Introduction
Biological invasions are one of the main threats to biodiversity and are responsible for species extinctions and ecosystem changes around the world (Walsh et al., Reference Walsh, Carpenter and Vander Zanden2016). The management of invasive species has required great efforts to implement measures to control the invasive vectors, such as initiatives for their early detection, monitoring and eradication, to avoid environmental disasters (Hewitt & Campbell, Reference Hewitt and Campbell2007; Creed et al., Reference Creed, Rocha, Hoeksema, Serrano, Rilov, Milazzo, Miranda, Sánches and Silva2020).
Marine bioinvasions are mainly related to two human activities, shipping and the aquarium trade. Bioinvasion vectors, found in ballast water and biofouling, have received substantial attention due to the marked increase in shipping activities in the past century (Carlton & Hodder, Reference Carlton and Hodder1995). However, in the last decades, the release of non-native aquarium species such as the lionfish and some cnidarians in marine natural habitats has also been considered another important source of bioinvasion (Padilla & Williams, Reference Padilla and Williams2004; Keller & Lodge, Reference Keller and Lodge2007).
Aquarium species can have invasive traits, such as the ability to tolerate broad variations in physiological conditions and a high reproductive capacity, which are favourable for logistical procedures of transport and commercialization, and consequently facilitate the establishment of their population in new environments (Padilla & Williams, Reference Padilla and Williams2004). They are also commonly traded in adult life-phases and as colonies, which allows greater capacity of survival and spread when released in natural non-native environments (Padilla & Williams, Reference Padilla and Williams2004). The risk of establishing success and potential impacts can be higher when these species have asexual reproduction, due to their ability to rapidly increase their population, as already described for ornamental plants and encrusting colonial cnidarians (Padilla & Williams, Reference Padilla and Williams2004; Ruiz-Allais et al., Reference Ruiz-Allais, Amaro, McFadden, Halász and Benayahu2014, Reference Ruiz-Allais, Benayahu and Lasso-Alcalá2021; Mantelatto et al., Reference Mantelatto, da Silva, dos Santos Louzada, McFadden and Creed2018).
Colonial octocorals are important components of the ornamental aquarium trade due to their colourful polyps. They reproduce sexually and asexually by budding and fission and have great potential to spread quickly (Kahng et al., Reference Kahng, Benayahu and Lasker2011). Recently, at least five cases of octocoral invasions in the Atlantic Ocean have been reported and associated with ecological impacts, including Unomia stolonifera (Gohar, 1938) in Venezuela (Ruiz-Allais et al., Reference Ruiz-Allais, Amaro, McFadden, Halász and Benayahu2014, Reference Ruiz-Allais, Benayahu and Lasso-Alcalá2021), Chromonephthea braziliensis van Ofwegen, 2005 (Lages et al., Reference Lages, Fleury, Ferreira and Pereira2006), Sansibia sp. and Clavularia cf. viridis (Mantelatto et al., Reference Mantelatto, da Silva, dos Santos Louzada, McFadden and Creed2018), and Erythropodium caribaeorum (Duchassaing & Michelotti, 1860) (Carpinelli et al., Reference Carpinelli, Cordeiro, Neves, Moura and Kitahara2020) in Brazil.
In 2018, a massive ‘blue carpet’ of octocorals was detected on the reef environments of Porto da Barra, Todos os Santos Bay, South-west Atlantic, occupying a large portion of these reefs. Careful analysis of images obtained from a scuba diving centre suggests that these octocorals have been in the region since 2015. Considering the risks of this invasion, the present study identifies and discusses the abundance and physical contact interactions of these species with local benthic organisms. The species were identified using morphology and DNA barcoding, and the benthic cover and contact interactions in two habitats were compared. Additionally, the study discusses the origin and evidence of the introduction of these species via the marine aquarium trade, and highlights the need to pay close attention to this introduction vector source that is often overlooked and neglected (Padilla & Williams, Reference Padilla and Williams2004; Keller & Lodge, Reference Keller and Lodge2007).
Materials and methods
Study area
This study was conducted in the rocky reefs of Porto da Barra, an area encompassing ~30,000 m2, located at one of the most popular urban beaches of the city of Salvador, Bahia, Brazil (13°0′14.41″S 38°32′2.38″W) (Figure 1). This area has high biodiversity and receives a large number of bathers and tourists year-round (Ferreira et al., Reference Ferreira, Coni, Medeiros, Sampaio, Reis-Filho, Barros, Loiola and Nunes2015b). The rocky reef environments under study are situated in the entrance of Todos os Santos Bay (TSB) (Figure 1), which is ~1200 km2 and contains several important and preserved ecosystems, although some portions of this bay have been historically subjected to environmental impacts, such as eutrophication, pollution and overexploitation of natural resources (Andrade & Hatje, Reference Andrade and Hatje2009).
