INTRODUCTION
Hydroides Gunnerus, Reference Gunnerus1768 is a large serpulid genus that includes several species of bioinvaders and foulers found in ports and harbours worldwide (Link et al., Reference Link, Nishi, Tanaka, Bastida-Zavala, Kupriyanova and Yamakita2009; Otani & Yamanishi, Reference Otani and Yamanishi2010). These common tubeworms may form dense aggregations of hard calcareous tubes on underwater structures (Qiu & Qian, Reference Pallas1997; Lewis et al., Reference Lewis, Watson and ten Hove2006), becoming important nuisances to marine aquaculture, navigation, shipping industries and power plants (Sun et al., Reference Sun, Kupriyanova and Qiu2012). For example, Hydroides ezoensis Okuda, Reference Okuda1934 is known to cause flotation problems for buoys and adds considerably to fouling of ships (Zibrowius, Reference Zibrowius1978; Zibrowius & Thorp, Reference Zibrowius and Thorp1989; Wang & Huang, Reference Wang and Huang1993), as well as forming large encrustations within pipes used for the sea-water cooling systems (Thorp et al., Reference Thorp, Pyne and West1987).
Foulers can also modify ecosystem dynamics and species assemblages through competition for space, oxygen and food. Outbreaks of introduced H. elegans (Haswell, Reference Haswell1883), one of the most common fouling invasive species (Streftaris & Zenetos, Reference Streftaris and Zenetos2006), caused serious damage to cultured oyster crops in Japan (Arakawa, Reference Arakawa1971), where a mass occurrence of this species has resulted in heavy economic costs (~ ¥10 billion) to the oyster culture industry (Hirata & Akashige, Reference Hirata and Akashige2004; Link et al., Reference Link, Nishi, Tanaka, Bastida-Zavala, Kupriyanova and Yamakita2009). On the other hand, foulers’ encrustations can provide suitable microhabitats for a diverse community of sessile and mobile invertebrates. Protozoans, bryozoans, sponges, polychaetes and amphipods, among other fouling organisms, live on the tangled tubes of H. ezoensis in British waters (Thorp et al., Reference Thorp, Pyne and West1987). The bulk of its massive aggregations (up to 30 cm thick) provides food to fish and other invertebrates (Eno et al., Reference Eno, Clark and Sanderson1997). However, dense settlement of this species may kill native sessile species such as oysters and mussels by growing over them (Eno et al., Reference Eno, Clark and Sanderson1997).
Mexico has more than 11 000 km of coast line with 47 ports in the Pacific Ocean and 43 in the Gulf of Mexico and Caribbean, and the risk of invasions by species of fouling Hydroides is high because the main pathways for dispersion are associated with maritime traffic. In Mexico, a number of taxonomic studies have addressed composition and distribution of Hydroides species from both the Pacific and Atlantic coasts: Rioja (Reference Rioja1941a, Reference Riojab, Reference Rioja1942, Reference Rioja1944, Reference Rioja1946, Reference Rioja1958, Reference Rioja1959), de León-González (Reference de León-González1990), Bastida-Zavala & de León-González (Reference Bastida-Zavala and de León-González2002), Bastida-Zavala & ten Hove (Reference Bastida-Zavala and ten Hove2002, Reference Bastida-Zavala and ten Hove2003), Bastida-Zavala (Reference Bastida-Zavala2008), Tovar-Hernández et al., (Reference Tovar-Hernández, Méndez and Villalobos-Guerrero2009a, Reference Tovar-Hernández, Villalobos-Guerrero, Yáñez-Rivera, Aguilar-Camacho and Ramírez-Santana2012). As a result, 27 species of Hydroides have been recorded in the country (Table 1), representing about 28% of the species recognized in the genus. Eleven of them were originally described from Mexico (type locality); and H. gairacensis Augener, Reference Augener1934, H. diramphus Mörch, Reference Mörch1863, H. elegans and H. sanctaecrucis Krøyer in Mörch, Reference Mörch1863 have amphi-American records. Exhaustive sampling has been conduced to examine the biodiversity of fouling communities associated with marinas and port structures (buoys, docks, ropes, pilings, among other substrates) and hulls of vessels in Mexico (Tovar-Hernández et al., Reference Tovar-Hernández, Méndez and Villalobos-Guerrero2009a, Reference Tovar-Hernández, Méndez and Salgado-Barragánb, Reference Tovar-Hernández, Suárez-Morales and Yáñez-Rivera2010, Reference Tovar-Hernández, Villalobos-Guerrero, Yáñez-Rivera, Aguilar-Camacho and Ramírez-Santana2012, Reference Tovar-Hernández, Yáñez-Rivera, Villalobos-Guerrero, Aguilar-Camacho, Ramírez-Santana, Low Pfeng, Quijón and Peters2014, Villalobos-Guerrero & Tovar-Hernández, Reference Villalobos-Guerrero and Tovar-Hernández2013, Reference Villalobos-Guerrero and Tovar-Hernández2014). As a result, an undescribed species of Hydroides was discovered from the southern Gulf of California.
