INTRODUCTION
The nurse shark, Ginglymostoma cirratum (Bonnaterre, 1788), is a coastal species found in tropical and subtropical waters of continental and insular shelves, often near patch reefs, both coralline and rocky (Compagno et al., 2005). This species is distributed in the West Atlantic Ocean from North Carolina (USA) to Southern Brazil; in the East Atlantic, from Cape Verde to Gabon; and in the East Pacific Ocean, from California (USA) to Peru (Castro, Reference Castro2000; Compagno et al., Reference Compagno, Dando and Fowler2005; Rosa et al., Reference Rosa, Castro, Furtado-Neto, Monzini and Grubs2006a). As stated by Rosa et al. (Reference Rosa, Castro, Furtado-Neto, Monzini and Grubs2006a), there is recent qualitative evidence of reduction in nurse shark populations in several parts of its distribution range. However, the actual levels of decline throughout most of the nurse shark's distribution range is still unknown and therefore, its conservation status is classified as ‘Data Deficient’ by the International Union for Conservation of Nature (Rosa et al., Reference Rosa, Castro, Furtado-Neto, Monzini and Grubs2006a).
Along the Brazilian coast, nurse sharks are more abundant in the northern and north-eastern regions (Rosa & Gadig, Reference Rosa, Gadig, Machado, Drummond and Paglia2008). In 2002, this species was classified as vulnerable by the Sociedade Brasileira para o Estudo de Elasmobrânquios (SBEEL, the Brazilian Society for the Study of Elasmobranchs), following the Red List criteria from IUCN (International Union for Conservation of Nature). This classification was due to: significant decreases (or even localized extinction) in the West Atlantic nurse shark population due to coastal fisheries (Rosa et al., Reference Rosa, Castro, Furtado-Neto, Monzini and Grubs2006b), and the recent interest for this species as an ornamental fish (Rosa & Gadig, Reference Rosa, Gadig, Machado, Drummond and Paglia2008). In Brazil, this species was recently considered as ‘threatened with extinction’ and its capture by fisheries forbidden (Annex I of Normative Instruction #05 from the Ministry of the Environment, 2004). Despite such legislation, nurse sharks continue to be caught as these laws are unenforced and fisheries are not monitored. The goal of the present study was to describe the population structure of nurse sharks caught off Ceará State, north-eastern Brazil, based on the following aspects: abundance and size, sex-ratio, fisheries incidence and seasonality of captures.
MATERIALS AND METHODS
Sampling
Nurse shark landings at the Mucuripe Embayment, Fortaleza, Ceará State, Brazil, were monitored weekly from October 2006 to September 2008. Monitoring was conducted from 5 to 6 am, primarily on Saturdays, but eventually on Fridays or Sundays (these are the days with largest fish landings and trade). The fishing area for the small-scale fleet is: 03°43′S/038°05′W; 03°23′S/038°05′W and 03°25′S/038°48′W; 03°01′S/038°49′W.
The fishermen landed specimens on the beach for trade either as whole sharks or carcasses (eviscerated specimens: head, viscera and fins removed). Specimens were identified following Compagno et al. (2005). Whole specimens were identified as male or female, and the total length (TL, in cm) and interdorsal distance (ID, in cm) were taken with a metric measuring tape. Only the ID was recorded from specimens presented as carcasses.
Data analysis
The Chi-square test (P < 0.05) was used to assess the monthly statistical differences in sex-ratios. To test for statistical differences between ID × TL of males and females, the statistical ‘w’ (P < 0.05) was used for comparison of straight lines. The Student's t-test (P < 0.05) was used for comparison of means of TL values for males and females (Zar, Reference Zar1999).
Descriptive statistics were used for TL mean and confidence interval (P < 0.05) of sampled specimens, including estimated TL for carcasses. After testing for normality and homoscedasticity, the ANOVA (P < 0.05) (Zar, Reference Zar1999) was used to test if there were significant seasonal variation of (1) monthly numeric abundance and (2) monthly mean TL values.
The maturity stage of nurse sharks was determined using the criteria proposed by Castro (Reference Castro2000) (e.g. newborn at 30 cm TL; sexual maturity at approximately 220 cm TL). The numeric abundance variation was expressed as a relation between numbers of specimens recorded per day of monitoring. Our intention was to take into consideration monthly variations of sampling effort (effort varied between 3 and 5 days in a single month).
RESULTS
Abundance and size
During 102 days of monitoring landings, 189 nurse sharks were recorded (1.85 individuals/sampling day): 116 were whole specimens and 73 were carcasses. The largest whole specimen was a female, 274 cm TL, caught in August 2008; the largest male was 252 cm TL, caught in July 2007. The smallest specimen was a 73 cm TL female, caught in February 2008; the smallest male measured 82 cm TL, caught in July 2008.
