INTRODUCTION
Sea turtles often act as hosts to a variety of marine plants and animals (Frick & Pfaller, Reference Frick, Pfaller, Wyneken, Lohmann and Musick2013), a phenomenon known as epibiosis (Wahl & Mark, Reference Wahl and Mark1999). These associations vary depending on the costs and/or benefits experienced by the associates (i.e. parasitism, commensalism or mutualism), the degree of interdependency (i.e. facultative vs obligate symbiosis), and the level of host specificity (i.e. generalists vs specialists). Epibiotic associations with sea turtles are primarily facultative commensalisms, in which organisms typically found associated with submerged inanimate structures – termed ‘free living’ – live on or attached to host turtles and do not provide benefits or derive nutrients from the turtle. Studies of sea turtle epibiosis have focused on the diversity and ecology of facultative, as well as obligate, commensalisms (Frick & Pfaller, Reference Frick, Pfaller, Wyneken, Lohmann and Musick2013). Less attention has been given to parasitic associations, in which the epibiont species derives nutrients from the tissue of the host turtle. Parasitic epibionts of sea turtles are not reported frequently (Williams et al., Reference Williams, Bunkley-Williams, Boulon, Eckert and Bruce1996), but these associations may have important consequences for the health of host turtles (Greenblatt et al., Reference Greenblatt, Work, Balazs, Sutton, Casey and Casey2004), of which all species are classified as at least vulnerable in the IUCN Red List of Threatened Species (IUCN, 2014).
Isopods are a highly diverse group that inhabit a wide variety of habitats and lifestyles (Poore & Bruce, Reference Poore and Bruce2012). Many species are free-living, while others form obligate and facultative associations with other marine organisms (e.g. ascidians, sponges, corals, arthropods, echinoderms and fishes; Poore & Bruce, Reference Poore and Bruce2012). A diverse array, however, are generalist and specialist parasites that attach either temporarily or permanently to various hosts (Bunkley-Williams & Williams, Reference Bunkley-Williams and Williams1998; Williams & Boyko, Reference Williams and Boyko2012). Isopods that cling to but do not feed on host tissue are not considered parasites, nor are isopods that occasionally feed on but do not attach to hosts (i.e. micropredators) (Bunkley-Williams & Williams, Reference Bunkley-Williams and Williams1998). Using grasping and piercing mouthparts, parasitic isopods attach either internally or externally and feed on the tissues of their host – blood and mucosal linings of vertebrate hosts or haemolymph in crustacean hosts (Bunkley-Williams & Williams, Reference Bunkley-Williams and Williams1998; Espinosa-Pérez & Hendrickx, Reference Espinosa-Pérez and Hendrickx2006; Williams & Boyko, Reference Williams and Boyko2012). Parasitic isopods of fishes are known to cause stunted growth, castration, injury, illness and even death of their hosts (Bunkley-Williams & Williams, Reference Bunkley-Williams and Williams1998). Parasitic isopods of commercial fishes have garnered special attention because infestations can lead to economic losses in the fishing industry (Bird, Reference Bird1981; Ravichandran et al., Reference Ravichandran, Rameshkumar and Balasubramanian2010; Poore & Bruce, Reference Poore and Bruce2012). Despite the ecological and economic significance of these associations, we know surprisingly little about the basic natural history and population distributions of parasitic isopods and their associations with vertebrate hosts (Bunkley-Williams & Williams, Reference Bunkley-Williams and Williams1998).
Records of isopods associated with sea turtles are relatively sparse. Bustard (Reference Bustard, Jones and Endean1976) and Schärer (Reference Schärer2003) reported isopods, including Eurydice sp. (Cirolanidae), on the carapaces of loggerhead (Caretta caretta), hawksbill (Eretmochelys imbricata) and green (Chelonia mydas) turtles inhabiting coral reefs, and Caine (Reference Caine1986) and Frick et al. (Reference Frick, Williams and Robinson1998) reported isopods (Sphaeroma quadridentatum and Cancrion carolinus) on the carapaces of nesting loggerhead turtles. Because these isopods were not attached to soft tissue and were likely not consuming tissue of the host turtles, these associations are not considered parasitic. Similar to most other sea turtle-epibiont associations, such associations would be considered facultative commensalisms (Frick & Pfaller, Reference Frick, Pfaller, Wyneken, Lohmann and Musick2013). Conversely, other authors report isopods, including two Excorallana species, attached to soft tissues (e.g. eyelids, shoulders and flanks) of leatherback (Dermochelys coriacea) and green turtles (Hendrickson, Reference Hendrickson1958; Monod, Reference Monod1975; Eckert & Eckert, Reference Eckert and Eckert1988; Delaney, Reference Delaney1989; Williams et al., Reference Williams, Bunkley-Williams, Boulon, Eckert and Bruce1996; Alonso, Reference Alonso2007), which suggests that some epibiotic isopods of sea turtles may be parasitic. Williams et al. (Reference Williams, Bunkley-Williams, Boulon, Eckert and Bruce1996) found blood in the digestive tracts of attached isopods, providing evidence that isopods were feeding on the tissues of turtle hosts. These records of parasitic isopods on sea turtles identify the host and isopod species and the body part to which the parasite is attached, but do not quantify other important biological characteristics of these associations (Williams et al., Reference Williams, Bunkley-Williams, Boulon, Eckert and Bruce1996). Thus, we know very little with respect to spatial and temporal patterns in the frequency and intensity of parasitism. Such information would allow us to identify source populations of parasites, evaluate the detrimental effects on host turtles and turtle populations, and understand the role that host turtles play as dispersal vectors for parasitic isopods.
