INTRODUCTION
Poecilochaetids represent a small family of spioniform polychaetes with a single genus, Poecilochaetus, and about 32 accepted species worldwide. Individuals can easily be recognized by the presence of an anteriorly projected cage of chaetae, ampullaceous cirri and by incredibly diverse types of chaetae.
The anatomy, body morphology and taxonomic history of poecilochaetids have been reviewed by several authors (e.g. Allen, Reference Allen1904; Pilato & Cantone, Reference Pilato and Cantone1976; Mackie, Reference Mackie and Morton1990; Santos & Mackie, Reference Santos and Mackie2008) and a recent phylogenetic analysis recovered it as a monophyletic group with two main clades composed of species with or without body papillae (Eibye-Jacobsen, Reference Eibye-Jacobsen2006).
The poecilochaetids from the western Pacific Ocean are the most diverse group of species with more than half of the described species (Okuda, Reference Okuda1935; Levenstein, Reference Levenstein1962; Gibbs, Reference Gibbs1971; Read, Reference Read1986; Miura, Reference Miura1988, Reference Miura1989; Imajima, Reference Imajima1989; Mackie, Reference Mackie and Morton1990). Fewer species have been reported in the eastern Pacific (Hartman, Reference Hartman1939; de León-González, Reference de León-González1992; Blake, Reference Blake, Blake, Hilbig and Scott1996; Brantley, Reference Brantley2009) and there is no published record for the occurrence of this family in the Hawaiian Islands. We provide herein the description of three poecilochaetid species collected around the island of Oahu, Hawaii; one species is newly described and two others are newly reported in Hawaiian waters.
MATERIALS AND METHODS
Specimens have been collected on the south and east shores of Oahu, Hawaii from sediments near the discharge pipe of sewage outfalls. Surface sediments were collected with a modified 0.1 m2 van Veen grab and fixed in a buffered solution of formalin and Rose Bengal. The fixed samples were elutriated over a 0.5 mm sieve, and the organisms were sorted and preserved in 70% ethanol. One larva in nectosomal stage was collected a mile off Honolulu Harbor with a plankton net and observed live prior to preservation in 92% ethanol.
Specimens were examined using a phase-contrast light microscope and a scanning electron microscope (SEM). Line drawings were made with a camera lucida attached to the light microscope and measurements of length and width were taken with an ocular micrometer. Width measurements were taken from chaetiger 10 including ampullaceous cirri. SEM preparations started with dehydration through a series of increasing concentrations of ethanol ending with 2 changes of absolute ethanol followed by critical point drying (in a SAMDRI-795). Worms were then mounted on stubs and coated with gold/palladium for 2 min at 5 nm thickness. SEM observations were carried out using the Hitachi S-4800 at the Biological Electron Microscopy Facility (BEMF), University of Hawaii at Manoa.
Holotype, paratypes and voucher specimens are deposited at the United States National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (USNM) and the Natural History Museum of Los Angeles County, Allan Hancock Foundation Polychaete Collection (LACM-AHF).
RESULTS AND DISCUSSION
SYSTEMATICS
Family POECILOCHAETIDAE Hannerz, Reference Hannerz1956
Genus Poecilochaetus Claparède
in Ehlers, 1875
Poecilochaetus anterospinus sp. nov.
(Figures 1–3)

Fig. 1. Poecilochaetus anterospinus sp. nov.: (A) anterior end, dorsal view; (B) anterior end, ventral view; (C) pygidium; (D) parapodium 1, posterior view; (E) parapodium 2, anterior view; (F) parapodium 3, anterior view; (G) parapodium 4, anterior view; (H) parapodium 6, anterior view; (I) parapodium 8, anterior view; (J) parapodium 11, anterior view; (K) parapodium 22, anterior view; (L) parapodium 45, anterior view; (M) parapodium 48, anterior view.
