Introduction
The caridean family Alvinocarididae is endemic to chemosynthetic environments in deep water (252–4960 m depth), and is currently represented by 36 species in nine genera, Alvinocaridinides Komai & Chan, Reference Komai and Chan2010, Alvinocaris Williams & Chace, Reference Williams and Chace1982, Keldyshicaris Vereshchaka, Kulagin & Lunina, Reference Vereshchaka, Kulagin and Lunina2015, Manuscaris Komai & Tsuchida, Reference Komai and Tsuchida2015, Mirocaris Vereshchaka, Reference Vereshchaka1997, Nautilocaris Komai & Segonzac, Reference Komai and Segonzac2004, Opaepele Williams & Dobbs, Reference Williams and Dobbs1995, Rimicaris Williams & Rona, Reference Williams and Rona1986, and Shinkaicaris Komai & Segonzac, Reference Komai and Segonzac2005 (Komai & Tsuchida, Reference Komai and Tsuchida2015; Vereshchaka et al., Reference Vereshchaka, Kulagin and Lunina2015; Komai et al., Reference Komai, Menot and Segonzac2016; Wang & Sha, Reference Wang and Sha2016, Reference Wang and Sha2017; Martin et al., Reference Martin, Wall, Shank, Cha, Seid and Rouse2018; Komai & Giguère, Reference Komai and Giguère2019). Vereshchaka et al. (Reference Vereshchaka, Kulagin and Lunina2015) proposed to recognize three subfamilies to reflect the phylogenetic pattern inferred by them, Alvinocaridinae (Alvinocaris), Mirocaridinae (Mirocaris and Nautilocaris) and Rimicaridinae (Alvinocaridinides, Manuscaris, Opaepele, Rimicaris and Shinkaicaris). Alvinocaris, which contains 16 species from the Atlantic, Pacific and Indian Oceans (Martin et al., Reference Martin, Wall, Shank, Cha, Seid and Rouse2018) is the most speciose genus of Alvinocarididae, and the discovery of new species is still continuing. These include: A. alexander Ahyong, Reference Ahyong2009, A. brevitelsonis Kikuchi & Hashimoto, Reference Kikuchi and Hashimoto2000, A. chelys Komai & Chan, Reference Komai and Chan2010, A. costaricensis Martin, Wall, Shank, Cha, Seid & Rouse, Reference Martin, Wall, Shank, Cha, Seid and Rouse2018, A. dissimilis Komai & Segonzac, Reference Komai and Segonzac2005, A. komaii Zelnio & Houdez, Reference Zelnio and Hourdez2009, A. longirostris Kikuchi & Ohta, Reference Kikuchi and Ohta1995, A. lusca Williams & Chace, Reference Williams and Chace1982 (type species), A. markensis Williams, Reference Williams1988, A. kexueae Wang & Sha, Reference Wang and Sha2017, A. methanophila Komai, Shank & Van Dover, Reference Komai, Shank and Van Dover2005, A. muricola Williams, Reference Williams1988, A. niwa Webber, Reference Webber2004, A. solitaire Yahagi, Watanabe, Kojima, Beedessee & Komai, Reference Yahagi, Watanabe, Kojima, Beedessee and Komai2014, A. stactophila Williams, Reference Williams1988 and A. williamsi Shank & Martin, Reference Shank and Martin2003. Four of these 16 species are known from the north-western Pacific: A. brevitelsonis, known only from hydrothermal vent on Minami-Ensei Knoll, Okinawa Trough and NW Eifuku Seamount in the Marian Arc (Komai & Segonzac, Reference Komai and Segonzac2005; Tsuchida et al., Reference Tsuchida, Yamaguchi, Komai, Watanabe, Fujikura, Okutani and Maruyama2012; Yahagi et al., Reference Yahagi, Watanabe, Ishibashi and Kojima2015); A. chelys, known only from hydrothermal vents offshore north-eastern Taiwan (Komai & Chan, Reference Komai and Chan2010); A. dissimilis, known only from hydrothermal vents on Minami-Ensei Knoll (Komai & Segonzac, Reference Komai and Segonzac2005; Yahagi et al., Reference Yahagi, Watanabe, Ishibashi and Kojima2015), and A. longirostris, known from hydrothermal vents of the Okinawa Trough and cold seep sites in Sagami Bay, Japan, and in the South China Sea (Komai & Segonzac, Reference Komai and Segonzac2005; Li, Reference Li2015; Yahagi et al., Reference Yahagi, Watanabe, Ishibashi and Kojima2015).
In this study, we describe a new species of Alvinocaris, A. marimonte sp. nov., from the Izu-Bonin and Mariana Arcs, on the basis of specimens collected from the Myojin Knoll in the Izu-Bonin Arc, and the NW Eifuku and NW Rota Seamounts in the Mariana Arc, at depths of 525–1580 m. Our identification of this new species is supported by both morphological comparisons and molecular genetic analyses based on partial sequences of mitochondrial COI and nuclear ITS-I genes. The new species that we report here represents the 17th species of the genus Alvinocaris.
Materials and methods
Specimens collection
Specimens were collected over the course of three dives of ROV ‘Hyper-Dolphin’ (RV ‘Natsuhima’): #1493 (16 March 2013) at Myojin Knoll (32°06.2′N 139°53.1′E, 1224 m depth); #492 (27 October 2005), #494 (29 October 2005) at NW Eifuku Seamount (21°29.2N 144°02.5′E, 1574–1582 m depth); and #1161 (26 July 2010) at NW Rota Seamount (14°36.0′N 144°46.5′E, 525 m depth) (Figure 1). Specimens were collected using a slurp gun and multiple canister bottles loaded on the ROV ‘Hyper-Dolphin’, and preserved in either 75 or 99.5% ethanol. Postorbital carapace length (CL) was used as a measurement of the size of specimens, and the morphological terminology employed in species description generally follows Komai & Segonzac (Reference Komai and Segonzac2005).

