Introduction
Latitudinal gradient paradigm
Body size, sexual maturity, longevity and reproductive period of animals are strongly influenced by the latitudinal gradient. One pattern commonly observed in nature, especially in a widely distributed taxonomic clade, is that body size increases with increasing latitude (Bergmann, Reference Bergmann1847; Partridge & Coyne, Reference Partridge and Coyne1997; Blanckenhorn et al., Reference Blanckenhorn, Stillwell, Young, Fox and Ashton2006). However, latitude is only a geographic location, and it is the environmental variables that change with the geographic scenario (Hawkins & Diniz-Filho, Reference Hawkins and Diniz-Filho2004). Despite the strong evidence that temperature influences the size of animals according to the latitudinal gradient, many ectothermal animals do not present this pattern (Hawkins & Lawton, Reference Hawkins and Lawton1995; Pincheira-Donoso et al., Reference Pincheira-Donoso, Hodgson and Tregenza2008).
Size and sexual maturity have also been reported as indirect effects of latitude. Sexual maturity is reached later and body sizes are larger at higher latitudes (Hines, Reference Hines1989; Castilho et al., Reference Castilho, Gavio, Costa, Boschi, Bauer and Fransozo2007; Monaco et al., Reference Monaco, Brokordt and Gaymer2010). Similarly, longevity increases with latitude (Defeo & Cardoso, Reference Defeo and Cardoso2002; Nilssen & Aschan, Reference Nilssen and Aschan2009). In crustaceans, the rate of growth tends to decrease as longevity increases, depending on factors such as taxonomic group, geographic scenario and habitat occupied (Vogt, Reference Vogt2012).
In tropical seas, the high water temperature year-round is the cause of continuous reproduction in a variety of marine invertebrate populations (Orton, Reference Orton1920). However, different reproductive strategies occur in Penaeoidea shrimps, with continuous reproduction in tropical seas, and seasonal in higher latitudes (Boschi, Reference Boschi1989; Bauer & Vega, Reference Bauer and Vega1992; Castilho et al., Reference Castilho, Gavio, Costa, Boschi, Bauer and Fransozo2007).
Oceanic circulation in the South-east Brazil Bight
The Southeast Brazil Bight (SBB) is strongly affected by two currents. The Brazil Current (BC) from the north, characterized by high salinity (>36) and temperature (>20 °C), and the Malvinas (Falkland) Current (MC) from the south, characterized by low salinity (<34) and temperature (<15 °C) (Castro-Filho & Miranda, Reference Castro-Filho, Miranda, Robinson and Brink1998). In addition, SBB is influenced by three distinct water masses: Coastal Water (CW), Tropical Water (TW) and South Atlantic Central Water (SACW) (Valentin et al., Reference Valentin, Monteiro-Ribas and Mureb1987; Silveira et al., Reference Silveira, Schmidt, Campos, Godoi and Ikeda2000). TW carries warm and nutrient-poor water, while SACW carries colder and nutrient-rich water. The region still has the presence of CW, which is influenced by the contribution of continental waters (Castro-Filho & Miranda, Reference Castro-Filho, Miranda, Robinson and Brink1998; Silveira et al., Reference Silveira, Schmidt, Campos, Godoi and Ikeda2000; Mahiques et al., Reference Mahiques, Silveira, Mello and Rodrigues2002; Calado et al., Reference Calado, Silveira, Gangopadhyay and de Castro2010; Cerda & Castro, Reference Cerda and Castro2014).
Between the latitudes of 23°S and 29°S, part of the subtropical gyre gives rise to the upwelling of Cabo Frio, mainly during spring and summer (October to March) (Castro et al., Reference Castro, Miranda and Miyao1987; Acha et al., Reference Acha, Mianzan, Guerrero, Favero and Bava2004). However, upwelling events can occur at any time of the year, favoured by north-east winds (Sumida et al., Reference Sumida, Yoshinaga, Ciotti and Gaeta2005; De Léo & Pires-Vanin, Reference De Léo and Pires-Vanin2006). Upwelling events favour the increase of nutrients in the water, changing the oligotrophic state of low levels of nitrogen and phosphorus in the region (Boltovskoy, Reference Boltovskoy1999). Consequently, the primary productivity of the SBB increases, particularly in Cabo Frio, Rio de Janeiro (23°S), with higher chlorophyll concentrations during SACW upwelling events in spring and summer or even during winter (De Léo & Pires-Vanin, Reference De Léo and Pires-Vanin2006).
