INTRODUCTION
The SW Atlantic is a vast area and the benthic ecology is mostly unexplored quantitatively. Argentina has an extended shoreline of about 4500 km in the South-western Atlantic Ocean. The Argentine littoral area comprises two large biogeographica provinces, one warm-temperate of northern Argentina (including Uruguay and southern Brazil) and the Patagonic or Magellanic cold-temperate (Boschi, Reference Boschi2000). Many coastal environments are poorly known or unknown, and shallow subtidal areas are practically unexplored. There have been no long-term studies due to the lack of a long-term policy. There have been only a few quantitative surveys on subtidal benthic communities (Elías et al., Reference Elías, Vallarino, Scagliola and Isla2004, Reference Elías, Palacios, Rivero and Vallarino2005; Centurion & López Gappa, Reference Centurion and Lopez Gappa2013).
All studies about polychaetes are scarce in Argentina and in particular taxonomy. The family Cirratulidae is often neglected due to the difficulties of identification. The first revision of the family Cirratulidae described six new species in a single locality of the warm-temperate area (Elías & Rivero, Reference Elías and Rivero2008, Reference Elías and Rivero2009a, Reference Elías and Riverob, Reference Elías and Rivero2011). New surveys (baseline studies for oil exploration or to evaluate environmental impact due to dredging), and the use of 0.5 mm mesh sieves allowed the collection of two previously unknown bitentaculate cirratulids of the genera Monticellina Laubier, Reference Laubier1961 and Chaetozone Malmgren, Reference Malmgren1867.
The genus Monticellina was re-instated by Blake (Reference Blake1991) as part of a revision of species that had been previously assigned to Tharyx Webster & Benedict, Reference Webster and Benedict1887. The main diagnostic character is the absence of spines and the presence of capillary chaetae with a distinct sawtooth (denticulate) edge, often basally expanded (Blake, Reference Blake, Blake, Hilbig and Scott1996). In Argentina, there are no previous records of Monticellina. Some previously described Tharyx species should be re-described as Monticellina (or Aphelochaeta Blake, Reference Blake1991). Here, we describe a new species of Monticellina from an estuarine environment of the Blanca Bay. The genus is morphologically similar to Aphelochaeta in prostomium, peristomium, parapodia and chaetal lengths, and it is postulated that may be interrelated, but new molecular studies will be required for the understanding of the relationships between these two genera (Dean & Blake, Reference Dean and Blake2009).
The genus Chaetozone is a bitentaculate characterized by the presence of acicular spines in neuro- and notopodia, forming posterior cinctures. The structure of these spines, their point of origin on the body, and whether they form cincture in posterior segments are crucial to species recognition (Blake, Reference Blake2015). The genus has been cited in Uruguay (Faget, Reference Faget1983), off Rio de la Plata (Roux & Bremec, Reference Roux and Bremec1996) and in northern Patagonia (Pastor de Ward, Reference Pastor de Ward2000).
Two new species are described, one belonging to Monticellina and the other to Chaetozone, both from south-western Patagonia, Atlantic shores. Diagnostic characters of all described species of Monticellina are discussed.
MATERIALS AND METHODS
The material was collected from two localities of the Argentinean coast (Figure 1), the shallow subtidal sand-muddy substrate of Cabeza de Buey Creek (near Ingeniero White harbour) in the Bahía Blanca Estuary (March 2012), R. Elias collector. The other came from subtidal muddy-sand bottom (70 m depth), 90 km off Comodoro Rivadavia in the San Jorge Gulf (October 2010), R. Elias & M.S. Rivero collectors.
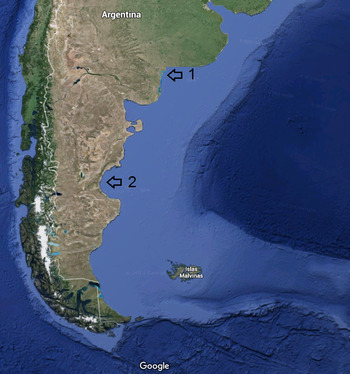
Fig. 1. Locations of the two sites where the new cirratulids were found, 1: Bahía Blanca estuary; 2: San Jorge Gulf, SW Atlantic.
Replicate van Veen grabs (0.05 m2) were taken and sieved on board through a 0.5 mm mesh. The material was fixed in 5% formaldehyde, and preserved in 70% ethyl alcohol.