Fig. 1. Map showing collection sites in the Izu-Bonin and Mariana Arcs in the North-west Pacific. Solid circles showed the hydrothermal vent fields: MK, Myojin Knoll; NWE, North-west Eifuku Seamount; NWR, North-west Rota Seamount.
Genetic analyses
For the genetic analyses, seven specimens (two from Myojin Knoll, four from NW Eifuku Seamount, and one from NW Rota Seamount) were used.
DNA was extracted from the pleon muscle using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). The partial sequences of the mitochondrial cytochrome oxidase subunit I (COI), and of the nuclear intergenic spacer region I (ITS-I) were amplified through polymerase chain reactions (PCR), using Ex Taq polymerase (TAKARA, Tokyo, Japan), with the universal primers LCO1490 (GGT CAA CAA ATC ATA AAG ATA TTG G; Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) and HCO2198 (TAA ACT TCA GGG TGA CCA AAA AAT CA; Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) for COI, and SP-1–3′ (ATT TAG CTG CGG TCT TCA TC; Chu et al., Reference Chu, Li and Ho2001) and SP-1–5′ 138 (CAC ACC GCC CGT CGC TAC TA; Chu et al., Reference Chu, Li and Ho2001) for ITS-1.
The amplification mixture (25 µl) contained 2.5 µl of 10× buffer reagent (100 mM Tris-HCl with a pH of 8.3, 500 mM KCL and 15 mM MgCl2 bovine serum albumin), 2.0 µl of dNTP, 1.0 µl of each of the primers, 0.125 µl of Taq DNA polymerase, 1.0 µl of template DNA, and sterile H2O to make up the balance volume. Thermal cycling conditions were 96.0 °C for 60 s, followed by 35 cycles of 96.0 °C for 30 s/48.0 °C for 30 s/72.0 °C for 60 s, and a final extension of 72.0 °C for 7 min. Sequencing was conducted using an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems Japan Ltd, Tokyo, Japan). The sequence strands for each gene were proofread and assembled using Codon Code Aligner version 3.7 (Codon Code Corporation, Dedham, MA, USA). In order to estimate the degree of similarity between the sequences of our specimens and other sequences deposited in GenBank, partial sequences of both genes were compared using the gapped BLAST search algorithm.
Maximum-likelihood (ML) and Neighbour-joining (NJ) trees using COI sequences were constructed with 11 species of Alvinocaris, and with Mirocaris fortunata (Martin & Christiansen, Reference Martin and Christiansen1995) as an outgroup, using MEGA7 (Kumar et al., Reference Kumar, Stecher and Tamura2016). The nucleotide divergences of COI and ITS-I were calculated based on the Kimura two-parameter (K2P) distance model (Kimura, Reference Kimura1980). Bootstrap values, for both ML and Neighbour-joining (NJ), were calculated from 1000 iterations.