Shrimp fishing
Knowledge about the onset of sexual maturity and reproductive periodicity is a key for understanding the life cycles of species. The study of these parameters in Penaeoidea shrimp populations is essential in the determination and implementation of management plans that favour species preservation (Bauer, Reference Bauer1992).
The pink shrimps Farfantepenaeus brasiliensis (Latreille, 1817), Farfantepenaeus paulensis (Pérez Farfante, 1967) and Farfantepenaeus subtilis (Pérez Farfante, 1967); the white shrimp Litopenaeus schmitti (Burkenroad, 1936); and the seabob shrimp Xiphopenaeus kroyeri (Heller, 1862) are among the most important penaeids commercially exploited in the south-eastern Brazilian coast (MPA, 2012). The fishing of the most lucrative species in recent decades (2005–2011) is considered stable due to investments in the sector. However, little explored species, such as Pleoticus muelleri (Bate, 1888), have been captured at an increasing rate (from 706 to 963 tons in the same period) (MMA, 2007; MPA, 2010, 2012, 2013).
Endemic to the South American coast, P. muelleri has a restricted distribution to the Western Atlantic from Rio de Janeiro, Brazil (23°S), to Santa Cruz, Patagonia, Argentina (50°S), and remains in the marine environment throughout its life cycle (Boschi, Reference Boschi1997). Several technical studies of P. muelleri in Uruguay and Argentina region (latitude up to 42°S) focused on the fishing opening period and specimens of fishing landings (Wyngaard & Bertuche, Reference Wyngaard and Bertuche1982; Fernández et al., Reference Fernández, Hernández and Roux2011, Reference Fernández, Iorio, Hernández and Macchi2012; Segura & Delgado, Reference Segura and Delgado2012; Fernández & Hernández, Reference Fernández and Hernández2013). Additionally, studies conducted by Boschi (Reference Boschi1989) and Castilho et al. (Reference Castilho, Costa, Fransozo and Negreiros-Fransozo2008, Reference Castilho, Wolf, Simões, Bochini, Fransozo and Costa2012) focused on the reproduction, growth, longevity and sex ratio of P. muelleri in longer and continuous sampling periods.
Studies on this species at latitudes above 42°S (Boschi, Reference Boschi1989; De la Garza, Reference De la Garza2003) indicated seasonal reproduction and maximum carapace sizes greater than 39 and 42 mm for males and females, respectively. In tropical regions (23°S), continuous reproduction was observed, with a maximum size of the carapace of ~17 and 37 mm for males and females, respectively (Castilho et al., Reference Castilho, Costa, Fransozo and Negreiros-Fransozo2008, Reference Castilho, Wolf, Simões, Bochini, Fransozo and Costa2012). Thus, these studies corroborate the theories regarding the paradigm of the latitudinal gradient.
This study presents results regarding the biology of P. muelleri in the South-western Atlantic in its northern distribution limit (22°S). Reproduction, body size, growth and longevity were determined and compared with literature in order to verify whether the theories for the latitudinal gradient pattern apply in a tropical region influenced by oceanic upwelling.
Materials and methods
Sampling
Shrimps were sampled monthly from March 2008 to February 2010 in six stations (5, 10, 15, 25, 35 and 45 m) in Macaé (22°22′ S, 41°46′ W), Cabo Frio region, northern coast of Rio de Janeiro State (Figure 1). A shrimp fishing boat equipped with otter-trawl nets (3.5 m mouth width, mesh sizes of 20 and 15 mm in the cod end) was used for trawling. The stations were trawled over a 15-min period at a constant speed of 2.0 nautical miles through 1 km.
