INTRODUCTION
The Magelonidae represents one of the most commonly encountered polychaete groups in Chinese coastal waters, and certain species obtain high densities. Until now, the species diversity of this family in China was poorly known. Previous taxonomic studies of Magelonidae in China were largely focused on species from Southern Chinese coastal waters (e.g. Wu & Sun, Reference Wu and Sun1978; Wu et al., Reference Wu, Sun and Chen1980; Paxton & Chou, Reference Paxton and Chou2000; Al-Hakin & Glasby, Reference Al-Hakin and Glasby2004; Mortimer & Mackie, Reference Mortimer and Mackie2009). The composition of magelonid species in northern Chinese waters was virtually unknown. Only two species (i.e. Magelona cincta Ehlers, Reference Ehlers1908 and M. japonica Okuda, Reference Okuda1937) have been recorded from this region in a compiled monograph on Chinese polychaete species by Yang & Sun (Reference Yang and Sun1988).
A total of five adult species have been recorded with sufficient taxonomic information in Chinese waters, they are Magelona cincta Ehlers, Reference Ehlers1908 (Magelona cf. cincta in Hong Kong waters) (Yang & Sun, Reference Yang and Sun1988; Mortimer & Mackie, Reference Mortimer and Mackie2009); M. japonica Okuda, Reference Okuda1937 (Yang & Sun, Reference Yang and Sun1988), M. cornuta Wesenberg-Lund, Reference Wesenberg-Lund1949 (Mortimer & Mackie, Reference Mortimer and Mackie2009), M. crenulifrons Gallardo, Reference Gallardo1968 (Mortimer & Mackie, Reference Mortimer and Mackie2009) and M. gemmata Mortimer & Mackie, Reference Mortimer, Mackie, Sigvaldadóttir, Mackie, Helgason, Reish, Svavarsson, Steingrímsson and Gudmundsson2003 (Al-Hakin & Glasby, Reference Al-Hakin and Glasby2004). Descriptions of new species and new records of Magelona species from other geographical regions in recent years (e.g. Fiege et al., Reference Fiege, Licher and Mackie2000; Mortimer & Mackie Reference Mortimer, Mackie, Sigvaldadóttir, Mackie, Helgason, Reish, Svavarsson, Steingrímsson and Gudmundsson2003; Mortimer et al., Reference Mortimer, Cassà, Martin and Gil2012) reveal that regional studies often yield a high number of species. Given this fact, species diversity of magelonid species may be higher than has already been reported for this region with a potential for a number of new species and new recorded species within the fauna.
Recently, a project was initiated, aimed at searching for a more productive and environmentally-friendly way to breed molluscs on a tidal flat of the Yellow Sea, People's Republic of China. During this work, several macrobenthic samples within the study area were collected, including a number of magelonid specimens. Careful examination of this material indicated the possibility of a new Magelona species. Thereafter, three additional field collections were carried out around the original localities of this species, covering both intertidal and shallow subtidal zones. The results of these collections confirmed the presence of the new species, herein described.
MATERIALS AND METHODS
Material examined within this study was collected on tidal flats and a shallow subtidal zone of the Yellow Sea, People's Republic of China (Jiangsu Province) (Figure 1). Sediment from the subtidal zone was obtained using a Van Veen grab with an area of 0.1 m2, and those from the subtidal flat were obtained by hand using a quantitative frame (area of 0.0625 m2 and depth of 30 cm) as a guide. The sediment obtained was then sieved using fine mesh sieves, the smallest mesh being 0.5 mm. All material was initially fixed in formalin/seawater, later washed with fresh water, and preserved in 75% alcohol.

Fig. 1. Sampling area where specimens of Magelona parochilis were collected.