The GenBank accession numbers for the partial sequences examined in this study are LC481565–LC481570, AB779505 (for COI) and LC481899–LC481904, AB914466 (for ITS-I).
Results
Species description
Systematic
Order DECAPODA Latreille, 1802
Infraorder CARIDEA Dana, 1852
Family ALVINOCARIDIDAE Christoffersen, 1986
Genus Alvinocaris Williams & Chace, Reference Williams and Chace1982
Alvinocaris marimonte sp. nov.
(Figures 2–7)
Alvinocaris brevitelsonis—Tsuchida et al., Reference Tsuchida, Yamaguchi, Komai, Watanabe, Fujikura, Okutani and Maruyama2012: 188, figure 11.15

Fig. 2. Alvinocaris marimonte sp. nov., paratype, female (CL 7.0 mm), JAMSTEC 2050041227, entire animal in lateral view. Scale bar = 5.0 mm.

Fig. 3. Alvinocaris marimonte sp. nov., holotype, female (CL 9.5 mm), JAMSTEC 1130036387: (A) anterior part of carapace and cephalic appendages, lateral view; (B) same, dorsal view; (C) second to sixth pleomeres, lateral view; (D) left antennal scale, dorsal view (marginal setae omitted). Scale bars = 1 mm.
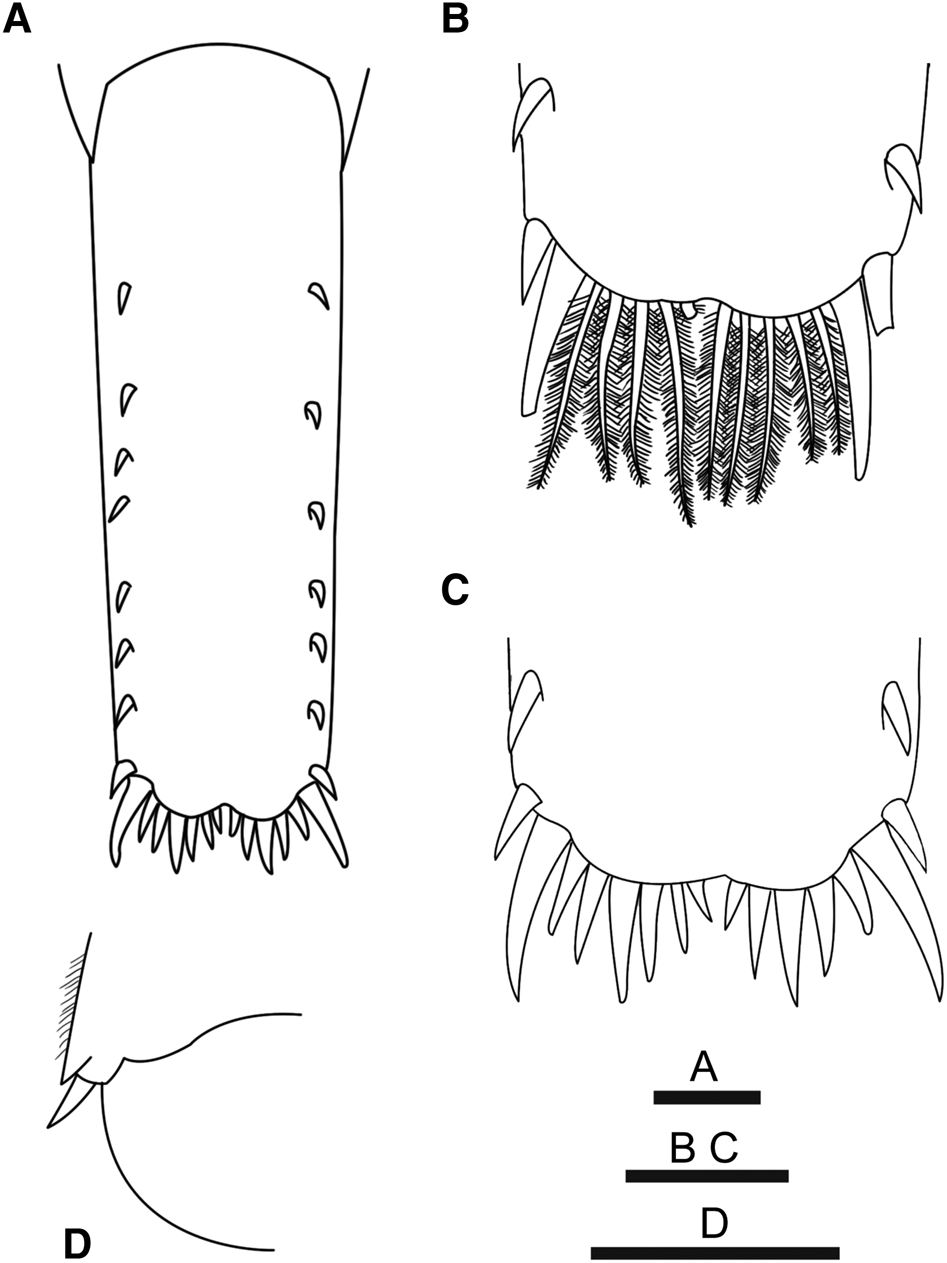
Fig. 4. Alvinocaris marimonte sp. nov. A, C, holotype female (CL 9.5 mm), JAMSTEC 1130036387, B, paratype, female (CL 7.0 mm), JAMSTEC 2050041227: (A) telson, dorsal view; (B, C) posterior margin of telson, dorsal view; (D) posterolateral part of left uropodal exopod, dorsal view. Scale bars = 1 mm.

Fig. 5. Alvinocaris marimonte sp. nov., paratype, male (CL 10.1 mm), JAMSTEC 2050041229: (A) left mandible, inner view; (B) right maxillule, outer view; (C) left maxilla, outer view; (D) left first maxilliped, outer view; (E) left second maxilliped, outer view; (E’) same, epipod and podobranch, inner view. Scale bars: A–B = 0.5 mm, C–E, E’ = 1 mm.
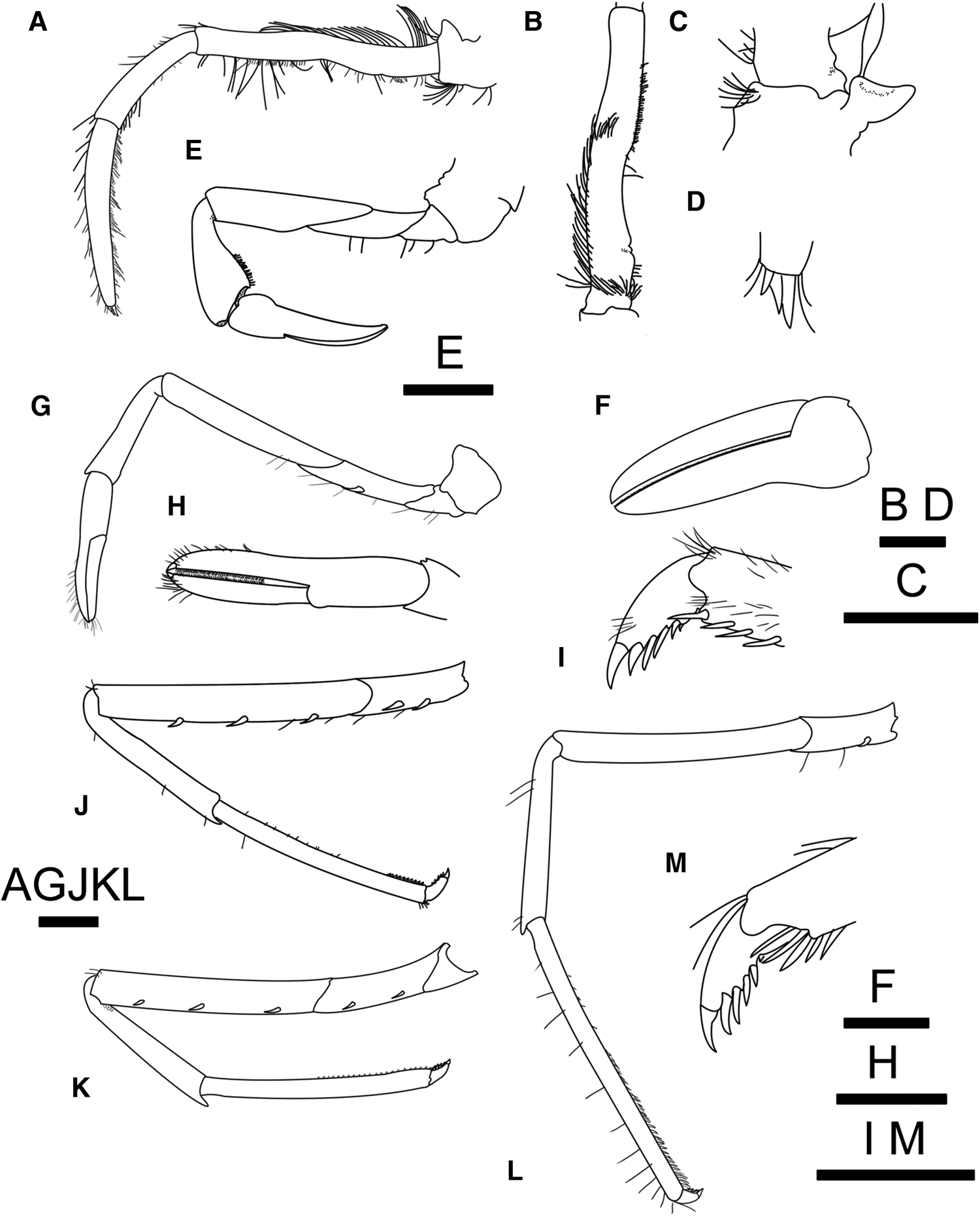
Fig. 6. Alvinocaris marimonte sp. nov., holotype, female (CL 9.5 mm), JAMSTEC 1130036387: (A) left third maxilliped, lateral view; (B) same, antepenultimate article, dorsomesial view; (C) same, coxa and epipod, ventral view; (D) same, distal part of ultimate article, lateral view; (E) left first pereopod, lateral view; (F) same, chela, mesial view; (G) left second pereopod, lateral view; (H) same, chela, extensor view; (I) left third pereopod, lateral view; (J) same, dactylus, lateral view; (K) left fourth pereopod, lateral view; (L) left fifth pereopod, lateral view; (M) same, dactylus and distal part of propodus. Scale bars = 1 mm.