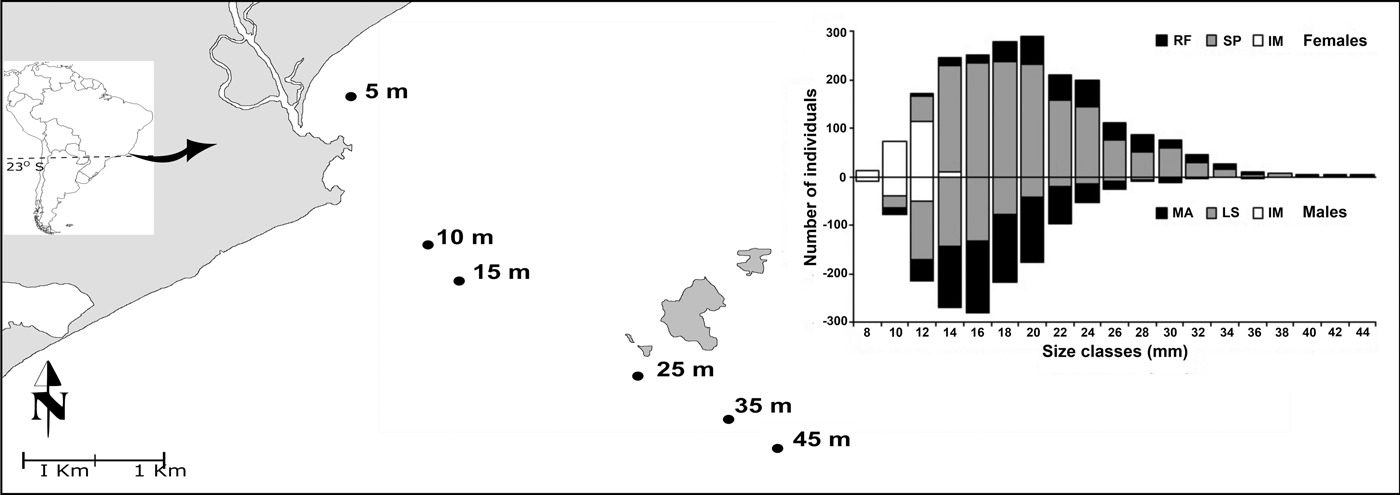
Fig. 1. Upwelling region and sampling area. Location of stations (5–45 m of depth) and major ocean currents of the South Atlantic. AC, Antarctic Circumpolar Current; MC, Malvinas Current; SAC, South Atlantic Current; SEC, South Equatorial Current; BC, Brazilian Current (Sancinetti et al., Reference Sancinetti, Azevedo, Castilho, Fransozo and Costa2014). Distribution of demographic categories of females and males among size classes from March 2008 to February 2014 in Macaé, Cabo Frio region (the categories are as follows: RF, reproductive females; SP, spent females; MA, mature males; LS, lacking spermatophore males; IM, juvenile males and females).
Bottom environmental parameters
Salinity and temperature (°C) were measured in bottom-water samples obtained monthly at each station using a Van Dorn bottle. In the laboratory, the salinity was verified with a manual salinometer calibrated with distilled water. The water temperature was verified with a mercury thermometer immediately after sampling in a thermic isolated container in the shade. The chlorophyll concentration was obtained monthly from the chlorophyll-a concentration database (MODISA_L3m_CHL v2014). The data were time series of area-averaged values, with spatial resolutions of 4 km that were obtained from the Giovanni NASA – The Bridge Between Data and Science, version 4.19 (NASA – Giovanni; accessed September 2018).
Reproduction
Shrimps were measured in relation to the carapace length (CL) (to the nearest 0.1 mm), corresponding to the distance from the orbital angle to the posterior margin of the carapace, and were classified by sex, according to the presence of a petasma in males or a thelycum in females (Costa et al., Reference Costa, Fransozo, Melo and Freire2003).
The stages of reproductive development were determined through macroscopic analysis of the male and female reproductive morphology. The female reproductive condition was assessed via macroscopic observation of the degree of ovarian development (according to colour and the volume occupied by the gonads). Ovaries were categorized as: immature (IM) – varying from thin, transparent strands to thicker strands; spent (SP) – white and much larger and thicker than those of immature females; developed – light green; and mature – green to dark green, occupying the entire dorsal portion of the abdomen, as well as part of the carapace (more details in Costa & Fransozo, Reference Costa and Fransozo2004). Females with gonads in the spent, developed and mature stages were defined as adults, while those with gonads in the developed and mature stages were considered reproductive females (RF). Females with gonads in the immature stage were defined as juveniles.