Table 1. Species of Hydroides recorded in Mexico (an asterisk denotes those species which type locality is placed in Mexico).

MATERIALS AND METHODS
Specimens of the new species of Hydroides were collected by hand from the fouling community on hard anthropogenic substrates (dock pilings, buoys and hulls of vessels) in six localities in the southern Gulf of California from 2008 to 2014 (permissions SAGARPA–CONAPESCA–DGOPA.14011.151012.3291 and DGOPA.05587.050608.1431). The fouling material from the Costa Rican Pacific was collected in a similar manner in 2012, where tube worms were removed from volcanic rocks (permissions No. ACG–PI–037–2012).
The samples were placed in a seawater-filled container for transportation to the laboratory and maintained in aquaria to document their live colouration and reproductive mode. Worms were anaesthetized with menthol crystals and fixed in 10% formalin in seawater or 95% ethanol. Specimens fixed in formalin were washed in the laboratory with tap water for 24 h and transferred to 70% ethanol for long-term preservation. In total, 234 specimens were examined for this study plus 12 specimens from museum collections.
A Leica MZ75 stereomicroscope and Olympus CH30 optical microscope were used for identification and digital photographs were taken with a Canon S5 camera. Longitudinal sections from one specimen of the new species were made to record spermatozoa morphology by means of scanning electron microscopy (SEM). Some opercula, fragments of thorax and abdomen were dissected and examined after final dehydration in two changes of 100% ethanol at the Laboratorio de Microscopía Electrónica de Barrido (Facultad de Ciencias, Universidad Nacional Autónoma de México). Specimens were critical point dried using CO2 before being mounted on stubs with platinum tape and coated with gold (200A° thickness). They were then viewed under a Cambridge 250 scanning electron microscope.
Terminology used in the description section follows ten Hove & Kupriyanova (Reference ten Hove and Kupriyanova2009). Holotype and complete paratypes were measured to record the lengths of body, radioles, filamentous tips, operculum, peduncle plus operculum, and thorax. We also counted the numbers of left and right radioles, radii funnel, verticil spines, thoracic and abdominal segments, and recorded the position of peduncle insertion (left or right), presence of pseudoperculum, operculum diameter, thorax width and presence of gametes. In the description section these measurements are based on holotype; measurements for paratypes are expressed between parentheses as mean values ± SD, number of paratypes analysed (N) and the range of such values. For example, radioles 4 mm long (2.66 ± 0.78, N = 11, 1.5–4 mm).
Additional material of H. dolabrus was examined from Colección de Referencia de Invertebrados, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México (EMU–ICML) and from Colección de Referencia, El Colegio de la Frontera Sur, Unidad Chetumal (ECOSUR). Type material and topotypes were deposited in the Australian Museum (AM), ECOSUR, in Colección de Referencia de Invertebrados (EMU–ICML) and Colección de Anélidos Poliquetos de México (CNP–ICML), both from Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. Comparative material of Hydroides species from the AM, ECOSUR, Zoological Museum of Amsterdam (ZMA), nowadays incorporated in the Naturalis Biodiversity Center, Leiden, the Netherlands and National Science Museum Tokyo (NSMT) with morphologically similar opercula is detailed in the ‘Comparative material examined’ section and included in Figures 4 & 5.