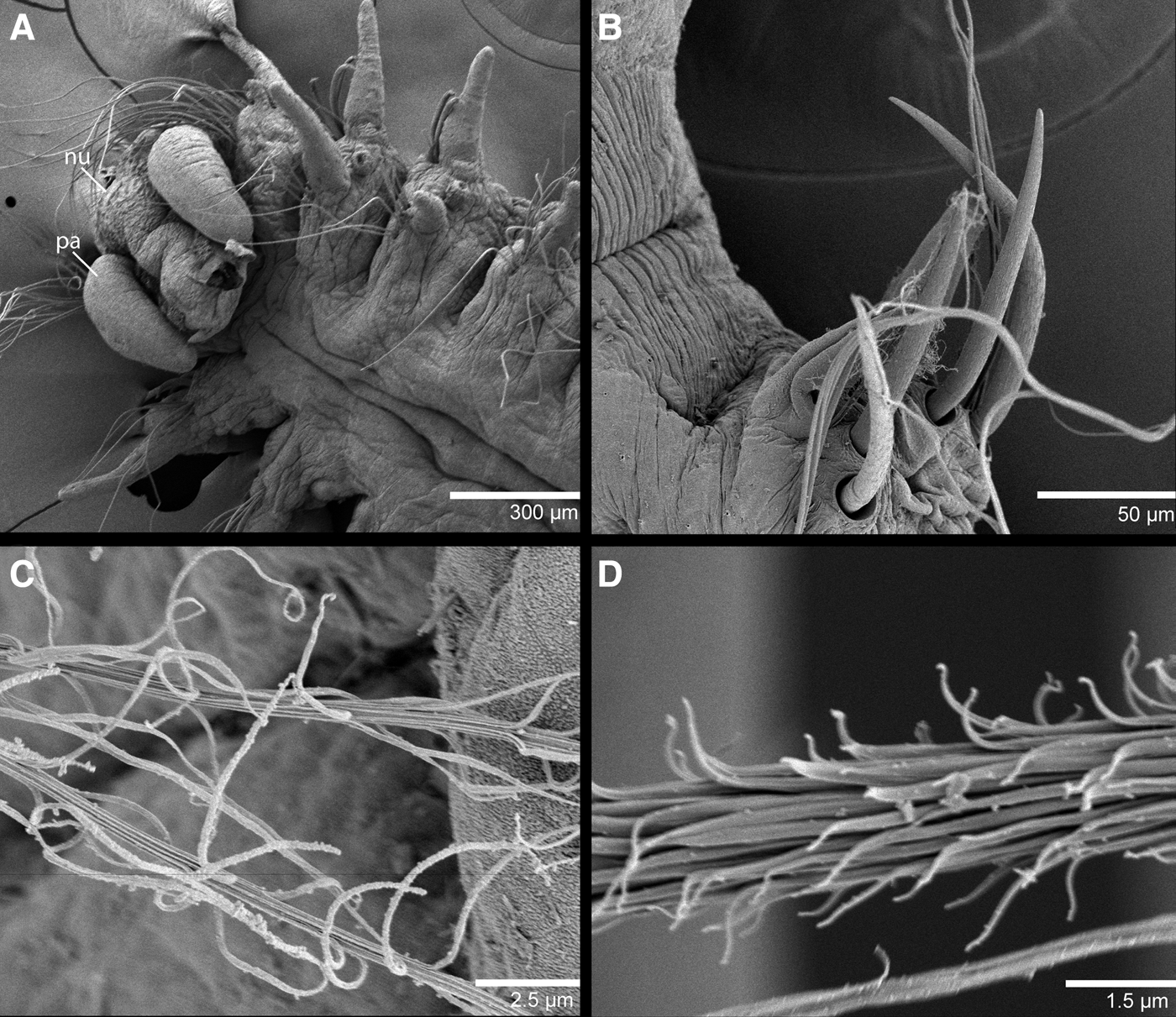
Fig. 2. SEM of Poecilochaetus anterospinus sp. nov.: (A) anterior end, dorsal view; (B) parapodium 20 showing spines from noto- and neuropodium; (C) distal region of slender plumose capillary; (D) proximal region of slender plumose capillary.

Fig. 3. Nectosomal larval stage of Poecilochaetus anterospinus sp. nov.: (A) live specimen in dorsal and ventral views; (B) anterior end showing large prostomial eyes and white arrow indicating heart body; (C) parapodia from anterior segments, white arrow showing interramal cup-like sensory papilla; (D) ventral region showing scattered papillae and melanophores; (E) ampullaceous parapodial cirri, white arrow showing beginning of spines.
TYPE MATERIAL
Holotype: Mamala Bay, Barbers Point outfall, south shore of Oahu, Hawaii, Station HB1R4, 21°16′50.5″N 157°59′19.7″W, 65.2 m, January 1995 (USNM 1231533).
