INTRODUCTION
The marine benthic environment is highly dynamic and its sessile fauna is constantly susceptible to physical injuries. These injuries, caused by predation or physical disturbances, may result in a decrease in the animal's feeding capacity and, if extensive, constitute a potential threat to survival (Jackson & Palumbi, Reference Jackson, Palumbi, Lévi and Boury-Esnault1979). Reconstitution and/or replacement of lost structures and tissues through regenerative processes are the way to recover from such harmful events. Regeneration can overlap and be complementary to asexual reproduction, but the first is involved in the development of a lost structure in a non-embryonic scenario, while the latter is involved in the modification of a part of the parent into a new and independent individual (Carlson, Reference Carlson2007; Kürn et al., Reference Kürn, Rendulic, Tiozzo and Lauzon2011). Among sessile marine invertebrates, sponges are known to possess great capacity for regeneration, maybe higher than any other living metazoan (Simpson, Reference Simpson1984; Duckworth, Reference Duckworth2003). Regeneration is probably a permanent event in the life history of adult sponges. In highly dynamic habitats, for example, where the presence of grazers is ecologically significant, and in tropical waters, where fishes and turtles are specialist sponge-predators, this regeneration capacity seems to be vital to guarantee the survival of sponge species (Ayling, Reference Ayling1983; Hoppe, Reference Hoppe1988).
There are many factors (intrinsic or extrinsic) that could influence positively or otherwise the regeneration rates in sponges, such as external morphology and place and size of the injury (for a review see Henry & Hart, Reference Henry and Hart2005). For example, when a wound occurs in an individual with a well-defined morphology (e.g. tubular) or when it affects a vital body region/structure (e.g. osculum), the individual should act rapidly to restore its optimal shape, otherwise it could cause structural failure and death (Jackson, Reference Jackson, Larwood and Rosen1979; Bell, Reference Bell2002). As it is a vital process for survival, regeneration rate is usually much faster than growth rate in sponges, reaching 2900 times growth velocity in some species (Ayling, Reference Ayling1983; Leys & Lauzon, Reference Leys and Lauzon1998; Turon et al., Reference Turon, Tarjuelo and Uriz1998; Wulff, Reference Wulff2010). It is also interesting to note that regeneration capacity is species specific and does not seem to follow any phylogenetic sense, meaning that even species with shared evolutionary history may present different regeneration rates (Wulff, Reference Wulff2010).
Although works concerning sponge regeneration have been published mainly in the last 30 years, the regenerative capacity of these animals has been known since the ancient Greek civilization, when the fishing tradition of bath sponges started and developed through the Mediterranean and later the Caribbean and Polynesia. Today, sponge-farming techniques continue to exploit the great capacity of these animals of regenerating a whole individual from a small fragment. This is even considered one of the solutions for the biotechnology industry seeking new metabolites and for the reconstitution of declining natural stocks due to disease and overfishing (Verdenal & Vacelet, Reference Verdenal, Vacelet and Rützler1990; Osinga et al., Reference Osinga, Tramper and Wijffels1999; Pronzato, Reference Pronzato1999).