The sexual maturity of male penaeids is usually indicated by fusion of the petasmal lobes (gonadal endopods), whereas immature males exhibit separated petasmal lobes (Boschi, Reference Boschi1989). The maturity of adult males was classified according to the development of spermatophores in the terminal ampoule (ejaculatory duct), since spermatophores are visible through the exoskeleton (Chu, Reference Chu1995). When spermatophores were not visible through macroscopic observation in an adult male, the individual was classified as lacking spermatophores (LS). If spermatophores were visible and occupied a portion of or all terminal ampoules, males were classified as spermatophore-bearing males (MA) (Castilho et al., Reference Castilho, Wolf, Simões, Bochini, Fransozo and Costa2012, Reference Castilho, Fransozo, Costa, Freire, Fransozo, Bauer and Grabowski2015). Males with gonads in the LS and MA stages were defined as adults, the MA stage was considered reproductive males, and those with gonads in the IM stage were defined as juveniles.
A Student t-test was used to verify if the size differed between the sexes. Normality was examined by Shapiro–Wilk test. The null hypothesis is that the size distribution between genders does not differ significantly.
Size at sexual maturity was determined for males and females using the proportion of juvenile and adult individuals in 0.5 mm CL size classes. The procedure employed to estimate sexual maturity was based on fitting a sigmoid logistic curve (Somerton, Reference Somerton1980). The equation y = 1/(1 + e(−r(CL − CL50))) was used, in which y is the estimated proportion of adult shrimps, CL50 is the carapace measurement at the onset of sexual maturity, and r is the coefficient for the slope of the logistic curve. The logistic curve was fitted via the least squares method to the previously indicated proportions for each size class of all individuals and samples using Maximum-likelihood iterations. After adjusting the regression model, sexual maturity (CL50) was estimated as the size at which 50% of males and females reached maturity. All analyses of CL50 values were conducted in Microsoft Office Excel 2010 using the Solver tool.
The reproductive period was determined based on the percentage of reproductive females over the studied months (Castilho et al., Reference Castilho, Costa, Fransozo and Negreiros-Fransozo2008). Juvenile recruitment was temporally characterized based on the frequency of the percentage of immature individuals of both sexes in the population. The onset of sexual maturity was determined based on the length of the smallest adult individual (Bauer, Reference Bauer1989).
For statistical analysis, the assumption of normality was verified via the Shapiro–Wilk test. When the data were not distributed according to a Gaussian curve, they were logarithmically transformed (Log x + 1). All tests were performed with a significance level of 5% (Zar, Reference Zar1999).
Cross-correlations (time series) were used to evaluate the correlation of the monthly abundance of reproductive females with recruits and with the mean values of temperature, salinity and chlorophyll-a concentration by month. In cross-correlations, two series of data are compared as a function of the time lag (n), using the Pearson correlation coefficient to measure relationships between values of the first data series and values of the second series from either earlier (in negative lags) or later months (in positive lags). Correlation coefficient values at lag 0 are equivalent to the standard Pearson correlation (Statsoft, 2011). According to the Dancey & Reidy (Reference Dancey and Reidy2011) categorization, the correlation with coefficient 1 is perfect, 0.99–0.7 is strong, 0.69–0.4 is moderate and 0.39–0.1 is weak.
Growth and longevity
Growth and longevity were analysed for males and females separately, based on the von Bertalanffy growth model (von Bertalanffy, Reference Von Bertalanffy1938) and using the methodology adopted by Simões et al. (Reference Simões, D'Incao, Fransozo, Castilho and Costa2013). Modal values were determined for each CL frequency using the software PEAKFIT (Automatic Peak Fitting Detection and Fitting, Method I-Residual, no Data Smoothing), with size classes of 1.0 mm, according to Fonseca & D'Incao (Reference Fonseca and D'Incao2003). The models were plotted on a scatter graph vs age, to analyse the growth rhythm of the cohorts. Growth parameters (CL∞: asymptotic carapace length; k: growth coefficient (day−1); t 0: theoretical age at size zero) were estimated by using the SOLVER supplement in Microsoft Excel (version 2010) for Windows 7, which applies the von Bertalanffy growth model: CLt = CL∞[1–exp−k(t−t0)] (CLt: carapace length at age t). The growth of a cohort was evaluated based on its similarity to values previously observed for this species (Campos et al., Reference Campos, Branco and D'Incao2011). Cohort data were pooled, and growth parameters were estimated. The estimated growth curves for males and females were compared by the F test (P = 0.05) (Cerrato, Reference Cerrato1990). Longevity was calculated using the inverse von Bertalanffy growth model, with a modification suggested by D'Incao & Fonseca (Reference D'Incao, Fonseca, von Vaupel Klein and Schram1998), which is given by longevity = 0−(1/k)Ln[1−(CLt/CL∞)] (considering t 0 = 0, and CLt/CL∞ = 0.99).