Fig. 7. Alvinocaris marimonte sp. nov. A, paratype, female (CL 7.0 mm), JAMSTEC 2050041227; B, C, D, paratype, male (CL 6.8 mm), JAMSTEC 2050041232 : (A, D) carapace and cephalic appendages, lateral view; (B) endopod of left first pleopod, ventral view; (C) appendices interna and masculina of left second pleopod, dorsomesial view. Scale bars = 1 mm.
Alvinocaris sp. —Yahagi et al., Reference Yahagi, Watanabe, Ishibashi and Kojima2015
Type material
Holotype: female, CL 9.5 mm, Myojin Knoll, Izu-Bonin Arc, 32°06.2′N 139°53.1′E, 1224 m depth ROV ‘Hyper-Dolphin’ #1493, 16 March 2013 (JAMSTEC 1130036387), GenBank accession numbers LC481565 (for COI) and LC481899 (for ITS-I).
Paratypes: one female, CL 9.0 mm, same data as holotype (JAMSTEC 1130041225), GenBank accession numbers LC481566 (for COI) and LC481900 (for ITS-I). Two males, CL 6.0, 6.8 mm, four females, CL 7.0–10.1 mm, one ovigerous female, CL 12.0 mm, NW Eifuku Seamount, Mariana Arc, 21°29.2′N 144°02.5′E, 1582 m depth, ROV ‘Hyper-Dolphin’ #494, 29 October 2005 (JAMSTEC 2050041226–2050041232). One female CL 7.2 mm, NW Rota Seamount, Mariana Arc, 14°36.0′N 144°46.5′E, 525 m depth, ROV ‘Hyper-Dolphin’ #1161, 26 July 2010 (JAMSTEC 1100023686). Accession numbers AB779505 (for COI) and AB914473 (for ITS-I).
Non-type: three females, CL 10.0–10.1 mm, one ovigerous female, CL 10.2 mm, NW Eifuku Seamount, Mariana Arc, 21°29.2′N 144°02.5′E, 1574 m depth, ROV ‘Hyper-Dolphin’ #492, 27 October 2005 (JAMSTEC061133–061136), GenBank accession numbers LC481567– LC481565 (for COI) and LC481901– LC481904 (for ITS-I).
Description. Body (Figure 2) moderately robust; integument thin and soft, but not membranous. Rostrum (Figures 3A, C & 7A, D) slightly descending or directed forward, reaching mid-length to distal margin of second article of antennular peduncle (0.38–0.60 times as long as carapace); dorsal margin armed with 13–17 moderately small teeth, including 5–7 postrostral teeth, posterior-most tooth arising at 0.23–0.39 of carapace length; ventral margin with 0–4 small teeth; ventrolateral margin sharply carinate, merging into orbital margin. Carapace (Figures 3A, C & 7A, C) 1.1–1.5 times longer than wide; postrostral median ridge moderately high, sloping anteriorly to rostrum; dorsal angle 160–165°; antennal tooth moderately strong, acuminate; pterygostomial tooth moderately produced anteriorly, extending as far as or slightly beyond antennal tooth; anterolateral margin between antennal and pterygostomial teeth concave; post-antennal groove shallow; branchial region moderately convex.
Pleon (Figure 3B) rounded dorsally; pleura of anterior 2 pleomeres broadly rounded; third pleuron with 0–8 minute marginal teeth, fourth pleuron with 4–8 minute marginal teeth posteriorly; fifth pleuron with 1 strong posteroventral tooth and 1–3 additional teeth on posterolateral margin; sixth pleomere 1.20–1.53 times longer than high.
Telson (Figure 5A) not reaching posterior margin of uropodal endopod, with lateral margins almost parallel; length 2.73–3.18 times anterior width and 4.25–5.25 times posterior width; armed with 6–8 dorsolateral spines, arranged in linear row, on either side; posterior margin (Figure 4B, C) usually weakly bilobed with shallow median notch, armed with 2 lateral unequal spines (inner pair longer than outer pair), and with row of 10–11 spines and 0–2 median plumose setae (specimens from Myojin Knoll) or with row of 10–15 plumose setae (all longer than inner pair of lateral spines) (specimens from NW Eifuku and NW Rota seamounts).
Eyes (Figure 3A, C) incompletely fused mesially, each with small tubercle on anterodorsal surface; cornea unfaceted.
Antennular peduncle (Figure 3A, C) reaching or slightly overreaching distal margin of antennal scale. First article with strong distolateral tooth, not reaching mid-length of second article; dorsal surface with distinct, obliquely longitudinal groove; stylocerite sharp, usually reaching (rarely overreaching) mid-length of second article. Second article 1.61–1.84 times as long as wide, with small distomesial spine. Lateral flagellum sub-equal in length to carapace; mesial flagellum longer than lateral flagellum.
