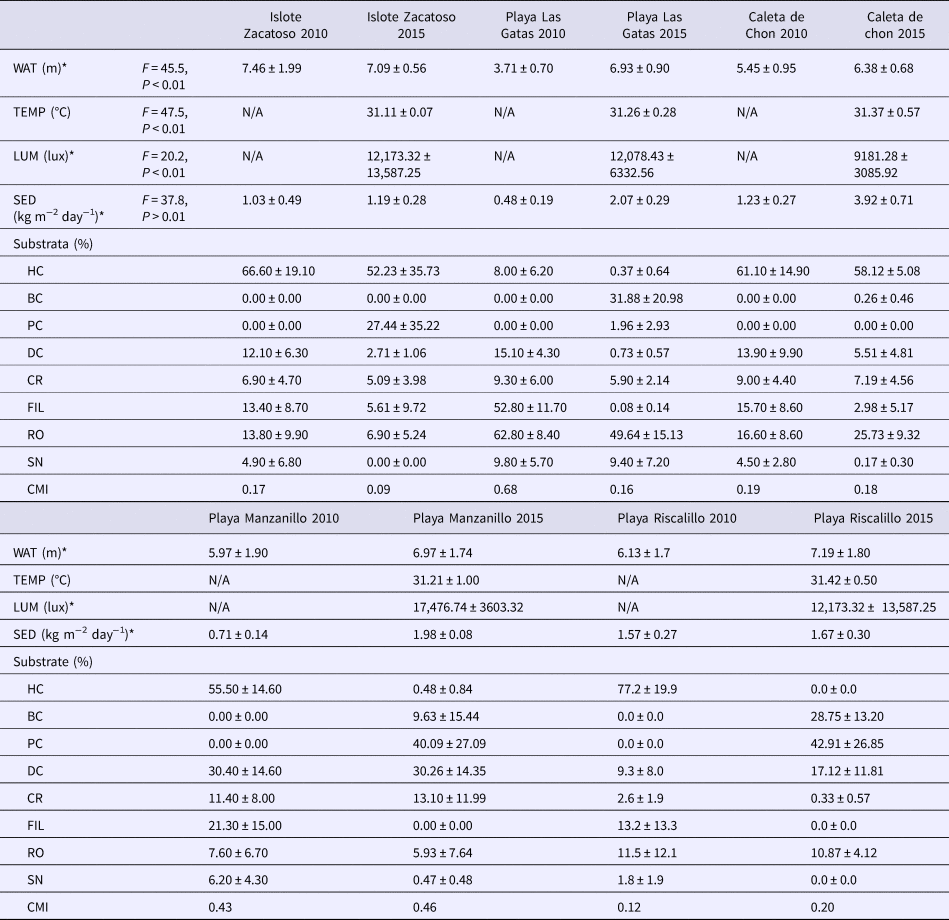
Significant spatial differences in 2015 when comparing environmental parameters across the sites by ANOVA are indicated by asterisk: water transparency (WAT), water temperature (TEMP), illumination (LUM) and sedimentation rate (SED). Healthy corals (HC), pale corals (PC), bleached corals (BC), attached dead corals (DC), coral rubble (CR), filamentous algae (FI), terrigenous rocks (RO) and sand (SN). Coral mortality index (CMI) and not available data (N/A).
Diversity and abundance of boring sponges
At the five sites described above, species richness and the abundance of boring sponges were recorded on pocilloporid corals, the major coral reef builders in the Mexican Pacific (Carballo et al., Reference Carballo, Bautista-Guerrero, Nava, Cruz-Barraza and Chávez2013). Environmental and coral health data were assessed simultaneously, following methods detailed in Nava & Carballo (Reference Nava and Carballo2008).
Three 50 m transects were oriented parallel to the shoreline along each reef at a depth of 5 m (Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). At every ~2 m along each transect, two substrate samples of ~9 cm3 each were taken. These samples included a fragment of attached dead coral substratum (DC), and a piece of coral rubble (CR), yielding 50 samples per transect (25 samples of each of the two types of coral substrate, following procedures outlined in Nava & Carballo, Reference Nava and Carballo2008, Reference Nava and Carballo2013; Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). By sampling 5 sites × 3 transects × 25 samples × 2 substrate categories, 750 samples of substrate were collected and investigated. All samples were broken into small pieces in the laboratory and examined for the presence of boring sponges, attempting to record every sponge species. When a sponge was found, a small portion of its tissue was sampled for identification. Sodium hypochlorite was used to digest the organic material in the sample (Carballo et al., Reference Carballo, Cruz-Barraza and Gómez2004), and the spicules were then examined under an optical microscope (Primo Star, Carl Zeiss Microscopy, Thomwood, USA). Species descriptions provided by Carballo et al. (Reference Carballo, Cruz-Barraza and Gómez2004, Reference Carballo, Hepburn, Nava, Cruz-Barraza and Bautista-Guerrero2007), Bautista-Guerrero et al. (Reference Bautista-Guerrero, Carballo, Cruz-Barraza and Nava2006) and Cruz-Barraza et al. (Reference Cruz-Barraza, Carballo, Bautista-Guerrero and Nava2011) were consulted to identify each sponge species to the highest taxonomic level possible. The incidence of each boring sponge species in each sample was considered as a record to calculate their abundance (FI) relative to the overall samples either per site or substrate category. FI per site was calculated considering the percentage of samples of both substratum types together (attached dead coral + coral rubble = 50 samples = 100%), averaged across transects. FI per substrate category (25 samples = 100%) considered the percentage of invaded samples in attached dead coral (FI-DC) and coral rubble (FI-CR), also averaged across transects. The same procedure was adapted to calculate the FI of each species of boring sponges, per site and by substrata type (Carballo et al., Reference Carballo, Bautista-Guerrero, Nava, Cruz-Barraza and Chávez2013; Nava & Carballo, Reference Nava and Carballo2013).
