INTRODUCTION
Shrimps of the suborder Dendrobranchiata are among the most important marine fishery resources in the world: the group includes several commercially important species constituting an important food resource for humans (Costa et al., Reference Costa, Fransozo, Freire and Castilho2007). Among this suborder, the family Penaeidae includes some of the most commercially important shellfish inhabiting the south-eastern and southern Brazilian coast, which have significant historical, social and cultural importance (D'Incao et al., Reference D'Incao, Valentini and Rodrigues2002; Branco, Reference Branco2005).
The shrimp fisheries of southern Brazil target the most profitable species, such as the pink shrimps Farfantepenaeus brasiliensis (Latreille, 1817) and F. paulensis (Pérez-Farfante, 1967) and the seabob shrimp Xiphopenaeus kroyeri (Heller, 1862). The increase in the fishing fleet and the decrease in landings of the customarily exploited species have contributed to an expanding interest in the shrimp Artemesia longinaris Bate, 1888, locally known as the barba-ruça (Costa et al., Reference Costa, Fransozo, Castilho and Freire2005; Castilho et al., Reference Castilho, Pie, Fransozo, Pinheiro and Costa2008a).
The geographical distribution of A. longinaris is restricted to the western Atlantic, from Brazil (Rio de Janeiro to Rio Grande do Sul), through Uruguay to the Province of Chubut, Argentina. This shrimp is found from shallow water to 30 m (Boschi, Reference Boschi1997; Costa et al., Reference Costa, Fransozo, Melo and Freire2003), and lives exclusively in the marine environment throughout its life cycle (Rodriguez et al., Reference Rodriguez, Gómez, Verdi and Muniz2002; Branco, Reference Branco2005; Costa et al., Reference Costa, Fransozo, Castilho and Freire2005).
Of the available information on A. longinaris, most accounts are drawn from populations in Argentinean waters (Boschi & Mistakidis, Reference Boschi and Mistakidis1966; Boschi, Reference Boschi1969, Reference Boschi1997; Boschi & Scelzo, Reference Boschi and Scelzo1977; Gavio & Boschi, Reference Gavio and Boschi2004). Some information about the biology of the species was obtained from the southern Brazilian coast, specifically in the States of São Paulo and Rio de Janeiro, including reports on population and breeding dynamics (Castilho et al., Reference Castilho, Gavio, Costa, Boschi, Bauer and Fransozo2007a, Reference Castilho, Costa, Fransozo and Boschib; Semensato & Di Beneditto, Reference Semensato and Di Beneditto2008) and ecological distribution (Costa et al., Reference Costa, Fransozo, Castilho and Freire2005). These authors found that age, maturity and carapace length of this species increase at higher latitudes. Additionally, Castilho et al. (Reference Castilho, Costa, Fransozo and Boschi2007b) observed that reproduction and juvenile recruitment are continuous for individuals of A. longinaris caught on the São Paulo coast (tropical regions = 23°S), but these events are seasonal in samples obtained in the Mar del Plata (cool-temperate = 37°S).
One of the main objectives in the study of the reproductive ecology of benthic invertebrates is to assess latitudinal trends in the timing of reproductive activity and recruitment of juveniles (Bauer, Reference Bauer1992; Costa & Fransozo, Reference Costa and Fransozo2004). The State of Santa Catarina is located between 25°5′741″–29°23′55″S and 48°19′37″–53°50′00″W, and has a subtropical climate. In this region, there are only a few accounts of the reproduction of dendrobranchiate shrimps. Almost all the available information deals with populations of Farfantepenaeus spp. (Branco & Verani, Reference Branco and Verani1998a, Reference Branco and Veranib) and Xiphopenaeus kroyeri (Branco et al., Reference Branco, Lunardon-Branco, Souto and Guerra1999; Branco & Fracasso, Reference Branco and Fracasso2004; Branco, Reference Branco2005). Information about the biology of A. longinaris is nonexistent.