Antenna (Figure 3A) with stout basicerite bearing moderately strong ventrolateral and weak ventral teeth. Carpocerite stout, not reaching mid-length of antennal scale. Antennal scale (Figure 3D) 1.61–2.13 times longer than wide, 0.40–0.50 length of carapace; lateral margin nearly straight; distolateral tooth directed anteriorly, falling short of broadly rounded distal margin of blade.
Mandible (Figure 5A), maxillule (Figure 5B) and maxilla (Figure 5C) typical of genus, without distinctive features. First maxilliped (Figure 5D) with exopod broad, with weak mesial projection probably representing reduced flagellum. Second maxilliped (Figure 5E) moderately stout; epipod with simple rod-like podobranch (Figure 5E). Third maxilliped (Figure 6A) usually overreaching antennal scale by 0.3–0.5 times length of ultimate article; ultimate article almost twice length of penultimate article, trigonal in cross section, subtruncate terminal margin with 3 or 4 small movable spines (Figure 6D); antepenultimate article flattened dorsoventrally, marginally setose (Figure 6B), ventrolateral distal angle with 1 slender spine; coxa stout; epipod directed laterally, slightly bi-lobed (Figure 6C).
First pereopod (Figure 6E, F) with fingers curved ventromesially; outer surface of both fingers convex, inner surface concave, cutting edges uniformly offset, closing without gap, each armed with row of closely set fine pectination; dactylus 2.0–4.0 times longer than palm, with longitudinal row of short setae on inner surface adjacent to cutting edge. Palm stout, length 0.57–0.97 of height. Carpus cupped distally to receive palm, with acute or subacute ventrodistal tooth; dorsodistal mesial margin rounded; ventromesial surface with grooming apparatus consisting of patch of dense setae and 1 minute spine. Merus and ischium unarmed, obliquely articulated.
Second pereopod (Figure 6G, H) shorter and more slender than first pereopod, not reaching distal margin of antennal scale. Fingers slightly longer than palm, each terminating in small corneous unguis crossing each other when closed; cutting edges without gap, each pectinated with a single row of small corneous teeth. Carpus subequal in length to chela. Merus and ischium obliquely articulated in lateral view. Ischium unarmed or armed with 1 spine ventrolaterally.
Third to fifth pereopods (Figure 6I–M) moderately slender, generally similar in structure and length although propodi becoming longer toward posterior, while meri becoming shorter. Dactyli (cf. Figure 6J) short (0.09–0.14 of propodal length in third, 0.10–0.12 in fourth, 0.08–0.11 in fifth), 1.92–2.55 times longer than high, each armed with 5–7 accessory spinules arranged in single row on flexor margin. Propodi of third and fourth pereopods with spinules arranged in 2 rows on flexor surface; propodus of fifth pereopod with numerous spinules arranged in 3 or 4 rows on distal half of ventral surface. Meri armed with 3 movable spines ventrolaterally in third, 2 or 3 spines or unarmed in fourth, unarmed in fifth. Ischia armed with 2 movable spines ventrolaterally in third, 1 or 2 spines or unarmed in fourth, and 1 spine or unarmed in fifth.
First pleopod in males with endopod (Figure 7B) about two-thirds length of exopod, distally bilobed; distomesial lobe with 2 or 3 spiniform setae directed mesially or distomesially on mesial margin; lateral margin with 8 or 9 stiff setae, and 10 or 11 setulose setae in proximal half. Appendix masculina on male second pleopod (Figure 7C) moderately robust, slightly shorter than appendix interna, with 6 or 7 spiniform setae terminally or subterminally. Uropod with exopod and endopod subequal in length, slightly overreaching telson; exopod with tiny posterolateral tooth and 1 small movable spine mesial to posterolateral tooth (Figure 4D).
Colouration in life: Body generally whitish translucent; carapace, tergites of anterior 4 pleomeres and first to fourth pleopods with tinge of pale orange (Figure 2).
Distribution: The present material was collected from Myojin Knoll (Izu-Bonin Arc, 1224 m depth), and NW Eifuku and NW Rota Seamounts (Mariana Arc; 525 m and 1574–1582 m depths, respectively). Yahagi et al. (Reference Yahagi, Watanabe, Ishibashi and Kojima2015) mentioned the occurrence of the new species (as Alvinocaris sp.) in the Suiyo Seamount in the Izu-Bonin Arc at a depth of 1387 m and Irabu Knoll in the Okinawa Trough at a depth of 1651 m.
Etymology: The specific epithet is the Latin ‘marimonte’ meaning ‘seamount’, referring to the occurrence of the new species in hydrothermal vents on seamounts.
Remarks: Tsuchida et al. (Reference Tsuchida, Yamaguchi, Komai, Watanabe, Fujikura, Okutani and Maruyama2012) recorded Alvinocaris brevitelsonis from the NW Eifuku Seamount at a depth of 1629 m. At present, however, only the occurrence of the present new species is confirmed from hydrothermal vents on the NW Eifuku Seamount, and thus it is likely that the previous record might actually represent A. marimonte sp. nov. As noted above, A. brevitelsonis is known with certainty only from the Minami-Ensei Knoll in the Okinawa Trough (Komai & Segonzac, Reference Komai and Segonzac2005). As mentioned above, Yahagi et al. (Reference Yahagi, Watanabe, Ishibashi and Kojima2015) mentioned the occurrence of the present new species in Suiyo Seamount, Izu-Bonin Arc and Irabu Knoll, Okinawa Trough, although we have had no opportunity to examine material from the Okinawa Trough.
