INTRODUCTION
The Paraonidae constitutes one of the more diverse and taxonomically complex families among polychaetes. This family has been investigated in the Mediterranean Sea by several authors (Cerruti, Reference Cerruti1909; Laubier, Reference Laubier1967; Laubier & Ramos, Reference Laubier and Ramos1974; Katzmann & Laubier, Reference Katzmann and Laubier1975; Castelli, Reference Castelli1985), but knowledge of the Mediterranean Paraonidae is far from being complete, and new species were recently described, even from shallow environments where polychaete fauna is better known (Sardá et al., Reference Sardá, Gil, Taboada and Gili2009; Çinar et al., Reference Çinar, Dağli and Açik2011; Çinar & Dağli, Reference Çinar and Dağli2013). Moreover, within Paraonidae, the taxonomy of several genera is unclear and the whole family needs a taxonomic revision based on the re-examination of existing type material and the study of new material, covering also poorly known areas, using both morphological and molecular data.
The genus Cirrophorus Ehlers, Reference Ehlers1908 in the Mediterranean Sea has been generally considered to be represented by two species, Cirrophorus branchiatus Ehlers, Reference Ehlers1908 and Cirrophorus furcatus (Hartman, Reference Hartman1957). Some authors list an additional species, Cirrophorus lyriformis Annenkova, Reference Annenkova1934 (Çinar et al., Reference Çinar, Dağli and Kurt Şahin2014), a species synonymized with C. branchiatus by Strelzov (Reference Strelzov1973) even if most of its Mediterranean records were later attributed to C. furcatus (see Katzmann & Laubier, Reference Katzmann and Laubier1975, and references therein). On the other hand, the identification of the Mediterranean C. furcatus has been more problematic, and several different species, or at least morphotypes, seem to be involved. The identity of Mediterranean individuals of the shallow water Cirrophorus nr furcatus, commonly found in brackish-water habitats and, in a more general way, organically-enriched environments, was questioned previously (Castelli et al., Reference Castelli, Abbiati, Badalamenti, Bianchi, Cantone, Gambi, Giangrande, Gravina, Lanera, Lardicci, Somaschini, Sordino, Minelli, Ruffo and La Posta1995), as the Mediterranean individuals show morphological and ecological differences with respect to those from California, the type locality of the species (Hartman, Reference Hartman1957).
Previous works on polychaetes have widely demonstrated that the combined use of morphological and molecular data is a powerful approach to disentangle complex systematic problems and make attempts to clarify evolutionary processes (e.g. Cadman & Nelson-Smith, Reference Cadman and Nelson-Smith1990; Wu et al., Reference Wu, Qian, Zhang, Petersen and Kirkegaard1991; Maltagliati et al., Reference Maltagliati, Camilli, Lardicci and Castelli2001, Reference Maltagliati, Casu and Castelli2004, Reference Maltagliati, Casu, Lai, Iraci Sareri, Casu, Curini-Galletti, Cantone and Castelli2005; Nygren & Pleijel, Reference Nygren and Pleijel2011). Within the frame of a systematic revision of the Mediterranean Paraonidae, and on the basis of morphological and molecular data, in this work we identify two divergent morphotypes that were historically identified as Cirrophorus furcatus. One of these morphotypes is described here as a new species, whereas the other represents a putative new species, but is not described due to the scarcity of the material available. Moreover, the synonymy between the genera Cirrophorus and Paradoneis Hartman, Reference Hartman1965, and the use of the generic names Paraonides Cerruti, Reference Cerruti1909 and Paraonella Strelzov, Reference Strelzov1973 are discussed based on the results from molecular data and the critical analysis of the literature.
