INTRODUCTION
Trochochaeta Levinsen, Reference Levinsen1883 is the only genus of the small family Trochochaetidae Pettibone, Reference Pettibone1963. Trochochaetids are spioniform polychaetes and, up to now, only nine species are described, while one species remains unnamed. Hernández-Alcántara & Solís-Weiss (Reference Hernández-Alcántara and Solís-Weiss2011) recently described a new species from the Eastern Pacific off Mexico after about twenty years without new species descriptions. Further, their work summarized almost all available informations about the known species, mapped the world distribution of each species, compared a dozen taxonomic features and provided an identification key to all known species.
Trochochaetids are sedentary polychaetes with numerous segments. The body is divided into a short thoracic and a long abdominal region, changing gradually with some transitional segments. Main diagnostic features within the genus are: the presence of notochaetae in chaetiger 2, the presence of stout acicular neurosetae on chaetiger 3, the occurrence of an antenna, eyes and abdominal papillae, the length of the nuchal crest and the shape of postchaetal lobes.
Up to now, trochochaetids were mostly known from the northern hemisphere. A single record of several unspecified Trochochaeta individuals from the southern hemisphere was given in an ecological study from Brazil at 11°S (Santos et al., Reference Santos, Santos and Oliveira1994). Trochochaetids occur rarely in the world and are widely recorded from temperate-cold waters from the western and eastern USA, from the North Atlantic and from the North and West Pacific. A few records have been made in warm waters. Trochochaeta diverapoda (Hoagland, Reference Hoagland1920) is recorded from several locations of the North Indian Ocean, T. cirrifera (Hartman, Reference Hartman1974) was found in the Arabian Sea and T. mexicana Hernández-Alcántara & Solís-Weiss, Reference Hernández-Alcántara and Solís-Weiss2011 were recorded in the Eastern Pacific. Trochochaeta kirkegaardi Pettibone, Reference Pettibone1976 inhabits the Eastern Pacific (Costa Rica) and is the only species found, up to now, in the Eastern Atlantic and off Africa (Liberia to Nigeria).
During a benthic investigation in the coastal shelf zone off Angola several specimens were identified to the genus Trochochaeta but differed from all known species of the genus in some morphological features and were therefore considered as a new species.
MATERIALS AND METHODS
Samples were collected at the shelf off Angola with a Van Veen grab (0.1 m2) during the cruise of the RV ‘Alexander von Humboldt' in 2004. Samples were sieved through a 1 mm screen and animals were preserved in 4% buffered formaldehyde. Sorting procedures were conducted at the laboratory with a stereomicroscope. The animals were then preserved in 70% ethanol and later examined using a compound microscope with up to ×800 magnification. Digital microphotographs were made by using an AxioCam ICC3 (Carl Zeiss MicroImaging GmbH, Jena) and the Software AxioVision Release 4.8.1 (Carl Zeiss Imaging Solutions GmbH, Jena). The resulting files were imported into Adobe Illustrator CS5 Release 15.0 (Adobe Systems Incorporated) and digital line drawings were made using a WACOM Intuos digitizer board. Scanning electron microscopy (SEM) studies were performed by means of Cam Scan 44WEX.
The type material was deposited at the zoological collection of the University of Rostock (ZSRO, Germany) and further material at the collection of the Leibniz Institute for Baltic Sea Research (Germany).
RESULTS
SYSTEMATICS
Class POLYCHAETA Grube, 1850
Family TROCHOCHAETIDAE Pettibone, Reference Pettibone1963
Genus Trochochaeta Levinsen, Reference Levinsen1883
Trochochaeta ankeae sp. nov.
(Figures 1–5)
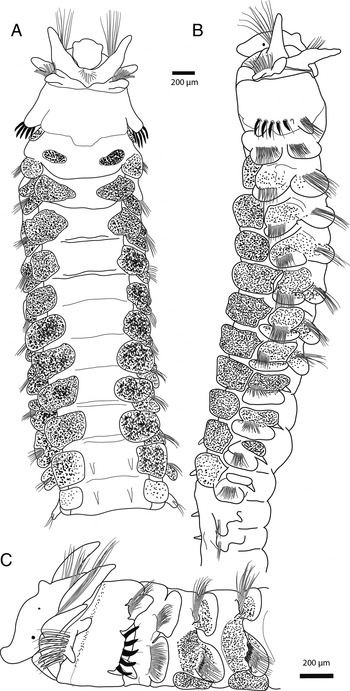
Fig. 1. Trochochaeta ankeae sp. nov.: (A) chaetigers 1–14 ventral view; (B) chaetigers 1–16 lateral view; (C) chaetigers 1–6 lateral view.

Fig. 2. Trochochaeta ankeae sp. nov.: (A) right parapodium 2, anterior view; (B) right parapodium 3, anterior view; (C) right parapodium 4, anterior view; (D) right parapodium 8, anterior view.