Paratypes: Mamala Bay, south shore of Oahu Island, Hawaii, underneath threadfin mariculture cages, Station ZR5, 21°17′17.7″N 158°00′26.5″W, 40.2 m, June 1992 (1, USNM 1231534); Kailua Bay, Mokapu outfall, east shore of Oahu Island, Hawaii, Station AR3, 21°27′45.6″N 157°42′43.6″W, August 1998 (2, LACM-AHF Poly 6366); Station DR3, 21°25′32.3″N 157°42′53.6″W, March 2013 (1, LACM-AHF Poly 6367).
NON-TYPE MATERIAL
South shore of Oahu, off Honolulu Harbor, collected with a plankton net at 25 feet depth, 13 February 2014, coll. Anuschka Faucci (1 larva in nectosomal stage, USNM 1231535).
DIAGNOSIS
Poecilochaetus with median length median nuchal organ, discoid lateral ones; neuropodial falcate spines on chaetigers 2 and 3; ampullaceous cirri on chaetigers 7–11; neuropodial and notopodial spines from chaetiger 11 and present thereafter; branchiae absent.
DESCRIPTION
ADULT MORPHOLOGY
Holotype posteriorly incomplete, 8 mm long, 0.6 mm wide for 38 chaetigers. One paratype complete, 9 mm long, 0.8 mm wide for 50 chaetigers (USNM 1231534), all other four paratypes incomplete, ranging from 7–11 mm long, 0.4–0.7 mm wide for 29–47 chaetigers.
Prostomium small, subrectangular to trapezoidal, anterior margin widest, concave, inserted between notopodia of chaetiger 1 (Figure 1A). Two pairs of black eyes, anterior pair large, oval and positioned on edges of prostomium; second pair rounded, minute and positioned on posterior margin (Figure 1A). Peristomium small and with three nuchal lobes posteriorly. Median lobe reaching posterior end of chaetiger 3 or anterior end of chaetiger 4, lateral lobes discoid (Figures 1A & 2A). Palps, when present, reaching up to chaetiger 5 (Figure 2A). Short cylindrical, blunt facial tubercle from upper margin of mouth (Figure 1B).
Ventral surface of chaetigers 1–3 densely covered with small, papilliform tubercles (Figures 1B & 3D); tubercles absent on chaetigers 4–5 and present again from chaetiger 6–9 and absent thereafter. Three longitudinal ventral grooves throughout; papillae present in grooves. Chitinous plate on dorsum of chaetiger 9 absent.
Chaetiger 1 large, directed forwards; neuropodial postchaetal lobes long, cirriform, notopodial lobes short, papilliform (Figure 1D). Neuropodial postchaetal lobes of chaetigers 2–6 short, lanceolate, longest on chaetiger 2, shortest on chaetigers 4–6 (Figure 1E–H). Notopodial postchaetal lobes of chaetigers 2–6 of similar size and shape, sometimes slightly longer than respective neuropodial lobes (Figure 1E–H). Parapodial lobes of chaetigers 4–6 adorned with short papillae (Figure 1G, H). Ampullaceous postchaetal lobes on chaetigers 7–11, with glandular and wide basal regions and smooth and slender distal regions (Figures 1I, J & 3E).
Postchaetal lobes from chaetiger 12 backward of similar shape and size as those on chaetiger 6; thereafter becoming shorter and more slender (Figure 1K–M).
Interramal cup-like sensory papillae on chaetigers 1–5 (Figures 1D–G & 3C), thereafter as very discreet tufts of cilia. Interramal cirri absent. Branchiae absent.
Pygidium with terminal anus and 3 short and slender cirri, as long as one posterior chaetiger (Figure 1C).
Chaetiger 1 with long, slender capillaries, fan-like in both rami, forming a cephalic cage; notochaetae twice as long as neurochaetae (Figure 1A, D). Notopodial and neuropodial capillaries smooth (at ×1000), with longitudinal striation. Neuropodia of chaetigers 2 and 3 with 3–4 smooth, falcate hooks (Figure 1E, F) and with 2–3 slender, short capillaries superior to hooks. Notopodia of same chaetigers with a few slender, short, smooth capillaries; notopodial capillaries longer than neuropodial ones. Following 7 chaetigers with only smooth capillaries.
Chaetae markedly different from chaetiger 11; one smooth spine and 3–4 smooth capillaries in notopodia, and two smooth spines and 7 smooth capillaries in neuropodia (Figures 1J, K, 2B & 3E). From chaetiger 19 or 20, 2–3 weakly plumose chaetae present in both rami (Figure 2C, D). Following chaetigers with 6–8 fully plumose chaetae and 2–3 smooth spines per rami.
