INTRODUCTION
The abundance and diversity of gelatinous zooplankton became evident when biologists began to dive in the open ocean (Hamner et al., Reference Hamner, Madin, Alldredge, Gilmer and Hamner1975; Madin & Harbison, Reference Madin and Harbison1978). In recent years, direct observations from submersibles have shown that these organisms are extremely abundant at depths of 200–400 m, where presumably they play an important role in pelagic processes (Angel, Reference Angel and Tyler2003; Robison, Reference Robison2004). Although knowledge of their ecological role in the oceans is scarce, they are considered important consumers of basal producers, both as grazers of phytoplankton and predators of fish larvae and other gelatinous zooplankton (Lucas et al., Reference Lucas, Jones, Hollyhead, Condon, Duarte, Graham, Robinson, Pitt, Schildhauer and Regetz2014). Because they also act as hydroclimatic indicators (Richardson, Reference Richardson2008), knowledge of species distribution is crucial to have well defined baseline information.
Isla del Coco (also known as Cocos Island) is located in the Eastern Tropical Pacific (ETP), a distinct epipelagic province where striking changes in species composition occur between the epi- and mesopelagic zones (Longhurst, Reference Longhurst and Steele2001; Spalding et al., Reference Spalding, Agostini, Rice and Grant2012). This island has been a site of intense scientific study due to its rich marine biodiversity, yet some animal groups, such as gelatinous zooplankton, remain poorly studied both locally at Isla del Coco (Cortés, Reference Cortés2012) and regionally in the ETP (Costello et al., Reference Costello, Coll, Danovaro, Halpin, Ojaveer and Miloslavich2010; Cortés et al., Reference Cortés, Enochs, Sibaja-Cordero, Hernández, Alvarado, Breedy, Cruz-Barraza, Esquivel-Garrote, Fernández-García, Hermosillo, Kaiser, Medina-Rosas, Morales-Ramírez, Pacheco, Pérez-Matus, Reyes-Bonilla, Riosmena-Rodríguez, Sánchez-Noguera, Wieters, Zapata, Glynn, Manzello and Enochs2017).
The ‘DeepSee’ submersible has explored the island to depths of 450 m since 2006 (Cortés & Blum, Reference Cortés and Blum2008) and has taken video footage of the local pelagic and benthic ecosystems. Here we present genera and species of cnidarians and ctenophores that have been opportunistically recorded around Isla del Coco National Park, along with images, species descriptions, and spatial and temporal distributions of these organisms.
MATERIALS AND METHODS
Images of gelatinous zooplankton were captured from videos taken with the ‘DeepSee’ submersible (Cortés & Blum, Reference Cortés and Blum2008) at several locations around Isla del Coco National Park (Figure 1). During some immersions, when a gelatinous organism was observed, the camera was turned on and recorded the animal for ~ 1 min. Videos were recorded with an AVI format on mini-DV tapes by a high definition Sony HDX7 camera (frame size: 1920 × 1080 pixels) and then transcoded with a 4:2:2 low codec to a .mov format. Image grabs were made in the laboratory using Imovie and FinalCut software (Apple Inc.). The videos were recorded on dives made from 2006 to 2012. Videos were recorded every year, but the depth varied, with 100 and 300 m usually being targeted.

Fig. 1. ‘DeepSee’ dive sites around Isla del Coco National Park, Costa Rica.
The descriptions of the observed organisms were supplemented with information from Kramp (Reference Kramp1961), Segura-Puertas (Reference Segura-Puertas1984), Suárez & Gasca (Reference Suárez and Gasca1991), Wrobel & Mills (Reference Wrobel and Mills1998), Bouillon (Reference Bouillon and Boltovskoy1999), Mianzan (Reference Mianzan and Boltovskoy1999), Mianzan & Cornelius (Reference Mianzan, Cornelius and Boltovskoy1999), Pugh (Reference Pugh and Boltovskoy1999) and Lindsay et al. (Reference Lindsay, Umetsu, Grossmann, Miyake, Yamamoto, Ishibashi, Okino and Sunamura2015). Our identifications were verified by experts on these groups: Dhugal Lindsay, Casey W. Dunn, Rebeca Gasca, Jun Nishikawa, Laurence P. Madin, Phil R. Pugh, Jennifer E. Purcell and Karina Rodríguez-Sáenz. Images of the species are included along with a brief description, distribution data at Isla del Coco National Park (e.g. location, depth, date, time of day), previous reports in the Eastern Tropical Pacific, and comments on their known distribution.