Data analysis
Environmental data recorded during September 2015 were analysed to test the assumptions of normality and homoscedasticity (Kolmogorov–Smirnov test, Sokal & Rohlf, Reference Sokal and Rohlf1995). Afterwards, each environmental variable was analysed with a series of separate one-way analyses of variance (ANOVA) and Student–Newman–Keuls post hoc tests (Zar, Reference Zar1984) to determine significant differences among five sites. A principal component analysis (PCA) based on Euclidian distances (Clarke & Warwick, Reference Clarke and Warwick1994) performed on the records of substrate types made during September 2015 to characterize the habitat conditions at each reef. Records of sponge abundance per site and substrate were not normally distributed (Kolmogorov–Smirnov test, Sokal & Rohlf, Reference Sokal and Rohlf1995) and were square root transformed prior to analysis with a two-way ANOVA with factors site (five levels) and substratum (two levels). Later, a Student–Newman–Keuls (SNK) post hoc test (Zar, Reference Zar1984) was applied to determine significant differences among means. To analyse the structure of boring sponge assemblages, cluster ordination and NMDS analysis (non-metric multidimensional scaling) were developed constructing a Bray–Curtis similarity matrix (Bray & Curtis, Reference Bray and Curtis1957) using the records of sponge abundance for each substratum from all sites (Kruskal & Wish, Reference Kruskal and Wish1978), which were square root-transformed beforehand. Similarity percentages (SIMPER) analysis was performed to detect which sponge species contributed to establish groups in cluster and MDS analysis (Warwick et al., Reference Warwick, Clarke and Suharsono1990; Clarke and Ainsworth, Reference Clarke and Ainsworth1993). To measure the strength of relationship between the environmental variables and the sponge abundance recorded per site, substrata and species; the Pearson correlation coefficient (R) and the coefficient of determination (R 2, Rao, Reference Rao1973) were employed using the square root-transformed data. Finally, using the same Bray–Curtis similarity matrix, a BIO-Env analysis was used to determine what combinations of environmental variables were correlated with the structure of boring sponge assemblages, using the records of the averaged environmental variables and the records made for sponge abundance per species at each site (Clarke & Ainsworth, Reference Clarke and Ainsworth1993).
Results
Environmental conditions
During September 2015, most environmental parameters significantly differed among sites. Caleta de Chon was characterized by particularly high sedimentation rates (F = 37.77, P < 0.01), high turbidity (F = 45.5, P < 0.01) and reduced light penetration (F = 20.2, P < 0.01), and it thus appeared to be a comparatively stressful environment for reef corals (Table 1). Islote Zacatoso stood out as the site with the lowest sedimentation rate, Playa Riscalillo had the clearest water column according to Secchi disk assessments, while illumination was most intense at Playa Manzanillo, also attesting to comparatively clear water. The environment at Playa Las Gatas showed high levels of sedimentation and low illumination. Water temperature recorded values near 31°C at all sites and showed significant differences (F = 47.5, P < 0.01). Maximum record was made at Playa Riscalillo and was lower at Islote Zacatoso (Table 1). The ONI index showed conditions of positive anomalies higher than 3°C throughout El Niño region 3 and thus warmer than normal water (Figure 2). This was related to the development of an El Niño event between April 2015 and April 2016.
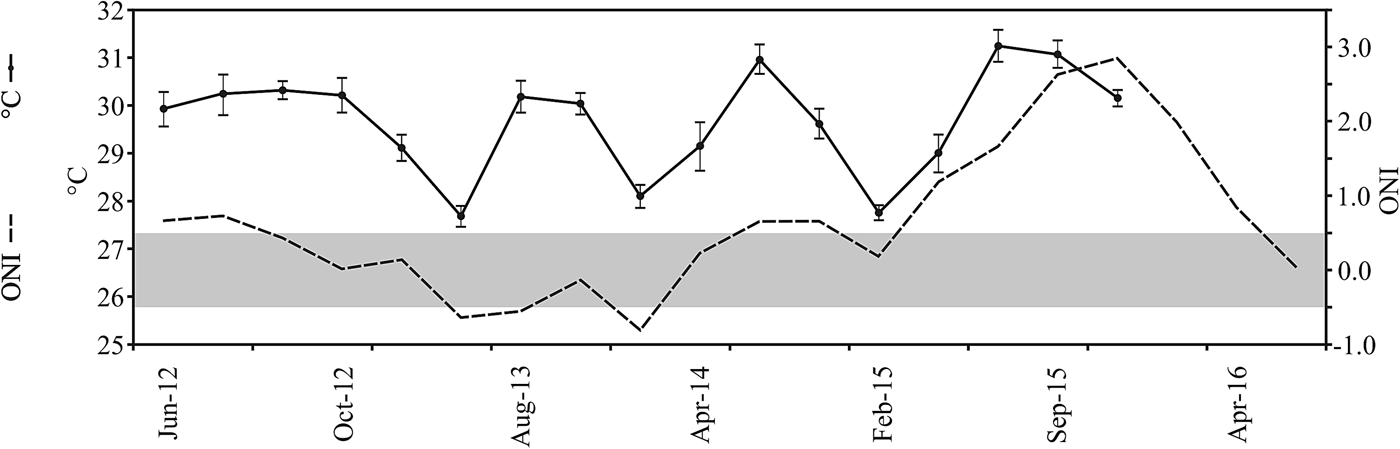
Fig. 2. Conditions of water temperature (black line, mean ± SD) recorded at Islote Zacatoso between June 2012 and December 2015. Temperature anomalies (dashed line), based on the records of the Oceanic El Niño Index made between June 2012 and May 2016 at El Niño 3 region (NOAA, 2017). Negative anomalies (La Niña conditions) and positive anomalies (El Niño conditions) are indicated as dashed line under and above grey bar, respectively.