Fig. 3. Trochochaeta ankeae sp. nov.: (A) frayed neurochaetae strongly curved backwards, basal with numerous very fine, short, hair-like projections anteriorly, tapered gradually to fine hairy capillary tips from chaetiger 8; (B) hairy capillary neurochaetae from posterior row of chaetiger 8; (C) stout acicular chaeta abdominal region; (D) slender capillary from abdominal region.

Fig. 4. Scanning electron microscopy micrographs of Trochochaeta ankeae sp. nov.: (A) anterior thorax (chaetigers 1–4) lateral view; (B) neuropodia (chaetiger 2) posterior view; (C) prostomium and anterior thorax (chaetigers 1–4) dorsal view; (D) notopodia (no) and neuropodium (ne) posterior thorax (chaetigers 5–6) dorso-lateral view; (E) notopodium (no) and neuropodia (ne) posterior thorax (chaetigers 8–9) dorso-lateral view; (F) transition region (chaetigers 10–12) dorsal view; roman numerals= number of chaetiger. Scale bars: B = 50 µm; A, C–F= 100 µm.

Fig. 5. Scanning electron microscopy micrographs of Trochochaeta ankeae sp. nov.: (A) neuropodia (chaetiger 8) anterior view; (B) anterior abdominal parapodia dorsal view; (C) two anterior abdominal parapodia dorso-lateral view; (D) abdominal region with paired round, white structures with conspicuously central opening dorsal view. Scale bar: 50 µm.
TYPE MATERIAL
Holotype: incomplete specimen with 50 chaetigers: length 12.7 mm, and 1.0 mm wide at 8th segment (ZSRO-P2203) (AHAB 8, BE11; south-east Atlantic, shelf coast off Angola; water depth 83.6 m, grab sampling; coordinates: 15°07.733S 12°06.508E; abiotic factors at the bottom: salinity 35.6; temperature 16.0°C; and oxygen 0.92 ml/l). Collected by M.L. Zettler, 17 May 2004.
Paratypes: 8 incomplete anterior fragments; 30–59 chaetigers; longest fragment with 17.3 mm in length (59 chaetigers) and 0.96 mm wide at 8th segment; from the same samples as the holotype (ZSRO-P2204).
Additional material: 24 incomplete anterior fragments; 90 middle fragments of 12–72 chaetigers.
DIAGNOSIS
Body slender, thorax with 10 chaetigers. Prostomium elongate, truncate anteriorly, with four slight lobes, with a small conical antenna and a nuchal crest projecting through chaetiger 2; two pairs of minute eyes. Second parapodia biramous, without notochaetae. Acicular spines on neuropodia 2 and 3. Postchaetal lobes entire, low, subrectangular to rounded. Abdominal region from chaetiger 15 without notochaetae, ventrally with pair of conical papillae from chaetigers 13–15. Chaetigers 5 to 10 with reddish areas ventro-lateral and at the base of parapodia. Transitional region from chaetigers 11 to 14 without notochaetae, with bundles of neurochaetae and with neuropodia showing anterior and ventral reddish areas.
DESCRIPTION
Prostomium elongate, truncate anteriorly, with four slight lobes, tapering posteriorly, with narrow nuchal crest extending posteriorly on chaetiger 2 to the anterior margin of chaetiger 3; with a small conical median antenna on the anterior region of the crest (Figures 1B, C & 4C). Two pairs of minute eyes in antero-lateral and postero-median position, posterior pair smaller than anterior pair, difficult to observe (Figure 1C).
Anterior four segments of thoracic region differing from one another (Figures 1A–C & 4A, C), with modified biramous parapodia. First chaetiger with parapodia shifted dorsally, directed anteriorly, notopodial and neuropodial lobes distinct. Notopodia with bundles of about 8–15 smooth capillary chaetae. Neuropodia with numerous (about 25) short and long smooth capillary chaetae, distinctly longer than notochaetae. Notopodial and neuropodial postchaetal lobes elongate, subconical, wider basally.
Second segment with parapodia shifted ventrally, biramous, without notochaetae (Figure 2A). Neuropodia with two kinds of paired chaetae: anterior row of 9 stout yellowish-brown acicular chaetae each accompanied postero-dorsal by one slightly longer, faintly hairy slender capillary (Figure 4B). Noto- and neuropodial postchaetal lobes small, subconical, with notopodial postchaetal lobe slightly longer.
Third segment with lateral parapodia, notopodial lobe small, with fan-shaped bundle of about 15 smooth capillary chaetae (Figure 2B), postchaetal notopodial lobe subconical. Neuropodial lobe low, wide, with fan-shaped bundle of 5 stout black acicular spines, clearly heavier than acicular spines from second chaetiger, alternating with equal number of slender capillary neurochaetae. Postchaetal neuropodial lobe low, conical, similar to notopodial lobe, shifted ventrally.