Last 8 chaetigers only observed in one specimen (from chaetigers 42–50), with up to 3 ancistroid spines, 2–4 straight smooth spines and 2–3 capillary chaetae in notopodia and 2–3 straight smooth spines and a reduced number of capillary chaetae in neuropodia (Figure 1L, M).
LARVAL MORPHOLOGY
One individual missing pygidium, 7 mm long, 0.25 mm wide for 29 chaetigers in the nectosomal stage (Figure 3A). Body transparent with a pair of ventral green melanophores in each segment, beginning from chaetiger 4 and present throughout (Figure 3A). Prostomium broadly rounded, with a pair of large red eyes and a pair of minute red eyes (Figure 3A, B). Median lobe of nuchal organ as long as pair of ciliate palps; lateral lobes hidden below palps. First chaetiger not projecting anteriorly and chaetae as long as chaetae from following segments. Distribution of chaetae, ventral papillae and shape of parapodial lobes as observed in adults (Figure 3C–E). Gut transparent and empty, with no apparent regionalization. Heart body present in middle of chaetiger 2 as a swollen, contractile region along a blood vessel (Figure 3B).
The larva kept active, swimming by serpentine movements if in contact with light as described for larvae of Poecilochaetus serpens (Nozais et al., Reference Nozais, Duchêne and Bhaud1997). Production of mucus was not observed. Larva rested on the bottom of the dish with the dorsal region facing down.
REMARKS
The described type material is considerably small and might be recently settled given that the described nectosoma is 7 mm long. The identification of the larva was possible by distribution of chaetae and ampullaceous cirri (Figure 3C, E).
Poecilochaetus anterospinus sp. nov. resembles P. tricirratus Mackie, Reference Mackie and Morton1990 by the presence of a median length median nuchal organ, discoid lateral ones, neuropodial falcate spines on chaetigers 2 and 3, ampullaceous cirri on chaetigers 7–11 and ancistroid posterior notopodial hooks. It differs most noticeably by the early appearance of neuropodial and notopodial spines, beginning on chaetiger 11 and present thereafter, lack of interramal cirri, robust plumose and aristate chaetae. The presence of noto- and neuropodial spines as early as chaetiger 11 is a unique feature of this new species. Neuropodial hooks are present in most species in chaetigers 2 and 3 only and, in rare cases, from chaetigers 2–4 (P. fulgoris Claparède, 1875, P. trachyderma Read, Reference Read1986 and P. vietnamita Gallardo, Reference Gallardo1968). Neuropodial spines are also present posteriorly in P. tricirratus (after chaetiger 44) but it differs from P. anterospinus because it is less robust and only slightly protruding (Mackie, Reference Mackie and Morton1990). Notopodial spines and modified hooks are restricted to posterior end segments in most described species with complete specimens. In addition, the absence of branchiae and aristate chaetae and the fact that notopodial lobes of chaetiger 5 are not noticeably longer than those of chaetigers 4 and 6 distinguish P. anterospinus sp. nov. from all other described species of Poecilochaetus.
ETYMOLOGY
The specific name ‘anterospinus’ refers to the anterior appearance of noto- and neuropodial spines, unique in this species.
DISTRIBUTION
Only known from the east and south shores of Oahu, Hawaii.
Poecilochaetus cf. koshikiensis
Miura, Reference Miura1988
(Figures
4–6)
Poecilochaetus koshikiensis: Miura, Reference Miura1988, pp. 671–675, Figs 1–3; Eibye-Jacobsen, 2005, Fig. 1B.

Fig. 4. Poecilochaetus cf. koshikiensis: (A) anterior end, dorsal view; (B) anterior end, ventral view; (C) segments 9 and 10 showing triangular chitinous plate; (D) posterior end segments and pygidium.

Fig. 5. Poecilochaetus cf. koshikiensis: (A) parapodium 1; (B) parapodium 2; (C) parapodium 3; (D) parapodium 4; (E) parapodium 5; (F) parapodium 6; (G) parapodium 7; (H) parapodium 12; (I) parapodium 16; (J) parapodium 40; (K) parapodium 60; (L) parapodium 70, chaetae omitted in neuropodium; (M) sympodial spinose capillary; (N) slender plumose capillary; (O) spinose capillary; (P) aristate chaeta; (Q) notopodial slightly curved spine from posterior chaetiger; (R) notopodial hook from posterior chaetiger; (S) slender notopodial spine from posterior chaetiger; (T) superiormost notopodial hook from posterior chaetiger.