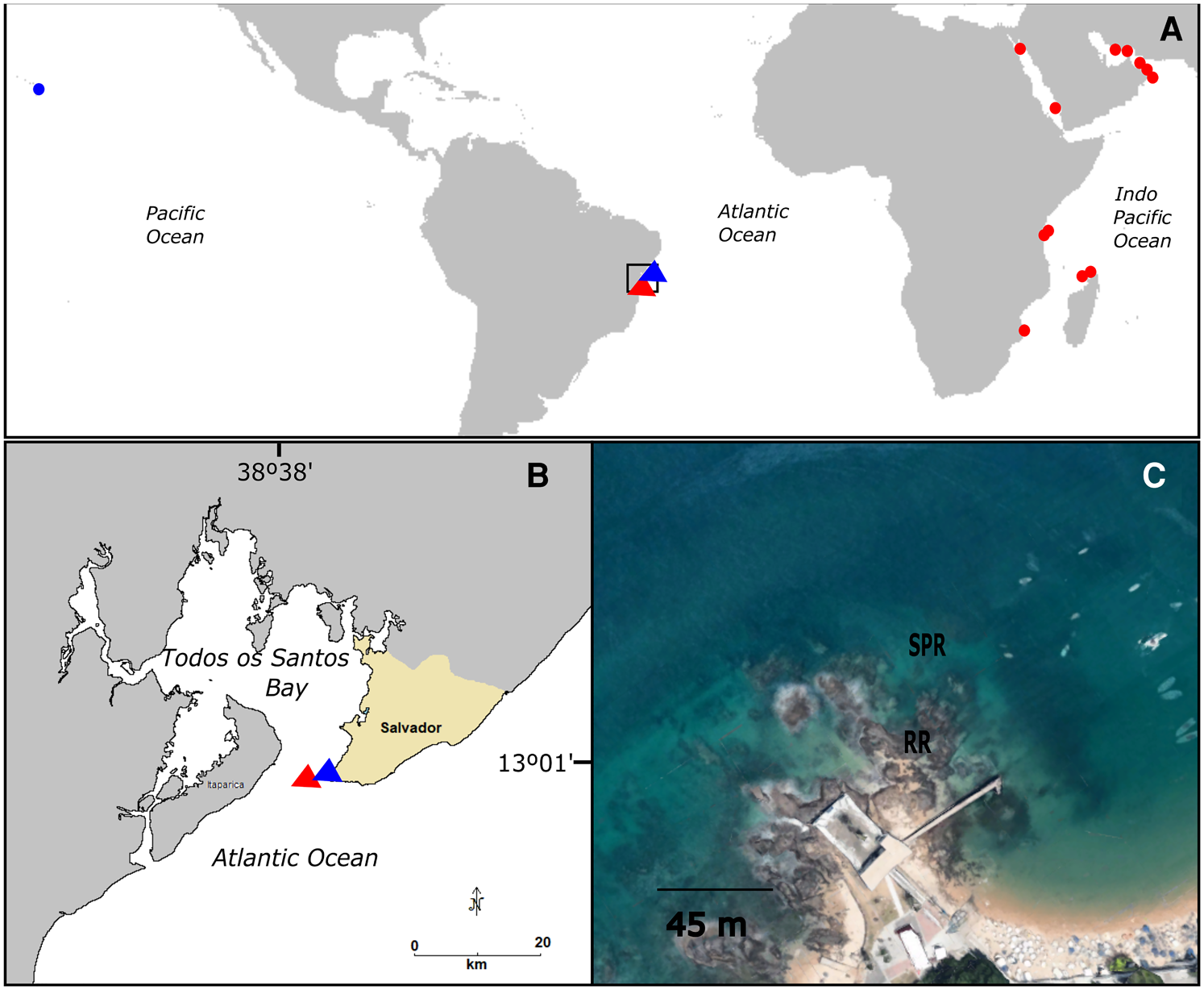
Figure 1. Maps with Briareum hamrum (red) and Sarcothelia sp. (blue) distribution. (A) Natural distribution (circle) and new records (triangles); (B) Locality where the non-native species were recorded – Porto da Barra, Todos os Santos Bay, Bahia, Brazil; (C) Reef environments where the species were found: ‘rocky reefs’ (RR), ‘sand and patch reefs’ (SPR).