In this study, we present the important biological characteristics of parasitic isopods found associated with sea turtles nesting in Bahia, Brazil. Three isopod species (Excorallana costata, Excorallana bicornis and Excorallana oculata) were found attached to loggerheads (Figure 1) and hawksbills, and one isopod species (E. costata) was found attached to olive ridley turtles (Lepidochelys olivacea). To our knowledge, each of these represents a previously undescribed association. The goals of this study are to quantify (1) the frequency and intensity of parasitism for each isopod species on each turtle species and (2) the temporal change in the frequency and intensity of parasitism across the turtle nesting season. Because parasitic isopods were observed only rarely prior to the 2009–2010 turtle-nesting season, it is important to gain a better understanding of these interactions and the reasons for their emergence.

Fig. 1. Photographs showing parasitic isopods (genus Excorallana) attached to the eyelids of sea turtles nesting on Praia do Forte, Bahia, Brazil. (A) Loggerhead infested with parasitic isopods (arrows indicate exposed posterior end of isopods). (B) Parasitic isopod being pulled with tweezers to show attachment location on underside of eyelid.
MATERIALS AND METHODS
Turtles were surveyed for parasitic isopods during the 2009–2010 nesting season (15 October to 15 February) on Praia do Forte, municipality of Mata de São João, Bahia, Brazil (12°34′39.60″S 38°0′6.21″W; Figure 2). Praia do Forte is a 14 km beach that supports a large nesting aggregation of loggerheads, and smaller numbers of nesting green turtles, hawksbills and olive ridleys (Marcovaldi & Marcovaldi, Reference Marcovaldi and dei Marcovaldi1999; Marcovaldi & Chaloupka, Reference Marcovaldi and Chaloupka2007). For this study, we focused our survey effort on a 5-km portion of Praia do Forte that supports the highest density of nesting turtle activity. Nocturnal patrols were conducted between 20:00 and 04:00 h to intercept all female turtles that emerged to deposit a clutch of eggs. Once encountered, each turtle was examined for and, if necessary, fitted with individualized inconel tags (National Band and Tag Co.), one in each front flipper. All observed isopods on each turtle were collected with surgical tweezers and immediately preserved in 70% ethanol for subsequent analysis and identification. Turtle species, date and the body part from which isopods were collected were also recorded. Isopods were identified based on Lemos de Castro (Reference Lemos de Castro1960), Lemos de Castro & Lima (Reference Lemos de Castro and Lima1971) and Delaney (Reference Delaney1984).

Fig. 2. Map showing the locations of the study site (Praia do Forte) in Bahia, Brazil and other Brazilian states/cities mentioned in this study (other state borders are not shown).
Frequency of parasitism (F P) was derived by dividing the number of turtles hosting isopods by the total number of turtles surveyed. Intensity of parasitism was the number of isopods per turtle and mean intensity (I P) was derived for turtles hosting at least one isopod. For individual turtles that were surveyed more than once during the nesting season, we pooled data for F P (i.e. each turtle was counted only once) and treated each occurrence for each turtle separately for I P. This was done because all isopods were collected for species identification at each observation. These parameters (F P and I P) were derived for (1) each isopod species for all turtle species combined, (2) all isopods for each turtle species, and (3) all isopods for all turtle species combined. The sample sizes among turtle species were too different to statistically compare F P and I P among turtles, but we were able to make qualitative comparisons.
We used binomial logistic regressions and Poisson regressions (log-link function) to test for temporal changes in isopod occurrence and intensity, respectively, across the nesting season (Bonferroni correction for 10 binomial logistic regressions and 10 Poisson regressions: corrected alpha for each set of 10 analyses = 0.005). Both regression analyses were performed for each turtle species (all isopod species were pooled together, when applicable) and each isopod species (all turtle species pooled together), as well as each isopod species on loggerheads and E. costata on hawksbills. Other possible relationships were not performed due to small sample sizes.
