INTRODUCTION
Crypsis is the ability of an organism to hide by having colours, patterns and shapes that avoid recognition or detection by other individuals. According to Wente & Phillips (Reference Wente and Phillips2003) and Ruxton et al. (Reference Ruxton, Sheratt and Speed2004), this is a common and effective mechanism to avoid predation in both aquatic and terrestrial environments. The most widespread form of crypsis is protective colouration or camouflage (Stevens, Reference Stevens2007). Colour change occurs over several timescales, in seconds, over hours, days, weeks and months (Duarte et al., Reference Duarte, Flores and Stevens2017). In all cases the vulnerability is bigger at shallow depths and basically minor at depths greater than 200 m (Johnsen, Reference Johnsen2003). Open ocean fish species exhibited camouflage that is superior to nearshore fish and also to mirror-like surfaces where polaro-crypsis has been recognized as an efficient model in 3D space with no objects to hide amongst (Brady et al., Reference Brady, Travis, Maginnisb and Cummings2013, Reference Brady, Gilerson, Kattawar, Sullivan, Twardowski, Dierssen, Gao, Travis, Etheredge, Tonizzo, Ibrahim, Carrizo, Gu, Russell, Mislinski, Zhao and Cummings2015). Usually, the dorsal surface of a fish is dark while the ventral face is light and often reflective. This body pigmentation may be an adaptation to high levels of ultraviolet radiation in the surface layer of the sea (Powles, Reference Powles1981), while the dorsal dark surface matches a dark substrate, prevents an organism from being seen when observed from above, and possibly reduces predation.
The Mugilidae family includes species of teleost fish that are distributed throughout littoral waters worldwide, but mainly in estuaries and coastal lagoons at nearly all latitudes, except for the Polar regions (Thomson, Reference Thomson1997). Mugil curema (Valenciennes, 1836), the white mullet, is essentially an American species with only a few reports from African waters (Alvarez-Lajonchere, Reference Alvarez-Lajonchere1976; Fischer et al., Reference Fischer, Krupp, Schneides, Sommer, Carpenter and Niem1995). It is a benthic fish that sucks silt or scrapes hard surfaces to consume mostly diatoms. Our research group has been studying fish mullets for the last two decades (Ibáñez-Aguirre, Reference Ibáñez-Aguirre1993; Ibáñez-Aguirre et al., Reference Ibáñez-Aguirre, Gallardo-Cabello and Chiappa-Carrara1999; Ibáñez-Aguirre & Gallardo-Cabello, Reference Ibáñez-Aguirre and Gallardo-Cabello2004; Pacheco-Almanzar et al., Reference Pacheco-Almanzar, Simons, Espinosa-Pérez, Chiappa-Carrara and Ibáñez2016) and our main interest was to analyse the effects on mullets of fish husbandry. Consequently, in order to explore the laboratory adaptation of this species we transported juveniles from the Gulf of Mexico to our installations at the Metropolitan University in Mexico City. During this process it was possible to document the presence of crypsis in M. curema juveniles under laboratory culture.
MATERIALS AND METHODS
Around 200 juveniles of the white mullet Mugil curema were collected on 16 June 2016 from the coastal area of the Cazones River, Mexico (20°43′30.72″N 97°12′12.96″W). Initially, fish size was 29.2 ± 3.50 mm (total length, TL) when captured. All specimens presented a complete pigmentation all over the head and the sides of the body.
Fish were cultivated in indoor recirculation systems using Fluval filter model MS306 with controlled temperatures in fresh saline water. The temperature and oxygen were checked twice weekly (the O2 with a photometer Hanna® HI83203). After the colour change was observed illuminance light (from Philips mercury-vapour gas-discharge lamp of 36 w, warm white) was measured three times weekly with a Foot Candle Lux Light Meter Extech 401025.
Three tanks were used, two brown tanks (brown tank 1 and 2: BT1 and BT2) of 0.70 m3 each and a white tank (WT) of 0.75 m3. The fish were fed to satiation three times a day with Nutripec Purina (protein 44% and crude fat 22%) with cod liver oil added. Initially, the 200 specimens were located in one of the brown tanks (BT1) however to reduce density and allow better growth, 110 specimens were moved after 19 days to a white tank in similar conditions to the ones in the brown tank. To determine the difference in temperature, oxygen, illuminance, total length (TL) and total weight (TW) among tanks, one-way ANOVA with level of significance of α < 0.05 was used.