The purpose of the present study was to determine the size at morphological maturity and the sex-ratio of A. longinaris, and to assess the seasonal variation in recruitment of juveniles off Pinheira Beach, Palhoça, Santa Catarina, in subtropical Brazil. Additionally, we compared our findings with those obtained for the same species in tropical and temperate regions. The sex-ratio and temporal distribution of the local population of this species were also evaluated.
MATERIALS AND METHODS
Shrimp were collected monthly from November 2003 through to October 2004 off Pinheira Beach, Palhoça, State of Santa Catarina, Brazil. The samples were collected in two areas where traditional fishermen customarily work (27°52′–27°51′S and 48°33′–48°29′W). One trawl was carried out in each area, at depths of 19 m (area I) and 30 m (area II). A boat (9 m long by 3 m wide) equipped with double-rigged nets (mesh 3.0 cm and 2.0 cm in the cod end) was used for trawling, which lasted for 1 hour in each area. Bottom salinity and temperature were monitored in each area. Detailed descriptions of the sampling methods and analysis of environmental factors for the period are available elsewhere (Branco, Reference Branco2005).
When a large number of individuals were caught in a trawl, a subsample of 500 g total biomass was separated randomly for examination of the sex and length of each individual. All the individuals sorted were sexed and measured (to the nearest 0.1 mm). When the catch did not exceed 500 g, all the shrimp were measured. The parameters used for the biometry of A. longinaris were: total length, including rostrum (TL, mm) and carapace length, excluding the rostrum (CL, mm). The reproductive status of males was assessed by examining the shape of the petasma, which is fused in adult individuals. The adult females were determined by macroscopic observation of the degree of ovarian development (colour and volume occupied by the gonads). Ovaries categorized as immature ranged from thin, transparent strands to thicker ones. Mature ovaries were much larger and thicker, and varied from yellow to light orange or light green, and green to olive-green (Dumont & D'Incao, Reference Dumont and D'Incao2004). Size–frequency distributions were constructed separately to estimate the seasonality for males and females, using 10-mm TL size-intervals.
From the total length, we obtained the maximum and minimum size-ranges for each sex, and plotted the relative frequencies (%) of adults in each size-class. The log function y = 1/1 + e r(TL−TL 50) was fitted to the data, where TL 50 corresponds to the size at which 50% of the individuals are considered adults, and r stands for the slope of the curve. The curves were fitted by the least-squares method (Vazzoler, Reference Vazzoler1996), which requires a size-range overlap of adults and young of at least two size-classes. The shrimp were therefore classed in 10-mm size-intervals.
Recruitment was defined as the percentage of juveniles of the total number of individuals of males and females in each month and season of the year. Size-classes of juveniles were defined separately for males and females. The sex-ratio in each month was compared using the χ 2-test (P < 0.05 and df N –1). The mean sizes (TL) of males and females were compared by Student's t-test (Zar, Reference Zar1984).
In order to assess the effect of the traditional fishery on juvenile and adult shrimp, we used as a basis, the size at first gonadal maturation and the frequency distribution by size-class of males and females (Branco et al., Reference Branco, Lunardon-Branco, Souto and Guerra1999).
RESULTS
Monthly mean bottom seawater temperature and salinity are shown in Figure 1. Mean bottom water temperature sampled between November 2003 and October 2004 varied between 15.9 and 26.9°C (21.1±3.6°C) in the bay and the water salinity varied between 29 and 35.8‰ (33.5‰±2.2), respectively
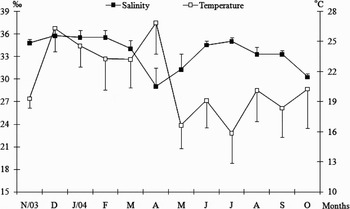
Fig. 1. Mean values of seawater bottom temperature and salinity recorded monthly from November 2003 through to October 2004 in the Palhoça region, Santa Catarina.
Of the total of 1099 specimens analysed, 23.93% were males and 76.07% females. The sex-ratio, calculated for all the individuals caught, was significantly different from 1:1 (P < 0.05, χ 2-test). Monthly sex-ratios were significantly biased towards females in November and December 2003, and March, May, and July through to October 2004. In the other months, the sex proportion was similar (Figure 2).