Material was examined with optical equipment (microscope and stereomicroscope) and also by scanning electron microscope (SEM) JEOL, JSM–6460 LV.
The description of the new species follows the bitentaculate cirratulid characters identified during two workshops with the collaboration of S. Doner, G. Paterson, J. Blake, S. Chambers, H. Dean and E. Soto.
The material for SEM was prepared after fixation for 24 h with 3% glutaraldehyde in a buffer of phosphate 0.2 mol with a pH = 7.4; followed by dehydration in ethyl alcohol (70, 80, 90, 95 and 100%). Samples were dried in HMDS (hexamethyldisilazane), mounted on aluminium stubs and metalized with Au–Pd.
Type material was deposited in the Invertebrate collection of the Museo de Ciencias Naturales de La Plata (MLP-OI, Argentina),
RESULTS
SYSTEMATICS
Family CIRRATULIDAE Ryckholt, Reference Ryckholt1851
Genus Monticellina Laubier, Reference Laubier1961
Monticellina morae sp. nov.
(Figure 2A–D)

Fig. 2. Monticellina morae sp. nov. (A) general view; (B) anterior region showing dorsal part of peristomium with dorsal tentacles, and first pair of branchiae in first chaetiger; (C) nuchal organ; (D) serrate chaetes.
TYPE MATERIAL
Holotype: SW Atlantic Ocean, Bahía Blanca Estuary, Argentina. Cabeza de Buey Creek Station 6, 38°48′14.31″S 62°17′37.37″W, 2 m depth in low tide, sand-muddy substrate, March 2012, near Ingeniero White harbour. Collected by R. Elias (MLP no. MLP-OI 4081).
Paratypes: SW Atlantic Ocean, Bahía Blanca Estuary, Argentina. Cabeza de Buey Creek Station 6, 38°48′14.31″S 62°17′37.37″W, 2 m depth at low tide, sand-muddy substrate, March 2012, near Ingeniero White harbour (2 complete, 5 anterior parts, 2 posterior parts). Collected by R. Elias (MLP no. MLP-OI 4082).
Another complete and 12 incomplete specimens for SEM from the same station.
DIAGNOSIS (After Dean & Blake, Reference Dean and Blake2009)
Prostomium long or short, sometimes pointed; peristomium elongated to short, with achaetigerous annulations; dorsal tentacles usually arising anterior to chaetiger 1; middle body segments sometimes beadlike; posterior segments usually expanded or enlarged. Chaetae include capillaries with distinct sawtooth (denticulate) edge, often basally expanded.
DESCRIPTION
Monticellina morae sp. nov., a moderate-sized species, holotype complete, 20 mm long, 0.1 mm wide for 115 chaetigers. Paratypes 7–20 mm long, 0.05–0.1 mm wide for 82–115 chaetigers. Elongate and thin organisms, expanded in anterior and posterior ends. Body divided into three regions (Figure 2A), (1) an anterior region of 21 crowded chaetigers, with each segment 12 times wider than long; inflated dorsally and expanded laterally, without shoulders (in paratypes anterior region extended between 15–25 crowded chaetigers; in some paratypes the first 4–10 anterior chaetigers narrow and then expanded laterally); (2) middle region with beadlike chaetigers, each about twice wider than long, rounded dorsally and flattened ventrally (75 chaetigers in holotype, and very variable in paratypes); (3) posterior region formed by 14 chaetigers (7–17 in paratypes), crowded, laterally expanded and dorsally inflated, each chaetiger about five times wider than long, ventrally grooved. Pygidium simple, anus dorsal. Colour in alcohol light tan.
Prostomium conical, short, acute (in some paratypes rounded), as long as first 2–3 chaetigers, without eyes, with a pair of oval nuchal organs dorsolaterally positioned at posterior border beneath overlapping peristomium (Figure 2C). Peristomium smooth, as long as first 10 anterior chaetigers (5–15 in paratypes); without secondary annulation; however, in methyl green staining, peristomium appeared as triannulated, with first two annuli shorter and the third longer (as long as the first two). SEM images of paratypes revealed also peristomium as clearly triannulate and third annulus being longer than other two. A small but well-defined crest originates in second half of the second annulus to end of peristomium. Dorsal tentacles arising from posterior part of peristomium; lateral to dorsal crest. First pair of branchial filaments posterolateral to dorsal tentacles on chaetiger 1 (Figure 2B); branchiae limited to dorsal part of notopodium in anterior region.