One fish per tank (WT, BT1 and BT2) was examined in three points of the lateral and dorsal view with a Minolta Chroma Meter CR-200b which is a tristimulus colour analyser for measuring reflective colours of surfaces. It uses an 8 mm-diameter measuring area that must be located directly on the surface of the object. A white reference standard was used to calibrate the instrument with the white calibration plate CR-A43. Chromaticity was measured in L*a*b* (CIE Commission Internationale de I'Eclairage/International Commission on Illumination) coordinates which is a colour scale based on the opponent-colour theory (Yonemura, Reference Yonemura1970). This theory assumes that the receptors in the human eye perceive colour as the following: L* scale: light vs dark where a low number (0–50) indicates dark and a high number (51–100) indicates light; a* scale: red vs green where a positive number indicates red and a negative number indicates green; b* scale: yellow vs blue where a positive number indicates yellow and a negative number indicates blue. All three values are required to completely describe an object's colour. As mentioned this chromaticity examination approximates the human eye perception and not what is perceived by the receiver, possibly a fish. However, many fish are trichromats and some have colour systems similar to humans (Cheney & Marshall, Reference Cheney and Marshall2009). A t-test was used to determine the differences between the fish and the background with level of significance α < 0.05.
RESULTS
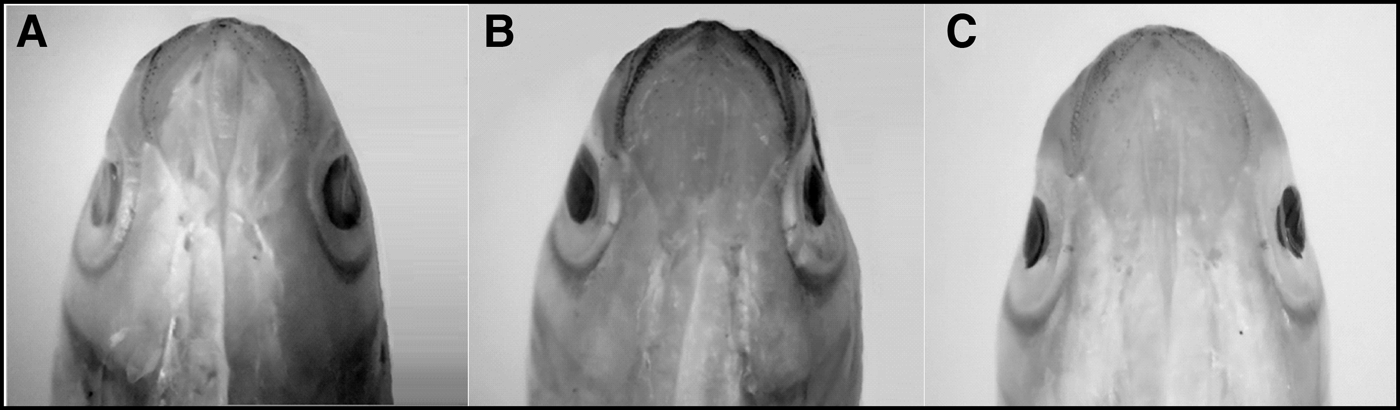
As soon as juveniles of Mugil curema were changed to the white tank they became completely white, with little pigmentation in the eyes, fins and along the sides or on the dorsal axes of the bodies (Figure 1A, B). The fish remained white for 79 days and, since they presented a 6% greater mortality than those in the brown tank, they were moved to the brown tank 2 (BT2) in order to observe whether they returned to their brown colour (converted fish), with a markedly silver side and a heavily pigmented dorsal axis (Figure 1C, D). The white juveniles that were placed in the second brown tank (BT2) returned to a browner state, but kept a few faint white spots on the dorsal area above the operculum (Figure 1E, F). The ventral head melanophore pattern also changed in the white specimens (Figure 2A), with no pigmentation in the gular area and few melanophores in the mandibular region. Heavy pigmentation was present in the mandibular and ventro-opercular surfaces, and very few melanophores were observed in the gular region of the juveniles with the brown original colour (Figure 2B). The white fish that were returned to a brown tank did not recover the brown colour (Figure 2C) and looked more like the white specimens.