Molecular methods
Genomic DNA samples were obtained from tissues preserved in 95% ethanol. Two individuals of Hydroides dolabrus sp. nov. (AM W46904, AM W49606), two of H. panamensis (AM W47427, AM W47428) and one of H. recurvispina (AM W46941) were used for molecular work. Before the extractions, the tissue was washed by 0.5 mL TE buffer three times to remove ethanol. Qiagen DNeasy Blood and Tissue Kit was used to extract the genomic DNA according to the manufacturer's protocol. An approximate 1100 bp fragment of 18S rRNA, an approximate 420 bp fragment of mitochondrial cytochrome b (cyt b) gene, and an approximate 460 bp fragment of mitochondrial cytochrome c oxidase subunit I (COI) gene were amplified with the primers TimA/1100R2 (Nóren & Jordelius, Reference Nóren and Jordelius1999), Cytb424f/cob825 (Burnette et al., Reference Burnette, Struck and Halanych2005) and Hydro-COIF/Hydro-COIR (Sun et al., Reference Sun, Kupriyanova and Qiu2012), respectively. Polymerase chain reactions were performed in a total volume of 15 μL with 10× PCR buffer (1.5 μL), 25 mM MgCl2 (1.8 μL), 10 mM each primer (0.25 μL), 2.5 mM dNTPs (1 μL), Milli-Q H2O (9.12 μL), Qiagen Taq DNA Polymerase (0.08 μL) and template (1 μL). PCR reactions were carried out in an Eppendorf Mastercycler® pro using the following PCR protocol: for 18S, 94 °C for 2 min, 40 cycles at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 80 s, final extension at 72 °C for 5 min; for cyt b, 94 °C for 2 min, 40 cycles at 94 °C for 30 s, 48 °C for 30 s and 72 °C for 30 s, final extension at 72 °C for 5 min; for COI, 94 °C for 2 min, 40 cycles at 94 °C for 30 s, 51 °C for 30 s and 72 °C for 30 s, final extension at 72 °C for 5 min. PCR products were separated by electrophoresis using 1.0% agarose gel. Successfully amplified products were purified with the ExoSAP-IT (Affymetrix). An Applied Biosystems 3730 xl DNA Sequencer was used for bidirectional sequencing done by Macrogen Inc, South Korea.
All sequences generated were checked for contamination with searches (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990). Sequences from both directions of target fragment were assembled using CodonCode Aligner (CodonCode Corporation). To detect the interspecific genetic distance among H. dolabrus sp. nov. and other species in Hydroides, all available 18S, cyt b and COI sequences of Hydroides from GenBank and some unpublished sequences from Sun's (Reference Sun2009) MPhil thesis were used in phylogenetic analyses. These sequences belong to 16 Hydroides species. The details of these taxa, including taxon name, collection localities, voucher numbers and GenBank accession numbers are given in Table 2. The alignment was performed with ClustalX (Larkin et al., Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm, Lopez, Thompson, Gibson and Higgins2007) with default settings (15 gap opening penalty and 6.66 gap extension penalty), and subsequently edited by eye using BioEdit (Hall, Reference Hall1999).
Table 2. List of species used in this study, along with localities, GenBank accession numbers, and references cited.

Bayesian analyses were performed with MrBayes ver. 3.1 (Ronquist & Huelsenbeck, Reference Ronquist and Huelsenbeck2003). Models for the sequence data partitions for Bayesian analysis were chosen using MrModelTest ver. 2.3 (Nylander, Reference Nylander2004) according to the hierarchical likelihood ratio tests (hLRTs). For 18S, TrNef + G model was chosen; for cyt b, HKY + G model; and for COI, TrN + G model. Then, MrBayes ver. 3.1 was used to perform the Bayesian analysis and reconstruct the tree under the selected models for sequences. The parameters were unlinked in different genes so the genes could have different parameters despite using the same model. Two simultaneous runs with four Markov chains each ran for 4 000 000 generations, sampling every 1000 trees. Parameter stationarity was confirmed using Tracer (Rambaut & Drummond, Reference Rambaut and Drummond2007) by examining when values for all parameters (including tree -lnL) converged across the two analyses. Based on this, the first 1 000 000 generations (1000 trees) were discarded as burn-in. The majority rule consensus tree of the remaining 3000 trees for each analysis gave the posterior probabilities for each clade.