Although rigorous, quantitative surveys for parasitic isopods were not conducted during the nesting seasons before and after the 2009–2010 season, the Brazil National Sea Turtle Conservation and Research Program – Projeto TAMAR/ICMBio – has monitored sea turtle nesting activity on Praia do Forte from 1982–2014 (Marcovaldi & Marcovaldi, Reference Marcovaldi and dei Marcovaldi1999) and fieldworkers make observations on the health of each turtle that is encountered, including notes on the presence of external parasites. Thus, observations of parasitic isopods made during the nesting seasons before (1982–1983 to 2008–2009) and after (2010–2011 to 2013–2014) the present study were collated by season and used to inform our results.
RESULTS
We recorded three different congeneric isopod species (Excorallana costata, E. bicornis and E. oculata) attached to loggerheads (N = 79) and hawksbills (N = 23), and one species (E. costata) attached to olive ridleys (N = 9). A small number of nesting green turtles (N = 3) were surveyed, but no isopods were detected. The majority of isopods was found attached to the eyelids of host turtles, while smaller numbers were found attached to the neck and/or flippers. On several occasions, blood was found in the digestive tracts of attached isopods.
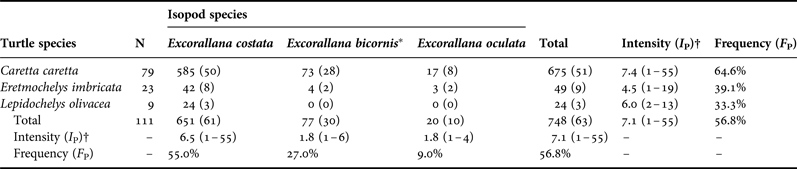
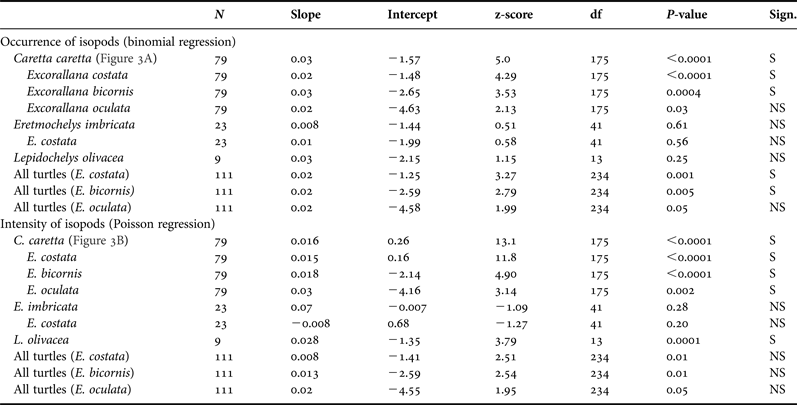
Table 1 shows the numerical distribution of the three isopod species on the three turtle species, and F P and I P for each isopod species for all turtle species combined, for all isopods for each turtle species, and for all isopods for all turtle species combined. During the 2009–2010 nesting season, we collected 748 individual isopods from 63 individual turtles (48 turtles did not host parasitic isopods). Excorallana costata was the most common isopod species, followed by E. bicornis and E. oculata. Qualitative patterns included: (1) E. costata exhibited a higher frequency and intensity of parasitism than E. bicornis and E. oculata; (2) loggerheads hosted parasitic isopods at a higher frequency and intensity than hawksbills and olive ridleys; and (3) hawksbills hosted parasitic isopods at a higher frequency, but lower intensity, than olive ridleys.