RESULTS
Nine species of medusae, ctenophores and siphonophores were observed at Isla del Coco National Park (Table 1), none of which had been previously recorded in this island. All of these species, except Pelagia noctiluca (Forsskål, Reference Forsskål1775) and the genus Praya are new records for Costa Rican waters. This study includes the first record of the species Modeeria rotunda (Quoy & Gaimard, Reference Quoy and Gaimard1827), Solmissus sp., Halitrephes maasi Bigelow, Reference Bigelow1909, Apolemia spp. and Thalassocalyce inconstans Madin & Harbison, Reference Madin and Harbison1978, in the Eastern Tropical Pacific.
Table 1. Species recorded in the videos taken by the ‘DeepSee’ submersible at Isla del Coco.

Due to the limitations of video resolution and the orientation of some organisms when they were recorded, only the genus could be determined for Solmissus sp., Praya sp., Apolemia spp. and Hormpihora sp. Also, because the ‘DeepSee’ is used mostly for tourism, this study does not include precise information of each observation made such as depth, temperature and salinity. Approximate depths for each observation were included only when the organism was observed near the sea floor (the maximum dive depths were recorded in the submersible log). Otherwise, a range is given based on the maximum dive depth, amount of light present, background terrain, and where one of us (JC) and others have observed them.
SYSTEMATICS
Phylum CNIDARIA Verrill, 1865
Class SCYPHOZOA Goette, 1887
Order SEMAEOSTOMEAE Agassiz, 1862
Family PELAGIIDAE Gegenbaur, 1856
Genus Pelagia Perón & Lesueur, 1810
Pelagia noctiluca (Forsskål, Reference Forsskål1775)
(N = 7)
(Figure 2A–C)

Fig. 2. Scyphomedusae recorded at Isla del Coco National Park. A–C: Pelagia noctiluca, (A) lateral view, (B) oral plane, (C) aggregation of P. noctiluca close to the bottom at the site ‘Wall 0475’. D–F: Phacellophora camtschatica, (D) whole animal, (E) close-up of the radial canals, (F) hyperiid amphipods on subumbrellar cavity.
RECORDED MATERIAL
Arena: 05°33.732′N 87°02.232′W, and Wall 0475: 05°34.753′N 87°03.504′W. One individual observed on 19 January 2008 at a depth of 200 m at Arena, and six individuals on 12 May 2007 at Wall 0475 at a depth of 80 m (Figure 2C).
DESCRIPTION AND DISTRIBUTION
Bell covered with numerous stinging warts. Eight marginal tentacles alternating with 7–8 rhopalia and 16 marginal lappets. Mouth arms also with numerous nematocyst warts and crenulated margins. Gonads well developed. The individuals observed had a golden brown colouration.
Pelagia noctiluca is a common epipelagic oceanic species found mostly in warm seas, from the coast of Mexico to Chile (Larson, Reference Larson1990; Wrobel & Mills, Reference Wrobel and Mills1998). This species is one of the most abundant in certain areas of the ETP, such as the shelf area off central Mexico during summer (Segura-Puertas et al., Reference Segura-Puertas, Franco-Gordo, Suárez-Morales, Gasca and Godínez-Domínguez2010). In Costa Rica, it has been found in the Gulf of Nicoya and the Gulf of Papagayo (Rodríguez-Sáenz & Segura-Puertas, Reference Rodríguez-Sáenz, Segura-Puertas, Wehrtmann and Cortés2009).
Class SCYPHOZOA Goette, 1887
Order SEMAEOSTOMEAE Agassiz, 1862
Family ULMARIIDAE Haeckel, Reference Haeckel1879–80
Genus Phacellophora Brandt, Reference Brandt1835
Phacellophora camtschatica Brandt, Reference Brandt1835
(N = 4)
(Figure 2D–F)
RECORDED MATERIAL
Wall 0475: 05°34.753′N 87°03.504′W; Everest: 5°33.950′N 87°02.862′W, Piedra Drop: 5°34.640′N 87°03.557′W; and Piedra 165: 05°34.670′N 87°03.473′W. Observed from April to November at a depth range between 50–300 m. A single individual observed each time.