SYSTEMATICS
Order SABELLIDA Latreille, Reference Latreille1825
Family SERPULIDAE Rafinesque, Reference Rafinesque1815
Genus Hydroides Gunnerus, Reference Gunnerus1768
Hydroides dolabrus sp. nov.
(Figures 1–3, 4I & 5B, D, F)
Hydroides recurvispina — Tovar-Hernández et al., Reference Tovar-Hernández, Méndez and Villalobos-Guerrero2009a: 13–15, figures 3m, 8d–f (non Rioja, Reference Rioja1941a).

Fig. 1. Photo of live Hydroides dolabrus sp. nov. (A) Entire worm in dorsal view in a tube fragment; (B) same in ventral view, tube removed; (C) male spawning; (D) collar and radiolar crown in ventro-lateral view; (E–F) operculum, dorsal view; (G) collar and radiolar crown in dorsal view. Scale bars: (A, F) 2.5 mm, (B–C) 4 mm, (D) 2 mm, (E) 0.5 mm. Holotype AM W47012 (A–B, E–F), Paratype AM W47099 (C–D).

Fig. 2. Photo of live Hydroides dolabrus sp. nov., chaetae and uncini. (A) Base of crown and thorax, dorsal view; (B) same, ventro-lateral view; (C) same, dorso-lateral view; (D) details of collar; (E) detail of thoracic membranes and tori; (F) details of anterior abdominal chaetigers; (G) collar chaetae; (H) saw-shaped uncini from thorax; (I) saw-shaped uncini from anterior abdomen; (J) rasp-shaped uncini from posterior abdomen. Scale bars: (A–C, E) 2 mm, (D) 1 mm, (F) 1.5 mm, (G) 100 μm, (H–J) 15 μm. Paratypes AM W47100 (A–B), AM W47098 (C–F), Holotype AM W47012 (G–J).
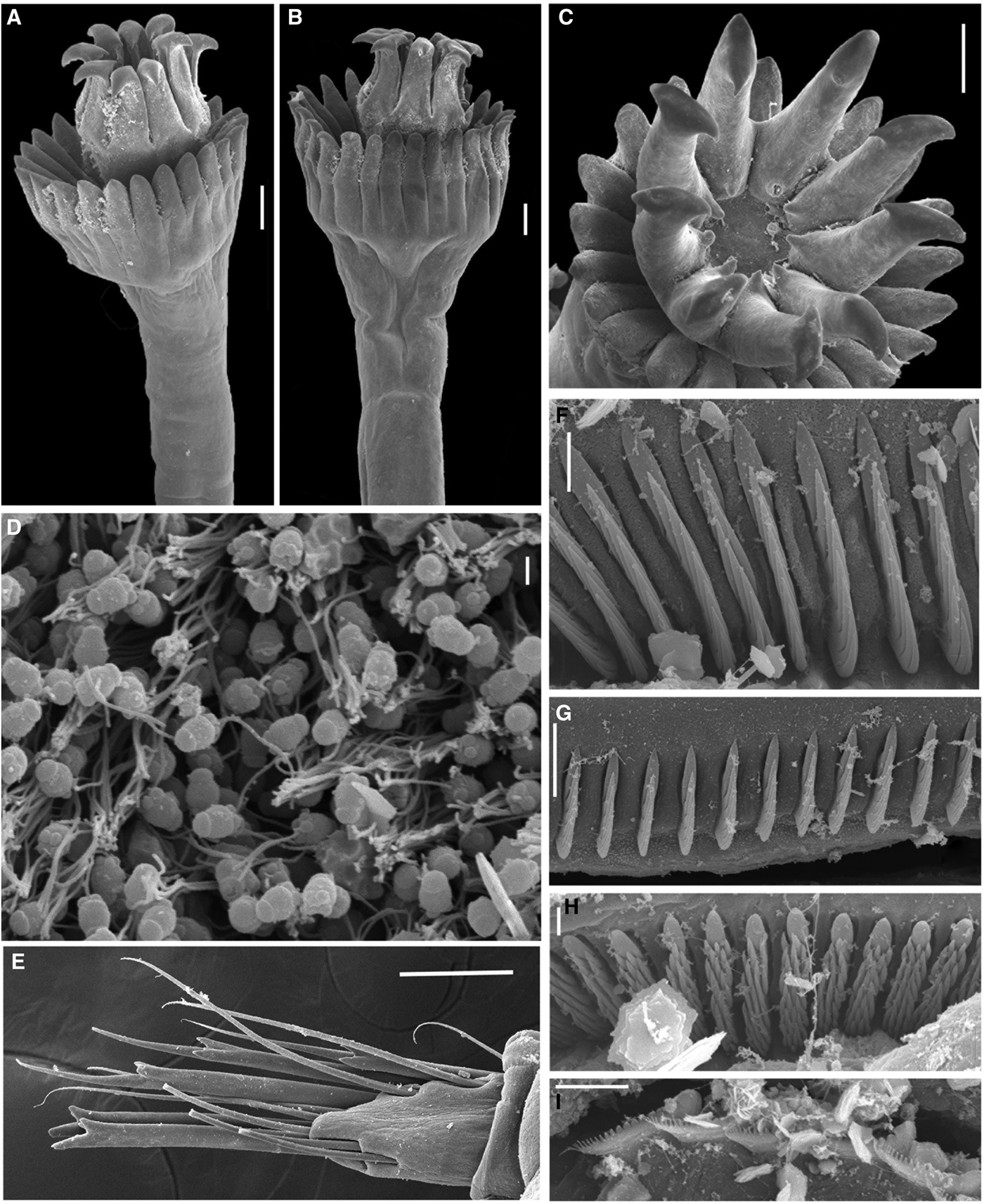