MATERIALS AND METHODS
Samples of Cirrophorus nr furcatus for morphological study were obtained from the collection of the University of Pisa. Specimens were fixed with 4% neutralized formaldehyde in seawater and subsequently preserved in 70% ethanol. Measurements and counts were performed with a Primo Star Zeiss light microscope equipped with an ocular micrometer; drawings were made from images taken with a digital camera, and refined with GIMP 2.8.18 (software downloadable and documentation available at http://www.gimp.org), following the guidelines in Montesanto (Reference Montesanto2015). Type specimens were deposited in the Museo di Storia Naturale of the University of Pisa (Italy) (MSNP); non-type material was deposited in the Natural History Museum of Los Angeles, USA (LACM – AHF) and in the collections of the Stazione Zoologica Anton Dohrn of Naples (Italy) (SZN – LAM). Live Paraonidae for molecular analyses were collected at several localities in the Mediterranean Sea and Atlantic Ocean (Table 1), fixed directly in 96% or 70% ethanol and preserved at 4 °C until DNA extraction. We included material of the species of Cirrophorus and Paradoneis Hartman, Reference Hartman1965 available to us, including respectively the type species C. branchiatus Ehlers, Reference Ehlers1908 and Paraonis (Paraonides) lyra Southern, Reference Southern1914. The type species of Aricidea Webster, Reference Webster1879 (Aricidea fragilis Webster, Reference Webster1879), Levinsenia Mesnil, Reference Mesnil1897 (Aonides gracilis Tauber, Reference Tauber1879) and Paraonis Grube, Reference Grube1873 (Aonides fulgens Levinsen, Reference Levinsen1884) were also included. DNA extraction was carried out using the GenElute™ Mammalian Genomic DNA Miniprep Kit distributed by Sigma-Aldrich, following the manufacturer's instructions. For phylogenetic reconstruction we amplified the genes for 16S rRNA and COI (mitochondrial) and 18S rRNA (nuclear). 16S rDNA amplification was obtained using the primer pair 16SarL (5′-CGCCTGTTTAACAAAAACAT-3′) and H3080 (5′-CCGGTCTGAACTCAGATCACGT-3′) (Palumbi et al., Reference Palumbi, Martin, Romano, McMillan, Stice and Grabowski1991), whereas for COI amplification we used the universal primers LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) and the annelid-specific primers POLYLCO (5′-GAYTATWTTCAACAAATCATAAAGATATTGG-3′) and POLYHCO (5′-TAMACTTCWGGGTGACCAAARAATCA-3′) (Carr et al., Reference Carr, Hardy, Brown, Macdonald and Hebert2011). 18S rDNA amplification was obtained using the primers F9 (5′-CTGGTTGATCCTGCCAG- 3′) (Medlin et al., Reference Medlin, Elwood, Stickel and Sogin1988) and R1513 (5′-TGATCCTTCYGCAGGTTC-3′) (Petroni et al., Reference Petroni, Dini, Verni and Rosati2002). Polymerase chain reaction (PCR) amplifications were carried out in 20 µl solutions using 1.5 mM of MgCl2, 0.2 mM of each dNTP, 0.1 µM of each primer, 1 U of DreamTaq DNA polymerase (Thermo Scientific), and ~2.5 ng of template DNA. For 16S rDNA and COI the PCR profile was set as follows: initial denaturing step at 94 °C for 3 min; 34 cycles of denaturing at 94 °C for 45 s, annealing at 54 °C for 1 min, and extending at 72 °C for 1 min, and a final extending step at 72 °C for 7 min. A negative control was included in each reaction. For 18S rDNA, PCRs were carried out in 45 µl using a protocol with low ramp speed, and annealing temperature set at 50 °C (Lorenz, Reference Lorenz2012). PCR products were precipitated with sodium acetate and absolute ethanol and sent to Macrogen Europe for sequencing.
Table 1. Paraonidae included in the molecular analysis.

Ad, Adriatic Sea; At, Atlantic Ocean; T, Tyrrhenian Sea.
Sequences from each gene were aligned with ClustalX 2.1 (Larkin et al., Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm, Lopez, Thompson, Gibson and Higgins2007), and alignments were edited in BIOEDIT version 7.2.5 (Hall, Reference Hall1999). The program jModelTest 2.1.6 (Guindon & Gascuel, Reference Guindon and Gascuel2003; Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012), based on the hierarchical likelihood ratio test, was used to assess the best model of evolution for the sequences under the Akaike Information Criterion (AIC) (Akaike, Reference Akaike1974). For molecular comparison and phylogenetic reconstruction, we used additional sequences downloaded from GenBank for Cirrophorus furcatus (accession numbers AY532349.1 and AY532330.1); moreover, we used Ophelina acuminata Örsted, Reference Örsted1843 as outgroup (accession numbers AY340471.1, AY340439.1 and HQ024164.1). The choice of the outgroup was based on Bleidorn's (Reference Bleidorn2005) remarks, who identified Opheliidae as a likely sister taxon of Paraonidae.
A Bayesian consensus phylogenetic tree based on the three concatenated markers was constructed using MrBayes 3.2 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2011), which allowed phylogenetic inference by treating each gene with its own substitution model. Four replicate runs were carried out with a total of three Markov chains per run for 2 × 106 generations. The chain was sampled every 100 generations to obtain 20,000 sampled trees. The first 5000 sampled trees (25%) were discarded as burn-in phase, with the remaining 15,000 trees used to estimate the Bayesian posterior probability (PP) of tree nodes. The convergence of Bayesian analyses was checked through the standard deviation of split frequencies, that should reach a value <0.01 at the end of the analysis (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2011).
RESULTS
SYSTEMATICS
Class Annelida
scolecida Rouse & Fauchald, Reference Rouse and Fauchald1997
Family paraonidae Cerruti, Reference Cerruti1909
Genus Cirrophorus Ehlers, Reference Ehlers1908
Cirrophorus nikebianchii sp. nov.