DESCRIPTION AND DISTRIBUTION
Bell with orange colouration. Marginal lappets 15–16, broad and curved, outer margin divided into sub-lappets. Rhopalia: 16 in deep clefts. Oral arms long, about twice the size of the bell diameter, also with orange colouration. Radial canals broad, 4–5 in each lappet, typically wider than the gaps between them. Present in all of the Eastern Pacific (Larson, Reference Larson1990), with a scattered distribution globally (Wrobel & Mills, Reference Wrobel and Mills1998).
Class HYDROZOA Owen, 1843
Order LEPTOTHECATA Cornelius, 1992
Family TIARANNIDAE Russell, 1940
Genus Modeeria Forbes, 1848
Modeeria rotunda (Quoy & Gaimard, Reference Quoy and Gaimard1827)
(N = 2)
(Figure 3A, B)
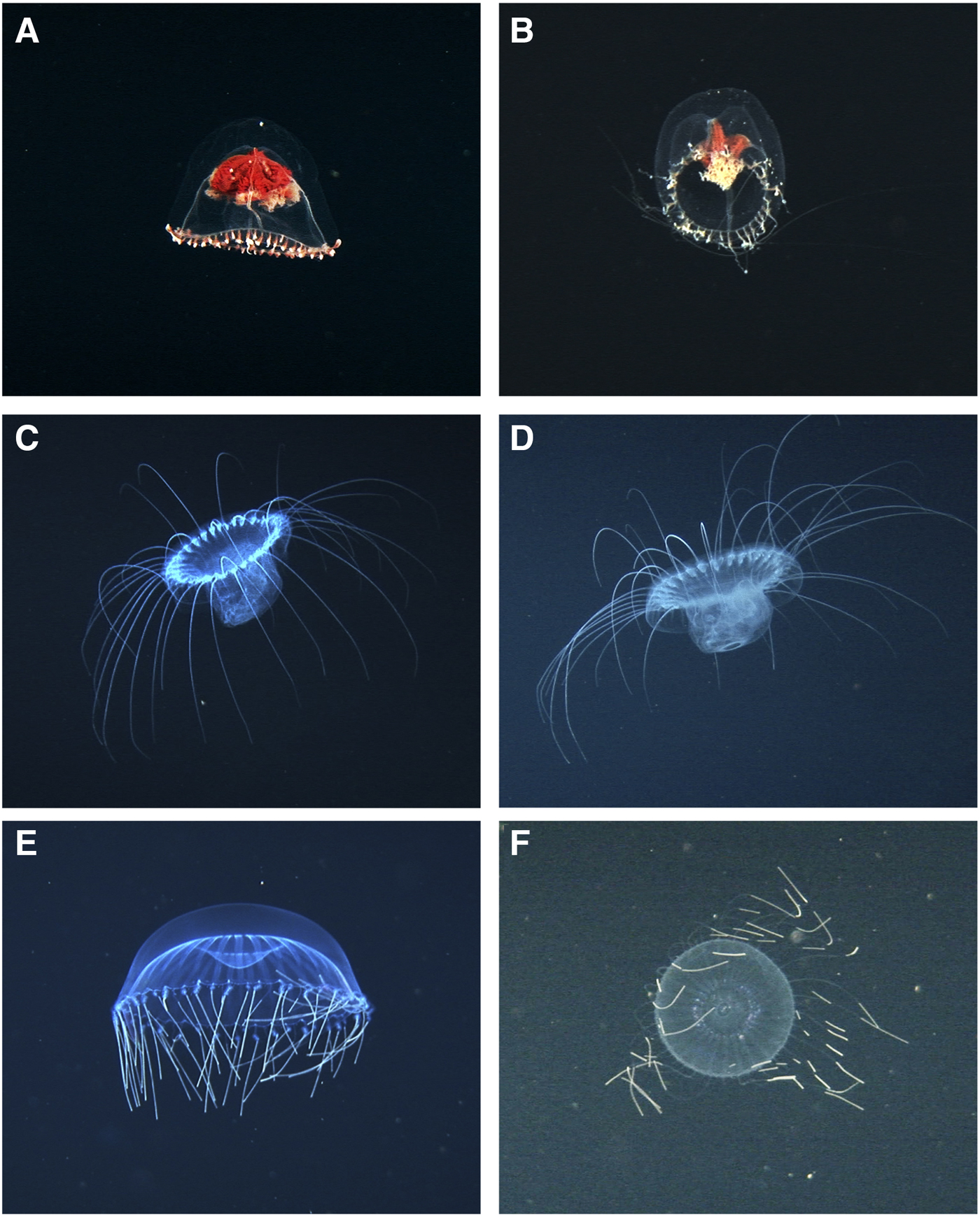
Fig. 3. Hydromedusae recorded at Isla del Coco National Park. A–B: Modeeria rotunda, (A) lateral view, (B) aboral plane, tentacles extended. C–D: Solmissus sp., (C) lateral view, (D) oral plane with gastrovascular cavity expanded, possibly due to prey capture. E–F: Halitrephes maasi, (E) lateral view with tentacles retracted, (F) aboral plane, tentacles extended.