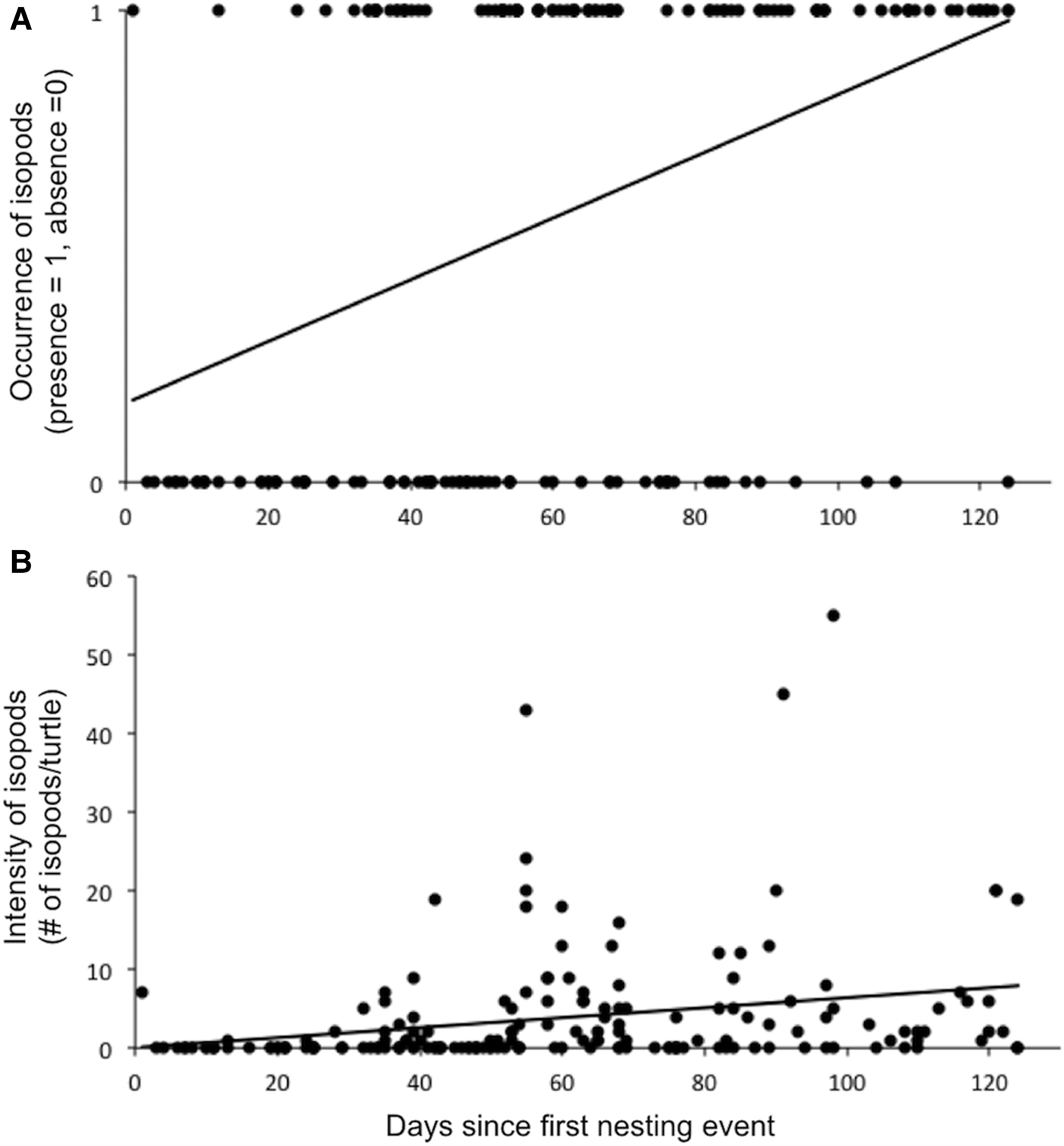
Fig. 3. Temporal shifts in (A) the occurrence of parasitic isopods (binomial logistic regression) and (B) the intensity of parasitic isopods (Poisson regression) on loggerhead turtles nesting at Praia do Forte, Bahia, Brazil during the 2009–2010 season. Statistical results for these analyses and similar analyses for other turtle and isopod species are shown in Table 2.

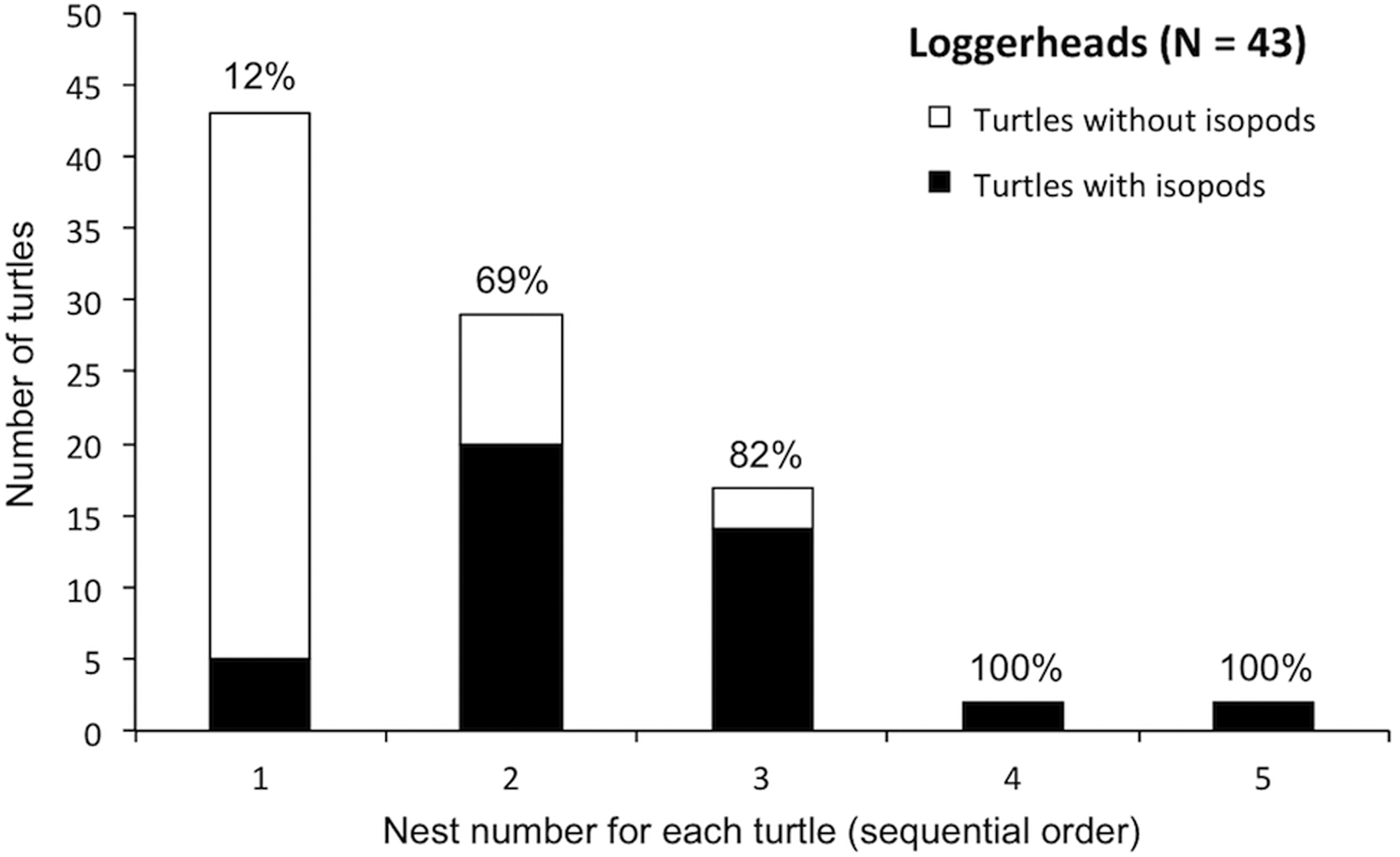
Fig. 4. Histogram showing the number of loggerhead turtles with 1–5 nests at Praia do Forte, Bahia, Brazil during the 2009–2010 season. White bars and data labels indicate the number and percentage of turtles hosting isopods at each sequential nesting event, respectively.
Table 1. Numerical distribution of parasitic isopods on nesting sea turtles on Praia do Forte, Bahia, Brazil during the 2009–2010 season.

Notes. Numbers in parentheses indicate numbers of turtles, except for Intensity, in which the numbers in parentheses indicate range.
*All specimens of E. bicornis were male.
†Each observation for each turtle was treated separately (i.e. data from turtles observed more than once were not pooled before calculating I P).
Table 2 shows the results of binomial logistic regressions and Poisson regressions testing for temporal changes in isopod occurrence and intensity, respectively, across the 2009–2010 nesting season. For loggerheads, we found significant increases in both the occurrence and intensity of parasitism for all isopod species pooled (Figure 3A, B), for E. costata alone and for E. bicornis alone. We also found significant increases in the occurrence of parasitism for E. costata and E. bicornis on all turtle species pooled, and in the intensity of parasitism for E. oculata on loggerheads and E. costata on olive