Specimen collection and identification
Specimen collection was conducted in October 2018 through snorkelling at between 1–3 m depth (Figure 1). Four fragments (3 cm) of each morphospecies were removed with a hammer and chisel, put in vials containing anhydrous ethanol and transported to the laboratory for molecular analysis. Species were initially identified to family and genus based on morphological characteristics (colony growth form and morphology of sclerites) (Fabricius & Alderslade, Reference Fabricius and Alderslade2001). DNA was extracted from preserved tissue using the Qiagen DNeasy Blood and Tissue Kit, followed by PCR amplification and sequencing of mtMutS, COI and 28S rDNA using published primers and protocols (e.g. McFadden et al., Reference McFadden, Brown, Brayton, Hunt and van Ofwegen2014). The multilocus DNA barcode that combines these three gene regions discriminates >80% of octocoral species (McFadden et al., Reference McFadden, Brown, Brayton, Hunt and van Ofwegen2014). Sequences were initially identified to known species using BLAST (NCBI GenBank), being subsequently aligned to existing published sequences of taxa that were close matches. Sequences were aligned using MAFFT (Katoh et al., Reference Katoh, Kuma, Toh and Miyata2005), and pairwise genetic distances among taxa were calculated using MEGA v.5 (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). All sequences were submitted to GenBank (accession numbers MT448879–MT448880; MT461403–MT461406). Voucher specimens were deposited in the Benthic Ecology Laboratory at Universidade Federal de Alagoas (UFALPEN/LEB-C019 and UFALPEN/LEB-C020). All individuals sampled were under permits granted by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio, permit no. 64559-3).
Benthic community surveys and physical contact interactions
The benthic community survey was conducted in June 2018 in reef environments of Porto da Barra. The benthic coverage was estimated in two reef environments: ‘rocky reefs’ (RR), predominantly composed of metamorphic rocks between 1–3 m depth, and ‘sand and patch reefs’ (SPR), predominantly composed of sand, but with some small reef outcrops around 3 m deep. Photoquadrats (25 × 25 cm) were haphazardly taken ~1 m apart by free-divers. We took 20 photographs at RR and 20 at SPR, in an area of ~24,200 m2, and the distance between these two environments was ~10 m. Images were analysed using the software Photoquad v1.4, where the mean percentage of benthic coverage was estimated through 30 randomly distributed points per photograph. The benthic invertebrates were identified at the lowest possible taxonomic level. The benthic macroalgae were grouped in functional groups: macroalgae (leafy macroalgae), turf and encrusting calcareous algae. We also used roving diver surveys to search for isolated octocoral colonies in the study region. It is an exploratory methodology commonly used for fishes and is efficient for finding rare species in the environment (Leão et al., Reference Leão, Minervino, Ferreira, Feitosa, Sampaio, Costa-Sassi, Neves, Freire, Silva, Strenzel, Sovierzoski, Oliveira, Mendes, Soares, Araujo, Oliveira, Maida, Correia, Rosa, Sassi, Jonhsson, Francini Filho, Kikuchi, Leite, Turra and Denadai2015). To test for potential differences between the benthic cover in the two habitats, we used a one-way permutational multivariate analysis of variance (PERMANOVA). This analysis was based on a Bray–Curtis similarity matrix with 999 permutations. The number of physical contact interactions between the non-native species and native benthic organisms was measured in each photoquadrat, and a proportion of contacts per native group was calculated. We performed a χ2 analysis to test whether the proportion of contacts differed from the proportion of different taxa in each reef environment, RR and SRP, and to detect preferences or antagonistic relationships between native organisms and the non-native species. For χ2 analysis, we excluded the benthic category ‘sediment’ since we are testing interaction contacts between organisms. All statistical analyses were performed with the R package vegan (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Gavin, Solymos, Stevens, Szoecs and Wagner2019).
Results
Species identification
The molecular analysis confirmed the new occurrence of two non-native octocoral species in the Atlantic Ocean. Initial morphological examination of specimens and their sclerites identified the two species to family and genus respectively. One species, observed in situ as small stoloniferous colonies with colours that vary from purple to fluorescent blue (Figure 2A, C, D), was identified to Xeniidae based on the presence of the minute, corpuscle-like sclerites that are a diagnostic character of this family of soft corals. It is difficult, however, to identify most xeniids to species (or even to genus) based on morphology alone (e.g. McFadden et al., Reference McFadden, Haverkort-Yeh, Reynolds, Halász, Quattrini, Forsman, Benayahu and Toonen2017). Sequences obtained for the mtMutS, COI and 28S rDNA barcode markers were 100% identical to a species of Sarcothelia previously reported from the marine aquarium trade in the USA (Parrin et al., Reference Parrin, Goulet, Yaeger, Bross, McFadden and Blackstone2016). The specimen from Brazil also shared the same COI sequence as Sarcothelia edmondsoni Verrill, 1928, but differed from that species by 0.4% (uncorrected p) for both mtMutS and 28S.