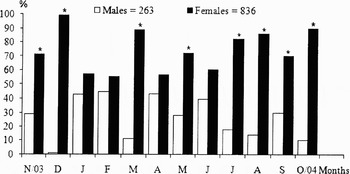
Fig. 2. Proportions (%) of males and females of Artemesia longinaris collected off Pinheira Beach, Palhoça, Santa Catarina, from November 2003 through to October 2004. *, significant difference, χ 2 (P < 0.05).
The relationship of CL and TL was represented by the expressions CL = 0.1645 TL (r = 0.80) for males; and CL = 0.1904 TL = (r = 0.88) for females. The TL50 was estimated as 56.38 mm (CL = 9.3 mm) for males, and 70.34 mm (CL = 13.4 mm) for females (Figure 3).
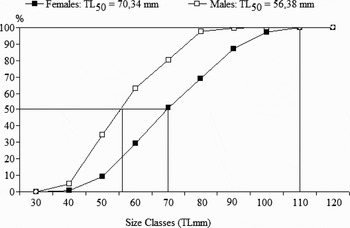
Fig. 3. Sexual maturity based on the TL50% (total length) of females and males of Artemesia longinaris collected off Pinheira Beach, Palhoça, Santa Catarina, from November 2003 through to October 2004.
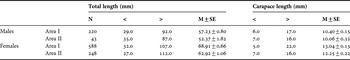
Seasonal and spatial size–frequency distributions for males and females for A. longinaris are shown in Figure 4. In both localities, females reached a larger size than males, and were most abundant in the size-classes from 60 to 120 mm TL (P < 0.05). No male was collected between the lengths of 110 and 120 mm. Overall, the mean size for males was 56.5±11.9 mm TL, ranging from 29 (CL = 4.8 mm) to 92 mm TL (CL = 15.3 mm); the mean size for females was 67.2±16.4 TL, ranging from 27 (CL = 5.1 mm) to 112 mm TL (CL = 21.3 mm). Females were significantly larger than males (t = 9.7, P < 0.001). The mean and minimum and maximum TL in each area for both sexes are listed in Table 1.
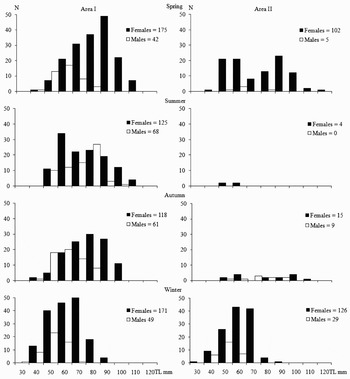
Fig. 4. Seasonal distribution of number of shrimps in each size-class (TL, mm) of males and females of Artemesia longinaris collected off Pinheira Beach, Palhoça, Santa Catarina, from November 2003 through to October 2004.
Table 1. Number (N), total length (mm) and carapace length (mm) of Artemesia longinaris in the two collecting areas, with their respective ranges, means (M) and standard errors (SE), off Pinheira Beach, Palhoça, Santa Catarina, from November 2003 through to October 2004.
Juveniles of A. longinaris were found in all months (Table 2). The highest percentage of juveniles in relation to adults occurred in November 2003, March, April and July to September 2004. In general, the traditional fishery effort at Pinheira Beach is mainly concentrated on the stocks of juveniles (Figure 4; Table 2), that is, 75.7% of the males, and 63.4% of the females caught were juveniles. A high predominance of juveniles in the areas sampled occurred in winter (July to September) for both sexes, and in spring for the females. In the summer and autumn, we observed a decrease in juveniles abundance in both areas, mainly, area II (Figure 4).
Table 2. Percentages of juveniles and adults of Artemesia longinaris in the two collecting areas, monthly sampled between November 2003 and October 2004 in the Pinheira Beach, Palhoça, Santa Catarina.