ridleys. For hawksbills, we found no significant change in both the occurrence and intensity of parasitism for all isopod species pooled and for E. costata alone. We also found no significant change in the occurrence of E. costata on olive ridleys and E. oculata on all turtles species pooled. We found no significant change in the occurrence and intensity of parasitism for each isopod species on all turtle species pooled. Lastly, we found that the percentage of loggerheads hosting isopods increased with additional nesting events (Figure 4). There were insufficient data to test this pattern for hawksbills and olive ridleys.
Table 2. Results of binomial and Poisson regressions testing for temporal shifts in occurrence and intensity of parasitic isopods across the sea turtle nesting season at Praia do Forte, Bahia, Brazil during the 2009–2010 season.

Notes. Temporal shifts were either significant (S) or not significant (NS) based on a Bonferroni corrected alpha of 0.005 (10 binomial logistic regressions and 10 Poisson regressions). Degrees of freedom (df) are greater than the sample size (N) because some individual turtles were observed multiple times throughout the nesting season.
During our study of nesting turtles at Praia do Forte in 2009–2010, two observations were made that are informative with respect to results of our study. First, a loggerhead that nested three times at Praia do Forte was found nesting at a different nesting beach (Arembepe) 30 km to the south (Figure 2). This turtle was found hosting isopods, which was the first record of parasitic isopods documented at this site. Second, a juvenile green turtle was captured in a foraging area off the coast of Praia do Forte (19 December 2009) that had seven isopods (E. costata and E. bicornis) attached to its eyelids.