Identity of the populations from Myojin Knoll, NW Eifuku and NW Rota Seamounts
Initially, it was thought that two species were represented in our collection from the Izu-Bonin and Mariana Arcs because the two morphs amongst our specimens differed in the ornamentation of the posterior margin of their telsons (spines vs plumose setae), a characteristic that is generally used to distinguish between species of Alvinocaris (cf. Kikuchi & Hashimoto, Reference Kikuchi and Hashimoto2000; Komai & Segonzac, Reference Komai and Segonzac2005). The first morph, represented by specimens from Myojin Knoll, has a row of spines on the posterior margin of the telson (Figure 4B), and the second morph, represented by specimens from NW Eifuku and NW Rota seamounts, has a row of plumose setae flanked by two unequal pairs of lateral spines on that margin (Figure 4C). It seemed that the variation was related to the geographic pattern. However, comparison of other morphological characters failed to detect any significant differences among the specimens of the two morphs. Furthermore, analyses based on partial sequences of the mitochondrial COI and nuclear ITS-I genes, representing molecular markers usually useful for species discrimination (Chu et al., Reference Chu, Li and Ho2001; Hebert et al., Reference Hebert, Cywinska, Ball and de Waard2003), showed that the two morphs could not be differentiated genetically (Tables 1 and 2): the genetic divergence between the two morphs was 0.0–1.1% (average: 0.5%) in COI (477 bp) and 0.0–0.5% (average: 0.2%) in ITS-I (552 bp), both included in ranges of the genetic divergence of specimens within respective morph. Although some examples of low genetic divergences between species in COI are known in Alvinocarididae (e.g. Vereshchaka et al., Reference Vereshchaka, Kulagin and Lunina2015), considering the evidence from both morphological comparison and genetic analyses using two markers, we came to a conclusion that the two morphs represent a single species exhibiting variation in the telson posterior margin ornamentation. Nevertheless, future study using more genetic markers may eventually reveal the significance of the variation in the telson ornamentation.
Table 1. K2P genetic divergence of the partial sequences of the mitochondrial COI gene among the nine individuals of A. marimonte sp. nov. Two sequences registered under the name Alvinocaris sp. (LCO029852, Suiyo Sea mount; and LCO029859, Irabu Knoll, Okinawa Trough) (Yahagi et al., Reference Yahagi, Watanabe, Ishibashi and Kojima2015) are also included in the analysis

MK, Myojin Knoll; NEW, North-west Eifuku Seamount; NWR, North-west Rota Seamount.
Table 2. K2P genetic divergence of the partial sequence of the nuclear DNA ITS-I gene among the seven individuals of A. marimonte sp. nov.

MK, Myojin Knoll; NEW, North-west Eifuku Seamount; NWR, North-west Rota Seamount.
Morphological comparison between Alvinocaris marimonte sp. nov. and other congeneric species
In general, species of Alvinocaris are morphologically very similar, and intraspecific variability often compromises the significance of supposed distinguishing characteristics (e.g. length and armature of the rostrum, development of the branchiostegal tooth of the carapace, and armature of the third to fifth pleura; cf. Komai & Segonzac, Reference Komai and Segonzac2005). The discovery of A. marimonte sp. nov. has revealed that the ornamentation of the posterior margin of the telson (spines vs plumose setae) can vary intraspecifically in species of Alvinocaris. Consequently, evaluation of the differentiating characters among species of the genus should be made very carefully.
Alvinocaris marimonte sp. nov. is characterized by (i) the moderately long rostrum, which does not overreach the distal margin of the second article of the antennular peduncle and is armed with 0–4 ventral teeth, (ii) the posterior-most tooth of the dorsal rostral series located at 0.23–0.39 of CL, (iii) the slightly elevated dorsum of the carapace even in adult females (the dorsal angle 160–165°), (iv) the weakly to moderately pronounced branchiostegal tooth of the carapace, (v) pleura of the third and fourth pleomeres which sometimes bear marginal denticles, (vi) the weakly bilobed posterior margin of the telson, (vii) the moderately stout second article of the antennular peduncle (1.6–1.8 times as long as wide), and (viii) the merus and ischium of the third pereopod armed with movable spines.
Below, comparison with other species presently assigned to Alvinocaris is given.
(1) Alvinocaris alexander is distinguished from A. marimonte sp. nov. by (i) the more strongly elevated dorsum of the carapace in females (the dorsal angle is ~145° in A. alexander), (ii) the more pronounced branchiostegal tooth, (iii) the convex posterior margin of the telson and (iv) the stouter second article of the antennular peduncle (length 1.25–1.31 times width) (cf. Ahyong, Reference Ahyong2009). Alvinocaris alexander is presently known only from the Brothers Caldera and Rumble V Seamount, Kermadec Ridge (south-western Pacific), at depths of 367–1346 m.
(2) Alvinocaris brevitelsonis is so far represented only by the holotype, and thus evaluation of intraspecific variation of the species is not possible. Nevertheless, A. brevitelsonis differs from the new species by (i) the relatively longer rostrum (reaching the distal margin of the second article of the antennular peduncle), (ii) more numerous ventral teeth of the rostrum (seven teeth are present), (iii) the more strongly elevated dorsum of the carapace in the adult female (dorsal angle of 150°) and (iv) the evenly convex posterior margin of the telson (Kikuchi & Hashimoto, Reference Kikuchi and Hashimoto2000; Komai & Segonzac, Reference Komai and Segonzac2005). Alvinocaris brevitelsonis is known only from the Minami-Ensei Knoll, Okinawa Trough (north-western Pacific), at a depth of 705 m (Kikuchi & Hashimoto, Reference Kikuchi and Hashimoto2000; Komai & Segonzac, Reference Komai and Segonzac2005).
(3) Alvinocaris chelys is distinguished from A. marimonte sp. nov. by (i) the ventral margin of the rostrum, which is unarmed or armed with one minute subterminal tooth, (ii) the more strongly elevated dorsum of the carapace in adult females (dorsal angle ~155° in A. chelys), (iii) the third and fourth pleonal pleura which are always unarmed in A. chelys, (iv) the stouter second article of the antennal peduncle (1.3–1.4 times as long as wide in A. chelys vs 1.6–1.8 times in A. marimonte sp. nov.), (v) fewer meral spines on the third pereopod (unarmed or only with one spine) and (vi) the unarmed ischium of the third pereopod (cf. Komai & Chan, Reference Komai and Chan2010). Alvinocaris chelys is known only from Gueishandao, off north-eastern Taiwan (north-western Pacific), at depths of 252–300 m (Komai & Chan, Reference Komai and Chan2010).