DISCUSSION
Sex-ratio was strongly skewed towards females in this population of A. longinaris. Monthly, and in most size-classes, females were always more abundant than males. Several hypotheses might be proposed to explain this observation. For instance, Cha et al. (Reference Cha, Oh, Hong and Park2002) attributed the annual sex-ratio in favour of Penaeus chinensis Osbeck, 1765 females to greater mesh-size selectivity. Additionally, we can suggest that the sex-ratio in favour of A. longinaris females may be related to greater vulnerability of females to fishing because of their size. However, in most of the size-classes that included juvenile females, the sex-ratio was still strongly skewed towards females. This suggests that sampling bias cannot account for the observed sex-ratio.
Kevrekidis & Thessalou-Legaki (Reference Kevrekidis and Thessalou-Legaki2006) postulated an operating factor ‘such as higher female catchability for Melicertus kerathurus (Forskål, 1775), due to longer foraging in order to meet increased food requirements during ovary maturation.’ Cha et al. (Reference Cha, Oh, Hong and Park2002) suggested that the predominance of adult females in P. chinensis might be related to a possible breeding period that caused higher natural mortality of males because of mating. Castilho et al. (Reference Castilho, Furlan, Costa and Fransozo2008b) proposed for Sicyonia dorsalis Kingsley, 1878, that the female-biased sex-ratio might be favoured in populations in which females are polygynous. Here we suggest another hypothesis for A. longinaris: differential migration between the sexes during the reproductive cycle. Copulation would occur in deep water, with subsequent migration of adult females to coastal waters (the area of the present study) to spawn. According to the results obtained, spawning is more intense in summer and autumn, thus favouring the greater occurrence of juveniles in the following seasons, i.e. winter and spring. Consequently, the male recruits could initiate their migration toward the offshore region before the females do. Boschi (Reference Boschi1969) observed that as individuals of A. longinaris become adults and reach commercial size, they begin to migrate to deeper regions in Argentinean waters. In their study, in the State of Rio Grande do Sul, Dumont & D'Incao (Reference Dumont and D'Incao2004) proposed a similar hypothesis and Gavio & Boschi (Reference Gavio and Boschi2004) confirmed that the main reason for this detour in the Mar del Plata region is the different breeding migrations of the males and females, because of the requirements for spawning.
Artemesia longinaris is sexually dimorphic in size, with females reaching a larger TL than males, indicating differential growth rates between sexes. The results obtained here followed the pattern obtained for this species in the States of Rio de Janeiro and São Paulo (Castilho et al., Reference Castilho, Gavio, Costa, Boschi, Bauer and Fransozo2007a, Reference Castilho, Costa, Fransozo and Boschib; Semensato & Di Beneditto, Reference Semensato and Di Beneditto2008), and Argentinean waters (Boschi, Reference Boschi1969; Gavio & Boschi, Reference Gavio and Boschi2004). Boschi (Reference Boschi1969) and Branco et al. (Reference Branco, Lunardon-Branco, Souto and Guerra1999) noted that differences in body length between sexes are a general rule among penaeoid shrimps. According to Costa & Fransozo (Reference Costa and Fransozo2004) and Castilho et al. (Reference Castilho, Costa, Fransozo and Negreiros-Fransozo2008c), this sexual dimorphism probably occurs because the large body size of females is an adaptation to increased egg production.
Boschi & Scelzo (Reference Boschi and Scelzo1977) and Rodriguez et al. (Reference Rodriguez, Gómez, Verdi and Muniz2002) have found individuals that still were juveniles between the lengths (TL) of 60 to 70 mm. If we compare the sexual maturity of females of A. longinaris estimated in the present study (27°S) with the findings of Dumond & D'Incao (2004) in the State of Rio Grande do Sul (32°S, 17.0 mm CL) and Castilho et al. (Reference Castilho, Gavio, Costa, Boschi, Bauer and Fransozo2007a) in the Mar del Plata, Argentina (37°S, 22.1 mm CL), we can perceive a trend toward an increase in the size at sexual maturity with increasing latitude. These authors did not mention the sexual maturity of the males. Nascimento (Reference Nascimento1981), along the coast of Rio Grande do Sul, estimated from morphological variations in the petasma, that at 10.7 mm (CL) all the males were functional adults. A similar trend appeared in relation to body size. Boschi (Reference Boschi1997) found females as large as 30 mm CL in Mar del Plata, and Boschi & Mistakidis (Reference Boschi and Mistakidis1966) recorded females as large as 37 mm CL in Chubut Province (43°S). In contrast, females caught on the coast of Rio Grande do Sul reached 28 mm. Similarly to the results obtained by Castilho et al. (Reference Castilho, Gavio, Costa, Boschi, Bauer and Fransozo2007a) in the State of São Paulo, we captured adult specimens of A. longinaris that were even smaller (<27 mm CL). (The authors cited did not mention the sexual maturity and body size of males.)