(Figure 1A–F)
Cirrophorus furcatus [non (Hartman, Reference Hartman1957)]: Castelli, Reference Castelli1985: 277; Castelli & Lardicci, Reference Castelli and Lardicci1985: 765–766; Castelli, Reference Castelli1987: 327; Rossi & Lardicci, Reference Rossi and Lardicci1995: 33; Como et al., Reference Como, Rossi and Lardicci2004: 165; Chessa et al., Reference Chessa, Scardi, Serra, Pais, Lanera, Plastina, Valiante and Vinci2007: 14–15; Schirosi et al., Reference Schirosi, Musco and Giangrande2010: 238–239. Paradoneis lyra [non (Southern, Reference Southern1914)]: Bonvicini Pagliai & Cognetti, Reference Bonvicini Pagliai and Cognetti1982: 55; Maggiore & Keppel, Reference Maggiore and Keppel2007: 193–194; Simboura et al., Reference Simboura, Reizopoulou, Arvanitidis and Basset2007: 57.

Fig. 1. Cirrophorus nikebianchii sp. nov., holotype (MSNP: P/3800): (A) dorsal view of the anterior region; (B) end of the branchial region (71th to 79th chaetiger); (C) left parapodium in the anterior region (6th chaetiger); (D) ventral part of a parapodium in the post-branchial region (110th chaetiger); (E) lyrate notopodial chaeta; (F) thickened neuropodial chaetae from the post-branchial region. Scale bar: 0.3 mm (A, B), 0.1 mm (C), 50 μm (D), 12 μm (E), 30 μm (F).
TYPE MATERIAL
Holotype: Sea of Sardinia, Italy, north-west Sardinia, Calich Pond (40°35.8′N 8°17.3′E), silty sand, brackish-water coastal pond, 1 m (March 1995) (MSNP P/3800).
Paratype: Locality and collection as holotype (MSNP P/3801).
COMPARATIVE MATERIAL EXAMINED
Balearic Sea, Spain: Ebro River Delta, Alfacs Bay (40°36.8′N 0°36.5′E), semi-closed inlet with freshwater inflow, 4 m: 3 individuals (September 1992) [1]; Sea of Sardinia, Italy: north-west Sardinia, Calich Pond (40°35.8′N 8°17.3′E), silty sand, brackish-water coastal pond, 1 m: 4 individuals (March 1988); 26 individuals (March 1995) [2]; Sardinian Channel, Italy: south-west Sardinia, Gulf of Cagliari (39°5.9′N 9°2.6′E), silty sand in fully marine environment, 20 m: 1 individual (1982) [3]; Tyrrhenian Sea, Italy: north-east Sardinia, Golfo Aranci, Gulf of Olbia (40°59.9′N 9°37.1′E), silty sand, under fish cages in fully marine environment, 5 m: 1 individual (January 1997) [4]; north Sardinia, Porto Pozzo (41°11.5′N 9°16.7′E), gravel, semi-closed inlet with brackish-water inflow, 0,8 m: 4 individuals (July 1987); 22 individuals (July 2015) [5]; eastern Sardinia, Tortolì Pond (39°56.9′N 9°41.2′E), gravel, brackish-water coastal pond, 1 m: 5 individuals (May 2016) [6]; Capraia Island (43°2.4′N 9°50.8′E), silt, under fish cages in fully marine environment, 33,5 m: 43 individuals (July 2003) [7]; Portoferraio Bay, Elba Island (42°48.7′N 10°18.5′E), silt, organically enriched environment, 8 m: 2 individuals (November 1982); 5 individuals (May 1990) [8]; Gulf of Follonica (42°55.1′N 10°44.3′E), fine sand in fully marine environment, 8 m: 8 individuals (December 1987) [9]; Livorno port (43°34.4′N 10°18.9′E), silt, organically enriched environment with brackish-water inflow, 3 m: 15 individuals (April 2016) [10]; Adriatic Sea, Italy: Southern Adriatic Sea, Acquatina Pond (40°26.7′N 18°14.3′E), silt, brackish-water coastal pond 2 m: 68 individuals (November 2014) [11]; Southern Adriatic Sea, Varano Lagoon (41°52.4′N 15°42.3′E), silty shell grit, brackish-water lagoon, 3 m: 4 individuals (November 2014); 7 individuals (October 2015) [12]; Northern Adriatic Sea, Venice Lagoon (45°29.2′N 12°29.4′E), silt, brackish-water lagoon system, 0,8 m: 66 individuals (March 2015) [13]; Aegean Sea, Greece: Agiasma Lagoon (40°52.9′N 24°37.1′E), brackish-water lagoon system, 2 m: 25 individuals (date unknown) [14].
Cirrophorus sp. B (see below in the Remarks section): Livorno port (43°34.4′N 10°18.9′E), silt, organically enriched environment with brackish-water inflow, 3 m: 1 individual (April 2016) [10].