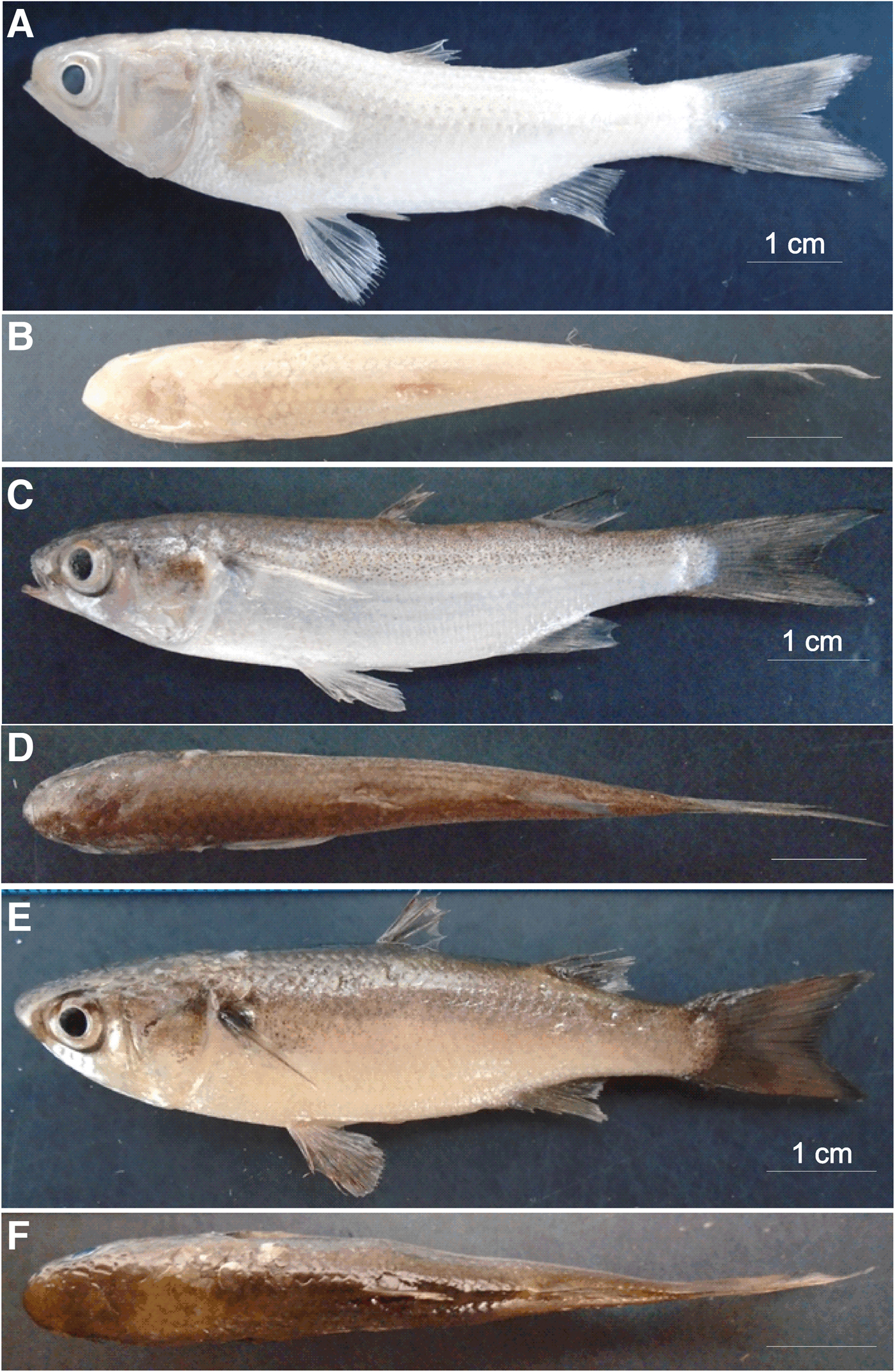
Fig. 1. White specimen from the white tank (WT), 8.6 cm total length (TL), showing crypsis: (A) lateral view, (B) dorsal view. Original brown pigmentation of juvenile from brown tank 1 (BT1), 7.5 cm TL: (C) lateral view, (D) dorsal view. White specimen, 7.8 cm TL, returned to a brown tank (BT2): (E) lateral view, (F) dorsal view.