Fig. 6. SEM of Poecilochaetus cf. koshikiensis: (A) anterior end, dorsal view; (B) sympodial spinose capillary; (C) spinose capillaries; (D) slender plumose capillaries; (E) hirsute capillary.
MATERIAL EXAMINED
Thirty examined specimens. Mamala Bay, Sand Island outfall, south shore of Oahu Island, Hawaii, Station B4R1, 21°17′01.4″N 157°54′23.8″W, 33 m, March 1990 (1); B4R1, 21°17′01.4″N 157°54′23.8″W, 33 m, May 1991 (1); B6R1, 21°17′04.7″N 157°53′25.3″W, 40 m, August 1990 (3); B4R6, 21°17′01.4″N 157°54′23.8″W, 33 m, August 1992 (1); B2R6, 21°17′23.4″N 157°55′23.9″W, 34 m, August 1993 (3, LACM-AHF Poly 6368); D1R3, 21°17′23.2″N 157°55′29.9″W, 48.8 m, March 1992 (1, LACM-AHF Poly 6369). Kailua Bay, Mokapu outfall, east shore of Oahu Island, Hawaii, Station AR3, 21°27′45.6″N 157°42′43.6″W, August 1998 (1); Station DR2, 21°25′32.3″N 157°42′53.6″W, August 1995 (1). Mamala Bay, Barbers Point outfall, south shore of Oahu Island, Hawaii, Station HB1R3, 21°16′50.5″N 157°59′19.7″W, 65.2 m, February 1990 (1 complete, USNM 1231536); Station HB4R1, 21°16′46.9″N 158°01′38.1″W, 63.7 m, February 1990 (1); Station HB6R1, 21°16′33.1″N 158°01′29.7″W, 62.8 m, February 1990 (1); Station HB2R3, 21°17′00.4″N 158°01′21.4″W, 59.4 m, February 1992 (2); Station HZR5, 21°16′53.7″N 158°01′29.7″W, 62.8 m, February 1992 (3, LACM-AHF Poly 6370); Station HB6R5, 21°16′33.3″N 158°01′47.9″W, 59.1 m, February 1992 (2); Station HB7R4, 21°15′30.0″N 158°03′11.3″W, 65.2 m, February 1992 (2); Station HB2R4, 21°17′00.5″N 158°01′21.2″W, 59.1 m, June 1993 (1); Station HZR1, 21°16′53.7″N 158°01′29.7″W, 62.5 m, February 1997 (3); Station HB3R3, 21°16′52.6″N 158°01′29.9″W, 67.1 m, March 2013 (1).
DIAGNOSIS
Poecilochaetus with medium length median nuchal organ, discoid lateral ones; neuropodial falcate spines on chaetigers 2 and 3; ampullaceous cirri on chaetigers 7–13; posterior notopodial spines robust curved; branchiae absent.
DESCRIPTION
One complete specimen 21 mm long, 1 mm wide for 67 chaetigers. Prostomium small, subrectangular with posterior incision, inserted between notopodia of chaetiger 1 (Figures 4A & 6A). Two pairs of black eyes, anterior pair large, oval and positioned on anterior prostomial edges; second pair minute, reniform and positioned on posterior margin (Figure 4A). Peristomium small and with three nuchal lobes posteriorly. Median lobe longer than lateral lobes; median lobe reaching posterior end of chaetiger 4 or 5, lateral lobes discoid (Figures 4A & 6A). Palps lost. Long and cylindrical facial tubercle from upper margin of mouth (Figure 4B).
Ventral surface of chaetigers 1–3 covered with papillae (Figure 4B); papillae absent on chaetigers 4–5 and present again from chaetiger 6 to anterior half of chaetiger 9 and absent thereafter. Papillae densely distributed on median region of chaetigers 1–3 and scattered on parapodial base; chaetigers 6–9 with papillae on venter and parapodial bases. Chitinous plate present on dorsum of chaetiger 9, weakly chitinized and triangular (Figure 4C).