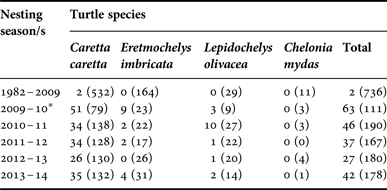
Table 3 shows the number of turtles that were observed hosting parasitic isopods prior to the 2009–2010 season and each year since the 2009–2010 season. Observations of turtles hosting parasitic isopods were very rare prior to our study (N = 2 observations) and have remained relatively frequent each year since then (N > 26 observations). Moreover, similar qualitative patterns have continued during each season since 2009–2010: (1) loggerheads tend to host parasitic isopods more frequently than hawksbills and olive ridleys; (2) hawksbills and olive ridleys tend to host parasitic isopods at similar, but low, frequencies; and (3) green turtles do not tend to host parasitic isopods.
Table 3. Interannual variation in number of nesting sea turtles with parasitic isopods on Praia do Forte, Bahia, Brazil.

Notes. Numbers in parentheses indicate the number of individual turtles surveyed during each nesting season.
*Season with rigorous, quantitative survey, in which all isopods were collected and identified to species.
DISCUSSION
In this study, we present the first report and description of the isopods Excorallana costata, Excorallana bicornis and Excorallana oculata on loggerhead and hawksbill turtles, and E. costata on olive ridley turtles. Previous studies report rare, mostly opportunistic observations of isopods on sea turtles (see review in Williams et al., Reference Williams, Bunkley-Williams, Boulon, Eckert and Bruce1996). Therefore, this study is the first quantitative survey analysing spatial and temporal patterns in the frequency and intensity of parasitic isopods on sea turtles. These data provide the basis for understanding the basic biology of these interactions, as well as identifying possible source populations of parasites, assessing detrimental effects on host turtles and turtle populations, and evaluating the role that host turtles play as dispersal vectors for parasitic isopods.
We found individual Excorallana firmly attached to the eyelids, and to a lesser extent the neck and flippers, of host turtles that emerged from the ocean to nest. Similar to Williams et al. (Reference Williams, Bunkley-Williams, Boulon, Eckert and Bruce1996), we also found blood in the digestive tracts of attached isopods. Although there is some debate as to whether corallanid (including Excorallana) and cirolanid isopods are parasites or ‘micropredators’ of marine vertebrates (Buckley-Williams & Williams, 1998), our observations strongly suggest that Excorallana spp. use modified mouthparts to attach to the skin of host turtles and feed on the tissue of the turtle, and therefore should be considered parasitic epibionts of sea turtles. While the duration of such parasitic interactions is unknown, the fact that Excorallana in this study were securely attached (see Figure 1B) and did not abandon the turtles during nesting emergences (1–2 h) suggests that the duration of these associations maybe somewhat extended. This is not consistent with ‘micropredator’ behaviour (Buckley-Williams & Williams, 1998) and further supports the idea that Excorallana are sea turtle parasites, at least temporarily.
The recent emergence of Excorallana isopods as parasitic epibionts of sea turtles nesting in Brazil is puzzling. The Brazil National Sea Turtle Conservation and Research Programme – Projeto TAMAR/ICMBio – has monitored sea turtle nesting on Praia do Forte for over 30 years (Marcovaldi & Marcovaldi, Reference Marcovaldi and dei Marcovaldi1999) and only twice were parasitic isopods detected prior to the 2009–2010 nesting season (Table 3). Other rare occurrences could have been missed in the past, but the high frequency and intensity of parasitism documented in 2009–2010, as well as subsequent nesting seasons, suggests that these parasitic associations may continue to be common and persistent occurrences into the future. To better understand the emergence of these parasitic associations and evaluate the implications of this potential threat, it is important to determine where, when and how such associations originate.
The genus Excorallana is speciose in the western Atlantic (15 species; Delaney, Reference Delaney1989) and all three species found in this study occur in Brazil (Pires-Vanin, Reference Pires-Vanin and Young1989). Although species ranges are not well defined, E. costata is known from Brazilian states south of our study site in Bahia (Espírito Santo, Rio de Janeiro and São Paulo; Figure 2), while E. bicornis and E. oculata are known from states north of Bahia (Pernambuco, Rio Grande do Norte and Amapá; Figure 2). Interestingly, the three species of Excorallana found in this study have not been documented off the coast of Bahia, which suggests that turtles may be parasitized in their foraging grounds and transport isopods to the nesting beach. If this is true and different isopod species are indeed geographically restricted, then our results suggest that loggerheads and hawksbills nesting in Bahia tend to use both northern and southern foraging areas (i.e. both northern and southern Excorallana species present), while olive ridleys nesting in Bahia tend to use only southern foraging areas (i.e. only E. costata present). This pattern is partially consistent with satellite-tracking data for post-nesting turtles. Loggerheads and hawksbills tracked after nesting in Bahia migrate to both northern and southern foraging areas (Marcovaldi et al., Reference Marcovaldi, Lopez, Soares, Lima, Thomé and Almeida2010, Reference Marcovaldi, Lopez, Soares and López-Mendilaharsu2012; Marcovaldi personal communication). Olive ridleys nesting in Bahia have not been satellite tracked, but those tracked after nesting in Sergipe (state north of Bahia; Figure 2) migrated to the north and south, as well as offshore (da Silva et al., Reference da Silva, dos Santos, das C. Oliveira, Weber, Batista, Serafini and de Castilhos2011).