Fig. 3. Selected structures and sperm of Hydroides dolabrus sp. nov., under SEM. (A–C) Opercula in different views; (D) sperm extracted from medium abdomen; (E) collar chaetae; (F) saw-shaped uncini from the fourth thoracic chaetiger; (G) saw-shaped uncini from anterior abdomen; (H) rasp-shaped uncini from posterior abdomen; (I) flat trumpet-shaped chaetae from medium abdomen. Scale bars: (A–C, E) 100 μm, (D) 1 μm, (F, H) 3 μm, (G, I): 10 μm. Paratypes ECOSUR–0171 (A–I).

Fig. 4. Opercula of Hydroides species morphologically similar to H. dolabrus n. sp. (A) Hydroides microtis, AM W43202, USA, Florida; (B) H. bandaensis, ZMA 313, Siboga Expedition; (C) H. diramphus, AM W27696, Australia, New South Wales; (D) H. sinensis, AM W45634, China, Qingdao; (E) H. trompi ZMA 3358, Panama, Pacific, Miraflores Locks; (F) H. bisectus, NSMT H256, Japan, Okinawa; (G) H. recurvispina, AM W46941, Costa Rica, Guanacaste; (H) H. panamensis, AM W43184, Panama, Paitilla Beach; (I) H. dolabrus sp. nov., AM W40547, Mexico, Mazatlán.

Fig. 5. Comparison of opercula of Hydroides panamensis and H. dolabrus sp. nov. (A, C, E) Hydroides panamensis, ECOSUR 0038, paratype, Panama, Paitilla Beach; (B, D, F) H. dolabrus sp. nov., ECOSUR–0171, paratype, Mexico, Mazatlán. Scale bars: (A–F) 0.5 mm. Arrows indicate dorsal spines.
TYPE MATERIAL
Holotype: AM–47012, Mexico, Gulf of California, Sinaloa, Mazatlán, Mazatlán Marina, 23°16′47.42″N 106°27′39.94″W, dock fouling, 0.3–0.5 m, 17 April 2014, coll. Tovar, fixed in 10% formalin.