Chaetiger 1 large, directed forwards; neuropodial postchaetal lobes long, cirriform, notopodial lobes short, papilliform (Figure 5A). Neuropodial postchaetal lobes of chaetigers 2–6 short, lanceolate, longest on chaetiger 2, shortest on chaetigers 4–6 (Figure 5B–F). Notopodial postchaetal lobes of chaetigers 2, 3, 4 and 6 of similar size and shape and those of chaetiger 5 longer than respective neuropodial lobe and all other anterior parapodial lobes (Figure 5B–F). Ampullaceous postchaetal lobes on chaetigers 7–13, with wide basal regions (Figure 5G, H).
Postchaetal lobes from chaetiger 14 backwards of similar shape and size as those on chaetiger 6; thereafter becoming shorter and more slender (Figure 5I–L).
Interramal cup-like sensory papillae on chaetigers 1–5 and 10–14, thereafter as very discreet tufts of cilia. Interramal cirri absent. Branchiae absent.
Pygidium with terminal anus and densely covered with pointed tubercles; two slender ventro-lateral cirri as long as two posterior segments and two short medio-ventral cirri, all covered with pointed tubercles (Figure 4D).
Chaetiger 1 with long, slender capillaries, fan-like, finely hirsute (×400) in both rami, forming cephalic cage; notochaetae twice as long as neurochaetae (Figure 5A). Neuropodia of chaetigers 2 and 3 with 3 finely hirsute (×400) falcate hooks (Figure 5B, C) and 2–3 short and slender capillaries superior to hooks. Notopodia of same chaetigers with 5–6 slender, short and smooth capillaries. Following 8 chaetigers with only smooth capillaries. Hirsute capillaries from chaetiger 12, one per ramus, increasing to up to 4 per ramus until chaetiger 16 (Figure 6E).
Chaetae markedly different from chaetiger 17; each ramus with 4–6 plumose capillaries (Figures 5N & 6D), 2–4 spinose capillaries (Figures 5O & 6C) and 2–3 sympodial spinose capillaries (Figures 5M & 6B). By chaetiger 50, chaetae less abundant and spinose capillaries replaced by 4–5 aristate chaetae with truncate hook-like tufted knob at base of plumose arista (Figure 5P). Later, aristate chaetae become less abundant, neuropodial aristates longer than notopodial ones; notopodia with one dorsal straight spine and up to three aristate chaetae, neuropodial with four aristates only (Figure 5K).
Last 8–10 chaetigers with robust curved notopodial hooks forming dorsal shield in addition to one smaller dorsalmost curved hook, 4 slightly curved spines and 2 smooth capillaries (Figure 5J, K, Q–T). Posterior neuropodia with 2 spines and few capillaries.
REMARKS
The Hawaiian specimens described here are identified as P. cf. koshikiensis because although it agrees well with the original description, there are small morphological differences. The Hawaiian specimens have ventral papillae on chaetigers 1–3 and 6, 7 and anterior region of chaetiger 9 while the original description states papillae on chaetigers 1–2 and 6–8. Membranous capillaries are reported to occur from chaetigers 2–3 in Miura (Reference Miura1988) while the Hawaiian specimens have smooth capillaries from chaetigers 2–11. Miura (Reference Miura1988) also describes specimens having basally fused anal cirri that were not observed in the material described here. The pygidium of the Hawaiian specimens also has pointed tubercles not described or illustrated in Miura (Reference Miura1988). This species is closely related to P. elongatus Imajima, Reference Imajima1989, P. fauchaldi Pilato & Cantone, Reference Pilato and Cantone1976 and P. polycirratus Santos & Mackie, Reference Santos and Mackie2008 by the presence of a medium length median lobe on the nuchal organ, discoid lateral lobes, neuropodial spines on chaetigers 2 and 3, and ampullaceous cirri on chaetigers 7–13. It differs most noticeably from these species by the absence of branchiae (present in P. polycirratus) and the presence of aristate hooks (absent in P. fauchaldi and P. elongatus).
DISTRIBUTION
The type locality of Poecilochaetus koshikiensis is Shimo–Koshiki Island, Japan (Miura, Reference Miura1988) but it has been recorded from Thailand, Indonesia, South China Sea (Eibye-Jacobsen, Reference Eibye-Jacobsen2006). Poecilochaetus cf. koshikiensis is newly recorded from Oahu, Hawaii.
Poecilochaetus sp.
(Figures 7 and 8)

Fig. 7. Poecilochaetus sp.: (A) anterior end, dorsal view; (B) anterior end, ventral view; (C) pygidium, dorsal view; (D) segments 9 and 10 showing chitinous plate with rounded edge.