An alternative explanation for the presence of these three Excorallana species on turtles nesting in Bahia is that there are undocumented, free-living populations of isopods off the coast of Bahia and turtles are parasitized after migrating to the nesting beach. All three turtle species in this study tend to remain within close proximity to nesting beaches during the nesting season (Marcovaldi et al., Reference Marcovaldi, Lopez, Soares, Lima, Thomé and Almeida2010, Reference Marcovaldi, Lopez, Soares and López-Mendilaharsu2012; da Silva et al., Reference da Silva, dos Santos, das C. Oliveira, Weber, Batista, Serafini and de Castilhos2011). The presence of temporal shifts in either the occurrence or intensity of parasitism across the nesting season on different turtle species and for different isopod species (all turtle species pooled) strongly suggests that turtles were parasitized by all three isopod species during their internesting periods. This interpretation is strengthened by the fact that all isopods were removed from each turtle during each encounter on the nesting beach. No temporal changes in parasitism were detected on hawksbill turtles, but the most frequent isopod on hawksbills (E. costata) showed temporal changes on other turtle species. We also found an increase in the percentage of loggerheads hosting isopods at each additional nesting event (Figure 4), providing further evidence that parasitic interactions originate in internesting habitats. These internesting habitats are also foraging grounds of sea turtles (especially juvenile hawksbills and green turtles) and during the study period we found a juvenile green turtle hosting Excorallana in a foraging ground adjacent to Praia do Forte. These results and observations strongly suggest that there are local, free-living populations of all three isopod species off the coast of Bahia, which constitute range expansions for E. costata to the north and E. bicornis and E. oculata to the south.
Where geographic ranges overlap, parasitism – like other forms of epibiosis – necessitates fine-scale spatial and behavioural overlap between the host turtles and potential parasites (Frick & Pfaller, Reference Frick, Pfaller, Wyneken, Lohmann and Musick2013). We have strong evidence that Excorallana isopods in this study colonize turtles in their internesting habitats, which satellite-tracking data indicate are fairly localized around the nesting beach (Marcovaldi et al., Reference Marcovaldi, Lopez, Soares, Lima, Thomé and Almeida2010, Reference Marcovaldi, Lopez, Soares and López-Mendilaharsu2012; da Silva et al., Reference da Silva, dos Santos, das C. Oliveira, Weber, Batista, Serafini and de Castilhos2011). Unfortunately, however, there is nothing known about the specific habitat preferences or behaviour of the three Excorallana species found in this study. Thus, we are limited in the inferences we can make with respect to the fine-scale habitat-use patterns of turtles during internesting periods and the process of colonization by these specific isopod species.
Isopods of the genus Excorallana occupy a wide range of marine habitats (e.g. coral reefs, hard and soft bottoms, open sand and mangroves) (Delaney, Reference Delaney1989), and most are either free-living or commensal with various benthic macroinvertebrates (e.g. sponges, cnidarians, ascidians and molluscs). However, several species will emerge from these cryptic refuges to temporarily parasitize larger motile crustaceans and vertebrates (e.g. fish, rays, shrimp and turtles) (Delaney, Reference Delaney1989). Excorallana are known parasites of 15 genera of marine fishes, ranging from relatively slow-moving, benthic species (Dasyatis and Diodon) to large, more pelagic species (Caranx and Sphyraena) (Delaney, Reference Delaney1984). Based on this information, Excorallana most likely colonize turtles that are resting on the bottom near benthic structures, but may also colonize turtles that are actively swimming. If inactive turtles are more frequently parasitized than active turtles, then this might explain why loggerheads in this study were more frequently and intensely parasitized by Excorallana spp. compared with hawksbills and olive ridleys: loggerheads may remain more sedentary during internesting periods (Marcovaldi et al., Reference Marcovaldi, Lopez, Soares, Lima, Thomé and Almeida2010), while hawksbills and olive ridleys may remain more active, possibly while foraging (Colman, Reference Colman2009; da Silva et al., Reference da Silva, dos Santos, das C. Oliveira, Weber, Batista, Serafini and de Castilhos2011; Marcovaldi et al., Reference Marcovaldi, Lopez, Soares and López-Mendilaharsu2012). Alternatively, different turtle species may occupy different microhabitats between nesting events that make each more or less susceptible to colonization by parasitic Excorallana. More studies are needed on the fine-scale habitat-use patterns of Excorallana and nesting sea turtles to further evaluate differences in the frequency and intensity of parasitism between Excorallana spp. and different turtle species.