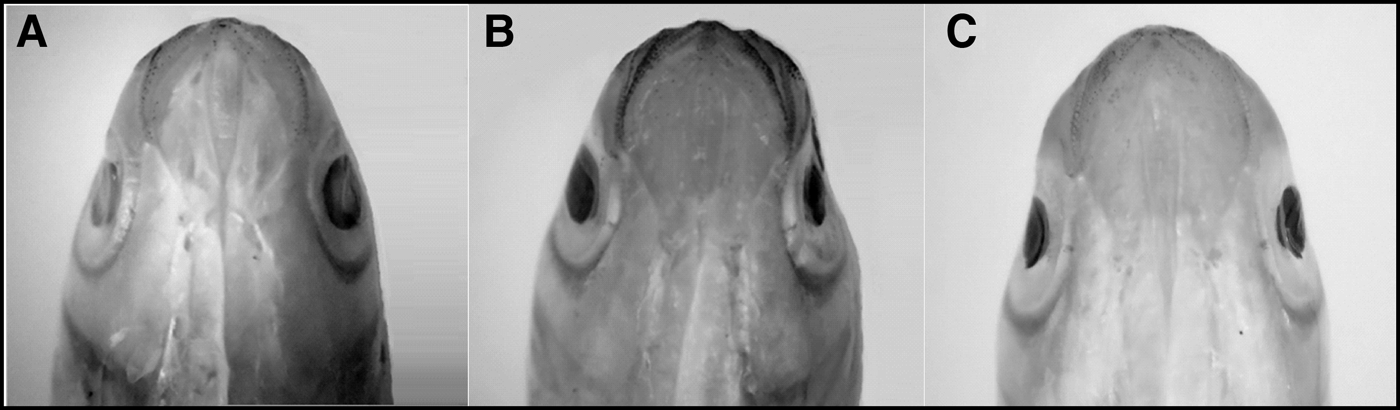
Fig. 2. Comparative photographs of the melanophore pattern on the ventral side of the head for: (A) an 8.6 cm TL white specimen showing crypsis from the white tank (WT), (B) brown original pigmentation of a 7.5 cm TL juvenile from brown tank 1 (BT1), and (C) 7.8 cm TL white specimens returned to a brown tank (BT2).
After 79 days of cultivation, 78 individuals (from the 110) died in the WT and 58 (from the 90) in BT1. Therefore 32 specimens remained in each tank and the 32 fish from the WT were moved to the BT2. Ten days later a massive mortality was observed as a consequence of electricity failure and only 17 individuals were left from both tanks (BT1 and BT2). Those individuals were changed to the 5 m3 tank painted with cream colour inside and all fish have remained clear.
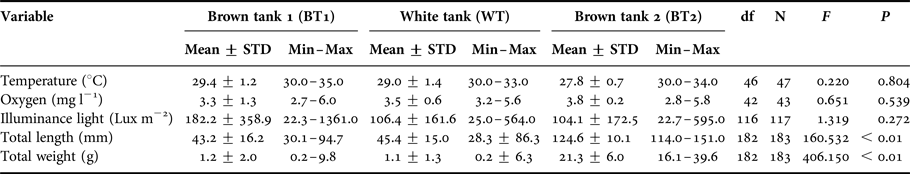
Mean of temperature, oxygen and illuminance were not different among the three tanks (Table 1). Minimum and maximum total length values varied from 30.1 to 94.7 mm and from 28.3 to 86.3 mm for BT1 and WT, respectively. For BT2, TL varied from 114.0 to 151.0 mm. Total length (TL) and total weight (TW) were different among the three tanks. However, a post hoc Tukey test showed that TL and TW of BT2 differed significantly from BT1 and Wt (P < 0.01 and P < 0.01, respectively) but BT1 and WT were not significantly different (P = 0.719 for TL and P = 0.968 for TW).
Table 1. Mean ± standard deviation (STD), minimum (Min) and maximum (Max) for temperature, oxygen, illuminance, total length and total weight for the brown tanks (BT1 and BT2) and the white tank (WT).
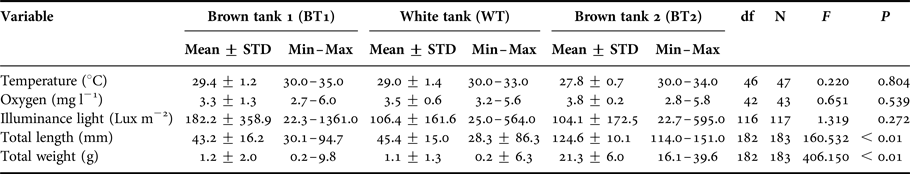
Results of the one-way ANOVA: total degrees of freedom (df), F value (F) and significance value (P).