Sex-ratio
Based on whole-specimens (predominantly juveniles), 63 were females and 53 were male. This difference was not statistically significant, the sex-ratio being 1.19♀:1♂. In addition, no trend of seasonal variation in sex-ratio (sexual segregation) was observed. One adult female landed as whole-specimen in August 2008 was 253 cm TL and had 20 fully developed embryos; sex-ratio in the embryos was 1.5♀:1♂.
Fisheries incidence
As opposed to juvenile sharks, which were landed as whole specimens, larger specimens were frequently eviscerated at sea. Consequently, the ratio of individuals landed as carcasses was positively correlated to TL(R2 = 0.8601).
The straight lines obtained from the linear regressions between TL and ID of males and females were not significantly different (P = 0.5918). Male and female data were then grouped (N = 101) and the following ID × TL equation was obtained: TL = 12.606ID + 14.24 (R2 = 0.9505). ID varied between 5.5 to 22 cm, while TL varied between 73 and 274 cm. Since males and females did not differ statistically, the ID × TL relation was used to obtain TL estimates for individuals landed as carcasses. The distribution of TL classes for nurse sharks (including estimated TL for carcasses) revealed a predominance of juveniles (86.2%) (Figure 1). Taking into consideration the TL of whole specimens and the estimated TL for carcasses (ntotal = 189), the mean TL was 154.6 cm ± 7.8 cm.
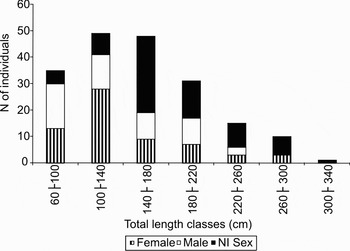
Fig. 1. Total length–frequency distribution for nurse sharks, Ginglymostoma cirratum, (N = 189) landed at the Mucuripe Embayment, Fortaleza, CE, Brazil, from October 2006 to September 2008.
Seasonality of captures
Nurse sharks did not show significant (P = 0.7354) seasonal variation of monthly numeric abundance (2.49 ± 1.6 numbers of specimens recorded per day of monitoring). Similarly, no seasonal pattern (P = 0.403) was observed for monthly mean TL values (154.86 ± 54.56).
DISCUSSION
Abundance and size
The nurse shark is considered an endangered species in Brazil (Rosa & Gadig, Reference Rosa, Gadig, Machado, Drummond and Paglia2008), and it has been extirpated from parts of its original distribution along the Brazilian coast (Shark Specialist Group, 2004). In the studied area, nurse sharks were landed quite often, as previously reported for landings in this same embayment between 1998 and 1999 (Arthaud, Reference Arthaud1999). However, Arthaud (Reference Arthaud1999) recorded landings for whole-specimens only and did not include carcasses in their data set. Considering only whole-specimens, an average of 1.18 whole specimen landings per day were observed in the present study, whereas Arthaud (Reference Arthaud1999) reported 0.79. Although whole-specimen landing comparisons can be made, Arthaud (Reference Arthaud1999) and the present study differed on their sampling week day. In the present study, monitoring was done primarily on Saturdays, the most important landing day in the week in terms of volume of landings, while Arthaud (Reference Arthaud1999) included other week-days with usually lower landings. Therefore, this difference in landing rates may simply reflect the different sampling schemes used.
The largest nurse shark recorded in the present study, a female 274 cm in TL, was close to the largest known TL for the species (280 cm in TL; Cadenat & Blache (Reference Cadenat and Blache1981)). More recently, a live specimen observed the Atol das Rocas was estimated to be 305 cm TL (Castro & Rosa, Reference Castro and Rosa2005). Based on the ID × TL equation obtained in the present study, one specimen had a TL estimated as 316.8 cm, which would make it the largest nurse shark reported. The mean nurse shark TL found (TL = 154.6 cm, including TL estimated from carcasses) is higher than that reported for Florida (USA) (TL = 124.6 cm) (Carrier & Luer, Reference Carrier and Luer1990), and lower than the mean TL observed among individuals at the Atol das Rocas Biological Reserve (TL = 186.7 cm) (Castro & Rosa, Reference Castro and Rosa2005).
Sex-ratio
The observed nurse shark sex-ratio (1.19♀:1♂) differed from data obtained by Arthaud (Reference Arthaud1999) for the same area: 2.1♀:1♂. It is not known what may explain this difference. Similar to our results, a relatively more even distribution of males and females were observed along the coast of Florida (USA): 1.36♂:1♀ (Carrier & Luer, Reference Carrier and Luer1990), 1.08♂:1♀ (Kohler et al., Reference Kohler, Casey and Turner1998), and 1.51♀:1♂ (Castro, Reference Castro2000). However, at the Atol das Rocas Biological Reserve, the sex-ratio was 3.77♀:1♂, probably due to sex segregation caused by habitat preferences (Castro & Rosa, Reference Castro and Rosa2005).