RECORDED MATERIAL
Piedra Drop: 5°34.640′N 87°03.557′W; Wall 0475: 05°34.753′N 87°03.504′W. One individual observed each time on 10 February 2010 at Piedra Drop and on 16 October 2009 at Wall 0475; at an approximate depth of 200 m in both cases.
DESCRIPTION AND DISTRIBUTION
Disc hemispherical, mesoglea thick, apex rounded, manubrium short, broad, cruciform. One individual with 25 tentacles (Figure 3A) and the other with 44 (Figure 3B). Mouth with four large, slightly crenulated lips. Gonads well developed and attached to the 4 radial canals. Mooderia rotunda is found in the North Atlantic, Mediterranean, Pacific and Antarctic oceans (Wrobel & Mills, Reference Wrobel and Mills1998). This species has been collected in the San Clemente Basin off San Diego, California (Wrobel & Mills, Reference Wrobel and Mills1998), but there are no previous records from the ETP.
Class HYDROZOA Owen, 1843
Order NARCOMEDUSAE Haeckel, Reference Haeckel1879–80
Family CUNINIDAE Bigelow, 1913
Genus Solmissus Haeckel, Reference Haeckel1879–80
(N = 2)
(Figure 3C, D)
RECORDED MATERIAL
Wall 0475: 05°34.753′N 87°03.504′W; and Piedra Drop: 5°34.640′N 87°03.557′W. One individual observed each time in June 2009 and July 2010 at an approximate depth of 150 and 300 m, respectively.
DESCRIPTION AND DISTRIBUTION
Bell flat. Mouth consists of a simple opening in the centre. Stomach pouches and lappets as numerous as tentacles, with a well-defined conical insertion base. One individual with 30 tentacles (Figure 3C, D) and the other with 27 (Supplementary material). Found previously in all warm and temperate seas, from the surface to abyssal depths (Kramp, Reference Kramp1961). There is no previous record of this genus in the ETP.
Class HYDROZOA Owen, 1843
Order TRACHYMEDUSAE Haeckel, 1866
Family HALICREATIDAE Fewkes, 1886
Genus Halitrephes Bigelow, Reference Bigelow1909
Halitrephes maasi Bigelow, Reference Bigelow1909
(N = 2)
(Figure 3E, F)
RECORDED MATERIAL
Piedra 165: 05°34.670′N 87°03.473′W; and Piedra Drop: 5°34.640′N 87°03.557′W. One individual observed at each site in December 2010 at an approximate depth of 300 and 100 m, respectively.
DESCRIPTION AND DISTRIBUTION
Bell thick, disc-shaped. One individual with 28 non-branching radial canals and 54 marginal tentacles (Figure 3E), another with 27 non-branching radial canals and 49 marginal tentacles (Figure 3F). All tentacles arranged in a single series, with a flexible proximal portion and a stiff spine-like distal portion. Halitrephes maasi is a deep-water species, probably cosmopolitan in warm and temperate regions (Wrobel & Mills, Reference Wrobel and Mills1998). There are no previous records of this species in the ETP.