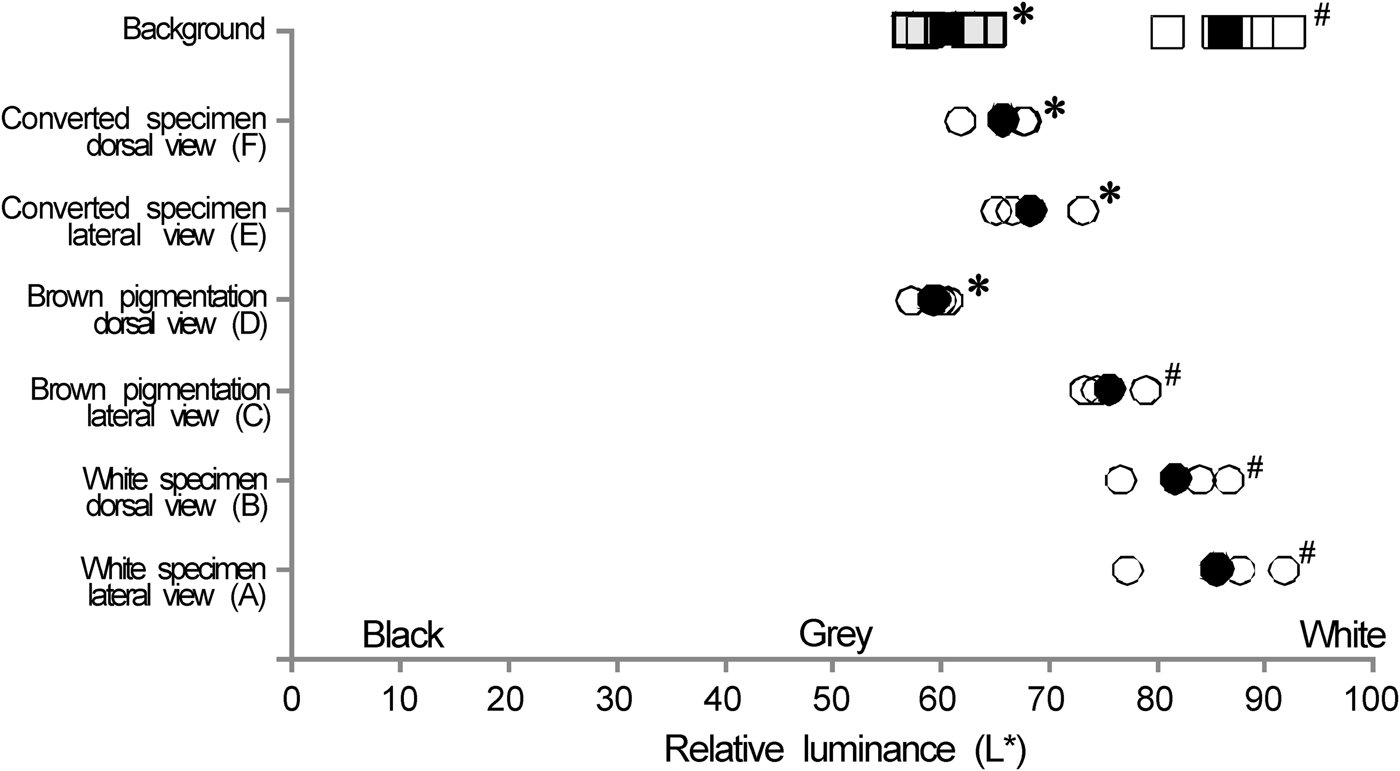
In white specimens relative luminance (L*) varied from 74.4 to 91.8 with mean and standard deviation of 83.7 ± 6.6 (Figure 3). For the brown original pigmentation specimen the dorsal area was darker than the lateral area with mean and standard deviation of 67.5 ± 9.2 and variation from 57.3 to 79.0. The brown original pigmentation specimen in the dorsal view showed the darkest L* while the lateral view was clearer with some tendency to silver colour.

Fig. 3. Relative luminance (L*) for the white specimens (A) lateral view, (B) dorsal view. Brown pigmentation of juvenile from brown tank 1 (C) lateral view, (D) dorsal view. White specimen, returned to a brown tank (BT2) termed converted specimens for (E) lateral view and (F) dorsal view. Black circle is the mean value by specimen. Squares are measures for the background; grey squares for the brown tanks and white squares for the white tank. Black squares are the mean values by background. Means with the same symbol are not significantly different from each other (P > 0.05). Same marks (*, #) are measurements that are not significantly different.
The relative luminance for the white specimens returned to BT2 (converted fish) was from 61.9 to 73.2 with mean and standard deviation of 67.1 ± 3.7. The white background relative luminance of the white tank and brown tank were 87.2 ± 4.2 and 60.3 ± 3.1, respectively. L* of the brown pigmentation specimens were different in the lateral and dorsal view (t (2) = 11.030, P = 0.008) while the converted and white individuals were similar in dorsal and lateral views (t (2) = 0.125, P = 0.912; t (2) = 1.935, P = 0.193, respectively). Converted specimens, in dorsal and lateral view, and brown pigmentation fish in dorsal view were similar with the brown background (t (2) = −2.680, P = 0.116 and t (2) = −0.904, P = 0.461, respectively) as well as white specimens with white background (t (2) = 0.433, P = 0.705). Therefore, the white specimens was similar to their background and the converted individuals to the brown background. The dorsal and lateral views of the brown pigmented fish were similar to the brown and white background, respectively.