Fisheries incidence
Although landed nurse sharks comprised almost all TL classes, most specimens (86.2%) were juveniles (smaller then 220 cm TL, following Castro (Reference Castro2000)). The fact that no specimen smaller then 60 cm TL was landed during surveys may indicate that small sharks are not captured by commercial fishing gear used locally or the biological recruiting of juveniles to the fishing area.
While monitoring landings of sharks in the study area, Arthaud (Reference Arthaud1999) recorded only whole sharks. In order to permit comparisons, only whole nurse sharks will be considered in the following discussion. The present study and Arthaud (Reference Arthaud1999) registered similar percentage of juveniles: 95.0 and 98.5%, respectively. In addition, the amplitude distribution of TL found in the present study was similar to those obtained by Arthaud (Reference Arthaud1999). However, one change in distribution of TL classes was observed. Present study captures peaked at lower modal TL classes (90–100 cm in TL) in comparison to those registered by Arthaud (Reference Arthaud1999) (140–150 cm in TL) (Figure 2). These results may represent changes in population structure at the studied area within a 10 year-period, possibly indicating overfishing. Overfishing may remove particular size-classes in harvested species, altering important demographic rates and increasing extinction risk (Jennings et al., Reference Jennings, Reynolds and Mills1998; Walker, Reference Walker1998; Bradshaw et al., Reference Bradshaw, Fitzpatrick, Steinberg, Brook and Meekan2008).
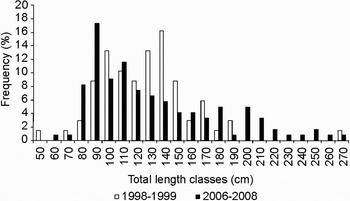
Fig. 2. Relative frequency distribution of total length-classes in nurse sharks, Ginglymostoma cirratum, landed at Mucuripe Embayment, Fortaleza, CE, Brazil, from 1998 to 1999 (Arthaud, Reference Arthaud1999) and from 2006 to 2008 (present study). The lowest value of each size-class was used as label for the x-axis.
The main fishing gears used by the small-scale fishing fleet in this study were gillnets and hook-and-line. However, nurse shark captures were done primarily with hook-and-line. Fishermen usually use hook-and-line after launching and before retrieving gillnets (Arthaud, personal communication). Rosa & Gadig (Reference Rosa, Gadig, Machado, Drummond and Paglia2008) did not cite hook-and-line among the fisheries leading to the decline in nurse shark populations in Brazil. Since hook-and-line is secondary during gillnet fishery, it is possible that the importance of hook-and-line in capturing nurse sharks in Brazil has been underestimated. The contrary may also be true, with the importance of gillnets being overestimated.
Seasonality of captures
The lack of seasonal patterns of variation of abundance and mean TL of nurse sharks may be due to either a true lack of seasonal variation in the study area, or selectivity of fishing gears. In support of the first hypothesis, studies have shown that nurse sharks have a low migratory activity (Carrier, Reference Carrier1985; Carrier & Luer, Reference Carrier and Luer1990; Kohler et al., Reference Kohler, Casey and Turner1998; Castro & Rosa, Reference Castro and Rosa2005). As for the second hypothesis, we may say that local fishing activities have their own dynamics, modifying the fishing effort throughout the year (i.e. lobster protection or periods of strong winds reduces fishing effort). Further studies are needed to interpret data obtained so far.
In south-eastern USA, nurse shark births tend to occur in November and December (autumn–winter) (Castro, Reference Castro2000). In Atol das Rocas, Brazil, the largest aggregation of pregnant nurse sharks was observed in August (winter) (Castro & Rosa, Reference Castro and Rosa2005). Around the oceanic islands of Fernando de Noronha, Brazil, nurse shark births occurred from July to September (winter) (Garla et al., Reference Garla, Garcia-Júnior, Veras and Lopes2008). The only pregnant female examined in this study carried fully developed embryos in August (winter), coinciding with the reproduction period in other areas as cited above. Nevertheless, data from additional adults are needed in order to fully investigate if there is any seasonal pattern for nurse shark reproduction in the studied area.
ACKNOWLEDGEMENTS
The authors are thankful to fishermen from Mucuripe Embayment, Fortaleza—CE, Brazil, for granting us access to their catch. We owe a great debt of gratitude to Isabelle D.B. Arthaud who kindly shared her data and experience in the studied area. We are also grateful to Toni L. Ferrara and an unknown referee for comments on this manuscript. This work was supported by the Brazilian National Research Council (CNPq) (V.F., grant number: 485120/2007-0) and the Ceará State Research Council (FUNCAP) (V.F., grant number: DCR 0039-2.04/07-FCPC 1514-52). J.S.N. and L.M.S. received scholarships from CNPq; M.F.N. received a research productivity fellowship from CNPq and V.F. received a research fellowship jointly from CNPq and FUNCAP.