DESCRIPTION
Holotype complete, ~12 mm long, 0.45 mm wide for 126 chaetigers (Figure 1A). Prostomium roughly trapezoidal, slightly longer than wider, antenna short, blister-like, with median insertion, ~1/6 of the prostomium length; in juveniles prostomial antenna extremely reduced and can be difficult to observe. Eyes absent, nuchal organs small, comma-shaped, difficult to examine in preserved individuals, more conspicuous in living specimens. Three pre-branchial chaetigers, with post-chaetal notopodial lobes gradually increasing in length towards the branchial region; Notopodial lobes are short, basally swollen, skittle shaped in anterior branchial region, then gradually increase in the posterior part, becoming distinctly longer, and tapered in the post-branchial region. Seventy-five pairs of branchiae from chaetiger 4; branchiae pointed, approximately as long as body width in anterior part, ~6–7 times length of notopodial lobes, then becoming gradually shorter, and in posterior branchial region almost tubercular, shorter than notopodial lobes (Figure 1B). In the additional material examined their number ranges from 24 to 72 pairs, more frequently 40–65; number of branchiae pairs coarsely correlated with size of specimen (Figure 2). Pygidium rounded, with three thread-like, elongated anal cirri, approximately twice the length of last notopodial lobes.
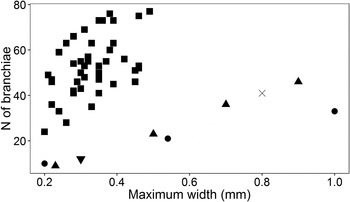
Fig. 2. Relationship between maximum width and number of pairs of branchiae in five Cirrophorus species with lyrate notopodial chaetae. Legend: ■: Cirrophorus nikebianchii sp. nov. (present study); ▴: Cirrophorus americanus Strelzov, Reference Strelzov1973 (from Strelzov, Reference Strelzov1973); •: Cirrophorus furcatus (Hartman, Reference Hartman1957) (from Strelzov, Reference Strelzov1973); X: Cirrophorus miyakoensis Imajima, Reference Imajima1973 (from Imajima, Reference Imajima1973); ▾: Cirrophorus sp. B (present study).
Parapodia biramous, composed of several thick, slightly curved capillaries (Figure 1C); 1–4 (usually 2–3) notopodial lyrate chaetae (Figure 1E) from chaetiger 3. In additional material, some individuals with lyrate chaetae from chaetiger 2. Lyrate chaetae with branches of sub-equal thickness, one approximately twice as long as other. Internal edge of each branch with several short, slender spines. Modified neurochaetae from chaetiger 96, gradually increasing in number from 1–2 to 6–7, intermixed with capillaries (Figure 1D). Modified chaetae are thickened capillaries, slightly curved, with or without tapered tips (Figure 1E). Starting point of modified neurochaetae at chaetiger 44–96, not always easy to identify.
Preserved individuals brownish to reddish; live colour bright orange, posterior part of the body often golden-green.
DISTRIBUTION
Mediterranean Sea. Collected in the Balearic Sea, Sea of Sardinia, Sardinian Channel, Tyrrhenian Sea, Adriatic Sea and Aegean Sea (Figure 3).

Fig. 3. Known distribution of Cirrophorus nikebianchii sp. nov. Localities: 1, Ebro River Delta; 2, Calich Pond (Type locality); 3, Gulf of Cagliari; 4, Gulf of Olbia, 5, Porto Pozzo; 6, Tortolì Pond; 7, Capraia Island; 8, Elba Island; 9, Gulf of Follonica; 10, Livorno port; 11, Acquatina Pond; 12, Varano Lagoon; 13, Venice Lagoon; 14, Agiasma Lagoon. See Systematics for locality details.
ECOLOGY
Present in organically enriched environments, from 0.5–3 m depth in brackish-water lagoons and coastal ponds, from 3.5 to ~30 m depth in marine environments. In brackish-water environments it is often associated with Zostera and Nanozostera meadows, whereas in marine environments its presence is typically related to anthropogenic organic enrichment, such as sewage pollution and fish farms. Cirrophorus nikebianchii sp. nov. shows remarkably high densities and a patchy distribution, typical of an opportunistic species occurring in enriched and polluted environments.
ETYMOLOGY
It is an honour to dedicate the new species to Carlo Nike Bianchi, in recognition of his important work on Mediterranean polychaetes.