Noto- and neuropodium very close, not elevated. Capillary chaetae in anterior region slender, finely serrated on one edge, not basally expanded; serrations observed under light microscope; capillaries in bundles of 10, long and short alternately. Long capillaries with fibrils (fine hairs) in addition to serrations (Figure 2D). In middle region, both types of capillaries shorter than those present from anterior region, decreasing in number to six per bundle. In posterior region capillaries decreasing to 2–3. Noto- and neuropodial capillaries similar in shape and number and emerging directly from the body wall.
METHYL GREEN STAINING PATTERN
The organisms stained the peristomium darker, an intersegment darker line between anterior segments, and a mid-ventral line anteriorly.
ETYMOLOGY
The species is dedicated to Mora Orensanz, unflagging polychaete collector. The youngest daughter of Lobo Orensanz, she often accompanied him to find polychaetes.
ECOLOGY
Monticellina morae sp. nov. is a subtidal species from shallow mixohaline waters, in soft-bottom substrates of mud and fine sand. The species reach densities up to 2000 ind. m−2, associate to the polychaetes Axiothella sp. and Kinbergonuphis dorsalis Ehlers, Reference Ehlers1897, and the ophiurid Amphiura sp.
DISTRIBUTION
It is known for the Blanca Bay estuary, near the Ingeniero White harbour, in 2 m depth (at low tide), Argentina.
REMARKS
Monticellina morae sp. nov. is closely related to Monticellina siblina Blake, Reference Blake, Blake, Hilbig and Scott1996 in having a similar shape of the anterior region, although the latter is flattened dorso-ventrally; the posterior region is also swollen and expanded, and the pygidium is like a papilla. The peristomium is short (as long as wide) and divided with three annuli in M. siblina while the peristomium is also divided in three (under SEM and MGSP) but as long as 10 chaetigers in M. morae sp. nov. The methyl green staining pattern is quite different in M. siblina, with the prostomium and peristomium unstained, body uniformly stained and parapodia and ventral bands stained across the anterior region; whereas, M. morae sp. nov. has the peristomium stained and a mid-ventral line in the anterior region. Bundles of capillaries are dorsal in both species, although capillaries are less numerous in M. morae sp. nov. Most described species have serrate capillaries from middle or posterior part of the body, while in M. morae sp. nov., capillaries are serrate from the first chaetigers (even in juveniles). Another related species Monticellina annulosa Hartman, Reference Hartman1965 has a long peristomium, up to five chaetigers while in M. morae sp. nov., the peristomium is 10 chaetigers long with a dorsal crest throughout. The anterior region is similar, with 10 crowded chaetigers, in M. annulosa and 21 in M. morae sp. nov. Body of M. annulosa is wider (0.7 mm) than M. morae sp. nov. (0.1 mm for similar length and chaetiger number). The posterior region is inflated and chaetigers crowded in both species, but in M. annulosa there are 20–25 chaetigers compared with 14 in M. morae sp. nov.).
Almost all species of Monticellina described are from the northern hemisphere. Only M. annulosa is mentioned for North-eastern Brazil and NE of South America (in deep waters), as well as Monticellina dorsobranchialis (Kirkegaard, Reference Kirkegaard1959) (Amaral et al., Reference Amaral, Nallin and Stainer2010). However, the validity of these identifications must be taken carefully, since the revision of the Brazilian cirratulids is in its initial stages (see Magalhães et al., Reference Magalhães, Seixas, Paiva and Elias2014). Monticellina morae sp. nov. is the first recorded species from shallow waters of the South-western Atlantic.
REVIEW OF MONTICELLINA SPECIES
Table 1 summarizes the 22 valid species. The data in our table are based on original descriptions only. The type material of Monticellina dorsobranchialis (Kirkegaard, Reference Kirkegaard1959) and Monticellina luticastella (Jumars, Reference Jumars1975) needs to be re-examined. In the first case, Blake (Reference Blake, Blake, Hilbig and Scott1996) recognized that the re-description of M. dorsobranchialis had included two valid species (previously passed to synonymy in the new combination made by Blake, Reference Blake1991). A new re-description is required. In the case of M. lutiscatella the original description (as Tharyx) shows a different number of annuli in the peristomium in the new combination made by Blake (Reference Blake, Blake, Hilbig and Scott1996). Also, Monticellina secunda and Monticellina serratiseta, both originally described by Banse & Hobson (Reference Banse and Hobson1968) but Blake (Reference Blake, Blake, Hilbig and Scott1996) made a new combination from paratypes. However, clarification is needed, because most characters (peristomial annulations, pygidium, origin of branchiae and serrate neurochaetae) do not agree with the original description of the species.