Paratypes: 19 paratypes (AM W46904, 1 specimen; AM W46905, 1 specimen; AM W46906, 1 specimen; AM W46907, 1 specimen; AM W46908, 1 specimen; AM W47097, 11 specimens; AM W47098, 1 specimen; AM W47099, 1 specimens; AM W47100, 1 specimen; fixed in 96% ethanol), 100 paratypes (ECOSUR–0171, fixed in 96% ethanol), 30 paratypes (EMU–10489, fixed in 10% formalin): Mexico, Gulf of California, Sinaloa, Mazatlán, Mazatlán Marina, 23°16′47.42″N 106°27′39.94″W, dock fouling, 0.3–0.5 m, 17 April 2014, coll. Tovar.
Topotypical material: CNP–ICML–POT–75–001, Mexico, Gulf of California, Sinaloa, Mazatlán, Mazatlán Marina, 23°16′47.42″N 106°27′39.94″W, dock fouling, 0.5 m, 26 April 2009, coll. Tovar, fixed in 10% formalin (3 specimens). Same, 3 November 2012, coll. Tovar, fixed in 96% ethanol (20 specimens). Same, 22 April 2013, coll. Tovar, fixed in 96% ethanol (8 specimens). EMU–8719A, C–J, L–R, T–Z, Mexico, Gulf of California, Sinaloa, Mazatlán, Urías Estuary, navigation channel, metallic buoys, 0.5 m, coll. Tovar, fixed in 10% formalin: site 27 (39 specimens) 23°12′13″N 106°24′31.4″W, 28 January 2009; site 28 (4 specimens) 23°12′13″N 106°24′30.1″W, 27 April 2009; site 29 (6 specimens) 23°11′48.7″N 106°24′31″W, 27 May 2009; site 30 (8 specimens) 23°11′31.1″N 106°24′43.9″W, 24 June 2009; site 31 (4 specimens) 23°11′8.9″N 106°24′55.8″W, 28 October 2009.
COMPARATIVE MATERIAL EXAMINED
Hydroides dolabrus sp. nov. (labelled as H. recurvispina): EMU–8521A, Mexico, Gulf of California, Sinaloa, Mazatlán port, ferry station, 23°11′10.9″N 106°25′12.2″W, 4 March 2008, metallic dock pilings, 18.7 °C, 37‰, coll. Tovar & Flores. EMU–8521B, same locality, 23°10′58.4″N 106°25′28.3″W, 21 July 2008, hull of a small vessel, coll. Tovar & Villalobos, fixed in 10% formalin (4 specimens). EMU–8521C, same, shipyard, 23°10′46.71″N 106°25′29.48″W, 4 August 2008, hull of a shrimp vessel, coll. Tovar & Villalobos, fixed in 10% formalin (3 specimens). EMU–8521D, same, dock ‘El Puma’ ship, 23°10′53.4″N 106°25′27.56″W, 14 August 2008, concrete dock pilings, coll. Villalobos, fixed in 10% formalin (2 specimens).
Hydroides bandaensis Zibrowius: ZMA 313, RV Siboga station 71 Sulawesi Makassar and surroundings, Pulu Barang Lompo, Samalona, shore exploration, down to 32 m, sand with mud, coral (1 specimen).
Hydroides bisectus Imajima & ten Hove: NSMT H256, Japan, Okinawa, Sesoko, coll. M. Imajima (1 specimen).
Hydroides diramphus Mörch: AM W27696, Australia, New South Wales, Port Jackson, Blackwattle Bay, 33°52′31″S 151°11′14″E, 18 April 2001, coll. A. Murray (1 specimen).
Hydroides microtis Mörch: AM W43202, USA, Florida, off Cape Canaveral to Ft. Pierce, 28°25′54″N 80°18′36″W, 15 October 1973, coll. D. Barber & H. A. ten Hove (1 specimen).