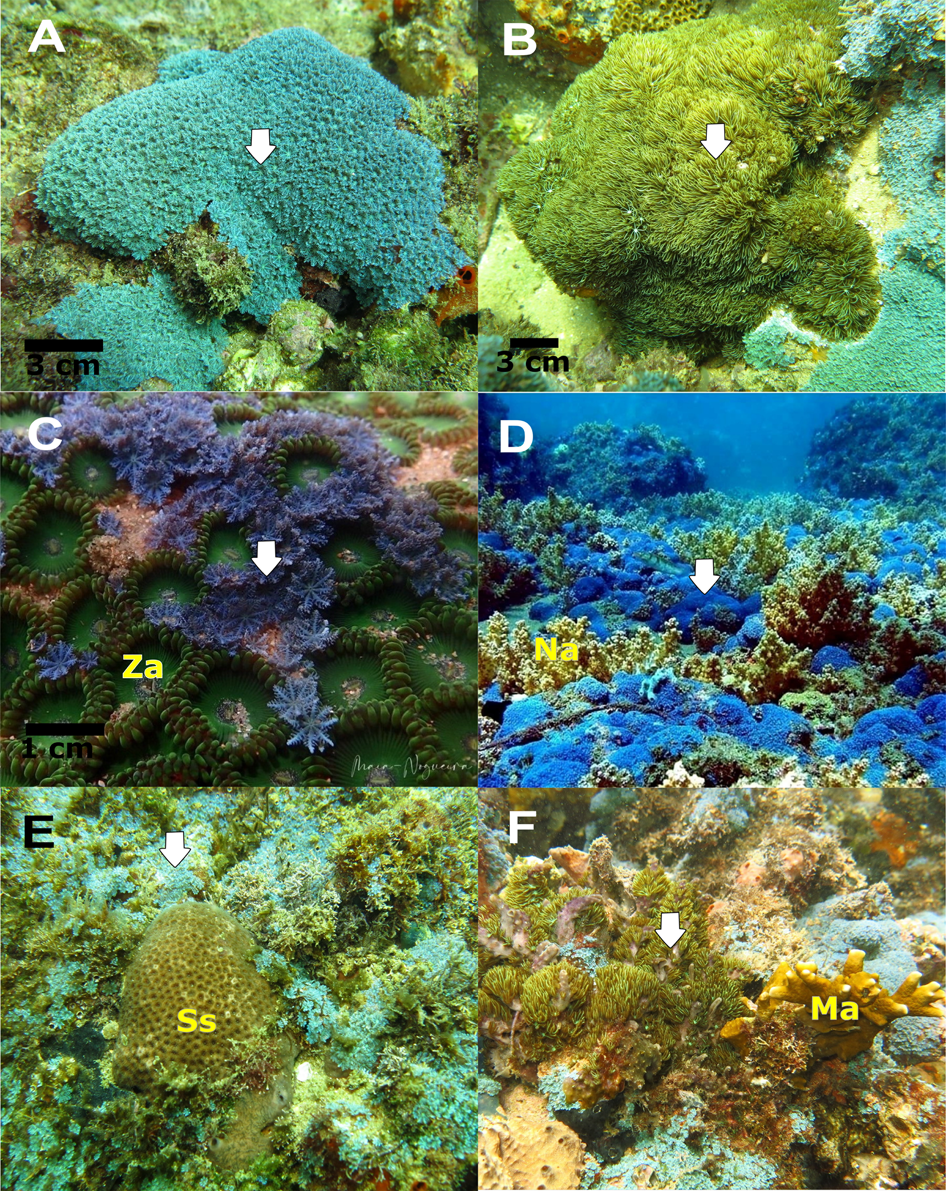
Fig. 2. Images of the non-native octocoral species on the rocky reef of Porto da Barra, Todos os Santos Bay, Bahia, Brazil. (A) Sarcothelia sp.; (B) Briareum hamrum; (C) Sarcothelia sp. overgrowing zoantharians; (D) ‘Carpet’ of Sarcothelia sp. in the sand and patch reef; (E) Physical contact between Sarcothelia sp. and Siderastrea sp.; (F) Physical contact between Briareum hamrum and Millepora alcicornis and sponges. Arrows indicate the non-native species in the figures; Za, Zoanthus sp.; Na, Neospongodes atlantica; Ss, Siderastrea sp.; Ma, Millepora alcicornis. Photographs: (A, B, C, E, F) Rodrigo Maia Nogueira; (D) Robson Oliveira.