Table 1. Species of Monticellina. Prost: prostomium; Pe. annul.: number of peristomial annulations; Nat. First Seg.: Nature of the first segment; First branchiae: Appearance of first pair of branchiae; Nature of denticulate chaetae: nature or type of denticulation in chaetes; Posterior end: form of posterior end and pygidium; Original description: region where the species was described.

a Monticellina annulosus is considered as Tharyx in Worms, but we agree with Blake who included it in Monticellina in 1991 (due to lack of acicular chaetes and denticulate capillaries). Morphologically is similar to M. morae sp. nov.
b Monticellina aphelocephalus was described as Tharyx, but Blake transferred it to Monticellina in 1991.
c Monticellina cryptica Blake (Reference Blake, Blake, Hilbig and Scott1996) was described as lacking peristomial annulations, but Dean & Blake (Reference Dean and Blake2009) mentioned two annulations. The pygidium is acute in 1996 and as conical papillae in 2009.
d Monticellina dorsobranchialis: there are two contradictory descriptions: the original showed the branchiae and palps together, but the re-description of Blake (Reference Blake1991) said first pair of gills in first chaetiger. In addition, Kirkegaard (Reference Kirkegaard1959) described the peristomium as triannulate, while Blake (Reference Blake1991) re-described as without pseudosegmentation.
e M. hanaumaensis without annulations in the peristomium, but 1–2 in the table. This species lacks serrations under optical microscope. The inclusion of the type in Monticellina is because of the blade shape of chaetae (Magalhães & Bailey-Brock, Reference Magalhães and Bailey-Brock2013).
f Monticellina morae sp. nov. has peristomium without annulation, however under methyl green staining appeared with 3 annulli.
g M. luticastellus the new combination of Blake (Reference Blake, Blake, Hilbig and Scott1996) is different from the original description of Jumars as Tharyx (1975), with differences in prostomium and peristomium, both biannulate in original description and re-described without annulations; the position where denticulate chaetes are present (in all the body in both rami according to Jumars, and limited to middle region neurochaetae in Blake); Jumars (Reference Jumars1975) defined chaetae as geniculate.
Blake (Reference Blake, Blake, Hilbig and Scott1996) noted that the first reports of cirratulid polychaetes were incomplete, which contributed to confusion in the taxonomy of the group. This was true for the northern hemisphere species, and even more for the southern hemisphere. Researchers during the European age of scientific exploration in the 19th century collected and classified the polychaete of southern regions based on surface observations. This ‘pro-European’ vision occurred not only for South America, but for the non-European world (Africa, Asia and Oceania). A researcher from North America also notes the same thing for that region (Tewari, Reference Tewari2015). This has contributed to the belief of widely distributed species but a re-examination of the material of the Southern Hemisphere may prove otherwise.
Table 1 shows that there is no agreed clear and precise character to define Monticellina. The uniqueness is the fine indentations in one edge of the modified capillaries, when observed at the optical microscope (Magalhães & Bailey-Brock, Reference Magalhães and Bailey-Brock2013), as are all serrated or finely denticulate under SEM (in Aphelochaeta and Monticellina).
The usual resolution to detect denticulations is around 400× (Magalhães & Bailey-Brock, Reference Magalhães and Bailey-Brock2013) but less than 1000×. However, in M. luticastella (Jumars, Reference Jumars1975), denticulation along the capillary chaetal blade have been observed at 1500× in the original description by Jumars (Reference Jumars1975). Also Monticellina setosa (Dean & Blake, Reference Dean and Blake2009) has denticulate chaetae barely visible under oil immersion (more than 1000×). In M. morae sp. nov., we observed it at 400×.