REMARKS
Currently, the genus Cirrophorus Ehlers, Reference Ehlers1908 comprises seven valid species; the majority of them are poorly known and need to be re-described. Cirrophorus branchiatus Ehlers, Reference Ehlers1908, which includes Cirrophorus lyriformis (Annenkova, Reference Annenkova1934) as a synonym (Strelzov, Reference Strelzov1973), and Cirrophorus aciculatus (Hartman, Reference Hartman1957) can be easily distinguished due to the presence of thick notopodial acicular chaetae and branchiae starting at chaetiger 5. The remaining species are characterized by the presence of a median antenna in adults (stated to be absent in juveniles or small specimens of Cirrophorus americanus Strelzov, Reference Strelzov1973, and Cirrophorus brevicirratus Strelzov, Reference Strelzov1973), three pre-branchial chaetigers and lyrate notopodial chaetae (Table 2). Cirrophorus nikebianchii sp. nov. clearly differs from C. brevicirratus Strelzov, Reference Strelzov1973 in the shape of the prostomium (triangular and elongated in C. brevicirratus, trapezoidal in C. nikebianchii), the segment of appearance of lyrate chaetae (chaetiger 6 in C. brevicirratus, chaetiger 2–3 in C. nikebianchii) and the number of branchiae (up to 14–15 pairs in C. brevicirratus, against usual 40–65 pairs in the new species). Cirrophorus americanus Strelzov, Reference Strelzov1973, Cirrophorus furcatus (Hartman, Reference Hartman1957), Cirrophorus longifurcatus (Hartmann-Schröder, Reference Hartmann-Schröder, Hartmann-Schröder and Hartmann1965) and Cirrophorus miyakoensis Imajima, Reference Imajima1973 are closer to Cirrophorus nikebianchii sp. nov. as regards the shape of the prostomium, but they show some differences, in particular in the shape and size of the antenna. The description of C. americanus is ambiguous and could refer to two different species, as the holotype shows the presence of thickened neuropodial chaetae in the posterior chaetigers and lacks the median antenna, whereas the remaining examined material has a well-developed median antenna and lacks modified neuropodial chaetae. Provisionally accepting that the holotype and the remaining material are conspecific, C. nikebianchii sp. nov. differs from C. americanus in the size of the median antenna (short, blister-like in C. nikebianchii, vs cirriform and ~1/3 of the prostomium length in C. americanus) and in the higher number of branchiae (up to 46 pairs in C. americanus, against 40–65 pairs in the new species). Moreover, in C. americanus notopodial lobes in the pre-branchial region are of the same size, while in C. nikebianchii sp. nov. they gradually increase in size. As with the new species, C. furcatus has lyrate chaetae from the 3rd chaetiger, notopodial lobes of increasing size in the pre-branchial region and a short median antenna; nevertheless, this species shows a remarkably larger size (up to 1 mm), a distinctly lower number of branchiae (up to 33 pairs), a slender and longer cirriform antenna, and neuropodial thickened chaetae are absent. In addition, in C. furcatus the size of the notopodial lobes decreases towards the pygidium (Blake, Reference Blake, Blake, Hilbig and Scott1996), whereas it increases in C. nikebianchii sp. nov. Cirrophorus longifurcatus, a species known from Chile, and rarely reported after the original description, has a long prostomial antenna, almost reaching the 2nd chaetiger (Hartmann-Schröder, Reference Hartmann-Schröder, Hartmann-Schröder and Hartmann1965), whereas it is very short, blister-like in the new species, and there is a large difference in length between the notopodial lobes in the pre-branchial and branchial region, compared with similar sized in C. nikebianchii sp. nov. Lastly, C. miyakoensis is similar to C. nikebianchii sp. nov., but the median antenna and notopodial lobes are distinctly slender, cirriform, and the size of the notopodial lobes decreases towards the pygidium (whereas it increases in C. nikebianchii sp. nov.). The Japanese species also shows a lower number of branchiae (up to 42 pairs for 0.8 mm maximum width). Another Mediterranean morphotype differs from C. nikebianchii sp. nov. mainly in the number of branchiae (usually 15–22 pairs) and in the pattern of notopodial lobes, that become very short in the posterior part of the body (whereas in C. nikebianchii they remain long and slender). This morphotype has been collected until now only in a marine organically enriched environment and morphologically corresponds to Cirrophorus lyriformis sensu Laubier & Ramos (Reference Laubier and Ramos1974) and to Cirrophorus furcatus sensu Katzmann & Laubier (Reference Katzmann and Laubier1975). Since molecular data did not support its assignment to Cirrophorus nikebianchii sp. nov., and given the scarce material available, we provisionally consider it as a putative new species, Cirrophorus sp. B.
Table 2. Comparison among species of Cirrophorus Ehlers, Reference Ehlers1908 with lyrate chaetae, highlighting the main differences in the most important taxonomic characters.

a Based on the debatable assumption that all material described by Strelzov (Reference Strelzov1973) should be referred to the same species.
b Corresponding to Cirrophorus cf. lyriformis sensu Laubier & Ramos (Reference Laubier and Ramos1974) and Cirrophorus furcatus sensu Katzmann & Laubier (Reference Katzmann and Laubier1975).