The results obtained for sexual maturity, type and period of reproduction, maximum size of carapace, growth coefficient, longevity and asymptotic carapace length were compared with previously published literature on the species in regions with latitudes higher than 22°S.
Results
Reproduction and recruitment
A total of 3554 individuals, 2109 females and 1445 males, were captured. The smallest individual had 8.0 mm of carapace length. The largest size for females was 45.0 mm, while the largest size for males was 36.3 mm, with a difference between sexes (Student t-test = 17.42, P < 0.001). Most females were found among size classes of 14–24 mm, with peak of reproductive females among 20–24 mm sizes. The smallest reproductive female was 12.9 mm, and sexual maturity (CL50) was observed when the shrimp were 12.45 mm in length. Most males were found among size classes of 12–18 mm, with peak of reproductive males among 14–20 mm, and CL50 estimated at 10.54 mm (Figures 1 and 2).

Fig. 2. Hypothetical graphical representation of the sexual maturity curve of individual males and females among size classes from March 2008 to February 2010 in Macaé, Cabo Frio region (CL, carapace length).
Reproductive female peaks were registered during austral winter and spring seasons (November–December 2008, and September 2009), after the main primary production peaks (chlorophyll concentration) of June–October 2008 and August 2009, respectively (Figures 3A and 3B). The mean bottom water temperature was below 23 °C. Lower temperature values (<20 °C) were registered during austral spring–summer seasons – October 2008, November 2008, November 2009 and January 2010 (Figure 3B).

Fig. 3. Monthly distribution of reproductive females, immature individuals, temperature, salinity and chlorophyll from March 2008 to February 2010 in Macaé region.
The juvenile recruitment periodicity was seasonal, with main peaks during April 2009 and August–October 2009, during or before the main spawning peaks (Figure 3A). The cross-correlation analysis of reproductive females and juveniles was weak (r < 0.21).
On the contrary, the cross-correlation analysis showed a moderate correlation (0.4 < corr < 0.69) a few months after or during the same time period between salinity and reproductive female (Lag = 0, r = 0.42 and Lag = −1, r = 0.49), temperature and chlorophyll (Lag = −2, r = 0.42), chlorophyll and reproductive female (Lag = −3; r = 0.50), chlorophyll and juveniles (Lag = −1, r = 0.52 and Lag = −2; r = 0.47) and temperature and juveniles (Lag = −3, r = 0.40).
Growth and longevity
Based on the growth analysis, 10 cohorts were identified for females and seven for males (Figures 4 and 5). A general average growth curve was constructed by grouping cohorts obtained for males and females separately. Based on these curves, general growth models were determined for each sex, which resulted in estimates of CL∞ = 40.21 mm, k = 0.004 and t 0 = −0.527 days for females, and CL∞ = 36.78 mm, k = 0.005 and t 0 = 0.144 days for males. Longevity was estimated to be 1041.2 days ( = 2.85 years) for females, and 860.1 days ( = 2.36 years) for males. Growth curves for males and females were marginally significant (F calculated = 2.45 < F tabulated = 2.717, P = 0.055).

Fig. 4. Frequency histograms of the carapace length (CL) of females of Pleoticus muelleri in Macaé region from March 2008 to February 2010. The lines represent the cohorts identified during the sampling months (Cont., Continuation).