Fourth segment with lateral parapodia, chaetal lobes low and wide, neuropodia and notopodial each with about 40 slender, capillary chaetae arranged in two rows (Figure 2C). Noto- and neuropodial postchaetal lobes similar, with outer margins smooth, low, wide, slightly rounded, meeting one another medio-lateral.
Following 6 thoracic segments (chaetigers 5 to 10) similar, with prominent biramous parapodia. Positions of neuropodia shift continuously from lateral, with wide parapodial gap to medio-lateral position on chaetiger 8 with lowest parapodial gap, positions of neuropodia shift back to lateral position until chaetiger 10. Reddish glandular areas present ventro-laterally, largest at chaetiger 8, anterior and posterior to parapodia. Notopodial chaetal lobes low, wide, with two rows of slender, smooth capillaries (Figure 4D). Notopodial postchaetal lobes entire, thick fleshy, broad and rounded, becoming subconical until end of thorax. Neuropodial chaetal lobes low, wide, with compact bundles of neurochaetae of two different types (Figures 2D, 3A, B & 4E), two anterior rows of about 8–9 strong backwards curved frayed chaetae, basally with numerous very fine, short hair-like projections on anterior margin, tapering gradually to fine hairy capillary tips (Figure 5A), one posterior row of about 10–12 hairy capillaries and also one bundle of 1–3 capillaries emerging ventro-anteriorly to first row of neurochaetae (Figure 2D). Neuropodial postchaetal lobes wide, increasing in size to posterior thoracic region, rounded in chaetigers 5–6, becoming subrectangular in chaetigers 8–10.
Parapodia of chaetigers 11–14 (transitional region) with notopodia represented only by small subconical papillae (Figure 4F), lacking notochaetae. Neuropodial postchaetal lobes of chaetigers 11–12 similar to last thoracic chaetiger, becoming smaller at segments 13–14, with reddish glandular areas of similar size ventro-lateral and anterior to neurochaetal lobe, disappearing at chaetiger 14. Neurochaetae of chaetiger 11 similar to those of preceding thoracic chaetigers, with frayed chaetae strongly curved backwards and with numerous very fine, short hair-like projections basally on anterior margin, modifying gradually to hairy capillaries at chaetiger 14. Chaetigers 13–14 with paired ventral papillae (Figure 1A).
Abdominal region beginning at chaetiger 15, slightly narrower than thoracic region, with paired rounded white structures, with conspicuous central opening dorso-lateral (Figure 5C, D), with paired papillae ventrally, without notopodia or notochaetae, neuropodial lobes small, elongated, with a few (1–3) slender capillaries, and 2–3 stouter acicular chaetae with or without subterminally attached aristae (Figures 3C, D & 5B).
Neuropodial postchaetal lobes small, digitiform.
Posterior abdominal region and pygidium unknown.
ETYMOLOGY
The new species is named after Anke Bochert, wife of the first author.
TYPE LOCALITY
South-west Atlantic, shelf off South Angola.
HABITAT
The specimens of T. ankeae sp. nov. were only found at the type locality at 84 m depth in muddy sediment. They live in fine sandy tubes of up to 2.7 mm in diameter with a wall thickness of 0.3–0.35 mm.
COLOUR
Specimens in alcohol: yellowish, with black aciculae on chaetiger 3, reddish lateral and ventro-lateral from chaetigers 4–14, white spots dorso-lateral on the abdomen.
REMARKS
Up to now only nine nominal species and one unnamed species are known all over the world (Hernández-Alcántara & Solís-Weiss, Reference Hernández-Alcántara and Solís-Weiss2011). Two main species groups are recognized, one presents noto- and neurochaetae in all thoracic chaetigers (T. watsoni, T. cirrifera and T. sp. A) and one (where chaetae are lacking in chaetiger 2) containing the remaining species.
Trochochaeta ankeae sp. nov. belongs to the second group, in which notochaetae are lacking in chaetiger 2. The new species have also acicular neurochaetae on chaetiger 2, differing from T. carica and T. pettiboneae which lack this feature. Most of the trochochaetid species, including T. ankeae sp. nov. have entire postchaetal lobes and are distinguishable from T. multisetosa and T. sp. A that present serrated or fimbriated postchaetal lobes. To compare the morphological characteristics of T. ankeae sp. nov. with those of other species an overview is given in Table 1 following the diagnostic features of Hernández-Alcántara & Solís-Weiss (Reference Hernández-Alcántara and Solís-Weiss2011).
Table 1. Diagnostic characters of Trochochaeta ankeae sp. nov. based on Hernández-Alcántara & Solís-Weiss (Reference Hernández-Alcántara and Solís-Weiss2011).