Substrate composition
Substrate composition was different at the different survey sites and provided insights into the local conditions of reef health. The first PCA component showed a general increase in reefal substrate, and the second component showed a differentiation among sites based in the cover of healthy corals. Together, they absorbed 55.9% of data variability; revealing important differences among all sites (Figure 3 and Table 1). Based on the calculated CMI index of 0.66 (Table 1), Playa Las Gatas showed an intermediate conservation status and was set apart from the other sites by its high cover of terrigenous rock, sand and bleached corals. This site also had a low amount of filamentous algae, pale corals, healthy corals and coral rubble. Coral reefs from Islote Zacatoso and Caleta de Chon had CMI indices close to 0 and appeared to be well conserved (Table 1). They were characterized by their high cover of healthy corals, but also by high incidence of pale corals and by a low cover of bleached corals, terrigenous rocks and sand (Table 1). Playa Riscalillo and Playa Manzanillo were the sites with the worst conservation status and highest CMI indices. Playa Riscalillo showed a high cover of pale corals and bleached corals; and low cover of healthy corals, coral rubble, terrigenous rocks and sand. At Playa Manzanillo the patterns were less obvious, although the most important components here were pale, bleached and dead corals.

Fig. 3. Principal components analysis ordination (PCA) based on the records of substratum cover made along the three transects at Islote Zacatoso (IZ1–3), Playa Las Gatas (PG1–3), Caleta de Chon (CC1–3), Playa Manzanillo (PM1–3) and Playa Riscalillo (PR1–3).
Diversity and abundance of boring sponges
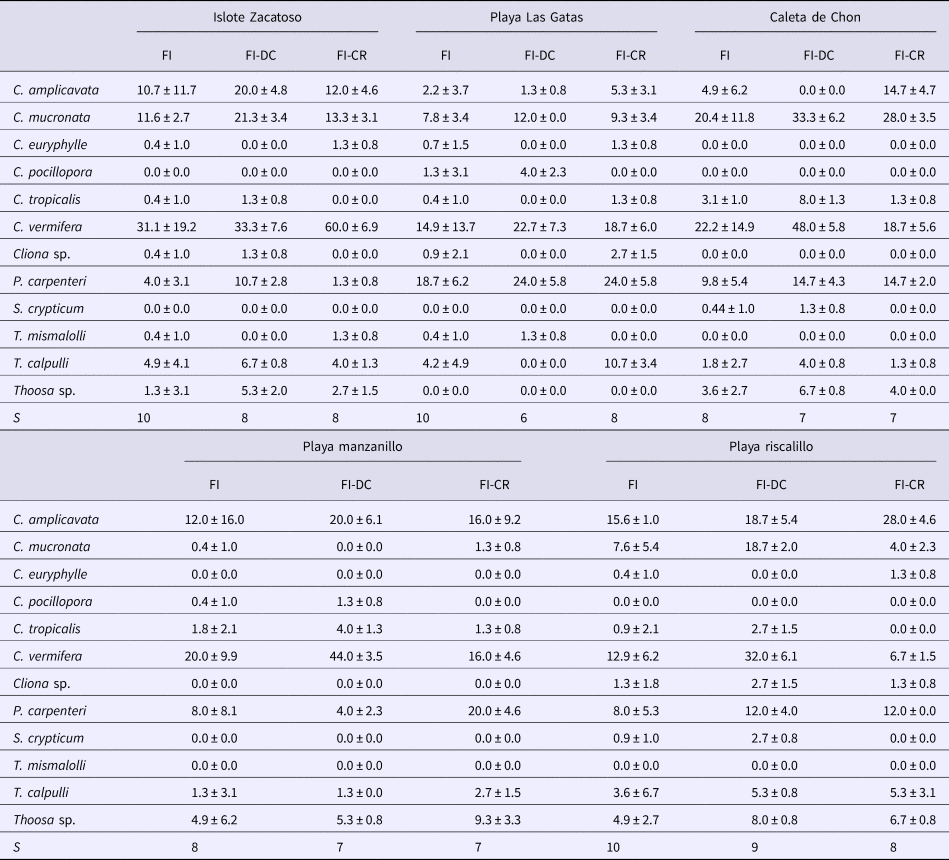
Twelve boring sponge species belonging to four genera (Cliona, Pione, Siphonodictyon and Thoosa) were recorded from 750 coral samples each of attached dead coral and coral rubble (Table 2). Across both substrates, Islote Zacatoso, Playa Las Gatas and Playa Riscalillo were the most diverse sites with 10 species each. In comparison, we found eight species at Caleta de Chon and Playa Manzanillo. When only attached dead coral is considered, Playa Riscalillo was again the most diverse and harboured nine species, Islote Zacatoso eight, while seven species were recorded at Caleta de chon and Playa Manzanillo and only six species were recorded at Playa Las Gatas. In coral rubble, eight sponge species were recorded at Islote Zacatoso, Playa Las Gatas and Playa Riscalillo. Seven species of boring sponges were recorded at Caleta de Chon and Playa Manzanillo. Species richness found in the rubble thus determined the overall ranking in boring sponge diversity per site.