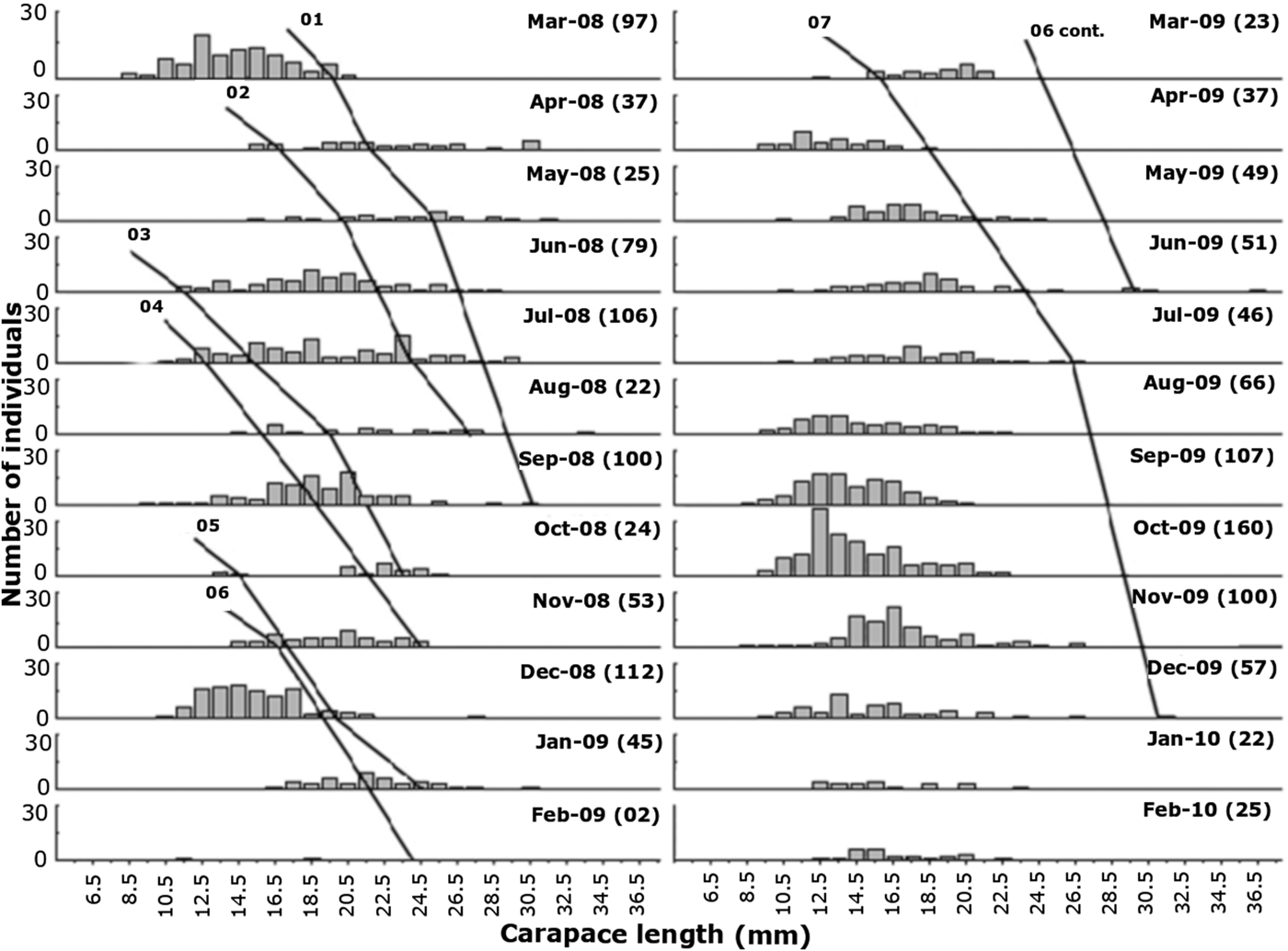
Fig. 5. Frequency histograms of the carapace length (CL) of males of Pleoticus muelleri in Macaé region from March 2008 to February 2010. The lines represent the cohorts identified during the sampling months (Cont., Continuation).
Regarding size at sexual maturity (Figure 2), cohorts of juveniles were identified: June, July and September of 2008, and May, August, September and October of 2009 (Figures 4 and 5).