The second species was observed as encrusting colonies that vary in situ from brownish to cream (Figure 2B). It was identified as a species of Briareum (Briareidae) based on the presence of sclerites of the unique form characteristic of that genus (i.e. spindles with tubercles arranged in distinct girdles, sclerites of purple inner layer; Samimi-Namin & van Ofwegen, Reference Samimi-Namin and van Ofwegen2016). Both mtMutS and COI sequences were 100% identical to B. hamrum (Gohar, 1948). It differed from the Indo-Pacific species B. violaceum (Quoy & Gaimard, 1833) and B. stechei (Kükenthal, 1908) by 0.4–0.5% at mtMutS, 0.3% at COI and 0.4–0.5% at 28S, and from the Caribbean species B. asbestinum (Pallas, 1766) by 0.4% at mtMutS, 1.2% at COI and 1.3% at 28S. No 28S sequences were available for B. hamrum.
Benthic community and physical contact interactions
The PERMANOVA analysis showed that benthic coverages were significantly different between rocky reefs (RR) and sand and patch reefs (SPR) (Figure 3, Table 1). The dominant taxon at the RR was the invasive species Sarcothelia sp. with per cent coverage reaching maximum values of 56.67% (mean 23.66% ± SD 21.46). At the SPR, the native octocoral Neospongodes atlantica Kükenthal, 1903 was the dominant taxon (24.33% ± 17.74) (Figure 2D). The second most abundant organism was algal turf for both habitats with mean values of 22.33% ± 17.74 and 21.50% ± 30.80 for RR and SPR, respectively. The per cent coverage of Sarcothelia sp. at SPR reached 43.33% (15.83% ± 15.81) and it was the third most abundant taxon. Zoantharians were found only at RR and were represented by Zoanthus sp. and Palythoa caribaeorum Duchassaing & Michelotti, 1860, totalling 11.16% ± 26.89. At SPR, 22.33% ± 23.47 of the bottom was represented only by sediments (Figure 3). The invasive species B. hamrum was not registered in photoquadrats, but three colonies were found through roving diver survey methods in RR. The maximum length × width (cm) of these colonies were 25 × 25, 30 × 25 and 22 × 20 cm.

Fig. 3. Boxplot of the benthic coverage (%) at both habitats (‘rocky reefs’ and ‘sand and patch reefs’) at Porto da Barra, Todos os Santos Bay, Bahia, Brazil. Red circles represent the position of the mean value. Black circles represent outliers.
Table 1. Results of PERMANOVA analysis for community comparison between ‘rocky reef’ (RR) and ‘sand and patch reef’ (SPR)

The results of physical contact interactions show that colonies of Sarcothelia sp. were directly contacting all the benthic groups used in our community analysis (Figure 2), totalling 54 and 41 contacts for RR and SRP, respectively. In the RR, Sarcothelia sp. were mainly in contact with the most abundant groups of turf, sponges and Neospongodes atlantica, representing 18.52, 25.93 and 27.78% of all encounters (Table 2), and the proportions of contacts amongst the observed taxa (observed) were not different from the proportional abundance of the taxa in the communities (expected) (χ2 = 11.199, P = 0.08). In contrast, in the SRP, we observed a significant difference between the proportion of contacts amongst the taxa and the proportion of abundance of taxa in the community (χ2 = 14.393, P = 0.008). The differential contacts were mainly observed for turf (a multi-species turf-forming algae) that represented 34.8% of abundance in the community, but only 19.5% of the contacts (Table 2). Although contacts with native scleractinian corals were not observed in the photoquadrats, some contacts were found during free-diving, as observed between Sarcothelia sp. and the scleractinian coral Siderastrea sp. (Figure 2E). Briareum hamrum was also in contact with native sponges and with the hydrozoan Millepora alcicornis (Figure 2F).