(4) Alvinocaris costaricensis is distinguished from A. marimonte sp. nov. by (i) the relatively longer rostrum (usually overreaching the distal margin of the antennular peduncle), (ii) the more numerous rostral ventral teeth (5–7) and (iii) the possession of only two movable spines on the merus of the third pereopod (vs three spines in A. marimonte sp. nov.). Alvinocaris costaricensis was recently described from a methane seep off Costa Rica, eastern Pacific, at depths of 1001–1800 m (Martin et al., Reference Martin, Wall, Shank, Cha, Seid and Rouse2018).
(5) Alvinocaris dissimilis can be differentiated from A. marimonte sp. nov. by (i) the more strongly elevated dorsum of the carapace (dorsal angle ~155°), (ii) the convex posterior margin of the telson and (iii) the always unarmed third pleuron (cf. Komai & Segonzac, Reference Komai and Segonzac2005). Alvinocaris dissimilis is known from the Minami-Ensei Knoll, Okinawa Trough, at depths of 691–705 m (Komai & Segonzac, Reference Komai and Segonzac2005; Yahagi et al., Reference Yahagi, Watanabe, Ishibashi and Kojima2015).
(6) Alvinocaris kexueae differs from A. marimonte sp. nov. in (i) the relatively longer rostrum (usually overreaching the margin end of the antennular peduncle), (ii) the more numerous rostral ventral teeth (five or six), (iii) the more posteriorly located posterior-most tooth of the dorsal rostral series (located at the 0.4 of CL) and (iv) the presence of a small median tooth on the posterior margin of the telson (vs no median tooth in A. marimonte sp. nov.) (cf. Wang & Sha, Reference Wang and Sha2017). Alvinocaris kexueae is known from hydrothermal vents on the Manus Basin, Bismarck Sea, south-western Pacific, at depths of 1714–1910 m.
(7) Alvinocaris longirostris is easily distinguished from A. marimonte sp. nov. by (i) the relatively longer rostrum (usually overreaching the distal margin of the antennular peduncle), (ii) the more numerous rostral ventral teeth (four to nine), (iii) the more posteriorly arising posterior-most tooth of the dorsal rostral series (arising at 0.38–0.48 of CL), (iv) the more elevated dorsum of the carapace (dorsal angle ~150°), (v) the more strongly pronounced branchiostegal tooth on the carapace and (vi) the convex posterior margin of the telson (Kikuchi & Ohta, Reference Kikuchi and Ohta1995; Komai & Segonzac, Reference Komai and Segonzac2005). Alvinocaris longirostris is known with certainty from the north-west Pacific localities, i.e. hydrothermal vents on the Iheya Ridge, Irabu Knoll and Hatoma Knoll, Okinawa Trough, and cold seeps in Sagami Bay (off Hatsushima Site) and in the South China Sea (Jiaolong Cold Seep I), at depths of 1053–1627 m (Yahagi et al., Reference Yahagi, Watanabe, Ishibashi and Kojima2015; Li, Reference Li2015). Webber (Reference Webber2004) identified specimens from the Brothers Caldera, Kermadec Ridge, New Zealand, at depths of 1200–1850 m, with A. longirostris, but this identification needs to be verified.
(8) Alvinocaris markensis and A. muricola differ from A. marimonte sp. nov. in (i) the relatively long rostrum (usually reaching or overreaching the distal margin of the second article of the antennular peduncle), (ii) the more numerous ventral teeth on the rostrum (six to nine), (iii) the more strongly elevated dorsum of the carapace (dorsal angle ~150°), (iv) the more strongly pronounced branchiostegal tooth on the carapace and (v) the convex posterior margin of the telson (cf. Komai & Segonzac, Reference Komai and Segonzac2005). Alvinocaris markensis has been reported from hydrothermal vents on the Mid-Atlantic Ridge, at depths of 1693–3650 m, and A. muricola has been reported from cold seeps on both sides of the Atlantic (viz., Gulf of Mexico, Barbados Accretionary Prism, Blake Ridge Diapir, and the West African equatorial margin in the Congo Basin), at depths of 1697–3277 m (Komai & Segonzac, Reference Komai and Segonzac2005). Teixeira et al. (Reference Teixeira, Karine, Decker, Cunha, Fuchs, Hourdez, Serrão and Arnaud-Haond2013) has shown that the two taxa are genetically identical based on partial sequences of the mitochondrial COI gene, suggesting that A. markensis and A. muricola are conspecific.
(9) Alvinocaris methanophila has (i) more numerous rostral ventral teeth (3–11), (ii) a more strongly elevated dorsum of the carapace (dorsal angle ~155° vs 160–165°), (iii) a more strongly pronounced branchiostegal tooth on the carapace and (iv) the convex posterior margin of the telson (Komai et al., Reference Komai, Shank and Van Dover2005). It is known only from the Blake Ridge Diapir, off North Carolina, north-western Atlantic, at depths of 2155–2167 m.
(10) Alvinocaris solitaire is easily distinguished from A. marimonte sp. nov. by (i) the relatively longer rostrum (overreaching the distal margin of the second article of antennular peduncle), (ii) the more posteriorly arising posterior-most tooth of the dorsal rostral series (located at the 0.45 of CL) and (iii) the more slender second article of the antennular peduncle (1.95 times as long as wide vs 1.6–1.8 times as long) (Yahagi et al., Reference Yahagi, Watanabe, Kojima, Beedessee and Komai2014). Alvinocaris solitaire is known only from Solitaire hydrothermal vent fields, Central Indian Ridge, Indian Ocean, at a depth of 2606 m.