Discussion
Reproduction, growth and longevity
The seasonal reproduction and the growth cohorts showed a seasonal juvenile recruitment, suggesting that it is influenced by temperature and mainly chlorophyll concentration periodicity. Previous studies showed that temperature induces maturation and spawning, highlighting the importance of environmental factors in the reproductive process (Castilho et al., Reference Castilho, Costa, Fransozo and Negreiros-Fransozo2008; Fernández et al., Reference Fernández, Hernández and Roux2011). According to Bauer (Reference Bauer1992), gametogenesis is favoured by temperature, defining the period of species reproduction. Laboratory experiments with P. muelleri indicated that temperatures between 10 °C and 19 °C are ideal for gonadal maturation and growth (Fenucci, Reference Fenucci1988). In studies from the Argentine coast, reproduction was favoured between 7.5 °C and 15.5 °C (Macchi et al., Reference Macchi, Iorio and Aubone1998; Fernández et al., Reference Fernández, Hernández and Roux2011). This pattern coincides with results obtained in the present study, in which reproductive females were caught in larger numbers in periods of temperatures below 22 °C.
The availability of food for the larval stages is a determining factor in the selection of breeding season for marine invertebrates (Thorson, Reference Thorson1950; Bauer & Lin, Reference Bauer and Lin1994). The increase in phytoplankton production favours a subsequent production of herbivorous zooplankton, resulting in a higher density of planktotrophic organisms (Pires-Vanin & Matsuura, Reference Pires-Vanin and Matsuura1993). Thus, the survival of the larval stages is favoured when it coincides with primary high productivity periods, which remain available for the first larval stages. In addition, chronobiology suggests that fish and crustaceans possess circa-annual endogenous biological clocks, which control the annual spawning repetitiveness (Naylor, Reference Naylor2005). In this biological clock, the photoperiod and water temperature together are the controlling factors proximal for spawning, while the availability of phytoplankton as food for the larvae acts as a final factor.
Our results indicate a higher number of juvenile individuals found after one or two months of higher concentration of chlorophyll in water. This fact can indicate that the planktonic larval phases of P. muelleri have available food to develop, as well as for later settlement in the benthos as juveniles. During periods with chlorophyll concentrations maintained for a longer time (first year of collection), reproduction and juvenile recruitment occurred for more than one consecutive season. In the second year, two peaks in the concentration of chlorophyll were observed, which consequently provided two non-consecutive seasonal peaks of reproductive females and juveniles.
The wide range of body size at all gonadal development stages indicated that P. muelleri complete their entire life cycle in Cabo Frio region, as proposed for the shrimp A. longinaris in the same region (Sancinetti et al., Reference Sancinetti, Azevedo, Castilho, Fransozo and Costa2015). The results of the present study suggest that the restriction period (March to May) is not consistent with the biology of the species. This fact is related to the species studied and regional oceanographic conditions (22°S), like ocean currents, environmental factors and chlorophyll concentrations. The fishing restriction period for P. muelleri was established based on another region (28°S) and another shrimp species (Farfantepenaeus sp.). Considering spawning and recruitment data, the adequate period to restrict P. muelleri fishing in Cabo Frio region would be during the winter and spring months. Therefore, we propose a more specific period from September to December, because the reproductive females and recruits are more abundant during this time of the year.
Dendrobranchiata shrimps appear to exhibit a sexual dimorphism pattern in size, with females larger than males, regardless of latitude and species of penaeid shrimps (Boschi, Reference Boschi1989; Castilho et al., Reference Castilho, Gavio, Costa, Boschi, Bauer and Fransozo2007, Reference Castilho, Costa, Fransozo and Negreiros-Fransozo2008, Reference Castilho, Wolf, Simões, Bochini, Fransozo and Costa2012; Sancinetti et al., Reference Sancinetti, Azevedo, Castilho, Fransozo and Costa2015; Silva et al., Reference Silva, Sancinetti, Fransozo, Azevedo and Costa2015). The larger carapace length in females would be an adaptation to higher egg production (Costa & Fransozo, Reference Costa and Fransozo2004). In addition, the longevity of females is greater than males enabling them to reach larger sizes. According to Vogt (Reference Vogt2012), this difference may be related to hormones and reproductive cost, but the mechanism for this difference is still not well understood. The greater longevity of females is a way to produce more eggs (Chacur & Negreiros-Fransozo, Reference Chacur and Negreiros-Fransozo1998), guaranteeing a more efficient stock replacement of the population. As observed in this study, it is common for males to have higher growth coefficient (k) and lower asymptotic growth compared with females (Gulland & Rothschild, Reference Gulland and Rothschild1981; Petriella & Boschi, Reference Petriella and Boschi1997). Thus, females maintain lower energy expenditure for growth, ensuring greater longevity.