Type and non-type material have been deposited at the East China Sea Fishery Research Institute (ECSFRI), Chinese Academy of Fishery Sciences and the National Museum Wales (NMW). Morphology was investigated using light and scanning electron microscopy (SEM). Drawings were made using a camera lucida attachment on a Leica MZ9.5 zoom microscope or Leica DM2000 compound microscope. Images of methyl green staining patterns were taken using a JVC KY-F7U3CCD camera on a Leica Z6 microscope. The resulting images were processed using syncroscopy, Automontage and Adobe Photoshop. Specimen descriptions are in the same format as those used in Mortimer (Reference Mortimer2010) and Mortimer et al. (Reference Mortimer, Cassà, Martin and Gil2012). Specimens are herein recorded as complete (c), anterior fragments (af), posterior fragments (pf) or fragments (f). Measurements were taken as detailed in Fiege et al. (Reference Fiege, Licher and Mackie2000). Staining patterns were observed using methyl green, as detailed by Nateewathana & Hylleberg (Reference Nateewathana, Hylleberg, Petersen and Kirkegaard1991) (although Nateewathana & Hylleberg used methyl blue) and Mackie & Gobin (Reference Mackie and Gobin1993).
SYSTEMATICS
Order: Spionida
Suborder: SPIONIFORMIA
Family MAGELONIDAE Cunningham & Ramage, Reference Cunningham and Ramage1888
Genus Magelona F. Müller, Reference Müller1858
Magelona parochilis sp. nov.
(Figures 2–5)
TYPE MATERIAL
Holotype: YELLOW SEA, CHINA–ECSFRI100532 (1c), 32°10.87′N 121°39.58′E, sandy mud, 23 August 2011, coll. Mei Jiang & Lei Li.
Paratypes: ECSFRI100533 (1c+ 2af), 32°10.98′N 121°39.93′E, sandy mud, 23 August 2011, coll. Mei Jiang & Lei Li; ECSFRI100534 (1c + 3af), 32°10.71′N 121°39.93′E, sandy mud, 23 August 2011, coll. Mei Jiang & Lei Li; ECSFRI100535 (4af), 32°10.63′N 121°40.00′E, sandy mud, 23 August 2011, coll. Mei Jiang & Lei Li; ECSFRI100589/ NMW.Z.2012.033.0001 (1af) 32°10′.42′N 121°39′56′E, sandy mud, 5 May 2012.
OTHER MATERIAL EXAMINED
YELLOW SEA, CHINA–ECSFRI100901 (3c + 8af), 32°10.89′N 121°39.93′E, sandy mud, 16 November 2011, coll. Mei Jiang & Lei Li; ECSFRI100902 (2af), 32°10.71′N 121°39.93′E, sandy mud, 16 November 2011, coll. Mei Jiang & Lei Li; ECSFRI100909 (2af), 32°10.63′N 121°40.00′E, sandy mud, 16 November 2011, coll. Mei Jiang & Lei Li; ECSFRI100914 (1af + 2pf), 32°10.87′N 121°39.58′E, sandy mud, 16 November 2011, coll. Mei Jiang & Lei Li; ECSFRI100916 (1c + 2af + 3pf), 32°10.71′N 121°39.93′E, sandy mud, 16 November 2011, coll. Mei Jiang & Lei Li; ECSFRI100922 (1c + 4af), 32°10.71′N 121°39.93′E, sandy mud, 5 May 2012, coll. Mei Jiang & Lei Li; ECSFRI100923 (1c + 1af), 32°10.63′N 121°40.00′E, sandy mud, 5 May 2012, coll. Mei Jiang & Lei Li; ECSFRI100930 (1c), 33°18.59′N 121°34.11′E, 7.4 m, sandy mud, 18 May 2012, coll. Jin Zhou; ECSFRI100931 (1af), 32°46.75′N 121°22.72′E, 3.9 m, sandy mud, 20 May 2012, coll. Jin Zhou; ECSFRI100932 (1af), 32°52.26′N 121°30.58′E, 2.9 m sandy mud, 17 May 2012, coll. Jin Zhou; ECSFR1100542/NMW.Z.2012.033.0002 (2af) 32°39.93′N 121°10.97′E, sandy mud, 5 May 2012, coll. Mei Jiang & Lei Li; ECSFR1102541 (1c), 33°11.03′N 121°16.59′E, 11.0 m, sandy mud, 22 September 2012, coll. Jin Zhou; ECSFR1102542 (2c + 4af), 32°40.32′N 121°19.67′E, 7.8 m, sandy mud, 23 September 2012, coll. Jin Zhou; ECSFR1102543 (1af), 33°26.45′N 121°03.01′E, 5.0 m, sandy mud, 22 September 2012, coll. Jin Zhou; ECSFR1102544 (2c + 12af), 33°24.69′N 121°14.46′E, 8.6 m, sandy mud, 22 September 2012, coll. Jin Zhou.