Since the pioneering work of Wilson (Reference Wilson1907), where he observed and discussed the cell aggregation capacity of sponges, many experiments exploring this issue and the regeneration of lost parts were undertaken in the class Demospongiae. Discussions about the independence between regeneration and growth processes (Reiswig, Reference Reiswig1973; Hoppe, Reference Hoppe1988) and regeneration speed related to sponge morphology (Hoppe, Reference Hoppe1988; Rohde & Schupp, Reference Rohde and Schupp2012) took place. In this context, more than 30 demosponge species with the most diverse growth forms and from different evolutionary lineages have been studied since then (Reiswig, Reference Reiswig1973; Ayling, Reference Ayling1983; Hoppe, Reference Hoppe1988; Bell, Reference Bell2002; Duckworth, Reference Duckworth2003; Gilliam et al., Reference Gilliam, Walker, Saelens, Fahy and Kosmynin2008; Wulff, Reference Wulff2010; Rohde & Schupp, Reference Rohde and Schupp2012). Regeneration in hexactinellid sponges was studied and discussed in recent works (Leys & Lauzon, Reference Leys and Lauzon1998; Leys et al., Reference Leys, Mackie, Reiswig and Sims2007), while the class Calcarea was the focus of such interest mainly in works published in the 1960s and 1970s (Korotkova, Reference Korotkova1961a, Reference Korotkovab, Reference Korotkova1963a, Reference Korotkovab, Reference Korotkova1969, Reference Korotkova1970, Reference Korotkova1972; Tuzet & Paris, Reference Tuzet and Paris1963). Since then, to our knowledge, the calcareous sponges were neglected as the subject of regeneration studies. In contrast to the number of demosponges studied, only five species of calcareous sponges were investigated concerning their regenerative capacity: Sycon lingua (Haeckel, 1870), S. raphanus Schmidt, 1862, S. ciliatum (Fabricius, 1780), Leucosolenia variabilis (Haeckel, 1870) and L. complicata (Montagu, 1814). All of them are syconoid and polarized (S. raphanus – solitary; S. lingua and S. ciliatum – several tubes) or asconoid and amorphous (L. variabilis and L. complicata – several tubes emerging from stolons).
In the present work, we investigated the regenerative capacity of an asconoid and polarized species concerning two body parts: osculum and choanosome. We compared our results with previous data and suggest whether the regenerative capacity is influenced by the polarity or body organization. Besides bringing new information about the regeneration in calcareous sponges, we also collected and reviewed the current knowledge about the regeneration process in the class Calcarea.
MATERIALS AND METHODS