Class HYDROZOA Owen, 1843
Order SIPHONOPHORAE Eschscholtz, Reference Eschscholtz1829
Family PRAYIDAE Quoy & Gaimard in de Blainville, 1834
Genus Praya Quoy & Gaimard, Reference Quoy, Gaimard and Tatsu1833
(N = 4)
(Figure 4A, B)
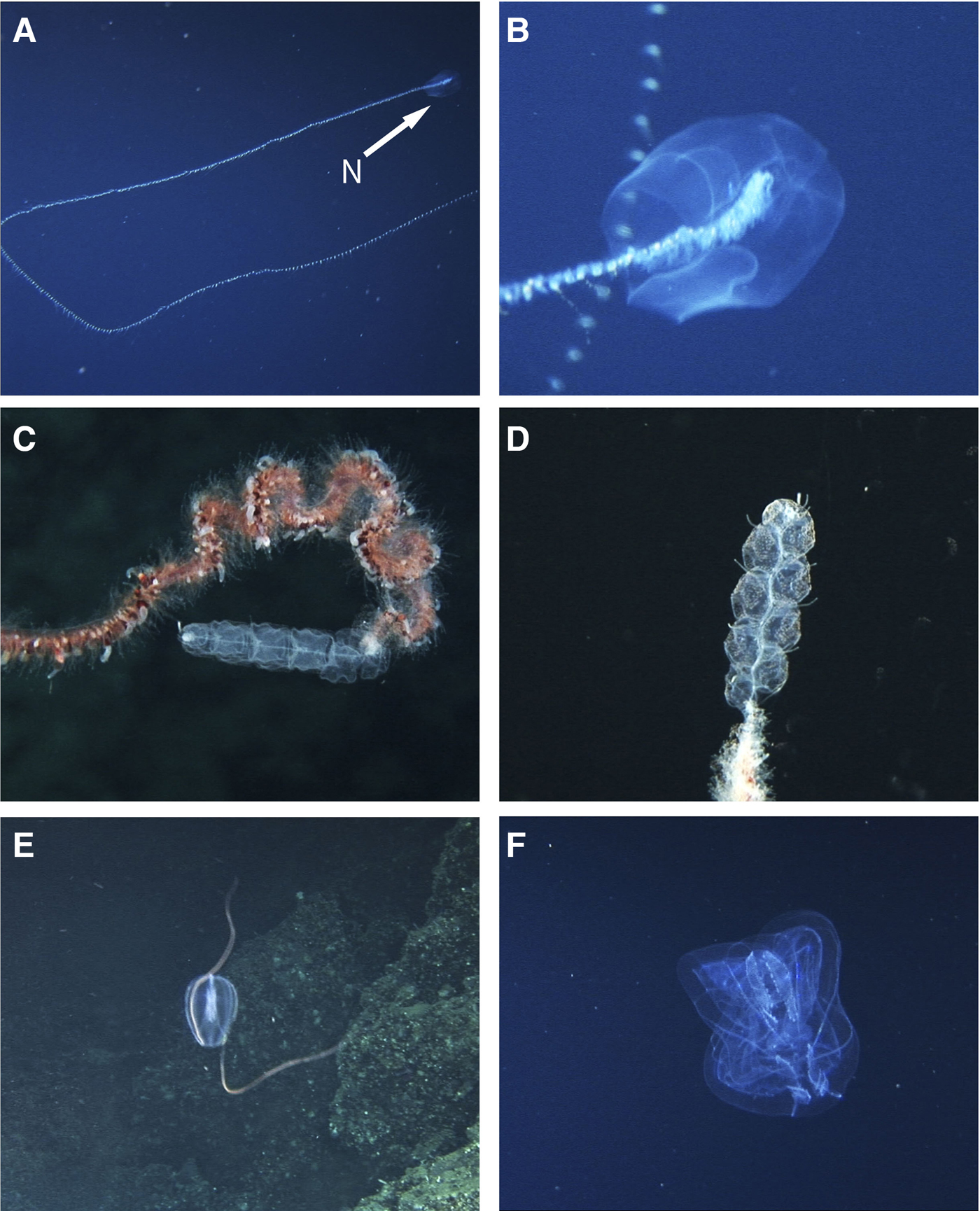
Fig. 4. Siphonophores and ctenophores recorded at Isla del Coco National Park. A–B: Praya sp., (A) whole colony, N: nectophore, (B) detail of the nectophores. C–D: Apolemia spp., (C) specimen without palpons and clear nectophores, (D) specimen with palpons and opaque spots on the nectophores, (E) Hormiphora sp. with tentacles extended. (F) aboral plane of Thalassocalyce inconstans.