Hydroides panamensis Bastida-Zavala & ten Hove: AM W43184, Panama, Paitilla Beach, Tetraclita zone, 8°58′24″N 79°31′05″W, 4 January 1972, coll. H. ten Hove (1 specimen). ECOSUR–0038, Paitilla Beach, 28 October 1970, coll. A. A. Reimer (1 paratype). AM W47427, Costa Rica, Guanacaste, Cuajiniquil, 9°58′27.35″N 84°49′52.71″W, 25 November 2012, volcanic rock, coll. T. Villalobos, fixed in 96% ethanol (1 specimen for DNA). AM W47428, same (1 specimen for DNA).
Hydroides recurvispina Rioja: AM W46941, Costa Rica, Guanacaste, Cuajiniquil, 9°58′27.35″N 84°49′52.71″W, 25 November 2012, coll. T. Villalobos (1 specimen).
Hydroides sinensis Zibrowius: AM W45634, China, Qingdao, Qingdao fish markets, 36°03′22″N 120°20′17″E, 1 August 2013, coll. X. Wu & J. Sui (1 specimen).
Hydroides trompi Bastida-Zavala & ten Hove: ZMA 3358, Panama, Pacific, Miraflores Locks, Lower Chamber, 8°58′8″N 79°35′78″W, 26 August 1974, coll. M. L. Jones (1 specimen).
DIAGNOSIS
Verticil with 8–11 spines all similar in shape and size, with one short basal internal spinule each; without either external or lateral spinules. Verticil without central tooth. Spine tips with medial projections, pickaxe-shaped, asymmetrical, triangular: internal ones short and pointing inwards verticil centre; external projection longer than internal, pointing outwards verticil centre (Figures 1E, 3A–C, 4I & 5B, D, F).
DESCRIPTION
Tube white and covered with hydrozoans, sponges and ascidians. Lack peristomes, transverse ridges inconspicuous. Thin and circular in cross-section, curved, sinuous, or straight (Figure 1A). External diameter 2.2 mm (1.69 ± 0.26 mm, N = 8, 1.3–2 mm), internal diameter 0.2 mm (1.89 ± 0.26, N = 8, 1.5–2.2 mm).
Total body length 19 mm (14.64 ± 2.98 mm, N = 11, 11–20 mm).
Branchial lobes arranged in semi-circles, with 20 radioles on left lobe, 19 on right lobe (15 ± 2 left radioles, N = 11, 10–19; 16 ± 2 right radioles, N = 11, 12–19). Radioles 4 mm long (2.66 ± 0.78, N = 11, 1.5–4 mm). Filamentous tips up to 5 mm long (0.46 ± 0.07 mm, N = 11, 0.25–5 mm). Radiolar eyes absent.
Peduncle cylindrical, smooth, not separated from opercular funnel by a constriction (Figures 1A, F & 3A, B). Peduncle plus operculum length 5 mm (3.61 ± 0.87 mm, N = 11, 2–5 mm). Operculum placed on the left side in holotype (right side N = 5, left side N = 6 in paratypes). Pseudoperculum present, on right side in holotype (right insertion N = 6: left insertion N = 5 in paratypes), pseudoperculum smaller than peduncular plus opercular length. Operculum length 1.5 mm (1.37 ± 0.44 mm, N = 11, 0.8–2 mm), opercular diameter 0.7 mm (0.57 ± 0.22 mm, N = 11, 0.4–1 mm). Funnel with 29 radii with rounded tips (Figures 1E & 3A, B), in holotype two radii are fused (26 ± 3.75 radii, N = 11, 18–30 radii). Verticil with amber-coloured spines (9 ± 1 spines, N = 11, 8–11 spines). All spines similar in shape and size (Figures 1E, 3A, B & 5B, F) with a short basal internal spinule each (Figure 3C); without external and lateral spinules (Figure 3A, B & 5B, F). Verticil without central tooth (Figures 3C, 4I & 5D). Verticil spines in lateral view: pickaxe-like appearance (Figures 1E, 3A–C, 4I & 5B).
Collar trilobed, subdivided into one long medio-ventral and two latero-dorsal lobes, the latter shorter than medio-ventral lobe. Thoracic membranes well developed forming a ventral apron, extending towards second abdominal chaetiger (Figure 2B, E).