Dean & Blake (Reference Dean and Blake2009) pointed out that the presence of serrated capillaries is a taxonomic tool and not a synapomorphic character. Therefore, further use of serrate capillaries to separate Aphelochaeta from Monticellina is questionable, and needs to be evaluated in a phylogenetic perspective. Dean & Blake (Reference Dean and Blake2007, Reference Dean and Blake2009) also questioned the validity of other characters to diagnose Monticellina (as well as Chaetozone and Caulleriella), such as the annulation of peristomium, prostomium, etc.
The species Monticellina hanaumaensis described by Magalhães & Bailey-Brock (Reference Magalhães and Bailey-Brock2013) has no denticulate capillaries under light microscope (a diagnostic character as mentioned by them), and yet included in this genus by the expanded bases of the capillaries. In many species the base of capillaries is expanded, but there are exceptions. Therefore, this character would not be diagnostic to generic level.
In the near future, a cladistics analysis should be performed in order to understand the relationships between the current species assigned to Monticellina and Aphelochaeta and possibly unify these genera because they are based on characters taxonomically comfortable, but with no evolutionary value (see Dean & Blake, Reference Dean and Blake2009).
Genus Chaetozone Malmgren, Reference Malmgren1867
Chaetozone larae sp. nov.
Figure 3A–F.
Chaetozone sp.: Faget, Reference Faget1983: t.4

Fig. 3. Chaetozone larae sp. nov. (A) dorso-lateral view, (B) pygidium and posterior region; (C) anterior region showing nuchal organ in the prostomium, peristomium annulations, and achaetous segment 1 bearing dorsal tentacles and first pair of branchiae, (D) capillaries; (E) acicular spines, (F) schematic view of partial cincture.
Roux & Bremec, Reference Roux and Bremec1996: t.2 [off La Plata River estuary (ecological survey)]
Pastor de Ward, Reference Pastor de Ward2000: 182 [San José Gulf (ecological survey)]
MATERIAL EXAMINED
TYPE MATERIAL
Holotype: SW Atlantic Ocean, San Jorge Gulf, Argentina. Station 15, 49°18′145″S 33°98′195″W, 70 m depth, muddy sand, November 2010; 90 km SE of Comodoro Rivadavia city (MLP no. MLP-OI 4083). Collected by R. Elias & M.S. Rivero.
Paratypes: SW Atlantic Ocean, San Jorge Gulf, Argentina. Station 15, 49°18′145″S 33°98′195″W, 70 m depth, muddy sand, December 2010, 90 km SE Comodoro Rivadavia (3 complete specimens, one anterior incomplete, one posterior incomplete. MLP no. MLP-OI 4084). Collected by R. Elias & M.S. Rivero.
Another 2 complete and 11 incomplete specimens were used for SEM, both from Station 15.
DIAGNOSIS (Re-described by Chambers, Reference Chambers2000; updated by Blake, Reference Blake2015).
Prostomium blunt to conical, usually lacking eye spots, with a pair of small nuchal organs at posterior edge; peristomium elongate to short, with a single pair of grooved dorsal tentacles arising from posterior edge of peristomium, or sometimes more posteriorly. First pair of branchiae arising behind dorsal tentacles. Body basically thick and fusiform over many segments, rarely with middle or posterior body segments beaded or moniliform; parapodia birramous with reduced lobes and a pair of branchiae on most anterior segments. Chaetae all simple and include fine capillaries of various lengths on most chaetigers, sometimes very long capillaries present in notopodia, and acicular spines in neuropodia and/or notopodia, spines typically concentrated in posterior segments, forming distinct cinctures with spines emerging from elevated membranes; cinctures with few to many spines and with none to many alternating capillaries; some species with posterior noto- and neuropodial sigmoid acicular spines numerous, encircling entire posterior parapodia; bidentate spines sometimes present in juveniles or occasionally in ventral most position of far posterior chaetigers of adults accompanying unidentate spines in cinctures; some species with long, natatory-like capillaries, sometimes limited to gravid individuals.
REMARKS
In the update of Blake (Reference Blake2015), it seems to say that the eyes and the nuchal organs are located in the peristomium, so this paragraph was rewritten. The form of the nuchal organs is variable, and the slit form could not be a diagnostic character (the same Blake, Reference Blake, Sardà, San Martín, López, Martin and George2006; Doner & Blake, Reference Doner, Blake, Sardà, San Martín, López, Martin and George2006; Dean & Blake, Reference Dean and Blake2007 described several species with oval or circular nuchal organs). The position of the dorsal tentacles and the first pair of branchiae are also so variable that these could not be considered a diagnostic character at the genus level, as well as the shape of the pygidium.