Within the genus Cirrophorus, C. nikebianchii sp. nov. can be easily identified based on the extremely small size of the median antenna, the relatively small body size (maximum width = 0.5 mm) and the high number of branchiae (up to more than 70 in large individuals, and an average number of 40–65). The ratio between the number of branchiae and maximum width of the animal is similar for C. americanus, C. furcatus, C. miyakoensis and Cirrophorus sp. B, but it is clearly different in C. nikebianchii sp. nov. (Figure 2). Finally, the occurrence in brackish-water environments at very shallow depths seems to be a peculiarity of this species.
Two species of Paradoneis, namely Paradoneis eliasoni Mackie, Reference Mackie, Petersen and Kirkegaard1991 and Paradoneis strelzovi de Léon-González & Díaz-Castañeda, Reference de Léon-González and Díaz-Castañeda2011, resemble C. nikebianchii sp. nov. in possessing both notopodial lyrate and neuropodial thickened chaetae. However, in both species there are only single (rather than multiple) thickened chaetae in each posterior neuropodium. In addition, both completely lack a median antenna, and have much fewer branchiae (7–12 and 6–7 pairs) than the new species. Paradoneis eliasoni and C. nikebianchii sp. nov., both have long anal cirri, but the former has tuberculate (rather than slender projecting) pre-branchial notopodial lobes. Paradoneis strelzovi differs markedly from the new species in having very short anal cirri.
MOLECULAR PHYLOGENETIC ANALYSIS
We obtained sequences of 471 bp for 16S rDNA (GenBank accession numbers: KX901418 to KX901433), 670 bp for COI (GenBank accession numbers: KX901434 to KX901446), and 1790 bp for 18S rDNA (GenBank accession numbers: KX901405 to KX901417). The best fitting nucleotide substitution models were GTR + G for 16S rDNA, and GTR + I + G for COI and 18S rDNA. The tree showed the presence of a well supported clade (clade I: posterior probability, PP = 1) with two separated lineages that corresponded morphologically to Paradoneis ilvana, a third one relative to Paradoneis armata (PP = 1), and a fourth with Cirrophorus sp. B (Figure 4). Another well supported clade (clade II: PP = 1) included Paradoneis lyra, C. furcatus and C. nikebianchii sp. nov. (Figure 4). Paradoneis lyra individuals, in their turn, grouped in a strongly supported clade (PP = 1) as well as C. furcatus and C. nikebianchii (PP = 1). Although C. furcatus and C. nikebianchii are very close and represent a well supported clade, the divergence between the two lineages is comparable to that observed between different species, thus supporting the distinction at species level. In a third clade Paraonis fulgens, and Aricidea fragilis grouped with high statistical support (clade III: PP = 1). Levinsenia gracilis represents the sister group of all remaining Paraonidae with high statistical support (PP = 1). The position of C. branchiatus, and the relationships among the three clades, are however not resolved in our reconstruction (Figure 4).

Fig. 4. Bayesian tree obtained from the concatenated 16S rDNA, 18S rDNA and COI sequences. Node values are Bayesian posterior probabilities; only statistically significant values are reported.
DISCUSSION
According to our results, the doubts on the identity of the Mediterranean individuals identified as Cirrophorus nr furcatus (Castelli et al., Reference Castelli, Abbiati, Badalamenti, Bianchi, Cantone, Gambi, Giangrande, Gravina, Lanera, Lardicci, Somaschini, Sordino, Minelli, Ruffo and La Posta1995) were well founded, since both morphological and molecular data supported the distinction between Cirrophorus nikebianchii sp. nov. and Cirrophorus furcatus. It is noteworthy, however, that not all Mediterranean reports of C. furcatus can be referred to C. nikebianchii; in particular, the descriptions of individuals referred to C. furcatus (or to C. lyriformis) in fully marine environments (Laubier, Reference Laubier1966; Laubier & Ramos, Reference Laubier and Ramos1974; Katzmann & Laubier, Reference Katzmann and Laubier1975) are morphologically different from C. nikebianchii, mainly as regards the number of pairs of branchiae (11–27 vs 24–75, respectively) and the length of the antenna. These individuals appear more similar to Cirrophorus sp. B. Unfortunately, the material available for this last putative species is scarce and not sufficient for a formal species description. On the other hand, the extremely small size of the median antenna led several authors to misidentify C. nikebianchii sp. nov. as Paradoneis lyra. However, P. lyra lacks thickened neuropodial chaetae (Mackie, Reference Mackie, Petersen and Kirkegaard1991). Such chaetae are present in P. eliasoni from northern European waters, but they are single, not multiple, and this species has not been found in the Mediterranean as yet. Shallow-water individuals, and in particular brackish-water records of P. lyra in the Mediterranean Sea probably can all be referred to C. nikebianchii. Paradoneis lyra is probably less widespread in the Mediterranean than commonly stated, and its distribution could be restricted to circalittoral and epibathyal bottoms.