DIAGNOSIS
Prostomium anteriorly rounded without prostomial horns. Thoracic notopodial lamellae smooth edged. Notopodia of chaetigers 1–8 with slender cirriform dorsal processes. Neuropodia of same chaetigers with ventral, slender triangular lamellae. Chaetigers 1–8 with capillary chaetae, chaetiger 9 with mucronate chaetae. Hooded hooks tridentate, unidirectional. Anteriorly and posteriorly open pouches present.
DESCRIPTION
Holotype (Figure 3), a moderate, complete specimen, abdomen thicker than thorax. Prostomium 0.68 mm long, 0.42 mm wide; thorax (including prostomium) 2.94 mm long, 0.44 mm wide at widest point; abdomen 11.62 mm long, 0.60 mm wide; total length 15.24 mm for 67 chaetigers. Dimensions of broadest specimen (ECSFRI100909): prostomium 0.84 mm long, 0.59 mm wide; thorax (including prostomium) 7.05 mm long, 0.63 mm wide; abdomen 0.73 mm wide; total length 19.86 mm for 39 chaetigers. Longest specimen (ECSFRI100533), 22.1 mm for 73 chaetigers. Other paratypes 11.4 mm–17.2 mm with 61–69 chaetigers.
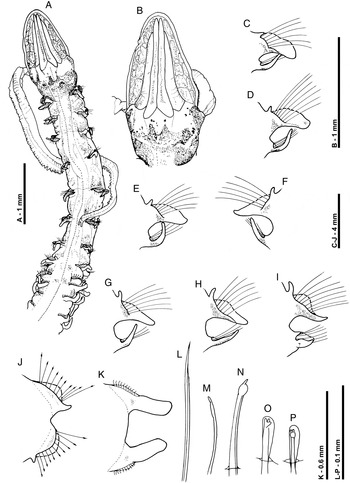
Fig. 2. Magelona parochilis sp. nov. (paratype, NMW.Z.2012.033.0001): (A) anterior region, dorsal view; (B) prostomium, dorsal view (showing partially everted proboscis to the right-hand side); (C–K) chaetigers 1, 2, 3, 5, 6, 7, 8, 9 and 10 (anterior views); (L) capillary chaeta, lateral view, from the notopodia of chaetiger 8; (M–N) specialized chaetae from the notopodia of chaetiger 9, lateral views (M, outermost lateral chaeta); (O–P) tridentate abdominal hooded hooks, oblique frontal and frontal views from chaetigers 10 and 11, respectively.

Fig. 3. Magelona parochilis sp. nov. (holotype ECSFRI100532): (A) anterior, dorsal view; (B) posterior, dorsal view.
Prostomium elongate, longer than wide (L:W 1.53–2.52), slender triangular, without prostomial horns, anterior margin smooth, rounded, eyes absent (Figures 2A, 3A, 5A). Two pairs of prominent longitudinal dorsal muscular ridges, outer pair (slightly shorter) abutting inners for entire length, inner pair almost reaching distal tip of prostomium, where they diverge only very slightly. Indistinct quadrangular areas present on either side of muscular ridges. Proboscis not everted on holotype but everted in five specimens, heart-shaped when fully everted, oval when partially everted (Figures 2B, 4A). Proboscis longitudinal ridged, ridging of upper surface much lighter, appearing smooth. Palps arising ventrolaterally from base of prostomium, robust, tapering to fine translucent tips, reaching chaetiger 10 on holotype but chaetigers 9–16 in other specimens, non-papillated region reaching chaetigers 1–2. Papillae very short proximally, increasing gradually in size, papillae long at distal tip. Initially 3–4 rows of papillae on either side of inconspicuous ventral groove, medially 2 rows, and distally 1–2 rows.