We investigated the regeneration capacity of Ernstia sp. (Clathrinida, Calcinea) concerning two regions of its body: (1) osculum and (2) choanosome. This yellow sponge has a well-defined globular shape, formed by regularly anastomosed tubes, asconoid aquiferous system and a clear polarized basal-apical symmetry with, in general, one apical osculum. It was referred by Klautau et al. (Reference Klautau, Azevedo, Cóndor-Lujan, Rapp, Collins and Russo2013) as Ernstia sp. nov. 2. The experiment was carried out in a tide pool (Falsa Barreta) in Rocas Atoll (Rio Grande do Norte state, north-eastern Brazil; 3°51′S 33°49′W). This species is considered endemic from the Rocas Atoll, where it is very abundant, being found in all tide pools, hanging from the ceiling of small caves or living in small crevices. Eight individuals were selected for each of the two regeneration experiments. (1) In the first experiment, only the osculum of each individual was totally removed with a razor blade. (2) For the second experiment, a longitudinal section of the choanosome was made, removing apical, central and basal regions. The 16 individuals were checked every other day for 8 days. Photographs were taken with a rule as a reference of size of the individuals. The measurements of growth were acquired with the software AxioVision 4.6 (Carl Zeiss Imaging Solutions). A one-way ANOVA was undertaken to compare regeneration and speed rates.
RESULTS
Experiment 1
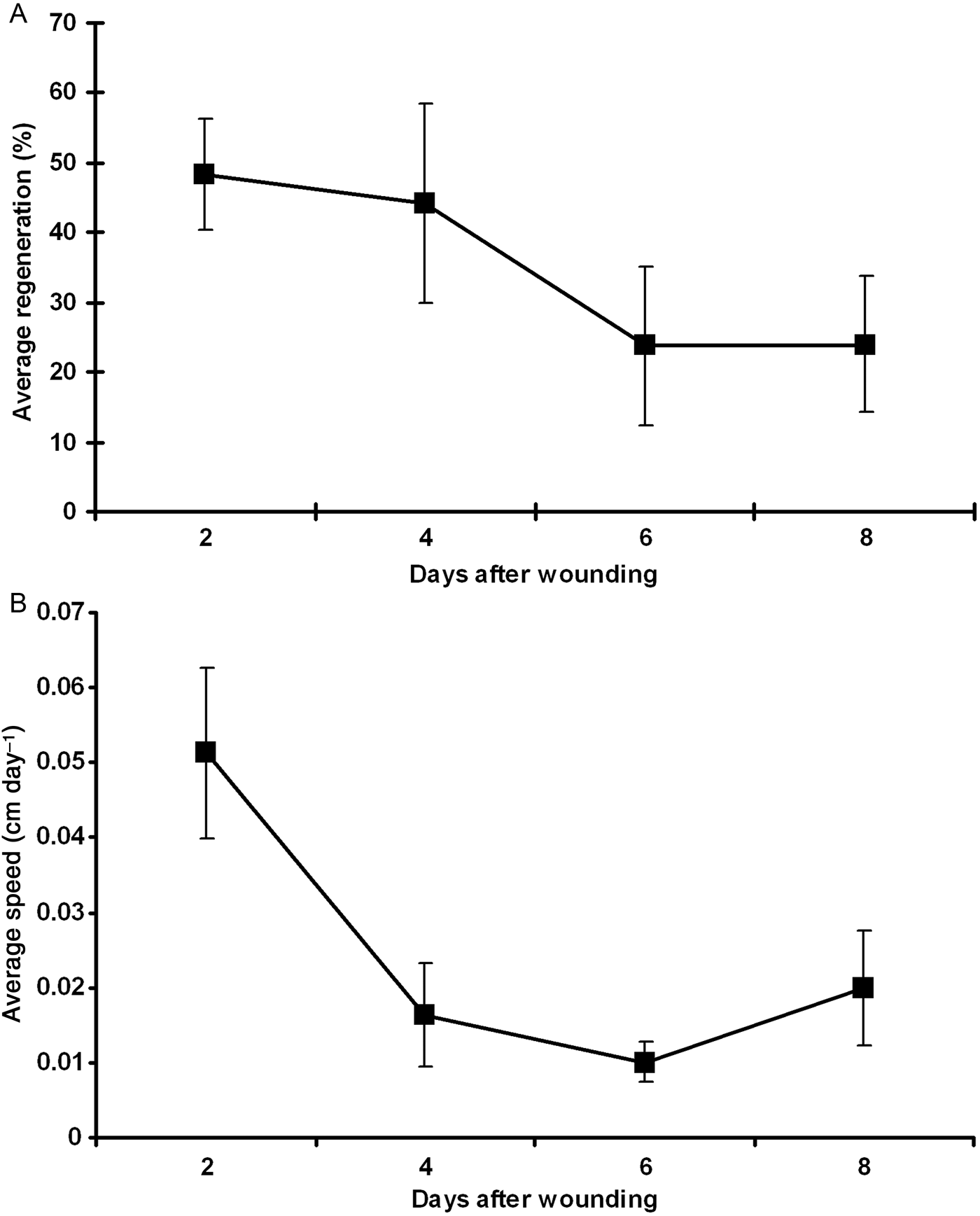
All the eight individuals studied fully regenerated their oscula after 8 days. Two days after the removal, a small growing osculum could be observed in all individuals (Figure 1). During this period (first 2 days), the osculum reached a regeneration rate that varied from 26.6 to 100% (48.3% in average ±8.0 of standard error (SE)) of its original size, with a regeneration speed varying from 0.02 to 0.12 cm day−1 (0.05 cm day−1 in average ±0.01 SE) (Figure 2). Between subsequent intervals (4, 6 and 8 days after being wounded), the regeneration rates slightly declined, reaching 44.2% (±14.3), 23.7% (±11.2) and 24% (±9.6) of average, respectively (Figure 2A). Nonetheless, differences in the regeneration rates among all intervals were not significant (ANOVA, P = 0.271). After the 8th day of the injury (and the end of the experiment), all individuals had regenerated their oscula on average 93.25% (±8.5 SE) compared with the original size (before the injury). The regeneration speed also declined after the first 2 days, reaching 0.01 (±0.007), 0.01 (±0.003) and 0.02 (±0.007) cm day−1 in the subsequent intervals respectively, and 0.02 cm day−1 (±0.002 SE) on average during the whole period. The average regeneration speed in the first 2 days after the wounding (0.05 cm day−1) was significantly higher when compared with the regeneration speed in the other intervals (ANOVA, P = 0.004; Tukey test, P < 0.05).

Fig. 1. Sequence of photographs following the osculum regeneration of one individual of Ernstia sp. (A) Before; (B) just after injury; (C) 2 days; (D) 4 days; (E) 6 days and; (F) 8 days after the induced injury. The white arrow points to the location and growth of the osculum. Scale bar: 0.5 cm.