The distinction between Cirrophorus Ehlers, Reference Ehlers1908 and Paradoneis Hartman, Reference Hartman1965, based on the presence of a median antenna in the former genus and absence in the latter, was questioned by Strelzov (Reference Strelzov1968, Reference Strelzov1973), who observed that in some species, such as Cirrophorus americanus and Cirrophorus brevicirratus, the antenna is present only in large adults, and absent in juveniles. The proposal of a synonymy between Cirrophorus and Paradoneis, however, was rejected by most of the subsequent authors (e.g. Laubier & Ramos, Reference Laubier and Ramos1974; Katzmann & Laubier, Reference Katzmann and Laubier1975; Mackie, Reference Mackie, Petersen and Kirkegaard1991; Blake, Reference Blake, Blake, Hilbig and Scott1996, Reference Blake, Westheide and Purschke2016; Aguirrezabalaga & Gil, Reference Aguirrezabalaga and Gil2009). Recently, Reuscher (Reference Reuscher and Reuscher2013) carried out a morphological cladistic analysis of Paraonidae and identified a clade including all species of Paradoneis and Cirrophorus. This cladistic analysis highlighted that the median antenna characteristic of Cirrophorus was acquired and lost several times in the evolutionary history of the clade and, therefore, it could not be considered a useful taxonomic character. In Reuscher's (Reference Reuscher and Reuscher2013) morphologically based phylogenetic reconstruction, the species with notopodial spines, Paradoneis spinifera (Hobson, Reference Hobson1972) and Paradoneis drachi Laubier & Ramos, Reference Laubier and Ramos1974, were the sister group of the remaining Cirrophorus/Paradoneis clade, and Reuscher (Reference Reuscher and Reuscher2013) assigned these two species to a new genus yet to be described. Species without notopodial modified chaetae and median antenna were assigned to the genus Paraonides Cerruti, Reference Cerruti1909 and are basal to the whole Cirrophorus/Paradoneis clade. Molecular data from our work did not support this reconstruction, and the tree obtained showed a very different topology, though the ‘Paradoneis-Cirrophorus’ clades in each analysis contained taxa with and without median antennae. Doubts about the reliability of the median antenna as a defining character led Strelzov (Reference Strelzov1973) to synonymize Paradoneis with Cirrophorus, and Reuscher (Reference Reuscher and Reuscher2013) came to the same conclusion following his analyses. In our analysis, species assigned to the genera Cirrophorus and Paradoneis were distributed in two different clades with high statistical support: the first clade includes C. furcatus, C. nikebianchii and P. lyra and the second clade includes Paradoneis armata Glémarec, Reference Glémarec1966, two strongly divergent lineages that were morphologically assigned to Paradoneis ilvana Castelli, Reference Castelli1985, and Cirrophorus sp. B, this last probably representing an undescribed species. Cirrophorus branchiatus Ehlers, Reference Ehlers1908 does not belong to either of the clades. Therefore the Cirrophorus/Paradoneis group was paraphyletic and the relationship between all the clades in our tree was unresolved.
Incongruence between molecular and morphological data has been observed in a number of polychaete studies (e.g. Bleidorn, Reference Bleidorn2005; Zanol et al., Reference Zanol, Halanych and Fauchald2014). Even though the results of the molecular phylogenetic reconstruction applied to the Cirrophorus/Paradoneis group were quite striking and statistically well-supported, we prefer to adopt a conservative approach, awaiting for a more complete phylogenetic reconstruction involving more taxa, and preferably based on both molecular and morphological data. However, some nomenclatural notes on the genera Cirrophorus, Paradoneis and Paraonides are in our opinion useful in anticipation of a necessary taxonomic revision of the family Paraonidae.