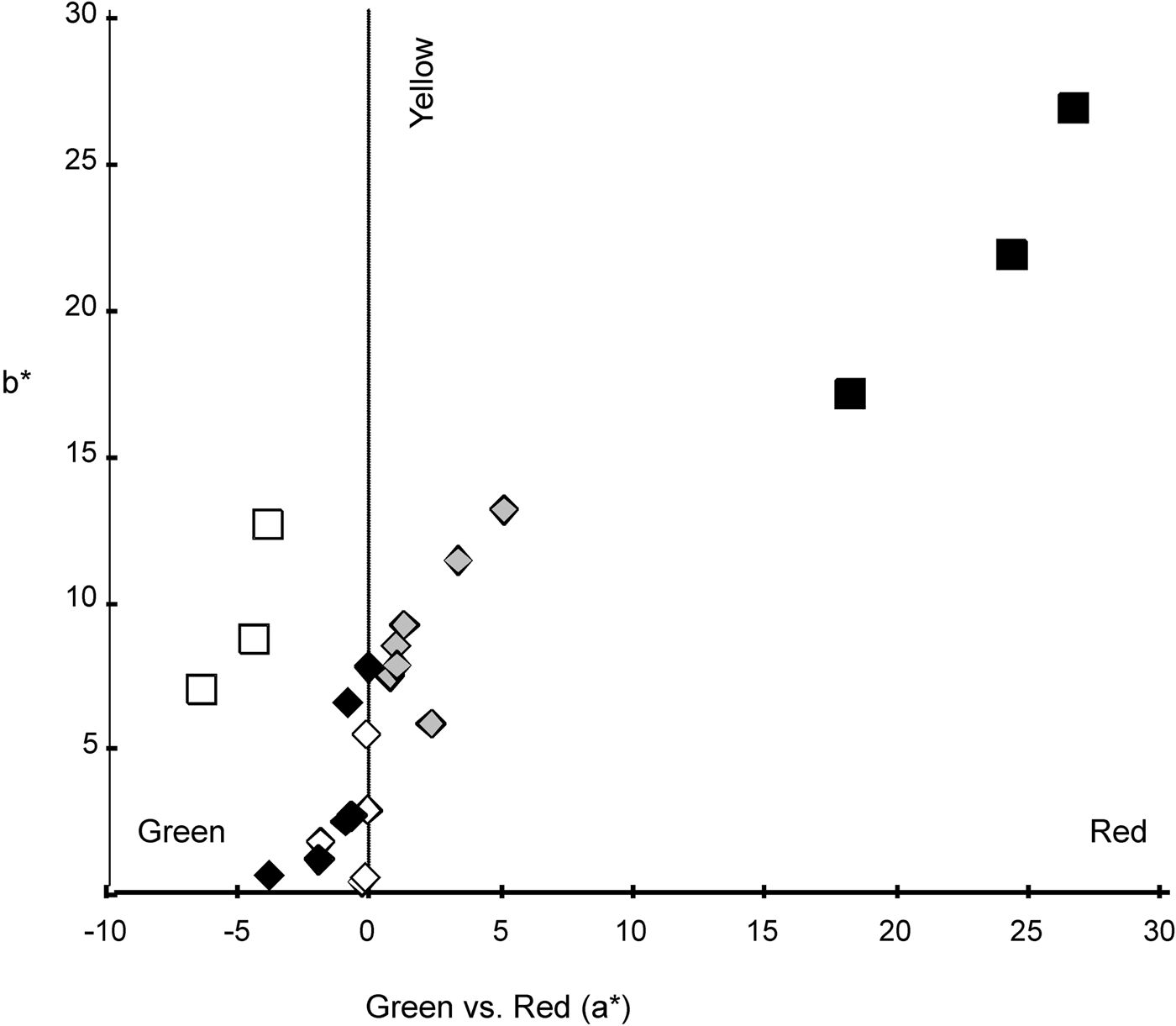
For all fish (white, brown pigmentation and converted specimens) b* depicted positive values, related with yellow colour while a* showed negative values (green) for the white and brown pigmentation specimens and positive values, related with red colour, for the converted fish (Figure 4). On the green-red coordinate (a*) white fish were different to converted fish (t (4) = −4.955, P = 0.008) but similar to brown pigmentation specimens (t (4) = 1.435, P = 0.225). Brown and converted fish were different (t (5) = −3.911, P = 0.011). None of the fish were similar to any background (white fish–Brown tank: t (2) = −6.344, P = 0.024; brown pigmentation fish–Brown tank: t (2) =−7.492, P = 0.017; converted fish–Brown tank: t (2) = −4.861, P = 0.040; white fish–White tank: t (2) = 8.549, P = 0.013; brown pigmentation fish–White tank: t (2) = 5.594, P = 0.031; converted fish–White tank: t (2) = 5.169, P = 0.035). On the yellow-blue axis (b*) white individuals were similar to brown pigmentation and converted fish (t (4) = −0.293, P = 0.784 and t (4) = −2.689, P = 0.055, respectively) but brown pigmentation and converted fish were different (t (5) = −2.878, P = 0.035). All fish were similar to white background (white fish–White tank: t (2) = −4.068, P = 0.055; brown pigmentation fish–White tank: t (2) = −3.046, P = 0.093; converted fish–White tank: t (2) = 0.535, P = 0.646) but different to brown background (white fish–Brown tank: t (2) = −6.380, P = 0.024; brown pigmentation fish–Brown tank: t (2) = −5.154, P = 0.036; converted fish–Brown tank: t (2) = −6.348, P = 0.034). No clear pattern was seen for the red-green and yellow-blue coordinates as was seen for the relative luminance. However, white and brown pigmentation specimens were similar in a* and b* coordinates.