Table 2. Sponge abundance (%, mean ± SD) and species richness (S) recorded per site (FI), in attached dead corals (FI-DC) and coral rubble (FI-CR)
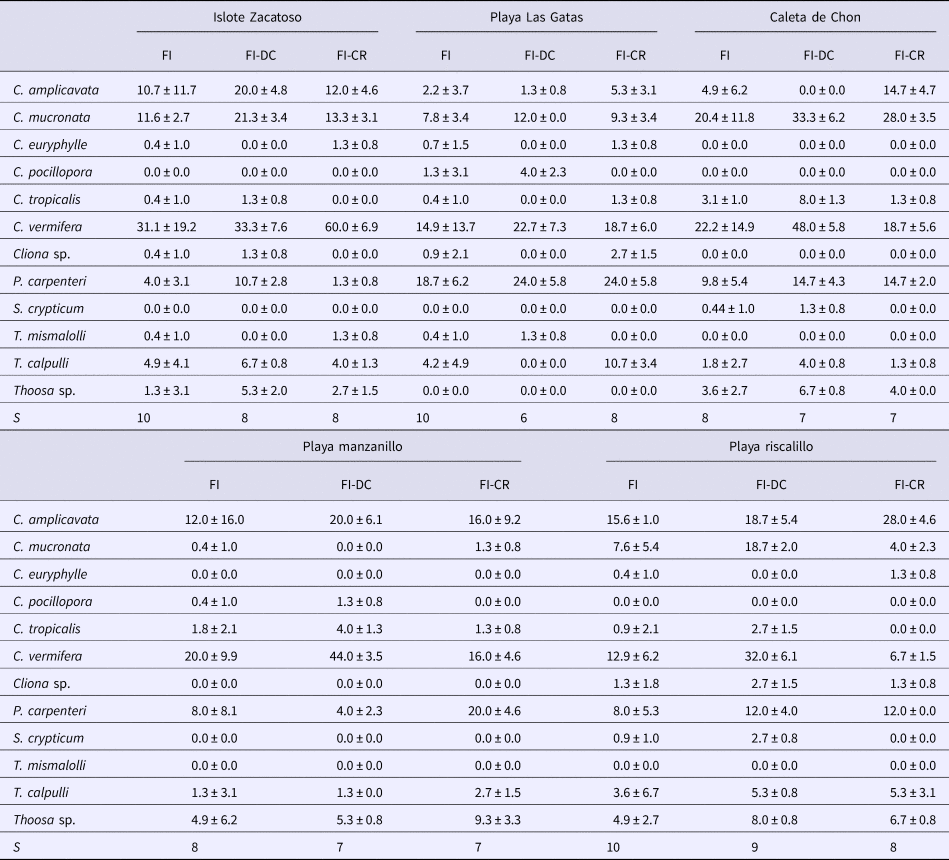
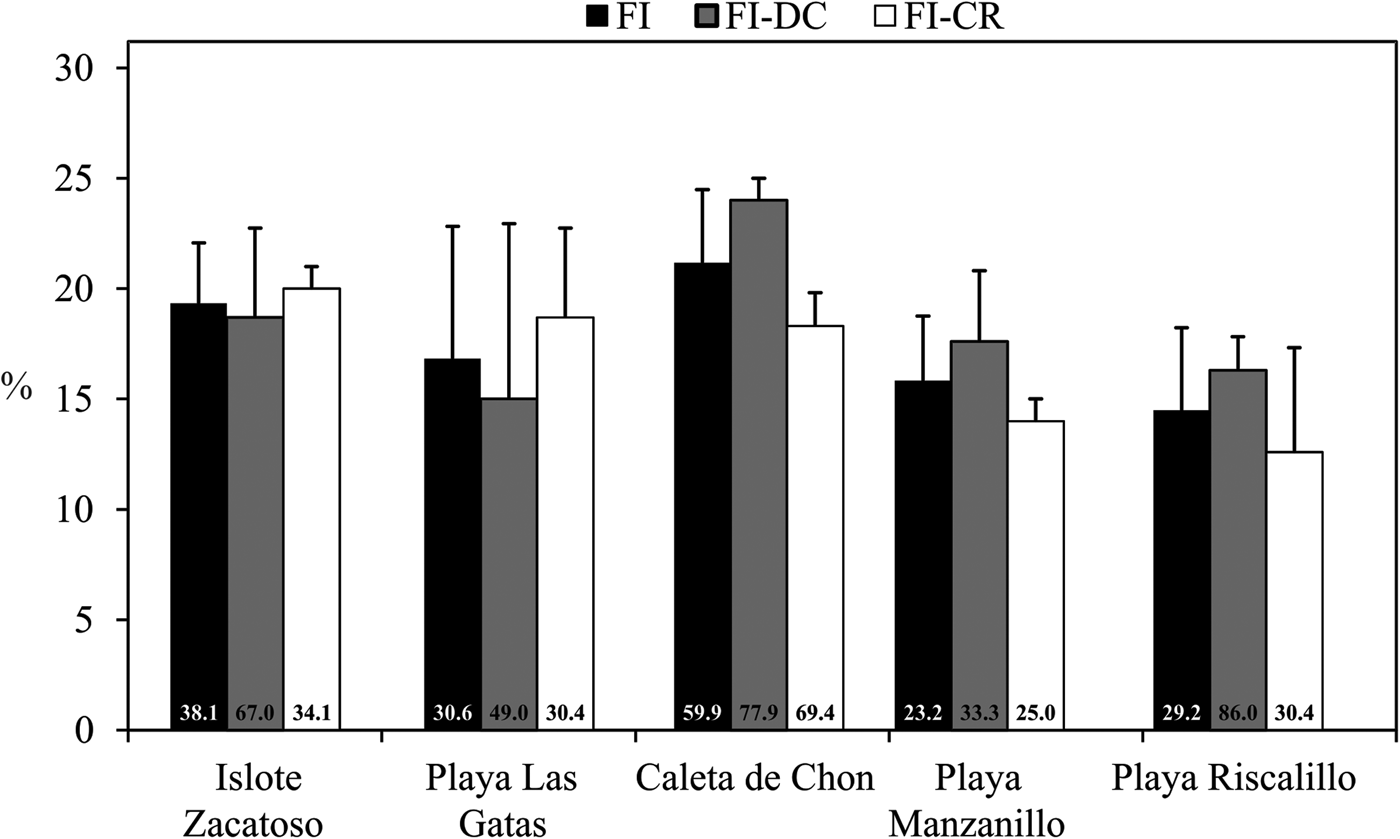
The species varied in their dominance of invading the substrates, and the most abundant species was Cliona vermifera. Cliona amplicavata, Cliona mucronata and Pione carpenteri were also quite common, but only about half as common as C. vermifera. Cliona tropicalis, Thoosa calpulli and Thoosa sp. were uncommon, and all other species were rare. Abundance of boring sponges did not depend on substrate type. Only at Caleta de Chon attached dead coral seemed to be more intensively invaded by boring sponges than coral rubble. When assessing both substrate types separately, we found no significant difference for sponge abundance among sites. However, when sponge abundance is analysed per site, the highest abundance of sponges occurred in Caleta de Chon and the lowest at Playa Riscalillo (F = 3.20, P < 0.05, Figure 4). Therefore, higher values of sponge abundance were found at the sites of lower sponge diversity and vice versa. The most dominant sponges at the lower-diversity sites were C. amplicavata and C. vermifera.

Fig. 4. Average abundance of boring sponges per site (FI), in attached dead coral (FI-DC) and coral rubble (FI-CR) recorded during this study (columns) and during 2010 (bottom of the columns). Significant differences were found only for FI (F = 3.20, P < 0.05, ANOVA). Bars above columns indicate the SD.
Boring sponge assemblages
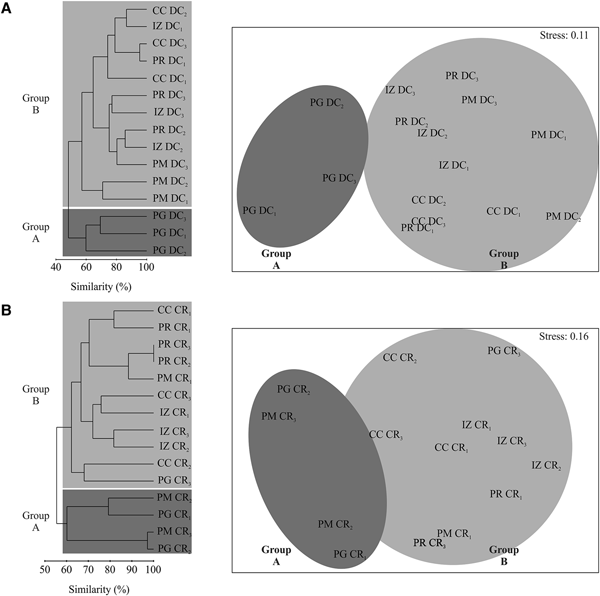