(11) Alvinocaris stactophila appears unique within the genus in the armature of the posterior margin of the telson (the mesial pair of the two posterolateral pairs of spines of the telson is noticeably incurved), and the flexor margin of the dactylus of the third pereopod (which bears penultimate and antepenultimate spinules that are distinctly longer than the ultimate and proximal spinules) (Komai & Segonzac, Reference Komai and Segonzac2005). In other species of Alvinocaris, including the present new species, the mesial pair of posterolateral spines is almost straight, and the accessory spinules on the dactylus of the third pereopod become gradually shorter, proximally. Furthermore, the convex posterior margin of the telson distinguishes A. stactophila from A. marimonte sp. nov. Alvinocaris stactophila is known from cold seeps on the Louisiana Slope, northern Gulf of Mexico, at depths of 534–650 m (Williams, Reference Williams1988; Komai & Segonzac, Reference Komai and Segonzac2005).
(12) Alvinocaris williamsi differs from A. marimonte sp. nov. in (i) the more robustly built body, (ii) the ventrally unarmed rostrum, (iii) the proportionally wider telson with dorsolateral spines arranged in a slightly curved row, (iv) the relatively stout antennal peduncle (the length–width ratio 1.3–1.4 in A. williamsi), (v) the relatively wider antennal scale (1.4–1.5 times as long as wide in A. williamsi vs 1.6–2.1 times as long in A. marimonte sp. nov.) and (vi) the unarmed ischia of the third and fourth pereopods (Shank & Martin, Reference Shank and Martin2003; Komai & Segonzac, Reference Komai and Segonzac2005). Alvinocaris williamsi is known only from hydrothermal vents on Menez Gwen, Mid-Atlantic Ridge, at depths of 850–865 m.
(13) Alvinocaris komaii and A. niwa are readily distinguished from all other species of Alvinocaris, including A. marimonte sp. nov., by having (i) two or more rows of accessory spinules on the flexor margins of the dactyli of the last three pairs of pereopods (Webber, Reference Webber2004; Zelnio & Hourdez, Reference Zelnio and Hourdez2009). Future study may reveal sufficient differences between these two species and all other congeners to assign them to a different genus altogether. Furthermore, A. niwa differs from A. marimonte sp. nov. in having (ii) a short rostrum that does not reach the distal margin of the first article of the antennular peduncle and (iii) unarmed meri of the third and fourth pereopods (Webber, Reference Webber2004). Alvinocaris komaii has (ii) very characteristic strong postrostral teeth on the carapace and (iii) a deeply bilobed posterior margin of the telson (Zelnio & Hourdez, Reference Zelnio and Hourdez2009; Komai & Tsuchida, Reference Komai and Tsuchida2015). Alvinocaris komaii occurs in the south-western Pacific hydrothermal vents (Lau Basin, Manus Basin, Vanuatu and Futuna Islands, at depths of 1406–2750 m) (Zelnio & Hourdez, Reference Zelnio and Hourdez2009; Komai & Tsuchida, Reference Komai and Tsuchida2015; Komai et al., Reference Komai, Menot and Segonzac2016); A. niwa is restricted to the Brothers Caldera and Rumble V Seamount, Kermadec Ridge, at depths of 367–1346 m.
(14) Alvinocaris lusca appears morphologically most similar to the new species. However, A. lusca differs from A. marimonte sp. nov. in that (i) it has relatively larger postrostral teeth on the carapace (cf. Komai & Segonzac, Reference Komai and Segonzac2005: figures 5A, C and figures 4A, 8A, D) and (ii) a relatively wider antennal scale (cf. Komai & Segonzac, Reference Komai and Segonzac2005: figure 5D and figure 4D). Furthermore, whereas in A. marimonte sp. nov., the third pleonal pleuron can have marginal denticles (although sometimes absent), in A. lusca, it is always unarmed. Alvinocaris lusca known from the East Pacific Rise 9°N, at depths of 2450–2520 m (Williams & Chace, Reference Williams and Chace1982; Komai & Segonzac, Reference Komai and Segonzac2005).
Genetic analyses of Alvinocaris
In this study, partial sequences of the mitochondrial COI gene (477 bp) of the 12 species of Alvinocaris were available for comparison, viz., A. chelys, A. costaricensis, A. dissimilis, A. kexueae, A. komaii, A. longirostris, A. lusca, A. markensis, A. muricola, A. solitaire and A. stactophila (Shank et al., Reference Shank, Black, Halanych, Lutz and Vrijenhoek1999; Zelnio & Hourdez, Reference Zelnio and Hourdez2009; Yahagi et al., Reference Yahagi, Watanabe, Kojima, Beedessee and Komai2014; Wang & Sha, Reference Wang and Sha2017; Martin et al., Reference Martin, Wall, Shank, Cha, Seid and Rouse2018). In the ML and NJ trees are almost congruent, and two major clades are evident (Figure 8). Alvinocaris marimonte sp. nov. is clustered with A. costaricensis, although the bootstrap values are rather low (68 and 76% in ML and NJ), and thus the relationship is not well supported. Indeed, morphology does not support such a close relationship between the two species (see above). Consequently, the sister relationship of A. marimonte with A. costaricensis might not actually be correct. The K2P genetic divergences between the new species and the other congeneric species range between 11.7–17.1%, well supporting the recognition of the present new species. The genetic divergence between the new species and the closest species, A. constaricensis, is 11.7%. The morphologically most similar species to the new species is A. lusca, but the genetic divergence between the two is 16.9%, suggesting that they might not be sister species (Table 3).

Fig. 8. The Maximum-likelihood (ML) tree of Alvinocaris and outgroup inferred from partial COI sequences (477 bp) using K2P distances. Mirocaris fortunata (AF125426) used as an outgroup. Bootstrap values in ML are given for branches with NJ values (ML/NJ) obtained by 1000 replicate sampling, respectively. MK, Myojin Knoll; NWE, North-west Eifuku Seamount; NWR, North-west Rota Seamount.
Table 3. K2P genetic divergence of the partial sequences of the mitochondrial COI gene among the 12 species of Alvinocaris

Acknowledgements
We thank the captain and crew of RV ‘Natsushima’ and the operation teams of the ROV ‘Hyper-Dolphin’ for their skilful collection of specimens. We are also grateful to Drs K. Fujikura and M. Kawato (Japan Agency for Marine-Earth Science and Technology), for providing technical support for this study.