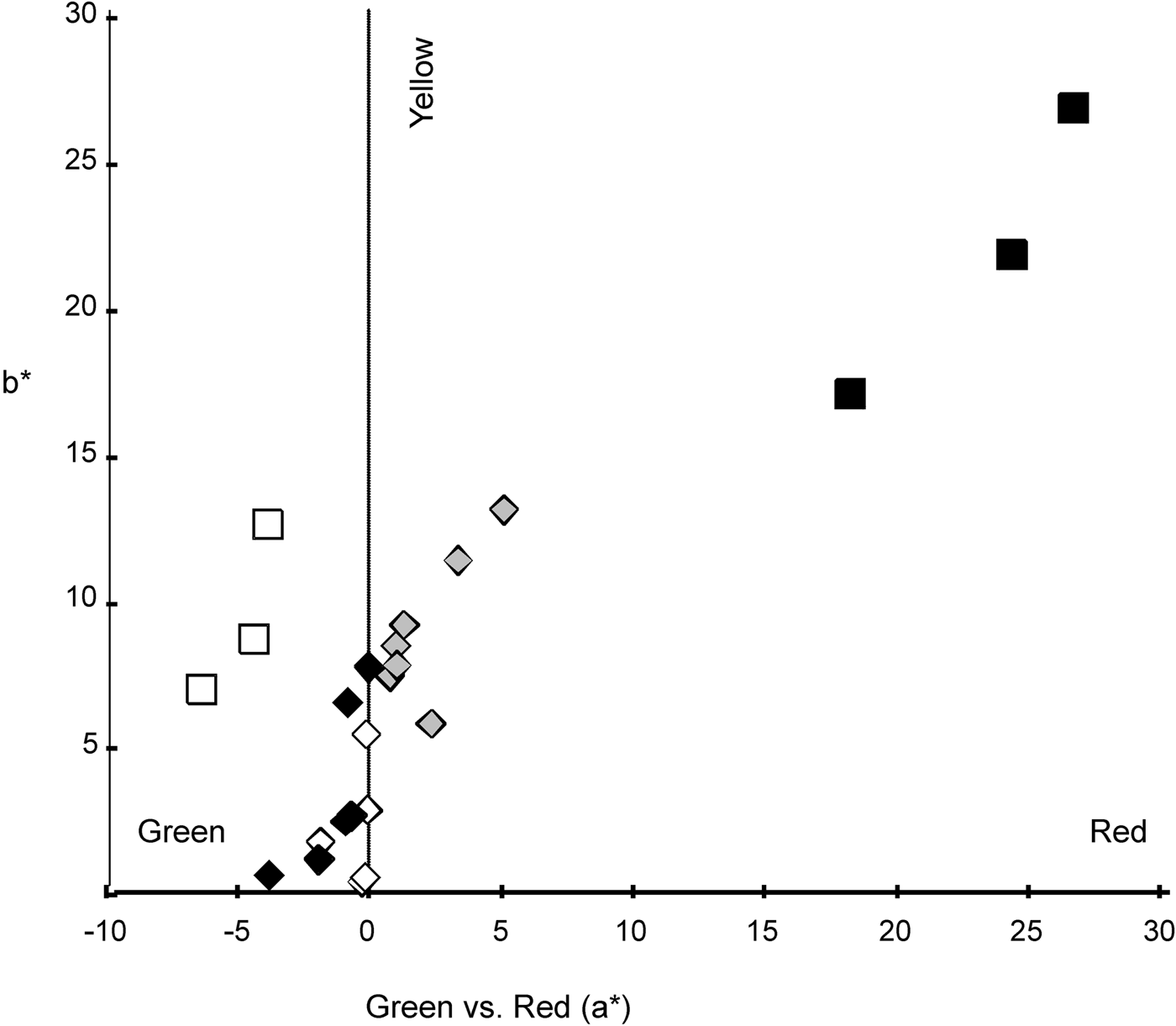
Fig. 4. Chromaticity coordinates. Axis 1: a* scale: positive number indicates redness and a negative number indicates greenness. Axis 2: b* scale: positive number indicates yellowness and a negative number indicates blueness. Black diamonds represent the brown original pigmentation of juvenile from brown tank 1, white diamonds represent the white specimens from the white tank; grey diamonds represent the white specimens returned to a brown tank (BT2) (termed converted specimens). Black rectangles represent measures of the background from brown tanks, white rectangles represent background from the white tank.