The value of the presence and development of cinctures also need to be re-evaluated at the generic level because as defined by Blake (Reference Blake2015) some species that lack cinctures like Chaetozone gracilis Moore, Reference Moore1923, Chaetozone armata Hartman, Reference Hartman1963 and Chaetozone vivipara Christie, Reference Christie1984 would need to be placed in a different genus.
DESCRIPTION
Chaetozone larae sp. nov., a moderate-sized species, holotype complete, 20 mm long, 0.1 mm wide, maximum width of 0.2 mm across chaetigers 14–26, for 94 chaetigers. Complete paratypes 87–99 chaetigers, 20–21 mm long, 0.1 mm wide, maximum width of 0.2 mm. Anterior region inflated dorsally, with 26 crowded chaetigers pear-shaped in dorsal view, first 12 chaetigers (6–16 in paratypes) not expanded, at least 15–20 times wider than long, and the other 14 laterally expanded (14–26 in paratypes), wider than long (25–30 times). The latest region shows a weakly developed narrow dorsal groove but only seen in paratypes under SEM. Middle region with rounded chaetigers in the dorsum; but sub-moniliform in some paratypes, about 3–4 times wider than long. Posterior segments moniliform, podial lobes forming a partial cincture, with segments 2–3 times wider than long (Figure 3A). Ventrally flat all along the body. Pygidium simple, rounded with dorsal anus (Figure 3B). Colour in alcohol pale tan.
Prostomium short, acuminate, without eyes; ciliated, oval nuchal organs present on lateral surface of prostomium just anterior to oral opening (not seen using light microscopy but obvious with SEM). Peristomium with one large and one narrow achaetous ring, followed by an achaetous segment between peristomium and chaetiger 1; achaetous segment most likely formed by fusion of third peristomial annulus with achaetous segment because it bears dorsal tentacles and first pair of branchiae. Peristomium overlain by low crest with no apparent annulations, overlapping prostomium anteriorly, narrowing posteriorly over achaetous segment between dorsal tentacles (Figure 3C). Following branchiae, one pair per chaetigers, dorsal to notochaetae, extended up to anterior middle region.
Parapodia rudimentary, with low rounded lobes (simple tori). Noto- and neuropodium very close to each other. Noto- and neurochaetae short and long simple lanceolate capillaries (bundles of 8–10 chaetae in each torus); with mid-region of one edge fimbriated (under optical stereomicroscope), with fine fibrils (under SEM), while smooth basally and distally (Figure 3D). Acicular spines smooth with curved distal tips (Figure 3E). Neuropodial acicular spines present from chaetigers 55 (in paratypes 43–74), 1–2 spines alternating with companion capillaries; 1–2 notopodial acicular spines from chaetiger 59 (in paratypes 40–75), also with companion capillaries. Partial cincture present from chaetiger 84 (66–77 in paratypes), with five acicular spines in notopodium and 6–7 acicular spines in neuropodium alternating with one capillary in each torus, arising from weakly developed membranes. Notopodial spines separated by small dorsal gap, and neuropodial spines leaving broad ventral gap between opposite parapodia (Figure 3F).
METHYL GREEN STAINING PATTERN
Uniform colouration.
ECOLOGY
This species inhabits subtidal bottoms (70 m depth) in muddy grey bottoms in the cold Magellanic region. It is accompanied by Leanira quatrefagesi Kinberg, Reference Kinberg1856, Prionospio sp., and several unidentified Paraonidae and Maldanidae. All species, including Chaetozone larae sp. nov., are present in low frequency and abundance.