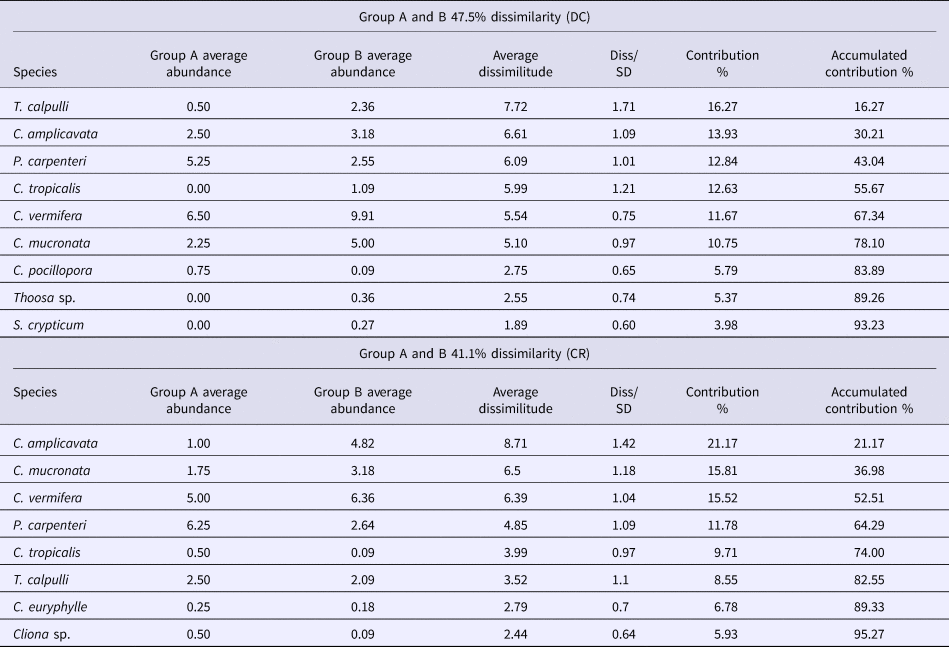
No clear, overarching spatial patterns were detected that determined sponge distributions, with the exception of an apparent influence of substrate unsuitable for clionaid recruitment. Boring sponge assemblages from dead coral substratum fell into two major clusters differentiated by 47.5% dissimilarity (Figure 5A). SIMPER analysis showed that these clusters were most strongly supported by the comparatively rare and patchily distributed species T. calpulli and C. amplicavata and in decreasing importance by P. carpenteri, C. tropicalis and C. vermifera, which together explained 78.1% of the cluster classification (Table 3). Group A was composed of the boring sponge assemblage of Playa Las Gatas, where C. pocillopora and especially P. carpenteri were more abundant than at the other sites, while abundance for C. amplicavata, C. tropicalis and C. vermifera were low (Table 2). While T. calpulli occurred at all other sites, it was missing in the attached substratum at Playa las Gatas. Group B contained the sponges that were more strongly associated to all other localities that had more coral substrate. These sites had the highest abundance of C. vermifera, C. mucronata, C. amplicavata, C. tropicalis and T. calpulli (Table 2). NMDS ordination (stress of 0.11) confirmed the segregation of groups A and B and showed that boring sponge assemblages found in dead corals from Playa Las Gatas, Playa Riscalillo and Playa Manzanillo were spatially heterogeneous (Figure 5A).