DISCUSSION
Our results suggest that the relative luminance of Mugil curema juveniles is similar to their background. Since numerous piscivorous land animals are visual predators, fish phenotypic attributes, such as performance and body colour luminance, are probably linked to predation as a vulnerability factor (Eriksson, Reference Eriksson1985). Sumner (Reference Sumner1934) showed that mosquitofish, Gambusia affinis (Bair & Girard, 1853), are approximately twice as likely to be consumed by the Galapagos penguin (Spheniscus mendiculus, Sundevall, 1871) when the fish's body colour contrasts with the background than when the fish's body colour matches the background. According to Northmore et al. (Reference Northmore, Volkmann, Yager and Mostofsky1978) an ability to detect low contrasts would be an advantage to a fish since its sensitivity to contrast determines the range at which avoiding action can be taken from a predator, or the highest distance that individuals may stray from a school without losing contact, to cite two examples.
In our results the dorsal area of prey fish has a similar colour luminance as the bottom, in this sense juveniles show crypsis possibly as a mechanism to avoid predation. To our knowledge this behaviour has not been reported before for any fish mullet in culture or living in the wild.
According to Harrison (Reference Harrison and Carpenter2002) M. curema specimens from the sea are bluish green or olive dorsally, flanks silvery, and abdomen off-white with yellowish blotch between eye and upper edge of operculum. These colours agree with the brown pigmentation and white specimens for b* and a* readings.
To our knowledge, there are no reports of crypsis for grey mullet species even though pigmentation patterns constitute an alternative set of characters for the identification of fry and juveniles (Koutrakis, Reference Koutrakis, Crosetti and Blaber2016). Zismann (Reference Zismann and Oren1981) and Cambrony (Reference Cambrony1984) proposed keys for 20–60 mm TL juveniles, based on the pattern of the pyloric caeca and pigmentation. Reay & Cornell (Reference Reay and Cornell1988) presented a key for British juvenile mugilid species based on the patterns of ventral head melanophores for three different sizes (25–45 mm). Minos et al. (Reference Minos, Katselis, Ondrias and Harrison2002) prepared a key for five species of Mediterranean mullets (Mugil cephalus Linnaeus, 1758; Liza aurata Risso, 1810; L. ramada Risso, 1827; L. saliens Risso, 1810; and Chelon labrosus Risso, 1827) based on the shape of the lower jaw and the melanophore patterns along the edge of the lower jaw and the ventral side of the head. Harrison et al. (Reference Harrison, Nirchio, Oliveira, Ron and Gaviria2007) has used colouration as a principal character to discriminate M. curema from M. rubrioculus. They mention the colour of the iris, the moderate to large goldish spot on opercle; flanks dark with ~ six longitudinal bands; large dark spot extending over most of base of pectoral fin; second dorsal fin usually uniformly dark; anal fin dark and the caudal fin usually dark. The colour of the lateral and dorsal axis could vary in juveniles and it could possibly vary for adults. Therefore, according to what we report here, the use of colour and the melanophore pattern as an identification character could possibly change in relation to the background, nevertheless our results were an adaptation in the rearing (cultivation) conditions of the white mullet fry and juvenile individuals and also length range reported here is to some extent bigger from the reported before. Therefore to validate that this phenomenon appears in nature in wild populations further studies are needed on this topic for this species.
ACKNOWLEDGEMENTS
We are grateful to Carmen Hernandez for his help in the reading of relative luminance.
FINANCIAL SUPPORT
This work was supported by the Universidad Autónoma Metropolitana.