Table 2. Taxa/functional group encounters (percentage) with Sarcothelia sp. at ‘rocky reef’ (RR) and ‘sand and patch reef’ (SPR) in Porto da Barra, Todos os Santos Bay, Brazil

Discussion
This study identified two new non-native encrusting octocoral species, their distribution and abundances in shallow reef environments of the Brazilian coast and some aspects of their interactions with native organisms. Sarcothelia sp. belongs to a monotypic genus composed by Sarcothelia edmondsoni Verrill, 1928. Although S. edmondsoni is relatively abundant in the Hawaiian Islands, Pacific Ocean (Fenner, Reference Fenner2005), the genus has not to date been confirmed to occur anywhere else, and has been considered to be endemic to Hawaii. However, our data show that the specimen from Brazil is not an exact genetic match to S. edmondsoni, but instead matches material previously sampled from the marine aquarium trade in the USA that may not be conspecific with S. edmondsoni (Parrin et al., Reference Parrin, Goulet, Yaeger, Bross, McFadden and Blackstone2016). Its native range remains unknown, but all members of the family Xeniidae are restricted to shallow and tropical waters of the Indian and Pacific Oceans (Janes & Mary, Reference Janes and Mary2012). The only records of this family in the Atlantic Ocean are the invasive species in Venezuela (Ruiz-Allais et al., Reference Ruiz-Allais, Amaro, McFadden, Halász and Benayahu2014, Reference Ruiz-Allais, Benayahu and Lasso-Alcalá2021) and south-eastern Brazil (Mantelatto et al., Reference Mantelatto, da Silva, dos Santos Louzada, McFadden and Creed2018), both of which have been confirmed to have been introduced via the aquarium trade.
The second identified species, Briareum hamrum (Gohar, 1948), is native to the Red Sea and the western Indian Ocean and has not previously been found anywhere in the Atlantic (Samimi-Namin & van Ofwegen, Reference Samimi-Namin and van Ofwegen2016). The only species of Briareum known from the Atlantic Ocean is B. asbestinum (Pallas, 1766), which is restricted to the north Atlantic reefs (S. Florida to Barbados; Bayer, Reference Bayer1961). Our sequence data for the species found in Brazil differed from B. asbestinum by >1% at both COI and 28S, genetic distances that are considered high and indicative of different species of octocorals (McFadden et al., Reference McFadden, Brown, Brayton, Hunt and van Ofwegen2014). The other four Briareum species, B. hamrum, B. cylindrum Samimi-Namin & van Ofwegen, Reference Samimi-Namin and van Ofwegen2016, B. stechei (Kükenthal, 1908) and B. violaceum (Quoy & Gaimard, 1833), are known only from the Indo-Pacific Ocean, and one or more of them has been confirmed to occur in the aquarium trade in the USA (C.S. McFadden, unpubl. data). We consider the co-occurrence of two species previously unreported in the Atlantic and both known in the aquarium trade, to be strong evidence of a recent introduction of these non-native species to Brazil.
Despite photographic records of Sarcothelia from 2015, previous ecological studies at TSB did not find evidence for the presence of any of the conspicuous, attractive and colourful octocorals cited here (Ferreira et al., Reference Ferreira, Coni, Medeiros, Sampaio, Reis-Filho, Barros, Loiola and Nunes2015b; Barros et al., Reference Barros, Almeida, Cavalcanti, Miranda, Nunes, Reis-Filo, Silva, Hatje, Dantas and Andrade2018). Ferreira et al. (Reference Ferreira, Coni, Medeiros, Sampaio, Reis-Filho, Barros, Loiola and Nunes2015b) provided a detailed benthic community description of the study site showing that turf complex was the dominant functional group at the rocky reefs, reaching a mean coverage of 34.75% ± 15.68. In this study, although turf complex is also an abundant component at RR, Sarcothelia sp. surpassed its abundance (i.e. reef cover). Sarcothelia sp. was more abundant on rocky reefs (RR) than on sand and patch reefs (SPR) but was able to successfully colonize both habitats, representing an imminent modification of the native assemblage structure. Briareum hamrum had low coverage, which could represent an excellent opportunity to implement management actions to eradicate this species from the region considering the low effort needed to manually remove it in the field.
The results of physical analysis encounters between Sarcothelia sp. and benthic groups showed different patterns in RR and SRP. In the former, Sarcothelia sp. had significantly more contact with the more abundant groups, indicating a generalist colonization pattern of this species, without preferential or antagonistic behaviour. In the SRP, the proportion of encounters did not reflect the proportion of local native organisms contributing to the community. The differences are mainly related to differential interactions with turf (a multi-species turf-forming algae) (Table 2). Turf usually traps and accumulates sediment, and it is probably accumulating much more sediment in the sand reef patch than in the rocky reef (Connell et al., Reference Connell, Foster and Airoldi2014). Octocorals such as Sarcothelia sp. usually avoid sediments, which may explain why Sarcothelia sp. is the most abundant species in RR but not in SRP. Despite these differences, Sarcothelia sp. is abundant in both regions (Figure 3), and exhibits aggressive strategies such as overgrowing other organisms, as cited for other invasive octocorals (Ruiz-Allais et al., Reference Ruiz-Allais, Amaro, McFadden, Halász and Benayahu2014; Mantelatto et al., Reference Mantelatto, da Silva, dos Santos Louzada, McFadden and Creed2018) and observed in Figure 2C. Thus, further investigations are necessary to support the management of these octocorals and avoid the increase of invasion problems in the region as pointed out by Barros et al. (Reference Barros, Almeida, Cavalcanti, Miranda, Nunes, Reis-Filo, Silva, Hatje, Dantas and Andrade2018).