The genus Cirrophorus Ehlers, Reference Ehlers1908 was created for C. branchiatus, and subsequently considered as a subgenus of Aricidea Webster, Reference Webster1879, which also had a median antenna (Hartman, Reference Hartman1957). This taxonomic arrangement was questioned by Day (Reference Day1963) and Laubier (Reference Laubier1966) and lastly rejected by Strelzov (Reference Strelzov1968), who stressed the similarity between Cirrophorus and Paradoneis. On the other hand, Paradoneis Hartman, Reference Hartman1965 was created for species lacking median antenna and with notopodial modified chaetae, with Paraonis (Paraonides) lyra Southern, Reference Southern1914 as type species. The genus Paraonides Cerruti, Reference Cerruti1909, created as subgenus of Paraonis Cerruti, Reference Cerruti1909 with Paraonis (Paraonides) neapolitana Cerruti, Reference Cerruti1909 as type species, was initially used for species with notopodial modified chaetae (Southern, Reference Southern1914; Fauvel, Reference Fauvel1927), but its diagnosis was subsequently emended (Hartman, Reference Hartman1965) and this genus was used to define species without modified chaetae. The question of the correct use of Paraonides has been long debated, and the main problem regarding this issue is the uncertain identity of the type species. In fact, P. neapolitana was described for the Gulf of Naples as a species without median antenna, with three pre-branchial chaetigers, nine pairs of branchiae and modified notopodial chaetae from chaetiger 12 (Cerruti, Reference Cerruti1909). The notopodial modified chaetae were apparently shorter than the capillaries, somewhat thicker and leaf-shaped, even though this kind of modified chaetae has never been reported in later descriptions of Paraonidae. Strelzov (Reference Strelzov1973) demonstrated that leaf-shaped chaetae could be an artefact of the fixation of lyrate chaetae in Canada balsam, since balsam and chaetae have a very similar refraction index that could prevent a clear distinction of the two branches of the lyrate chaeta. For this reason, Strelzov (Reference Strelzov1973) considered Paraonides a synonym of Cirrophorus and Paradoneis. Since the type material is lost (Castelli, Reference Castelli1987), Strelzov (Reference Strelzov1973) re-described Cirrophorus neapolitanus on the basis of North and South Atlantic, and Black Sea individuals; an act considered highly questionable without the examination of topotypic material (Katzmann & Laubier, Reference Katzmann and Laubier1975). The rejection of this re-description maintained Paraonides as diagnosed by Hartman & Fauchald (Reference Hartman and Fauchald1971), even with the possibility that Strelzov's (Reference Strelzov1973) remarks on the peculiar chaetae of P. neapolitana were correct. Topotypic material in good condition would be necessary to properly clarify the identity of P. neapolitana. It may be noted, however, that this species has never been re-described in any taxonomic work from the Mediterranean (Laubier & Ramos, Reference Laubier and Ramos1974; Katzmann & Laubier, Reference Katzmann and Laubier1975; Castelli, Reference Castelli1985; Çinar et al., Reference Çinar, Dağli and Açik2011; Çinar & Dağli, Reference Çinar and Dağli2013). In recent years this species has been cited only in species checklists of soft bottom ecology works (Gambi et al., Reference Gambi, Conti and Bremec1998; Simonini et al., Reference Simonini, Ansaloni, Bonini, Grandi, Graziosi, Iotti, Massamba N’Siala, Mauri, Montanari, Preti, De Nigris and Prevedelli2007; De Biasi & Pacciardi, Reference De Biasi and Pacciardi2008). Thus, it is likely that P. neapolitana is actually a species with lyrate chaetae, and that the genus Paraonides is not suitable for species lacking of modified notopodial chaetae. Because of the unclear identity of the type species of Paraonides, we suggest to precautionarily use Paraonella Strelzov, Reference Strelzov1973 for species lacking both notopodial and neuropodial modified chaetae and prostomial antenna, although we recognize that this group may turn out to be artificial, since the loss of modified chaetae could have happened several times in the evolutionary history of Paraonidae.
The paraphyletic condition of the Cirrophorus/Paradoneis group makes taxonomic revisions challenging. In our analysis, the type species of Cirrophorus was not included in any of the two highly supported clades and its relationships with the other groups within Paraonidae were unclear. Moreover, the uncertainty about the identity of Paraonides neapolitana does not allow us to settle the question of its relationships or possible synonymy with Paradoneis. As observed in other polychaete families, morphological traits of the family Paraonidae may be misleading with regard to the actual evolutionary history of the group, and their evolutionary meaning should be, therefore, critically evaluated. An important contribution to Paraonidae systematics can be provided by molecular tools alongside critical morphological investigations in order to obtain a more sound classification of this taxonomically complex family.
ACKNOWLEDGEMENTS
We would like to thank F. Aguirrezabalaga, G. Benedettini, O. Bresciani, T. Darbyshire, A. Giangrande, A.S.Y. Mackie, D. Martin, A. M. Pastorelli, A. Pavia and K. Vasileiadou for providing useful material, both for the morphological and the molecular works; M. Casu, A.M. De Biasi, I. Guarneri, M. Oliva, L. Pacciardi, M. Pertusati, E. Pollonara, C. Pretti, F. Scarpa, M. Sigovini, D. Tagliapietra and A. Vannucci for their invaluable help in sampling Mediterranean Paraonidae from several environments; G. Di Giuseppe, F. Erra, G. Montesanto and F. Verni for their technical support in microscope measurements and scientific drawing; T. Ravaglia and F. Squarcia for their help in molecular laboratory work; J.A. Blake and K. Meißner for providing literature; and two anonymous referees for their valuable comments and corrections.