Fig. 5. Cluster analysis and two-dimensional MDS ordination of the records of boring sponge abundance of attached dead coral (DC) and coral rubble (CR) from the study sites (IZ, Islote Zacatoso; PG, Playa Las Gatas; CC, Caleta de Chon; PM, Playa Manzanillo and PR, Playa Riscalillo).
Table 3. Result tables for SIMPER analyses abundance and average dissimilarity of the boring sponge species that contributed to the formation of groups A and B for attached dead coral substratum (DC) and coral rubble (CR). The groups were sorted to 93.23% and 95.27% respectively, of the accumulative contribution (last column)
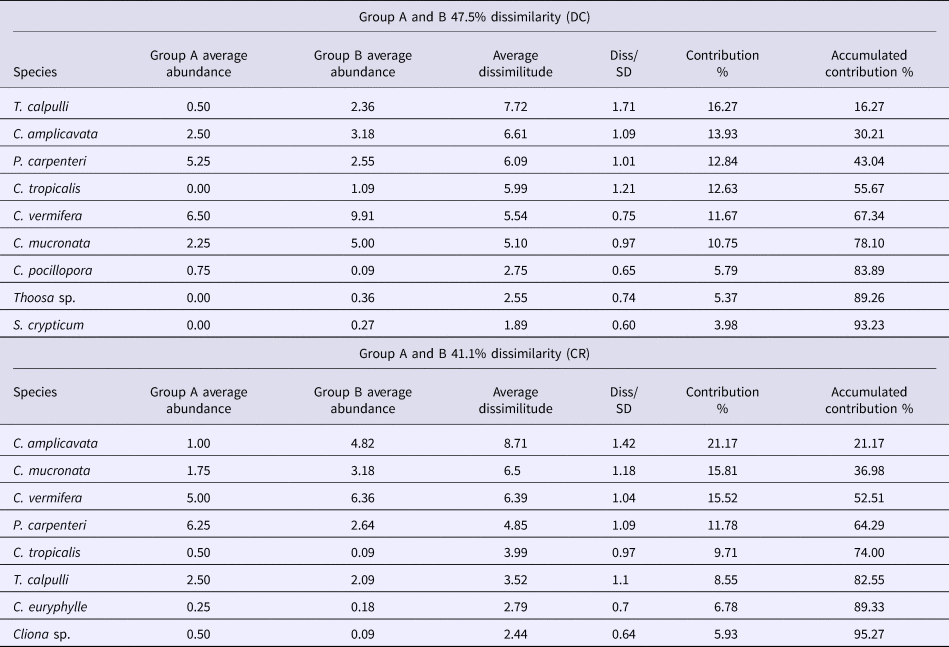
With regards to boring sponge assemblages from coral rubble, the clusters were not clearly segregated by sample site, even though two major clusters were differentiated with 41.4% of dissimilarity (Figure 5B). SIMPER analysis indicated that the following sponges drove together the 82.6% of these cluster patterns in decreasing importance: C. amplicavata, C. vermifera, P. carpenteri, C. tropicalis and T. calpulli (Table 3). Group A was made up of two transects each of Playa Las Gatas and Playa Manzanillo and it was not immediately obvious why the sponge community grouped differently on these four transects compared with the remaining transects. Sponge assemblages of this group exhibited low abundance of C. mucronata and C. vermifera, which were otherwise very common, while P. carpenteri was especially abundant at both sites (Table 2). Group B differed from A by high abundance of C. amplicavata and C. vermifera (the latter especially at Islote Zacatoso), and low abundance of T. calpulli (Table 2). NMDS ordination (stress of 0.16) showed a high heterogeneity of boring sponge assemblages at each site (Figure 5B).