REMARKS
Chaetozone larae sp. nov. is similar in shape to Chaetozone hartmanae Blake, Reference Blake, Blake, Hilbig and Scott1996 because of the crowded and expanded anterior region and narrow middle and posterior region. However, C. hartmanae has posterior region that is concave ventrally, while in C. larae sp. nov., it is ventrally flat. The prostomium is short in C. larae sp. nov. and long in C. hatmanae, and a similar crest over the peristomium is present in both species. First pair of branchiae are posterior to dorsal tentacles in both species, however, branchiae are limited to anterior part in C. larae sp. nov. but extended all over the body in C. hartmanae. Pygidium is rounded and simple in C. larae sp. nov. while in C. hartmanae it bears a ventral triangular lobe. Chaetae are of equal length in C. larae sp. nov., alternating short and long simple lanceolate capillaries, while in C. hartmanae notochaetae are thicker and longer than neurochaetae, both tori having chaetae of the same length. Bundles of chaetae have between 5–6 capillaries in C. hartmanae but 8–10 in C. larae sp. nov. Acicular spines begin in the anterior region in C. hartmanae and in the middle region in C. larae sp. nov. Acicular notoaciculae are straight but recurved neuroaciculae bear a crest of fine fibres or serrations in C. hartmanae, but smooth and always with curved tips in C. larae sp. nov. Both species have partial cincture. Chaetozone hartmanae is distributed in the Pacific ocean (California), while C. larae sp. nov. specimens were collected from the South-western Atlantic.
Chaetozone larae sp. nov. is also similar to Chaetozone pigmentata Blake, Reference Blake2015, Chaetozone brunnea Blake, Reference Blake, Sardà, San Martín, López, Martin and George2006 and Chaetozone spinosa Moore, Reference Moore1923 in the position of the first pair of branchiae behind the dorsal tentacles in the first achaetous segment. By the presence of the achaetous first segment, C. larae sp. nov. is also similar to Chaetozone pigmentata and Chaetozone malmgreni Blake, Reference Blake2015. Chaetozone larae sp. nov. is similar to C. pigmentata by having a low crest over the persitomium and no dorsal annulations. The description of the lectotype of Chaetozone setosa by Blake & Petersen (in Blake, Reference Blake2015) shows a weakly and narrow dorsal groove on the dorsum of the anterior region like most SEM paratypes of C. larae sp. nov. (Figure 3C); The segmental origin of acicular spines is similar to Chaetozone anasimus Doner & Blake, Reference Doner, Blake, Sardà, San Martín, López, Martin and George2006, Chaetozone ruffi Blake, Reference Blake2015, Chaetozone pugettensis Blake, Reference Blake2015 and also to the lectotype of Chaetozone setosa. The partial cincture is also present in Chaetozone michellae Magalhães & Bailey-Brock, Reference Magalhães and Bailey-Brock2013 and C. anasimus. Chaetozone larae is subantarctic while all these Chaetozone species (C. pigmentata, C. brunnea, C. spinosa, C. malmgreni, C. anasimus, C. ruffi, C. pugettensis, C. setosa and C. michellae) are from the northern hemisphere.
ETYMOLOGY
The species is dedicated to Lara Elías-Franco. She is a tireless fighter for human rights and a committed journalist.
DISTRIBUTION
This species is only known for the type locality, off Comodoro Rivadavia city, in San Jorge Gulf, Patagonia, in 70 m depth.
DISCUSSION
The cirratulids of the south-western Atlantic are poorly known because of a combination of factors. In addition to the extension of the coastline and the lack of specialists already mentioned, the problem with the Cirratulidae is also methodological, because of the methods used historically to collect samples. The samples collected during the two samplings made in both the Blanca Bay estuary and the continental shelf were sieved through a 0.5 mm mesh, resulting in the collection of cirratulids and other small invertebrates. Most of the studies in the continental shelf are carried out by sieving through a 1 mm mesh size. The cirratulids are probably lost during this process due to their small size. This was precisely what happened in the large survey carried out in the Bahia Blanca estuary in the 1980s, where several polychaetes were identified and quantified except for the cirratulids, because of the large mesh size (2 mm) used (Elías, Reference Elías1992).
Furthermore, the very few described species in the southern hemisphere made it difficult to make comparisons. For Monticellina, there are no described species from the SW Atlantic. In Brazil, a different warm biogeographic region, there is a single Monticellina species mentioned, but the family needs revision. In the case of Chaetozone, any of the species described are similar to the new species.
The diversity of cirratulids will most likely be increased when more studies are conducted in the region. This could be important for environmental assessments, because the presence and abundance of Cirratulidae is an indicator of poor environmental quality (Pearson & Rosenberg, Reference Pearson and Rosenberg1978; Chambers & Woodham, Reference Chambers and Woodham2003; Dean, Reference Dean2008; Elías & Rivero, Reference Elías and Rivero2008, Reference Elías and Rivero2009b, Reference Elías and Rivero2011; Sánchez et al., Reference Sánchez, Jaubet, Garaffo and Elías2013).