Achaetous region behind prostomium, about one and half times the size of chaetiger 1 (Figures 2A, 3A). Chaetigers 1–7 similar; parapodia biramous (Figure 2C–H). Notopodia prechaetal lamellae low triangular, increasing in size and becoming well-developed by chaetiger 5, confluent with larger spatulate postchaetal lamellae. Point of connection between pre- and postchaetal lamellae becoming closer to distal lamellar tips towards the posterior thorax. Postchaetal lamellae becoming marginally longer and thinner along thorax, somewhat triangular by chaetiger 5. Upper edges of notopodial postchaetal lamellae relatively smooth. Slender, tapering cirriform prechaetal superior processes (DML) present on all thoracic chaetigers (except chaetiger 9), increasing in size along thorax. Those of chaetiger 1 relatively small. Neuropodia of chaetigers 1–7 with slender triangular lamellae directly underneath chaetae (VNL), becoming slightly prechaetal in position by chaetiger 6. pre- and postchaetal lamellae not developed.
Chaetiger 8 parapodia (Figures 2I, 4B): Notopodial prechaetal lamellae well-developed, confluent with slightly larger, rounded triangular postchaetal lamellae. Point of connection between these lamellae, near distal tips, thus appearing as though lamellae are sub-chaetal. When viewed laterally, lamellae form a U-shaped structure around chaetae. Prechaetal superior processes long, digitiform (DML). Neuropodial prechaetal lamellae low, confluent with triangular postchaetal lamellae, additional digitiform prechaetal processes present. Chaetae of chaetigers 1–8 simple winged capillaries (Figures 2L, 4C).
Chaetiger 9 (Figure 2A): shorter and narrower than preceding chaetigers. Notopodial prechaetal lamellae rounded, smooth, confluent with higher rounded triangular postchaetal lamellae (Figure 2J). Dorsal superior processes (DML) absent. Neuropodia similar to notopodia, however, prechaetal lamellae inferiorly developed as small triangular processes. Chaetae mucronate (Figures 2M–N, 4D), arranged in arcs; chaetae longer but with narrower distal tips towards margins of each fan.
Abdominal chaetigers (Figure 2K) with spatulate lateral lamellae, of about equal size in both rami, bluntly rounded. Lateral lamellae not overlapping. Lamellar shape shows some variation; from rounded, subrectangular to slightly reniform. Postchaetal extension of lateral lamellae, not well-developed, apparent only in anterior abdomen. Dorsal and ventral processes (DML & VML) at inner margins of chaetal rows absent.
Abdominal chaetae (Figures 2O–P, 4E) tridentate hooded hooks of similar size, superior two fangs parallel, above main fang. Hooks in each ramus unidirectional, main fangs pointing laterally. A few hooks closest to the lamellae slightly twisted in some parapodia. Initially around 10–14 hooks per rami, decreasing to approximately 8–10 hooks per rami on posterior fragment.
Paired anteriorly open pouches present between chaetigers 11–12 and 14–15 (∑ configuration pouches of Fiege et al., Reference Fiege, Licher and Mackie2000: well-developed, large membrane, often extruded, bounded between two cuticular flaps) (Figures 4F–G, 5D). In some specimens, this type of lateral pouch is also present between chaetigers 17–18. Unpaired posteriorly open pouches present in middle and posterior abdomen, on alternate chaetigers (C configuration pouches of Fiege et al., Reference Fiege, Licher and Mackie2000: often quite large, more expanded both dorsally and ventrally, often convoluted) (Figure 4H). Pouches observed on holotype on 21L, 23R, 25L, 27R, 29L, 31R, 33L, 35R, 37L, 39R, 41L, 43R, 45L, 47R, 49L, 51R, 53L and 55R.