Fig. 2. (A) Average regeneration of the osculum (in %; with standard error) and (B) average speed of regeneration (in cm day−1; with standard error) along the days after the wounding of the eight individuals of Ernstia sp. analysed.
Experiment 2
In contrast to the osculum experiment, all the sponges that had a fragment of their choanosome removed did not fully regenerate the lost part after the 8th day of the study. However, 2 days after the injury, the sponges managed to heal it completely, closing the opened wound. As they only cicatrized the wound, no statistical analysis was done concerning choanosome regeneration.
DISCUSSION
Since the beginning of the last century it was known that calcareous sponges are capable of regeneration after a wound and reaggregation after cellular dissociation (Maas, Reference Maas1910; Huxley, Reference Huxley1912), but it was especially between the 1950s and the 1970s that studies with the class Calcarea were published (Jones, Reference Jones1957, Reference Jones1958; Korotkova, Reference Korotkova1961a, Reference Korotkovab, Reference Korotkova1963a, Reference Korotkovab, Reference Korotkova1969, Reference Korotkova1970, Reference Korotkova1972; Tuzet & Connes, Reference Tuzet and Connes1962; Korotkova & Gelihovskaia, Reference Korotkova and Gelihovskaia1963; Tuzet & Paris, Reference Tuzet and Paris1963). Since then little attention was given to this topic.
Some works that preceded the ones cited above focused on the ‘level of integration’ of different sponge species and the differences between regeneration and somatic embryogenesis (Korotkova, Reference Korotkova1963a). Tokin (Reference Tokin1963) dedicated one of his works to establish both terms among sponges and other organisms due to misuse. Concerning Porifera, he concluded that the wound healing process should be called regeneration, while the formation of new individuals after cell aggregation or deep injury is a product of somatic embryogenesis because it consists of a radical cellular reorganization, which alters the existing correlations among cells in a process similar to asexual reproduction. In this work he also stated that ‘weakly integrated’ (usually amorphous, multioscular) sponges often show somatic embryogenesis. Instead, ‘highly integrated’ (polarized, unioscular) sponges would restore their tissue via regeneration, recovering their original shape and functioning. The ‘integration level’ in sponges was sometimes discussed together with its concept of individuality. In those multioscular sponges (that sometimes were called colonies) composed of several tubes (units) connected by stolons at the base (e.g. Leucosolenia), the level of individuality and integration of each ‘unit’ and the whole sponge was considered very low. On the other hand, in single, polarized (unioscular) species the level of integration and individuality was considered higher. In those single forms the regeneration power should be higher and the restoration faster than in multioscular forms due to a higher individuality, integration and polarized body (Korotkova, Reference Korotkova1963a, Reference Korotkova1970).
The regeneration differences depending on the morphology can be demonstrated even in species of the same genus, suggesting that (as in Demospongiae; Wulff, Reference Wulff2010) this event does not obey a phylogenetic sense. When Sycon lingua and S. raphanus were cut longitudinally in two or four parts, individuals of S. lingua bent inwards, closing into a bean-shape, and a new osculum was formed in a position different from the original, while in S. raphanus the opposite margins joined, closing the individuals again and maintaining the original shape with the original osculum (figure 2 in Korotkova, Reference Korotkova1963b). In these experiments, the majority of specimens of S. lingua changed polarity, while in S. raphanus it was maintained. When individuals of S. lingua, S. raphanus and S. ciliatum were cut transversally in two or three parts, the fragments regenerated the lost parts (osculum and base), maintaining the original polarity. Only in a few individuals of S. lingua (15%) did a distortion of polarity occur (Figure 6 in Korotkova, Reference Korotkova1963b). These differences may be due to the level of ‘integration and individuality’ observed in each species. Tuzet & Paris (Reference Tuzet and Paris1963) found the same results cutting S. raphanus longitudinally and transversally, but when the cut passed over two-thirds of the sponge body, two tubes with one osculum each were originated.