A first view of Table 1 shows no clear diagnostic differences for Monticellina and Aphelochaeta species. The denticulation visible through the light microscope in Monticellina is considered a tool rather than a character with phylogenetic meaning (see Dean & Blake, Reference Dean and Blake2009), while basally expanded blades could be present or not. In this context, the validity of both genera should be re-evaluated.
The methyl green staining pattern has proven to be useful for species differentiation in the northern hemisphere, allowing the separation of morphologically similar species. However, for the few southern hemisphere species, it has not proven to be successful.
On the other hand, the presence of acicular unidentate spines could be a valid character to differentiate Chaetozone from other bitentaculate cirratulids. As mentioned by Blake (Reference Blake, Blake, Hilbig and Scott1996) in the Hartman's system, species of Tharyx had only capillary-tipped chaetae; species of Caulleriella had acicular chaetae with bifid tips; whereas species of Chaetozone had acicular spines with distally entire tips. This concept differed from definitions of Fauvel (Reference Fauvel1927), Berkeley & Berkeley (Reference Berkeley and Berkeley1952) and Day (Reference Day1967), who defined Chaetozone on the basis of whether or not the spines formed cinctures encircling the posterior part of the body. However, this character (partial or complete cincture) must be revisited, because some species included in the genus lack cincture. In the case of types of spines as a diagnostic character to generic level, it must be revisited, because there are cases in which a species has a combination of two or three spines (see Caulleriella trispina Elías & Rivero, Reference Elías and Rivero2011). Another aspect of morphology of Chaetozone that must be revisited is the existence of an achaetous segment between the peristomium and the first chaetiger, which has been suggested but not proved. The presence of a first pair of branchiae could be an indicator of the existence of a segment that has lost its chaetae (we follow the criteria of Blake, Reference Blake2015). In this work, we remove this feature as a diagnostic character of the genus because it is too variable. In the near future, all described species of the genus must be revisited in order to clarify the existence of this segment, and histological studies must be conducted to find evidence that prove it.
It is clear that the taxonomy of cirratulids may be clarified with the help of new techniques (such as molecular biology, histology, etc.). However, it would fall into an extreme reductionism to rely solely on molecular characters, as benthic ecology, monitoring and evaluation of sites subject to environmental impact assessment (where cirratulids are often considered as important indicator organisms) require one or more morphological characters that allow separation of different morphotypes without liquefying and pass by a molecular scanner.
As Blake (Reference Blake, Blake, Hilbig and Scott1996) states, little work has been done so far in the field of systematics of this group. Day (Reference Day1967) conducted one of the few existing reviews of this family (in South Africa), and found many species of wide geographic distribution. However, it is unlikely that so many cosmopolitan species exist. It is most likely that, when studies are deepened, we discover that the diversity of Cirratulidae polychaetes is much higher.
It is probable that cosmopolitanism exists in large geographic areas of homogeneous environmental conditions, such as abyssal plains, but at the level of coastal ecosystems it is more likely that there is a broad diversification. Currently, the taxonomy and systematics of the Cirratulidae is based on material from the northern hemisphere. We hypothesize that the examination of material from the southern hemisphere could provide new taxonomic characters by which to make a better systematic and make the taxonomic identification of new specimens somewhat less problematic.
ACKNOWLEDGEMENTS
We thank Drs W. Magalhães and S. Chambers for critical comments on manuscript. SEM images were obtained by Lic. Mónica Oppedisano from the Laboratorio de Microscopía Electrónica of Facultad de Ciencias Exactas y Naturales of Universidad Nacional de Mar del Plata. Figures were drawn by RE. We thank E. Soto and J. Blake for sharing the list of characters of bitentaculate cirratulids. Lobo Orensanz passed away on 5 January 2015. He was involved in the present work since 2013. We lost a great person and researcher, the ‘father’ of the polychaetes in Argentina. In life, he decided to honour his daughter with a new species.
FINANCIAL SUPPORT
The consultants Environmental Resource Management and Serman & Associated S.A. financed the surveys (Comodoro Rivadavia and Bahía Blanca estuary, respectively) that allowed the discovery of these new species. This work was supported by loan BID – PICT 2013 no 1511 (to R. Elias).