Fig. 4. Magelona parochilis sp. nov. (paratype, ECSFRI 100535): (A) anterior region, lateral view; (B) chaetiger 8, lateral view (specimen orientated so that the prostomium is towards the top of the picture); (C) capillary chaetae from chaetiger 6; (D) modified chaetae from chaetiger 9, postchaetal view (notopodia); (E) tridentate hooded hooks from chaetiger 42, anterior view; (F) anteriorly open lateral pouch between chaetigers 11 and 12, lateral view; (G) anteriorly open lateral pouch between chaetigers 14 and 15, lateral view; (H) posteriorly open lateral pouch between chaetigers 52 and 53, lateral view.
Two short anal cirri present (Figure 2B).
COLOUR
Specimen cream–white in alcohol. Staining with methyl green diffuse, however when much of the stain has dissipated (MBA as defined by Nateewathana & Hylleberg, Reference Nateewathana, Hylleberg, Petersen and Kirkegaard1991) a distinct pattern on the prostomium and thorax noticeable, particularly in the anterior thorax. Light, speckled longitudinal staining of the dorsal prostomial ridges present as well as heavy dense staining of the areas either side (Figure 5A). Staining darkest on achaetous region, generally comprising large speckles. Thorax stained weakly all over (Figures 5B–C), however dorsally, areas surrounding parapodia and on mid-dorsal region in anterior thorax showing less stain. Ventral thoracic staining similar to dorsal, staining strongest on prostomial edges and in the anterior thorax. Thoracic lamellae and those of the anterior abdomen possessing stain near to the lamellar bases (Figure 5D). Interparadodial staining in mid and posterior abdomen. Staining pattern weaker on some specimens. Two green parallel lines between notopodial and neuropodial lobes on abdominal chaetigers present. However, these lines are absent on thoracic chaetigers.

Fig. 5. Magelona parochilis sp. nov. (paratype, NMW.Z.2012.033.0001 methyl green staining patterns;): (A) prostomium, dorsal view; (B) anterior, dorsal view; (C) anterior, ventral view; (D) lateral abdominal pouch between chaetigers 11–12.
ETYMOLOGY
From the Latin meaning ‘limited area’, referring to the species habitat being somewhat restricted during these present investigations (see Remarks section below).
REMARKS
During the current investigations Magelona parochilis sp. nov. appeared to be restricted to one particular region of the tidal flat (32°10.63′N–33°26.45′N) of the Yellow Sea, China. No other records of species matching this description have been found for tidal flat or subtidal zones in other Chinese coastal waters, even those directly adjacent to the type locality. Additional material from other studies has been systematically examined (extending from the Bohai Sea to the South China Sea, covering both northern and southern Chinese coastal waters). The results revealed that Magelona cincta was the most frequently encountered magelonid species. Other magelonid specimens in Chinese coastal waters were commonly identified as Magelona crenuliforms or Magelona cornuta. Futhermore, no other specimens of M. parochilis were found in this additional material.