Correlations between environmental conditions and the structure of boring sponge assemblages
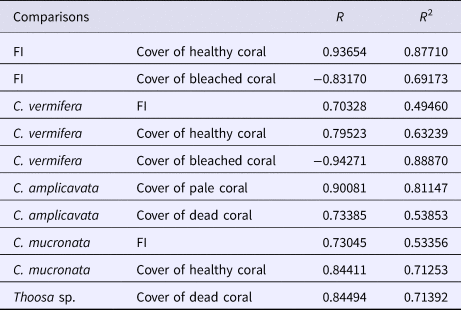
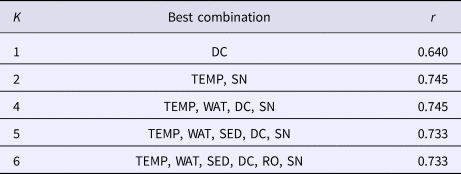
Boring sponge abundance at the different sites was positively correlated with cover of healthy corals, and they seemed to be negatively correlated with cover of bleached corals. Throughout all sample sites and substrates sampled, C. vermifera was the most dominant sponge. Changes in the distribution of C. vermifera would therefore affect the entire community of boring sponges. Its abundance was positively correlated with total abundance of boring sponges, but correlations were highest with cover of living corals (Table 4). This species seemed to be negatively correlated with cover of bleached corals. Another important species, C. mucronata also seemed to be more common where cover of healthy corals was higher. Abundance of C. amplicavata was positively correlated with cover of pale and dead corals and Thoosa sp. seemed to be positively correlated with coverage of dead corals (Table 4). Combinations of water transparency, sedimentation and cover of bleached corals, dead corals, terrigenous rocks and sand correlated with the variation in sponge assemblages (Table 5). Nonetheless, cover of dead corals by itself showed the highest correlation with the structure of boring sponge assemblage (Table 5).
Table 4. Correlations among the records of boring sponge abundance and the records of substrate cover. Pearson correlation coefficient (R) and coefficient of determination (R 2)

Table 5. Effects of environmental variables on boring sponge distributions as evidenced by Spearman rank correlation (r)
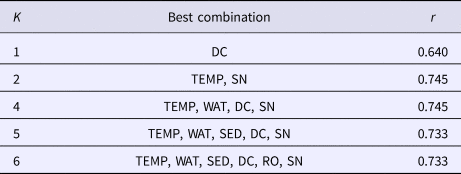
Cover of attached dead corals (DC), water temperature (TEMP), water transparency (WAT), sedimentation rate (SED), cover of terrigenous rocks (RO) and cover of sand (SN).
Discussion
Extensive mortality of reef-building corals caused by environmental deterioration and climate change is a major challenge for the permanence of coral reef structures (Sheppard et al., Reference Sheppard, Spalding, Bradshaw and Wilson2002; Schönberg & Ortiz, Reference Schönberg, Ortiz, Riegl and Dodge2009). Sponge bioerosion may slow or impede reef recovery after disturbances that enhance the availability of dead coral substrata (Schönberg & Ortiz, Reference Schönberg, Ortiz, Riegl and Dodge2009; Carballo et al., Reference Carballo, Bautista-Guerrero, Nava, Cruz-Barraza, Hernández-Zanuy and Alcolado2010). Based on our data, we observed an increase in sedimentation rate at Playa Las Gatas, Caleta de Chon and Playa Manzanillo. Water transparency was low, especially at Playa Las Gatas and Caleta Chon, apparently a common occurrence on Mexican Pacific reefs near densely populated coastal areas and river mouths (Reyes-Bonilla et al., Reference Reyes-Bonilla, Carriquiry, Leyte-Morales and Cupul-Magaña2002; Granja-Fernández & López-Pérez, Reference Granja-Fernández and López-Pérez2008; Nava & Carballo, Reference Nava and Carballo2013; Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). Positive anomalies of water temperature higher than 3°C were related to the 2015–16 El Niño event (NOAA, 2017). With regard to records of substratum cover made in 2010 (Table 1), all sites presented a reduction in cover of healthy corals (Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). Nonetheless, Playa Riscalillo and Playa Manzanillo showed the worst effect of such thermal anomaly, Playa Las Gatas only suffered an increase in cover of bleached corals and both Caleta de Chon and Islote Zacatoso maintained their high coverage of healthy corals. Deteriorating conditions at coral reefs including elevated nutrient levels and an increased availability of dead coral substrata can at times lead to an increased abundance of boring sponges (Schönberg et al., Reference Schönberg, Fang, Carreiro-Silva, Tribollet and Wisshak2017a and references therein). Nonetheless, at least during our study, such conditions did not lead to an escalation in the invasion of substrata by boring sponges. Moreover, assemblages of sponges displayed highly complex patterns. Species richness showed low difference between substrata and sites, which was in accordance with previous records (Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). This indicated that the local species composition of boring sponges remained stable across time, despite current conditions. However, sponge invasion in dead coral and coral rubble was almost three times lower than from previous observations at the same sites (Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). This was despite species such as C. vermifera, C. amplicavata, C. mucronata and P. carpenteri becoming much more abundant in comparison to their abundances recorded on 2010. Apart from C. amplicavata these species seemed to be tolerant of sedimentation and turbidity (Table 2), and might have been able to adapt to the changes on the reefs and benefit from the larger amount of suitable