RECORDED MATERIAL
Wall 0475: 05°34.753′N 87°03.504′W; Everest: 5°33.950′N 87°02.862′W; Piedra Drop: 5°34.640′N 87°03.557′W; and The Edge: 05°34.920′N 87°03.161′W. A single colony was recorded each time. Nectophores were visible on all colonies. Species observed from July to December in 2006, 2007, 2010 at variable depths (0–400 m).
DESCRIPTION AND DISTRIBUTION
Two large cylindrical nectophores, with a baso-ventral extension below the ostium of the nectosac. Recorded for the Northern Pacific, Atlantic, Antarctic and Indian Oceans (Mapstone, Reference Mapstone2009). Alvariño (Reference Alvariño1971) reports the species Praya reticulata (Bigelow, 1911) in several locations in the Eastern Pacific and Angulo et al. (Reference Angulo, Naranjo-Elizondo, Corrales-Ugalde and Cortés2014) has recorded this species for Isla del Coco. The genus Praya has also been collected on the Oceanographic Cruises to the Costa Rica Thermal Dome, DOMO I to IV between 1979 and 1982 (Vicencio-Aguilar & Fernández-Álamo, Reference Vicencio-Aguilar and Fernández-Álamo1996).
Class HYDROZOA Owen, 1843
Order SIPHONOPHORAE Eschscholtz, Reference Eschscholtz1829
Family Apolemiidae E. Huxley, 1859
Genus Apolemia Eschscholtz, Reference Eschscholtz1829
(N = 2)
(Figure 4C, D)
RECORDED MATERIAL
Wall 0475: 05°34.753′N 87°03.504′W; and Piedra Drop: 5°34.640′N 87°03.557′W. A single colony was recorded each time. Observed on 13 November 2009 and 1 December 2010, close to the bottom at 300 and 400 m, respectively.
DESCRIPTION AND DISTRIBUTION
Nectophore hollowed axially, forming a pair of large axial wings. The nectosac is extensive and its lateral radial canals follow an S-shaped course of varying complexity. One of the colonies with 10 smooth nectophores and without palpons (Figure 4C), the other colony has 9 nectophores with numerous warts and palpons (Figure 4D). Apolemia is widely distributed in all oceans (Alvariño, Reference Alvariño1971). There is no previous record of this genus in the ETP.
Phylum CTENOPHORA Eschscholtz, Reference Eschscholtz1829
Class TENTACULATA Eschscholtz, 1825
Order CYDIPPIDA Gegenbaur, 1856
Family PLEUROBRACHIIDAE Chun, 1880
Genus Hormiphora L. Agassiz, Reference Agassiz1860
(N = 1)
(Figure 4E)
RECORDED MATERIAL
Wall 0475: 05°34.753′N 87°03.504′W. Only one organism observed on 28 May 2007 at Wall 0475, at a depth of 300 m.
DESCRIPTION AND DISTRIBUTION
Body oblong, moderately compressed. Broadly rounded to flattened at the aboral end. Comb rows equal, extending from near the aboral pole to at least 4/5 the distance to the mouth. Canals underlying the comb rows without branches. Tentacle bulbs are about 1/3 of the body, located midway along the body and close to the pharynx. Tentacle sheaths parallel to the pharynx and exiting the body near the aboral end. Transparent body.
This genus has been recorded worldwide in warm and temperate seas. The species Hormiphora cf. palmata (Chun, 1898) has been reported in the Galápagos Islands (Tirado, Reference Tirado, Bungartz, Herrera, Jaramillo, Tirado, Jiménez-Uzcátegui, Ruiz, Guézou and Ziemmeck2012), but the author states that the identification and occurrence of this species in the archipelago is doubtful.
Class TENTACULATA Eschscholtz, 1825
Order THALASSOCALYCIDA Madin & Harbinson, Reference Madin and Harbison1978
Family THALASSOCALYCIDAE Madin & Harbinson, Reference Madin and Harbison1978
Genus Thalassocalyce Madin & Harbinson, Reference Madin and Harbison1978
Thalassocalyce inconstans Madin & Harbinson, Reference Madin and Harbison1978
(N = 3)
(Figure 4F, Supplementary Figure)
RECORDED MATERIAL
Wall 0475: 05°34.753′N 87°03.504′W; Piedra 165: 05°34.670′N 87°03.473′W. Three individuals observed between July and December in 2007, 2009 and 2010, at a depth of ~200 m at Wall 0475 site and near the bottom (326 m) at Piedra 165 and Wall 0475.