Fig. 8. Poecilochaetus sp.: (A) parapodium 1; (B) parapodium 2; (C) parapodium 3; (D) parapodium 4; (E) parapodium 5; (F) parapodium 6; (G) parapodium 7; (H) parapodium 11; (I) parapodium 12; (J) parapodium 47; (K) parapodium 52; (L and M) ventral region, right side of two different specimens showing chaetiger 12 with shorter ampullaceous cirri.
MATERIAL EXAMINED
Twenty examined specimens. Mamala Bay, Barbers Point outfall, south shore of Oahu, Hawaii, Station HB3R2, 21°16′53.2″N 158°01′29.8″W, 67.1 m, January 1999 (1 complete, LACM-AHF Poly 6371); Station HB2R6, 21°17′00.4″N 158°01′21.5″W, 59.4 m, February 1990 (1 incomplete); Station HB7R5, 21°15′30″N 158°03′11.3″W, 65.2 m, July 1991 (1 incomplete); Station HB1R4, 21°16′50.5″N 157°59′20.2″W, 64.9 m, January 1995 (3 incomplete, USNM 1231537); Station HB6R4, 21°16′33.1″N 158°01′48.2″W, 59.4 m, January 1999 (1 incomplete); Station HB6R4, 21°16′33.1″N 158°01′48.2″W, 59.4 m, March 2003 (1 incomplete); Station HB1R2, 21°16′50.7″N 157°59′19.8″W, 65.5 m, March 2004 (1 incomplete). Mamala Bay, Sand Island outfall, south shore of Oahu Island, Hawaii, Station B2R2, 21°17′23.4″N 157°55′23.9″W, 35 m, August 1990 (1 incomplete); Station B4R4, 21°17′01.4″N 157°54′23.8″W, 35 m, August 1990 (2 complete, LACM-AHF Poly 6372); Station B1R1, 21°17′30.2″N 157°55′44″W, 33 m, August 1991 (1 complete); Station D1R1, 21°17′23.4″N 157°55′30.1″W, 48.8 m, October 1993 (1 complete).
DIAGNOSIS
Poecilochaetus with medium length median nuchal organ, discoid lateral ones; neuropodial falcate spines on chaetigers 2 and 3; ampullaceous cirri on chaetigers 7–12 or 7–11 with shorter cirri on chaetiger 12; posterior notopodial spines robust, curved; branchiae absent.
DESCRIPTION
Four complete specimens ranging from 5–12 mm long and 0.4–1 mm wide for 35–51 chaetigers. Prostomium small, subrectangular to trapezoidal, anterior margin widest, concave, inserted between notopodia of chaetiger 1 (Figure 7A). Two pairs of black eyes, anterior pair large, oval and sickle–shaped, positioned on anterior edge of prostomium; second pair minute and rounded, positioned on posterior margin (Figure 7A). Peristomium small and with three nuchal lobes posteriorly. Median lobe longer than lateral lobes; median lobe reaching posterior end of chaetiger 4, lateral lobes discoid. Palps lost. Long, cylindrical facial tubercle from upper margin of mouth (Figure 7B).
Ventral surface of chaetigers 1–3 densely covered with small papillae (Figure 7B); papillae absent on chaetigers 4–5 and present again from chaetiger 6 to 9 and absent thereafter. Chitinous plate present on dorsum of chaetiger 9, weakly chitinized and with rounded edge (Figure 7D).
Chaetiger 1 large, directed forwards; neuropodial postchaetal lobes long, cirriform, notopodial lobes short, papilliform (Figure 8A). Neuropodial postchaetal lobes of chaetigers 2–6 short, lanceolate, slightly longer on chaetiger 2, shorter on chaetigers 4–6 (Figure 8B–F). Notopodial postchaetal lobes of chaetigers 3, 4 and 6 of similar size and shape and notopodial lobes of chaetigers 2 and 5 slightly longer than respective neuropodial lobe and all other anterior parapodial lobes (Figure 8B–F). Ampullaceous postchaetal lobes on chaetigers 7–12 or 7–11, with glandular and wide basal regions and smooth distal regions (Figure 8G–I, L, M). Short conical cirri or some degree of development of ampullaceous cirri on chaetiger 12 on both sides of segment.
Postchaetal lobes from chaetiger 13 backwards of similar shape and size as those on chaetiger 6; thereafter becoming shorter and slender (Figure 8J, K).