Korotkova (Reference Korotkova1961a) carried out several regeneration experiments using Leucosolenia complicata, obtaining different results. When doing partial longitudinal cuts in the osculum, most specimens regenerated the original shape, but some doubled oscula and deformed regeneration were also observed. When the osculum was totally removed, only 15% regenerated, while up to 60% closed the opening and new lateral buds appeared. Korotkova & Gelihovskaia (Reference Korotkova and Gelihovskaia1963) also did experiments with L. complicata and L. variabilis and observed 100 and 87% of regeneration respectively when the oscula were cut. An inversion of polarity was observed in 12 and 50% of the cases when the apical and basal parts of the tubes were cut. The orientation of spicules and, sometimes, the change of the oscular rim position were also observed in L. complicata and L. variabilis (Jones, Reference Jones1958).
The level of integration and the polarity of sponges related to their external morphologies are the main issues discussed by many authors (Korotkova & Gelihovskaia, Reference Korotkova and Gelihovskaia1963; Tokin, Reference Tokin1963; Tuzet & Paris, Reference Tuzet and Paris1963; Korotkova, Reference Korotkova1970; Tuzet, Reference Tuzet and Grassé1973). Many of the examples suggest that the mechanisms of integration in calcareous sponges are very unstable and the changes in polarity after an injury may depend on the degree of disintegration of the system caused by the trauma (Tuzet, Reference Tuzet and Grassé1973). The influence of the morphology on the capacity of regeneration in calcareous sponges seems very clear. Sycon raphanus usually is found in a solitary form, with a single tube and well-defined body symmetry, while S. lingua and S. ciliatum, when achieving a certain size, are found with more than one tube arising with indistinct boundaries at the same base (Korotkova, Reference Korotkova1970). Leucosolenia complicata and L. variabilis are formed by several branching tubes arising from a common base spreading on the substrate with several stolons (Korotkova, Reference Korotkova1961a). Unioscular and polarized species (as S. raphanus) are more capable of restoring their original shape, while in multioscular species (S. lingua, S. ciliatum, L. complicata and L. variabilis) it seems to be a random process.
Like S. raphanus, the species studied here (Ernstia sp.) clearly shows a well-defined polarized body but with an asconoid aquiferous system and a single, apical osculum, which certainly exerts a crucial role in the functioning of the aquiferous system of the individuals. In the present work, we observed a fast regeneration oriented to the apical region (osculum) in order to restore the shape of the individuals, while the choanosome did not reconstitute the lost part but, at least in the short term, regenerated the injured tissue (Carlson, Reference Carlson2007). Osculum regeneration in demosponges also tends to be faster than choanosome regeneration (Hoppe, Reference Hoppe1988; Walters & Pawlik, Reference Walters and Pawlik2005; Rohde & Schupp, Reference Rohde and Schupp2012). As a ‘solitary’ and unioscular species with a polarized external morphology, the osculum regeneration in Ernstia sp. was complete (not random) and directed to restore the optimal shape of the individuals. It can then be considered the true regeneration process cited by Tokin (Reference Tokin1963) driven by the clear polarization of the body and by the importance of the structure. The same was observed by Korotkova in her works with S. raphanus.
Differently from the osculum, Ernstia sp. did not regenerate the entire lost choanosome, but healed the opened wound in less than 2 days. It is known that some calcareous species may take longer periods to heal lost pieces of the choanosome after an induced injury: Leucosolenia complicata took up to 10 days (Korotkova, Reference Korotkova1961a), while S. raphanus did not regenerate nor cicatrized but, after up to 6 days, the sponge retreated at the level of the cut and separated in two (Tuzet & Paris, Reference Tuzet and Paris1963). It is possible that, if followed for a longer period, the individuals studied here would regenerate the lost part to regain the lost substrate in a regular process of growth. The non-immediate recovering of the lost part of the choanosome in Ernstia sp. could be explained by its asconoid aquiferous system. Possibly the lost part did not compromise the functioning and survival of the individuals, and the fast healing was necessary only to avoid, for example, the settlement of particles or organisms in the exposed parts that could preclude the normal function of the aquiferous system (Hoppe, Reference Hoppe1988; Leys & Lauzon, Reference Leys and Lauzon1998).