DESCRIPTION AND DISTRIBUTION
Body shaped like a broad medusa, up to 15 cm when fully expanded. Slit-like mouth on a central conical peduncle. Eight short comb rows. Tentacle bulbs located on sides of central peduncle. Tentacles with simple lateral filaments that are not enclosed in tentacle sheaths.
Known to occur in the Atlantic Ocean, Bahamas, Mediterranean and several locations off California (Wrobel & Mills, Reference Wrobel and Mills1998). There is no previous record of this species for the ETP.
DISCUSSION
Observations of gelatinous zooplankton were not made systematically because the ‘DeepSee’ submersible is used for tourism, and recordings focused mostly on large, conspicuous animals. Therefore, small hydromedusae and siphonophores may have been overlooked. However, this study complements and increases existing information of these organisms in the Eastern Tropical Pacific (Morales-Ramírez, Reference Morales-Ramírez2008; Angulo et al., Reference Angulo, Naranjo-Elizondo, Corrales-Ugalde and Cortés2014). For instance, the scyphozoans Pelagia noctiluca and Phacellophora camtschatica are distributed from California to Chile (Larson, Reference Larson1990), and were observed several times during the ‘DeepSee’ inmersions. Pelagia noctiluca has been previously found in Costa Rica (Rodríguez-Sáenz & Segura-Puertas, Reference Rodríguez-Sáenz, Segura-Puertas, Wehrtmann and Cortés2009), whereas P. camtschatica has not been recorded in Costa Rican waters. At Isla del Coco, aggregations of P. noctiluca (N = 6) were observed close to the bottom at the site ‘Wall 0475’ (Figure 2C).
Hydromedusae are well studied in the region (e.g. Segura-Puertas, Reference Segura-Puertas1984). Costa Rican waters contain at least 76 species of hydromedusae (Rodríguez-Sáenz & Segura-Puertas, Reference Rodríguez-Sáenz, Segura-Puertas, Wehrtmann and Cortés2009), with 53 species in the Gulf of Papagayo alone, a seasonal upwelling region in the ETP (Rodríguez-Sáenz & Vargas-Zamora, Reference Rodríguez-Sáenz and Vargas-Zamora2012). Mooderia rotunda has not been previously recorded in the region, which may be due to its deeper vertical distribution in the tropics: although this species is present in shallow waters of temperate regions (Wrobel & Mills, Reference Wrobel and Mills1998), it is probably limited to deeper waters in the tropics, a phenomenon also common in coronate medusae (Lucas & Critch, Reference Lucas and Critch1974; Pielou, Reference Pielou1979).
Rodríguez-Sáenz & Gasca (Reference Rodríguez-Sáenz, Gasca, Wehrtmann and Cortés2009) estimated that ~90 species of siphonophores inhabit the Pacific Ocean adjacent to Costa Rica, based on Alvariño (Reference Alvariño1972, Reference Alvariño1974), Stepanjants (Reference Stepanjants1977) and Gasca (Reference Gasca2002). Although Praya was frequently observed with the ‘DeepSee’, only P. reticulata has been recorded with enough detail to identify the species based on morphological characteristics of the nectophores (Angulo et al., Reference Angulo, Naranjo-Elizondo, Corrales-Ugalde and Cortés2014). Regarding Apolemia, we suggest that there may be additional species in the ETP, because the specimens recorded here had different numbers of palpons (Figure 4C, D) and one specimen had opaque spots on its nectophores (Figure 4D). Apolemia lanosa Siebert, Pugh, Haddock & Dunn, Reference Siebert, Pugh, Haddock and Dunn2013, recently described from Monterey Bay (Siebert et al., Reference Siebert, Pugh, Haddock and Dunn2013), has both characteristics previously mentioned, but collection of specimens is needed to verify this identification.