There are several examples of octocoral invasions recently reported for the Atlantic Ocean, which have resulted in reductions of local biodiversity. Lages et al. (Reference Lages, Fleury, Ferreira and Pereira2006) recorded a species of non-native octocoral, Chromonephthea braziliensis Ofwegen, 2005, which, although restricted to sandy bottoms of the south-eastern Brazilian coast, has caused severe damage to Phyllogorgia dilatata (Esper, 1806), a Brazilian endemic octocoral. Ruiz-Allais et al. (Reference Ruiz-Allais, Amaro, McFadden, Halász and Benayahu2014, Reference Ruiz-Allais, Benayahu and Lasso-Alcalá2021) reported a xeniid from Indonesia, recently identified as Unomia stolonifera (Gohar, 1938), invading coral reef communities of Venezuela, occupying both soft and coral reef substratum as well as eelgrass beds. It has reached a coverage of 30–80% and spread >10 km, growing over scleractinian corals and hydrocorals, such as Colpophyllia natans (Houttuyn, 1772), Diploria strigosa (Dana, 1846), Orbicella annularis (Ellis & Solander, 1786), Montastraea cavernosa (Linnaeus, 1767) and the hydrocoral Millepora alcicornis Linnaeus, 1758. Mantelatto et al. (Reference Mantelatto, da Silva, dos Santos Louzada, McFadden and Creed2018) detected two non-native octocorals, the xeniid Sansibia sp. and the clavulariid Clavularia cf. viridis, both from the Indo-Pacific, occupying shallow subtidal tropical rocky reefs along 170 m of shoreline at Ilha Grande Bay, south-east Brazil, dominating benthic communities and interacting negatively with native species. Recently, Carpinelli et al. (Reference Carpinelli, Cordeiro, Neves, Moura and Kitahara2020) also found the non-native species Erythropodium caribaeorum (Duchassaing & Michelotti 1860) dominating some substrates in Praia Vermelha, Angra dos Reis, state of Rio de Janeiro, south-eastern Brazil.
There is an imminent risk of the spread of these species on coral reefs in TSB and other regions in north-east Brazil, as has occurred for the invasive coral Tubastraea spp., a species that can cause tissue necrosis in native corals and changes in the benthic community structure and reef processes by competition (Miranda et al., Reference Miranda, Cruz and Barros2016). In the case of the octocorals identified in this study, some consequences of their establishment could be the reduction of coral recruitment and changes in reef-associated species because of allelopathic metabolites and anti-predatory chemicals found in some octocorals (Raveendran et al., Reference Raveendran, Mol and Parameswaran2011). In an extreme case, these non-native species can affect the ecosystem function of carbonate deposition on reefs through a coral reef phase shift – a process that occurs following a sudden change from the dominance of benthic organisms that build the calcareous structure of the reef, such as scleractinian corals, to the dominance of a non-reef-building organism, such as octocorals (Edmunds & Lasker, Reference Edmunds and Lasker2016). Cases of phase shifts with benthic invasive species have already been observed in Caribbean reefs with the seaweed Caulerpa brachypus (Lapointe et al., Reference Lapointe, Bedford and Baumberger2006).
The octocoral B. hamrum, commonly called ‘the Neon Green Star Polyps’, is a well-known species in the ornamental marine trade for their fast growth in aquarium tanks (Borneman, Reference Borneman2004). The distance between the study area (Brazil) and its native region (Indian Ocean) suggests that it is unlikely that their transport has been due to ballast water or biofouling, although it needs to be better investigated. Sarcothelia sp. has only been previously recorded in aquariums from the US ornamental trade, but without further information about its origin (Parrin et al., Reference Parrin, Goulet, Yaeger, Bross, McFadden and Blackstone2016). Although biological data for this species is still lacking because of its cryptic condition, the genus is considered a pest in the aquarium trade and it must be controlled regularly to avoid growing over other ornamental species (Borneman, Reference Borneman2004). Thus, we suggest that the introduction of B. hamrum and Sarcothelia sp. to the Brazilian coast probably occurred due to aquarium release, a similar pathway to that concluded by Mantelatto et al. (Reference Mantelatto, da Silva, dos Santos Louzada, McFadden and Creed2018) and Carpinelli et al. (Reference Carpinelli, Cordeiro, Neves, Moura and Kitahara2020), further south for other ornamental octocorals.