Latitudinal pattern rule
In regions where seasonal climatic differences are small, there is a continuous flow of birth, with year-round reproduction (Thorson, Reference Thorson1950). However, reproduction is seasonal in some species (seasonal births), occurring in a short period of time in reproductive peaks. These breeding seasons (in reproductive peaks) may occur once or twice a year (every 6 months) to optimize the survival of offspring (Colihueque et al., Reference Colihueque, Cárdenas, Ramírez, Estay and Araneda2010; Simões et al., Reference Simões, D'Incao, Fransozo, Castilho and Costa2013).
According to Bauer (Reference Bauer1992), shrimps have different reproduction processes according to the latitudinal gradient. Comparing the results obtained in this study (22°S) with previous studies on P. muelleri (22–48°S), it is observed that the population studied herein does not corroborate the reproductive and growth patterns expected in a latitudinal gradient. Nevertheless, the population found at 22°S presents characteristics similar to latitudes 34–48°S (see Table 1).
Table 1. Reproductive parameters of different populations of Pleoticus muelleri along Western South Atlantic

Lat (S), southern hemisphere latitude; CL, carapace length; K, growth coefficient; BR, Brazil; ARG, Argentina; URU, Uruguay.
Seasons in bold: longer reproductive period.
According to the research on the latitudinal gradient, it was expected that the population studied herein would present reproduction tending to continuous, smaller sizes of carapace length, less longevity and faster growth compared with shrimps along the rest of the Brazilian coast (23–33°S). However, in the studied region, P. muelleri presented larger sizes when compared with latitudes of 23°–33°S. Carapace length did not follow the latitudinal trend of smaller sizes at lower latitudes. Moreover, the shrimp sizes were similar to shrimps found in higher latitudes (above 42°S) in Patagonia, Argentina.
Our results of longevity and growth rates were higher than those in regions between latitudes 23°S and 33°S, and similar to those of higher latitudes (above 33°S) (Table 1). Bauer (Reference Bauer1992) observed an opposite pattern for three species of Sicyonia Edwards 1830, with an increase in longevity and size at which individuals reach sexual maturity in higher-latitude species. Such results may be associated with the water temperature and consequent body temperature of invertebrate animals. The low temperature in invertebrates (i.e. ectotherms) is generally associated with a slower life history, including a slower rate of development (Gillooly et al., Reference Gillooly, Charnov, West, Savage and Brown2002; Trudgill et al., Reference Trudgill, Honek, Li and van Straalen2005). The interaction of processes involved suggests a direct effect of low temperatures on low metabolism (e.g. oxidative reduction and/or DNA damage), as well as neuroendocrine mechanisms of ageing and longevity (Keil et al., Reference Keil, Cimmings and Magalhães2015).
Reproduction and recruitments appear to show seasonal patterns due to more evident reproductive peaks at certain times of the year, as well as in higher latitudes. There was a large reproductive season favoured by lower water temperatures and greater availability of food, mainly during the austral spring season. In the highest-latitude populations of the Southern Argentine littoral (latitudes from 38°S to 46°S), the reproductive period of P. muelleri is seasonal, including two consecutive seasons of the year or two distinct and separate seasons in the year (Table 1).
We conclude that P. muelleri population established around the region of Cabo Frio presents reproductive and growth characteristics similar to cold temperate populations. This similarity is due to temperature and food availability (phytoplankton) being compatible with the life history in the past, at the point of species dispersion in higher latitudes. This way, latitudinal patterns should not be generalized. At lower taxonomic levels (species), and principally with short-lived species, more exceptions than patterns are expected.
Acknowledgements
We thank the co-workers (NUPEM and LABCAM) for their help with fieldwork, and Universidade Federal do Rio de Janeiro/NUPEM. This study complies with current applicable state and federal laws (Authorization of the Instituto Chico Mendes de Biodiversidade/ICMBio no11274).
Financial support
We are grateful to the ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ for providing financial support (FAPESP: #09/54672-4 and 010/50188-8 – RCC), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Research Scholarships #306672/2018-9 and 304784/2011-7 RCC), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj) and to the Financiadora de Estudos e Projetos (Finep/MCT).