Records of ctenophores in the Eastern Pacific are scarce compared to other taxa of gelatinous zooplankton because their fragile bodies are usually destroyed by net trawls (Swift et al., Reference Swift, Hamner, Robison and Madin2009). Mathews (Reference Mathews1954) recorded in Hawaii only Hormiphora palmata, Beroe forskalii and the platycteniid ctenophore Coeloplana duboscqui Dawydoff, 1930. Wrobel & Mills (Reference Wrobel and Mills1998) recorded 28 species of ctenophores between the Gulf of Alaska and the Baja California Peninsula. In the Eastern Tropical Pacific, specifically in the Galapagos Islands, Tirado (Reference Tirado, Bungartz, Herrera, Jaramillo, Tirado, Jiménez-Uzcátegui, Ruiz, Guézou and Ziemmeck2012) lists Beroe sp., Hormiphora cf. palmata and Cestum sp. Eight species of ctenophores have been recorded in the Pacific coast of Mexico, where Ocyropsis maculate (Rang, 1828), Bolinopsis vitrea (L. Agassiz, Reference Agassiz1860) and B. forskalii have been recently found in surface waters of the Oaxacan coast (Ruiz-Escobar et al., Reference Ruiz-Escobar, Valadez-Vargas and Oliveira2015). Thalassocalyce inconstans is a unique ctenophore that presents a medusoid bell (Supplementary Figure B) without any discrete lobes (Madin & Harbison, Reference Madin and Harbison1978).
Behavioural and ecological information of the recorded species is limited due to the short time lapses (0.5–3 min) in which the organisms were observed. Some specimens of P. camtschatica had symbiotic hyperiid amphipods on the subumbrella (Figure 2F). This association has been reported in other regions of the Pacific Ocean (Towanda & Thuesen, Reference Towanda and Thuesen2006). Solmissus sp. shows the typical tentacle-first foraging behaviour (Mills & Goy, Reference Mills and Goy1988; Raskoff, Reference Raskoff2002) and an ingested prey (Figure 3C, D). On the other hand, the radial canals of H. maasi seem to be empty (Figure 3E) compared with other individuals that might have oil droplets in those structures, which has been interpreted as evidence of recent prey ingestion (Wrobel & Mills, Reference Wrobel and Mills1998). The images of T. incostans show individuals with their feeding disc retracted (Figure 4F, Supplementary Figure A) and extended (Supplementary Figure B), which relates to the type of prey captured by the ctenophore. Big, fast swimming prey such as euphausiids are engulfed by the contracted feeding disc (Swift et al., Reference Swift, Hamner, Robison and Madin2009) while small copepods are entrained in the mucus at the inner side of the feeding disc when it is expanded (Madin & Harbison, Reference Madin and Harbison1978). The videos taken by the ‘DeepSee’ have also led to the description of the association between a fish larva of the genus Paracaristius sp. with the siphonophore P. reticulata (Angulo et al., Reference Angulo, Naranjo-Elizondo, Corrales-Ugalde and Cortés2014).
The use of submersibles has provided new information on the diversity and natural history of gelatinous organisms in meso- and bathypelagic environments (Russell, Reference Russell1967; Larson et al., Reference Larson, Madin and Harbison1988; Matsumoto et al., Reference Matsumoto, Raskoff and Lindsay2003; Raskoff & Matsumoto, Reference Raskoff and Matsumoto2004). However, most studies of gelatinous zooplankton in the Eastern Tropical Pacific have sampled only the epipelagic stratum. The videos recorded by the ‘DeepSee’ submersible have been useful so far to broaden our knowledge on gelatinous species occurrence in this region, but the lack of a systematic sampling through time by the submersible impedes the use of the videos for relative abundance and seasonality estimations for the species in the area. New records will increase as more studies targeted on gelatinous zooplankton are conducted in the Eastern Tropical Pacific. Information about their behaviour and ecology can also increase as more systematic sampling and/or observations with submersibles are conducted in this region. Future research should also be focused on obtaining detailed environmental parameters and quantitative information about the behaviour and abundance of these organisms.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315417000558.
ACKNOWLEDGEMENTS
We acknowledge the support provided by the Vicerrectoría de Investigación (project 808-B0-654) and CIMAR, Universidad de Costa Rica. We thank the owners, captains and crews of the Undersea Hunter Group, also Shmulik Blum and his pilots of the ‘DeepSee’ submersible for the video recording. We appreciate the help of Astrid Sánchez-Jiménez, Alex Rodríguez-Arrieta and Cinthya Pérez with the video analysis, and Laurence P. Madin, Jennifer E. Purcell, Agustín Schiariti and an anonymous reviewer for their comments and corrections to this manuscript. This is a contribution of the Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Universidad de Costa Rica.