The reef environments where these two non-native species were found are on accessible and popular urban beaches (Porto da Barra), another indication that the species was probably released. This site, particularly, is an important area for the capture of ornamental organisms, which has supported local and national trade for decades (Rosa et al., Reference Rosa, Sampaio and Barros2006). Therefore, there is a possibility that aquarists take this water to their home aquariums and when discarded through the sewage they accidentally contaminate the region. Sites with clear waters and easy access have been previously reported to be used for farming purposes by commercial aquarists as mentioned by Ruiz-Allais et al. (Reference Ruiz-Allais, Amaro, McFadden, Halász and Benayahu2014) in Venezuela and previously discussed by Carpinelli et al. (Reference Carpinelli, Cordeiro, Neves, Moura and Kitahara2020) in south-eastern Brazil.
Along the Brazilian coast, the number of introduced non-native aquarium species is not very high compared with other regions such as Florida, USA (Padilla & Williams, Reference Padilla and Williams2004). Nevertheless, there are several Indo-Pacific species recorded on our coast such as the red lion fish, Pterois volitans (Linnaeus, 1758) (Ferreira et al., Reference Ferreira, Luiz, Floeter, Lucena, Barbosa, Rocha and Rocha2015a); the whitetip reef shark, Triaenodon obesus (Ruppell 1837) (Bornatowski et al., Reference Bornatowski, Loose, Sampaio, Gadig, Carvalho-Filho and Domingues2018); and the octocorals Sansibia sp. and Clavularia cf. viridis (Mantelatto et al., Reference Mantelatto, da Silva, dos Santos Louzada, McFadden and Creed2018). All are native to the Indo-Pacific and were recorded on the Brazilian coast. However, the number of non-native species of octocorals recently recorded, and highly likely to have been introduced from aquarium trade activities, is of great concern since many aquarium species can become potentially invasive (Padilla & Williams, Reference Padilla and Williams2004).
The popularization of the aquarium hobby has increased with the recent construction of several large public aquariums in Brazil, therefore more attention is needed in the control of the marine aquarium trade to avoid environmental impacts (Brasil, Reference Brasil2008). For this, three main actions are recommended: First, the inclusion of aquarium octocorals in the list of the harmful invasive species proposed by the Federal Government. The Ministry of Environment (MMA) determines which marine invertebrates cannot be commercialized due to their invasive history in other countries. Currently, it only prohibits the species Tubastraea spp. (http://www.ibama.gov.br/biodiversidade-aquatica/aquariofilia/listas-de-invertebrados-para-importacao). Second, education campaigns about good aquarium practices for aquarist shopkeepers. For instance, clear explanations that it is preferable to donate (to a public aquarium with environmental education programmes) or sacrifice anything withdrawn from an aquarium than to discard it in the natural environment. The third is the need of critical studies to support control or eradication programmes for these two invasive species. Species with low abundance can be manually removed, as long as it is possible to avoid the spread of small propagules. In contrast, for species with high abundance, a removal action needs to be planned more carefully. It would involve a thorough description of the species' distribution, research and tests on removal techniques, evaluation and monitoring of areas after removal. Research laboratories, museums, public aquariums and non-governmental organizations with environmental education programmes are the best destinations for donating samples. All of these recommendations have been pointed out to Brazil's federal environmental agency, IBAMA, however, for the moment, no action has been taken.
Acknowledgements
We are grateful to Prof. Dr Igor Cruz and BSc Tatiane Aguiar (Laboratório de Oceanografia Biológica – UFBA) for helping with field-sampling collection and suggestions. We thank Prof. Dr Henrique Batalha and his team (Laboratório de Evolução e Biogeografia – UFBA) for helping with initial laboratory tests for molecular analysis, Rodrigo Maia-Nogueira (SharkDive Diving Center) and Robson Oliveira (Submerso Diving Center) for their contribution with information and support. We also thank the funding agencies Fundação de Amparo à Pesquisa do Estado de Alagoas – FAPEAL (Process numbers: 60030 01270/2017 and 60030001275/2017); and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPQ (Process numbers: 380171/2020-1 and 304907/2017-0).
Financial support
This study was funded by Fundação de Amparo à Pesquisa do Estado de Alagoas – FAPEAL (Process numbers: 60030 01270/2017 and 60030001275/2017).