substrate. Abundance of boring sponges was higher in attached dead corals than in unattached rubble (Carballo et al., Reference Carballo, Bautista-Guerrero and Leyte-Morales2008a; Nava & Carballo, Reference Nava and Carballo2013, present paper). Boring sponges in coral rubble are assumed to be susceptible to be buried and smothered in sediment and under the risk of being carried into unfavourable areas by water currents (Carballo et al., Reference Carballo, Bautista-Guerrero and Leyte-Morales2008a; Schönberg, Reference Schönberg2015). Then the instability and the possibility of being occasionally transported to different places may explain the difficulty in detecting clear patterns of sponge assemblages in this substratum. Availability of dead coral substratum seemed to be important for explaining the variation in the configuration of sponge assemblages across sites, but it was not directly correlated with boring sponge abundance. Among the boring sponge species C. amplicavata was an important species that seemed to be positively influenced by the cover of pale and dead coral. Occurrence of this species has previously been related to eutrophic conditions (Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). It is interesting to note that the abundance of boring sponges was higher at the sites with higher cover of healthy corals and low cover of bleached corals. This pattern seemed to rely on the high occurrence of C. vermifera and C. mucronata in the sponge assemblages. Both species were previously found at sites with low sedimentation rate and high water transparency (Nava & Carballo, Reference Nava and Carballo2013), but now also occurred at sites with intensive sedimentation and high turbidity (Tables 1 and 2). Cliona vermifera, which was on average 10 times more abundant than most other species in the assemblages, is prevalent at all coral reefs studied along the eastern Pacific coast, attesting to its wide ecological plasticity (Carballo et al., Reference Carballo, Bautista-Guerrero and Leyte-Morales2008a, Reference Carballo, Cruz-Barraza, Nava and Bautista-Guerrero2008b; Nava & Carballo, Reference Nava and Carballo2013; Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014; Table 2). Its importance in sponge assemblages across this region encouraged the study of its biology (Nava & Carballo, Reference Nava and Carballo2008; Bautista-Guerrero et al., Reference Bautista-Guerrero, Carballo and Maldonado2014). Seasonal warming triggers its reproductive activity, thereby possibly indirectly enhancing its potential as a threat for the health of coral reefs (Bautista-Guerrero et al., Reference Bautista-Guerrero, Carballo and Maldonado2014). Severe episodes of thermal stress and El Niño events can lead to massive coral bleaching and mortality, favouring increased settlement opportunities for boring assemblages that take advantage of the enhanced availability of exposed substrate (Brown, Reference Brown1997; Rützler, Reference Rützler2002; Schönberg & Ortiz, Reference Schönberg, Ortiz, Riegl and Dodge2009). As it appears, reproductive activity of C. vermifera and some other species of bioeroding sponges could not be affected during this time, taking advantage of any newly available substrate (Bautista-Guerrero et al., Reference Bautista-Guerrero, Carballo and Maldonado2014). However, the present results seem to indicate that reefs dominated by species such as C. vermifera, may not be highly invaded immediately after episodes of thermal stress. It is important to note, however, that in the absence of this species, the other next important ones, such as C. amplicavata, could influence different responses of boring sponge assemblages at coral reefs affected by high coral mortality, especially under eutrophic conditions (Nava et al., Reference Nava, Ramírez-Herrera, Figueroa-Camacho and Villegas-Sánchez2014). Boring sponges are a relevant component of coral reefs, but their response to environmental stress and natural impacts such as El Niño events seems to be more complex than previously realized. The Eastern Pacific coral reefs are especially vulnerable environments, not only facing climate change, but also having earlier been identified as habitats with weak calcification and elevated levels of bioerosion (Glynn, Reference Glynn1988; Eakin, Reference Eakin2001; Manzello et al., Reference Manzello, Kleypas, Budd, Eakin, Glynn and Langdon2008; Alvarado et al., Reference Alvarado, Grassian, Cantera-Kintz, Carballo, Londoño-Cruz, Glynn, Manzello and Enochs2017). Although more studies are necessary, our results imply that El Niño events in the Mexican Pacific are not likely to cause immediate invasion outbreaks by boring sponges.
Acknowledgements
We are grateful to Thierry Durand and Capitan ‘Chilolo’ for the logistical support provided during the sampling.
Financial support
The authors acknowledge the following sources of funding: CONACYT-SEP No.CB-2012-01-177537 and the Research Council of Scientific Research of the Universidad Michoacana de San Nicolás de Hidalgo.