However, this tendency was not confirmed in comparing the results obtained for populations farther north (tropical climate, 23°S). Only the sexual maturity of females was similar when our results were compared with those of Castilho et al. (Reference Castilho, Costa, Fransozo and Boschi2007b) on the São Paulo coast. Semensato & Di Beneditto (2008) found that at latitude 22°S, females reached sexual maturity at 16.4 mm CL; this is 3.0 mm CL larger than the size at maturity observed here, and also by Castilho et al. (Reference Castilho, Costa, Fransozo and Boschi2007b).
In relation to maximum body length, our estimates were the lowest in comparing two tropical regions. Perhaps, different sampling or differential migration between juveniles and adults of the populations of the two localities (south-east and south) may have contributed to this trend. Semensato & Di Beneditto (Reference Semensato and Di Beneditto2008) carried out their sampling in locations where the fishing fleets worked most, and therefore, few juveniles were caught. On the coast of São Paulo, Castilho et al. (Reference Castilho, Costa, Fransozo and Boschi2007b) sampled in a similar depth-range to this work; however, juveniles and also adults approached the coast in greater abundance only in spring or the beginning of summer. At these times, the cold water mass (South Atlantic Coastal Waters (SACW)) intrudes, and the bottom temperatures were lower than 21°C. In the other seasons, most of the population moved to deep areas with the retreat of the SACW. In the present study area, during the winter and spring, we observed mean monthly values lower than 21°C, which may have caused the juveniles to remain longer. Here, the adult population probably moves to shallow waters only to spawn in the first six months of the year. This may have led to similar sizes at maturity between these regions.
According to the present regulations, the fishery is closed between March to May, because this is the main recruitment period of juvenile pink shrimps (Farfantepenaeus spp.) in the entire south-east and south regions of Brazil (Costa et al., Reference Costa, Lopes, Castilho, Fransozo and Simões2008). Our results indicate that the traditional fishery at Pinheira Beach acted with greatest intensity on juveniles, mainly, in winter (July to September) on both sexes. In this way, we suggest that during all winter months the region up to 30 m of depth should be included in the present proposed off-season.
One of the main objectives of the study of the reproductive ecology of benthic invertebrates is to assess latitudinal trends in the timing of reproduction and recruitment of juveniles (Bauer, Reference Bauer1992). In Pinheira Beach, the presence of juveniles throughout the year suggests that this species breeds continuously. However, there is evidence for more intense breeding activity in summer and autumn. Similar results were obtained by Castilho et al. (Reference Castilho, Costa, Fransozo and Boschi2007b), when the reproductive period was highest in spring and summer. In cool-temperate Argentinean waters, Christiansen & Scelzo (Reference Christiansen and Scelzo1971) observed seasonal breeding and recruitment. These results, too, corroborate the classical paradigm of seasonal reproduction at higher latitudes, and continuous reproduction at lower latitudes.
In general, it can be concluded that the areas in the present study are of maximum importance for the establishment and growth of juveniles, and are a breeding ground of this species. These results can be used to develop a more appropriate fishery policy in the study region. Future studies on migration and reproduction, focusing on deeper-water sites, are especially important to clarify the hypotheses presented on the population aspects of Artemesia longinaris.
ACKNOWLEDGEMENTS
Thanks to the Universidade do Vale do Itajaí (UNIVALI) for financial support, and to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for a scientific productivity grant awarded to Dr Joaquim Olindo Branco. Dr Janet W. Reid revised the text.