Magelona parochilis sp. nov. belongs to a ‘Magelona mirabilis group’ of species, which are characterized by possessing a rounded prostomium lacking frontal horns, and mucronate chaetae on chaetiger 9. There are 14 members of this group, including: M. mirabilis (Johnston, Reference Johnston1865), Magelona johnstoni Fiege, Licher & Mackie, Reference Fiege, Licher and Mackie2000, Magelona crenulata Bolívar & Lana, Reference Bolívar and Lana1986, Magelona pitelkai Hartman, Reference Hartman1944, Magelona sacculata Hartman, Reference Hartman1961, Magelona riojai Jones, Reference Jones1963, Magelona conversa Mortimer & Mackie, Reference Mortimer, Mackie, Sigvaldadóttir, Mackie, Helgason, Reish, Svavarsson, Steingrímsson and Gudmundsson2003, Magelona pectinata Nateewathana & Hylleberg, Reference Nateewathana, Hylleberg, Petersen and Kirkegaard1991, Magelona sachalinensis Buzhinskaja, Reference Buzhinskaja1985, Magelona tinae Nateewathana & Hylleberg, Reference Nateewathana, Hylleberg, Petersen and Kirkegaard1991, Magelona debeerei Clarke, Paterson, Florence & Gibbons, Reference Clarke, Paterson, Florence and Gibbons2010, Magelona obockensis Gravier, Reference Gravier1905 and two unnamed species (Magelona sp. A and Magelona sp. B) from the Gulf of Mexico (Uebelacker & Jones, Reference Uebelacker, Jones, Uebelacker and Johnson1984).
Magelona debeerei and M. johnstoni differ from the new species by the absence of dorsal superior processes in the anterior thorax, in M. parochilis sp. nov. they are present from chaetigers 1–8. Dorsal superior thoracic processes are absent in M. mirabilis. Three species (M. conversa, Magelona sp. A and M. sachalinensis) differ from the new species by possessing bidentate instead of tridentate abdominal hooded hooks. Magelona crenulata and Magelona sp. B differ from M. parochilis sp. nov. by possessing crenulate lamellae of chaetiger 9. In possessing smooth edged thoracic notopodial lamellae, M. parochilis sp. nov. differs from M. obockensis, M. tinae and M. pectinata in which they are minutely crenulate and bilobed on several chaetigers in the two first species and pectinate in the latter species. Magelona pitelkai differs from the new species in not possessing lateral abdominal pouches in the anterior abdomen, possessing posteriorly open pouches only. It is reported for M. sacculata that ‘conspicuous pouched membranes, first present behind the modified ninth segment, occur also between segments 10 and 11’. In M. parochilis sp. nov. the abdominal lateral pouches first appear between chaetigers 11–12. Magelona riojai differs from the new species by possessing a squared prostomial anterior margin, whilst in M. parochilis sp. nov. it is rounded, and not possessing neuropodial processes on chaetiger 9. Magelona parochilis sp. nov. differs from all of the above species, except M. debeerei by not possessing small triangular processes (DML & VML) present at inner margins of chaetal rows in the abdomen.
Of all these species, M. conversa, M. mirabilis, M. johnstoni, M. sacculata, M. riojai and M. debeerei have unidirectional facing abdominal hooded hooks as in the new species (orientation for M. sachalinensis and M. pectinata unknown, however, for the latter species, in figure 7L of the original description they are shown as unidirectional).
The shape of the thoracic notopodial lamellae of chaetigers 7 and 8 in M. parochilis sp. nov, where the pre-chaetal and postchaetal lamellae meet to form U-shaped structures around the chaetae is a character which was described for M. riojai. Jones (Reference Jones1963) when describing this species stated ‘Dorsal lateral lamellae are U-shaped in cross section, and presetal and postsetal portions meet ventrally’. This is a character which the new species seems also to share with the following members of this group: M. mirabilis, M. pitelkai, Magelona sp. A, Magelona sp. B and M. obockensis.
The new species can be readily distinguished from other known Chinese magelonid species in having a rounded anterior prostomial margin without horns.
DISTRIBUTION
The species is currently known only from tidal flats and shallow subtidal waters of Yellow sea, China.
ACKNOWLEDGEMENTS
This study was funded by grants from the National Natural Science Foundation (No. 40906084) and Key Laboratory of Marine Bio-resources Sustainable Utilization (LMB) South China Sea Institute of Oceanology, Chinese Academy of Sciences (LMB111007). We are grateful to Dieter Fiege (Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Sektion Marine Evertebraten II, Senckenberganlage) for providing valuable comments on the manuscript. Thanks are also due to Mr Lei Li and Mrs Mei Jiang for the collections of the samples.