The emergence of Excorallana isopods as parasitic epibionts of sea turtles may have important implications for turtle health and the health of turtle populations. Consistent with observations by Hendrickson (Reference Hendrickson1958) and Monod (Reference Monod1975), we found Excorallana attached primarily to the eyelids of host turtles. Presumably, this attachment site is more favourable compared with the neck and flippers because the epidermis around the eyes is thinner and the tissue is more vascularized, allowing for stronger attachment and greater feeding efficiency. In some cases, isopods were even attached to the mucous-lined underside of the eyelid (see Figure 1B). Attachment to the eyelid may directly affect the vision of host turtles while isopods are attached. However, physical damage caused during parasite attachment may also expose host turtles to secondary infection or other more dangerous pathogens (George Reference George1997). Similar to Alonso (Reference Alonso2007), turtles were occasionally found with blood associated with attached isopods. More alarmingly, on several occasions, we found nesting turtles with large quantities of blood exuding from the eye (Figure 5), suggesting that the parasites can cause substantial trauma to the tissue around attachment sites. Parasitic epibionts may also act as disease vectors of pathogens. Parasitic marine leeches (Ozobranchus sp.) have been linked to the dispersal of the fibropapilloma-associated herpes virus found in latent tumours that often cover, deform and debilitate host turtles (Greenblatt et al., Reference Greenblatt, Work, Balazs, Sutton, Casey and Casey2004). More work is needed to better understand the potentially detrimental physical and pathological effects of parasitic isopods on turtles in these nesting populations.

Fig. 5. Loggerhead showing blood exuding from wound likely caused by parasitic isopods attached to the eyelid.
Lastly, the emergence of Excorallana isopods as parasitic epibionts of sea turtles may have important implications for isopod dispersal and the potential for future parasitic invasions. Female isopods retain ova in a brood pouch, as in other peracarid crustaceans, and release fully formed offspring that bypass a pelagic larval phase (Poore & Bruce, Reference Poore and Bruce2012). For this reason, the dispersal ability of isopods is considered severely limited (Espinosa-Pérez & Hendrickx, Reference Espinosa-Pérez and Hendrickx2006). However, sea turtles are well known for their wide-ranging migrations as adults and especially as juveniles (Musick & Limpus, Reference Musick, Limpus, Lutz and Musick1997; Plotkin, Reference Plotkin, Lutz, Musick and Wyneken2003). After nesting in Bahia, individual turtles migrate between 200–2500 km to foraging areas, where they remain during non-reproductive years (Marcovaldi et al., Reference Marcovaldi, Lopez, Soares, Lima, Thomé and Almeida2010, Reference Marcovaldi, Lopez, Soares and López-Mendilaharsu2012; da Silva et al., Reference da Silva, dos Santos, das C. Oliveira, Weber, Batista, Serafini and de Castilhos2011). Even during the nesting season, turtles may introduce isopod parasites to other nesting aggregations. This is exemplified by a turtle that nested on Praia do Forte and later became the first nesting turtle in Arembepe (30 km to the south; Figure 2) to host isopods. Because foraging areas along the coast of Brazil support turtles from many different rookeries (Naro-Maciel et al., Reference Naro-Maciel, Bondioli, Martin, Almedia, Baptistotte, Bellini, Marcovaldi, Santos and Amato2012; Proietti et al., Reference Proietti, Reisser, Marins, Rodriguez-Zarate, Marcovaldi, Monteiro, Pattiaratchi and Secchi2014), transport of parasitic isopods to these areas may have effects that extend beyond the turtles nesting in Bahia. Moreover, while infestations of Excorallana isopods have never been implicated in economic losses to the commercial fishing industry, it is possible the turtle-mediated dispersal of parasitic isopods may also have important ecological, as well as possible economic, implications for fish populations.
ACKNOWLEDGEMENTS
We thank Paulo Lara, Zenilton Pereira and Mariana Oshiro for help in fieldwork, and Luciano Soares and Karen Bjorndal for helpful comments that improved the quality of the manuscript. Projeto TAMAR, which supported this study and has coordinated sea turtle research at Praia do Forte for over 30 years, is a conservation programme of the Brazilian Ministry of the Environment, affiliated with ICMBio, co-managed by Fundação Pró-TAMAR and officially sponsored by Petrobras.