Interramal cup-like sensory papillae on chaetigers 1–5 and 10–14, thereafter as very discreet tufts of cilia. Interramal cirri absent. Branchiae absent.
Pygidium with terminal anus; two long and slender dorsal cirri and two short ventral cirri, joined basally (Figure 7C); all cirri covered with pointed tubercles (Figure 7C). Ventral region of pygidium with dark pigmentation.
Chaetiger 1 with long, slender capillaries, fan-like, finely hirsute (×400) in both rami, forming cephalic cage; notochaetae longer than neurochaetae (Figure 8A). Neuropodia of chaetigers 2 and 3 with 2–3 finely hirsute (×400) falcate hooks (Figure 8B, C), and 2–3 short and slender capillaries superior to hooks. Notopodia of same chaetigers with 5–6 slender, short, smooth capillaries. Following 8 chaetigers with only smooth capillaries.
Chaetae markedly different from chaetiger 16; each ramus with 4–6 plumose capillaries, 2–3 spinose capillaries with plumose endings and 2 sympodial spinose capillaries. By chaetiger 20 chaetae more abundant and spinose capillaries replaced by 2–3 aristate chaetae with truncate, hook-like tufted knob at base of plumose arista (Figure 8J).
By chaetiger 52 chaetae less abundant, notopodia with 3 curved hooks, middle one larger and striated, one slightly curved hook, 2 straight spines and 2 short capillaries; neuropodia with one long aristate chaeta and later on last 5 chaetigers replaced by a straight spine and 3–4 long capillaries (Figure 8K).
REMARKS
This species closely resembles P. ishikariensis Imajima, Reference Imajima1989, P. bermudensis Hartman, 1965 and P. aff. bermudensis sensu Read (Reference Read1986) by the distribution of ampullaceous cirri on chaetigers 7–12. Both P. bermudensis and specimens identified as P. aff. bermudensis have the median nuchal organ as short as one segment and lack a chitinous plate; they are therefore distinct from P. ishikariensis and Poecilochaetus sp. The specimens described in Read (Reference Read1986) and Hartman (1965) are anterior fragments and the chaetae of middle and posterior segments are unknown.
Poecilochaetus ishikariensis differs most noticeably from the species herein designated as Poecilochaetus sp. by: (i) length of median nuchal organ (reaching anterior part of chaetiger 3 in P. ishikariesis and 2 segments longer in Poecilochaetus sp.); (ii) shape of chitinous plates (triangular in P. ishikariensis and with rounded edge in Poecilochaetus sp.); (iii) number and size of neuropodial hooks on chaetigers 2–3 (3–4 enlarged hooks in P. ishikariensis sensu Eibye-Jacobsen, Reference Eibye-Jacobsen2006 and 2–3 smaller hooks in Poecilochaetus sp.); and (iv) presence of harpoon-like knobbed chaetae on posterior notopodia.
Most examined specimens of Poecilochaetus sp. did not present fully formed ampullaceous cirri on chaetiger 12 (see Figure 8L, M). This was observed in more than one specimen on both sides of the same parapodium, so regeneration would not be the explanation. Hannerz (Reference Hannerz1956) provided a description of the pelagic larva of Poecilochaetus serpens and noted that the ampullaceous cirri were absent on chaetigers 12 and 13 while it is present in adults of the same species. The postlarval development of ampullaceous cirri from conical cirri needs further investigation because the number of these cirri is extensively used to distinguish species or groups of species.
DISTRIBUTION
Only known from the south shore of Oahu, Hawaii.
ACKNOWLEDGEMENTS
This is contributed paper WRRC-CP-2015-04 of the Water Resources Research Center and contributed paper 2014-14 of the Department of Biology, University of Hawaii at Manoa, Honolulu. We thank Tina W. Carvalho (BEMF) for assistance with SEM procedures. Specimens from several localities in Oahu, Hawaii would not have been available for study without the collecting efforts of the City and County of Honolulu Oceanographic Team, and collection permits. Dr Anuschka Faucci kindly provided the plankton samples where the larva of P. anterospinus was found. Wormlab staff from the University of Hawaii at Manoa elutriated and sorted the sewage outfalls samples. Two anonymous reviewers greatly improved this manuscript. Linda Ward (USNM) and Leslie Harris (LACM-AHF) kindly organized the deposition of specimens and provided catalogue numbers.