*, the text of diagnostic features from table 1 in Hernández-Alcántara & Solís-Weiss (Reference Hernández-Alcántara and Solís-Weiss2011) refers to ‘anterior thorax', but this must be a mistake because specialized neurochaetae occur from chaetiger 5 (posterior thorax); **, the text of diagnostic features from table 1 in Hernández-Alcántara & Solís-Weiss (Reference Hernández-Alcántara and Solís-Weiss2011) refers to ‘thorax anterior', but this must be a mistake because their table lists features of chaetiger 3 (anterior thorax) and chaetiger 5 or chaetiger 11 (posterior thorax).
The new species is similar to T. japonica, T. mexicana, T. diverapoda and T. kirkegaardi by having entire postchaetal lobes, chaetiger 2 with stout acicular neurochaetae and without notochaetae. The presence of 4 eyes in T. ankeae sp. nov. is shared by T. japonica and T. mexicana, whereas the other two species lack eyes. Trochochaeta japonica does not present antenna, in contrast to T. ankeae sp. nov., and T. mexicana lacks abdominal papillae on midventral line and has the nuchal crest extending only to the end of chaetiger 1, instead of to the end of chaetiger 2 as seen in T. ankeae sp. nov. In contrast, the nuchal crest of T. diverapoda extends to the end of chaetiger 3.
The only known species from the East Atlantic off Africa is T. kirkegaardi. There are no other records of trochochaetids off Africa except that of this species off Liberia and off Nigeria (4°N–6°N) (Pettibone, Reference Pettibone1976). Trochochaeta ankeae sp. nov. can easily be distinguished from the latter by the presence of eyes, by the abdominal region on chaetiger 15, with papillae on midventral line from chaetigers 13–15, by the presence of a small, subconical antenna and by the nuchal crest reaching to chaetiger 2. In T. kirkegaardi eyes are lacking, the abdominal region begins on chaetiger 16, papillae on midventral line appear from chaetiger 16, the antenna is small, subtriangular and continuous with the nuchal crest, which extends at least to middle of chaetiger 3.
KEY TO THE SPECIS OF TROCHOCHAETA LEVINSEN, 1883 (ADAPTED FROM HERNÁNDEZ-ALCÁNTARA & SOLÍS-WEISS (2011))
1 With noto- and neurochaetae in all thoracic parapodia…………2
— Without notochaetae in chaetiger 2……4
2 Postchaetal lobes thick and subconical……3
— Postchaetal lobes cirriform………… T. cirrifera (Hartman, Reference Hartman1974)
3 Postchaetal lobes serrated or fimbriated………… T. sp. A Gilbert, Reference Gilbert, Uebelacker and Johnson1984
— Postchaetal lobes entire, not serrated or fimbriated………… T. watsoni Pettibone, Reference Pettibone1976
4 Stout acicular neurochaetae on chaetiger 2……5
— Stout acicular neurochaetae on chaetigers 2 and 3…6
5 13–15 thoracic chaetigers; 4–8 filiform anal cirri………… T. carica (Birula, Reference Birula1897)
— 15–19 thoracic chaetigers; 10 digitate anal cirri………… T. pettiboneae Dean, Reference Dean1987
6 Postchaetal lobes serrated or fimbriated………… T. multisetosa (Örsted, Reference Örsted1843)
— Postchaetal lobes entire, not serrated or fimbriated…7
7 Prostomium without antenna; median crest projects on chaetiger 1………T. japonica Imajima, Reference Imajima1989
— Prostomium with antenna; median crest arising upwards from the prostomium…………8
8 With eyes; prostomium with nuchal crest extending on chaetiger 1 or 2…………9
— Without eyes; prostomium with nuchal crest extending on chaetiger 3…………10
9 Without abdominal papillae on midventral line; prostomium with nuchal crest extending on chaetiger 1…… T. mexicana Hernández-Alcántara & Solís-Weiss Reference Hernández-Alcántara and Solís-Weiss2011
— With abdominal papillae on midventral line; prostomium with nuchal crest extending on chaetiger 2………… T. ankeae sp. nov.
10 Prostomium with filiform antenna………… T. diverapoda (Hoagland, Reference Hoagland1920)
— Prostomium with small subtriangular antenna, continuous with nuchal crest… T. kirkegaardi Pettibone, Reference Pettibone1976
ACKNOWLEDGEMENTS
We thank the crew of RV ‘Alexander von Humboldt’ for assistance during sampling. We wish also to thank I. Glockzin (Rostock) for analysis of benthic samples in the laboratory. The authors are very grateful to R. Bahlo (Rostock) for technical assistance with the scanning electron microscope. We thank the anonymous referee for the critical arguments that improved the manuscript and Sergio Salazar for discussion of the Trochochatea key.