As the mesohyl of calcareous sponges is almost acellular (except for sclerocytes), the totipotency of choanocytes and their possible role in the regeneration process of sponges were already proposed (Funayama, Reference Funayama2010, Reference Funayama2012). Research shows that cells that will work in the regenerative process probably are derived from the neighbouring tissue around the wounded area (Tuzet & Paris, Reference Tuzet and Paris1963; Boury-Esnault, Reference Boury-Esnault1976) and the role of choanocytes and sclerocytes are evident in Calcarea, while in Demospongiae the archaeocytes are more important (Korotkova, Reference Korotkova1963a, Reference Korotkova1972). Choanocytes originate oocytes and spermatozoa in some calcareous sponges (Gaino et al., Reference Gaino, Burlando and Buffa1987; Lanna & Klautau, Reference Lanna and Klautau2010) and may also be the main source of stem cells in the class (Funayama, Reference Funayama2012). In a histological study of the regeneration process in Sycon ciliatum, a decrease in the number of choanocyte chambers and an increase of amoebocytes around the regenerative area were observed (Tuzet & Paris, Reference Tuzet and Paris1963). These amoebocytes may be derived from mobile cells of the mesohyl or from the differentiation of nearby choanocytes (Tuzet & Paris, Reference Tuzet and Paris1963) and could be acting in the regenerative process. It has already been observed that choanocytes may detach from choanocyte chambers, lose their flagella, differentiate into amoeboid cells, and move into the mesohyl to act as stem cells in calcareous sponges (Korotkova, Reference Korotkova1972; Gaino et al., Reference Gaino, Burlando and Buffa1987; Funayama, Reference Funayama2012). Nevertheless, Korotkova (Reference Korotkova1970) also proposed that, in asconoid species, the regenerative process takes place with the pinacocytes and the posterior spread of underlying choanocytes to the recovered area.
Another issue of sponge regeneration that is not commonly investigated are the biochemical mechanisms involved in the process. Basile et al. (Reference Basile, Cerrano, Radjasa, Povero and Zocchi2009) studied the activity of ADPRC (ADP-ribosyl cyclase) in sponges during 2 years and observed that it can increase up to 10 times in regenerating specimens of the calcareous sponge Clathrina clathrus (Schmidt, 1864). It is known that the signal transduction pathway that involves ADPRC is related to physiological activities in sponges, such as stem cell duplication, respiration, water filtration and protein synthesis (Zocchi et al., Reference Zocchi, Carpaneto, Cerrano, Bavestrello, Giovine, Bruzzone, Guida, Franco and Usai2001, Reference Zocchi, Basile, Cerrano, Bavestrello, Giovine, Bruzzone, Guida, Carpaneto, Magrassi and Usai2003).
In conclusion, the simple morphology of calcareous sponges together with the totipotency of their choanocytes may be mainly responsible for the efficiency of the regenerative process in these animals (Jackson & Palumbi, Reference Jackson, Palumbi, Lévi and Boury-Esnault1979). Besides, the body's polarity probably conducts and streamlines the regenerative process in order to restore the species’ optimal shape (Jackson, Reference Jackson, Larwood and Rosen1979; Hoppe, Reference Hoppe1988; Bell, Reference Bell2002; Walters & Pawlik, Reference Walters and Pawlik2005; Rohde & Schupp, Reference Rohde and Schupp2012). Although more studies are needed using species with different external morphologies and a broader phylogenetic range, the studies undertaken with calcareous sponges (including this one) suggest that regeneration in this class is strongly dependent on morphology and body polarity.
ACKNOWLEDGEMENTS
We are grateful to Raquel Berlandi and Josiel Vasconcelos for helping during the field study and to Alexander Ereskovsky for sending us several of the Russian papers. We would also like to thank the ICMBio and the head of the Reserva Biológica Marinha do Atol das Rocas for the required licences to develop this study in the Rocas Atoll.
FINANCIAL SUPPORT
We were funded by grants from the Grupo Fundação O Boticário de Proteção à Natureza (no. 0858_20101) and from the Brazilian National Research Council (CNPq) (no. 557162/2009-2; no. 476597/2013-7). M.K. was funded by a research fellowship from the CNPq (no. 302442/2011-1).