Collar chaetae bayonet with two blunt-elongate teeth (Figures 2G & 3E); distal blade smooth and limbate chaetae present (Figure 3E). Six thoracic chaetigers with narrowly limbate chaetae of two sizes; saw-shaped uncini with 6–7 teeth, all equal in size (Figures 2H & 3F).
Abdomen with 104 chaetigers (78 ± 14, N = 9, 54–97 chaetigers). Anterior and mid-abdominal chaetigers with flat trumpet-shaped chaetae; asymmetrical with distal margin lobed, proximal margin pointed (Figure 3I); denticulate edge (distal half with festooned teeth, basal half with pointed teeth); uncini saw-shaped with 5–7 teeth, equal size (Figures 2I & 3G). Posterior chaetigers with capillary chaetae and uncini rasp-shaped with 7–9 rows of teeth, equal in size (Figures 2J & 3H).
LIVE COLOUR
Base of branchial crown purple (Figure 1B, D, F). Radioles with purple bands (occupying the space of 3–4 pinnules) alternating with white bands (2–3 pinnules), purple and white colour extending towards pinnules (Figure 1A, B, D, F). Peduncle with purple basal band alternating with white and orange bands until the base of verticil (Figure 1F). Base of funnel and base of verticil black (Figure 1E). Body pale with collar and thoracic membrane translucent (Figures 1A & 2A–C, E, F). A large green olive area from the base of collar chaetiger extending towards the end of third thoracic chaetiger (Figure 2B, C). Ventral shields whitish with spongy-like texture (Figure 2D, E). A transverse brown band present anterior to each thoracic uncinigerous torus; brownish pigmentation present transversely across ventral side of abdominal segments, fainter bands dorsally in posterior part of abdomen. Posterior abdominal segments with dark spots below chaetigers. Live colour identical in all specimens from different locations in the Gulf of California.
POST-FIXATION COLOUR
Specimens from 2008 have grey funnel and verticil amber-coloured. Specimens from 2012–2014 have black funnel, base of verticil black and spines amber-coloured. Transverse brown bands present anterior to each thoracic uncinigerous tori in live specimens are still present in samples collected in 2008.
REPRODUCTION
Gonochoric broadcast spawning species lacking sexual dimorphism. Holotype and many paratype males with mature sperm in posterior abdominal segments (Figure 1C); more than 30 paratype females with oocytes in abdomen. Fully developed oocyte diameter measured in 10 females: 54.0 ± 10.9 μm (N = 95 oocytes; 41.5–66.4 μm). Spermatozoa with spherical nucleus, rounded acrosome, four mitochondria and a long flagellum (Figure 3D). Reproductive season occur during spring and summer (April to August).
HABITAT
Fouling worm in buoys, docks and on vessel hulls (Gulf of California). Inhabiting shallow waters (0.3–0.5 m deep). Salinity 32.1–34.8‰, temperature 21.5–31.5 °C and dissolved oxygen 3.15–6.66 mg L−1. Sharing habitat with 12 species introduced to the Gulf of California: polychaetes Branchiomma bairdi (McIntosh, Reference McIntosh1885), Hydroides diramphus (Mörch, Reference Mörch1863), H. elegans (Haswell, Reference Haswell1883), H. sanctaecrucis Krøyer in Mörch, Reference Mörch1863; ascidians Botryllus schlosseri (Pallas, Reference Pillai1766), Botrylloides nigrum (Herdman, Reference Herdman, Thompson and Murray1886), Botrylloides violaceus Oka, Reference Oka1927, Didemnun perlucidum Monniot, Reference Monniot1983 and Polyclinum constellatum Savigny, Reference Savigny1816; bryozoans Bugula neritina (Linnaeus, Reference Linnaeus1758) and Zoobotryon verticillatum (delle Chiaje, Reference delle Chiaje1822); and copepod Haplostomides hawaiiensis Ooishi, Reference Ooishi1994.
DISTRIBUTION
Southern Gulf of California.
ETYMOLOGY
According to ten Hove (Reference ten Hove, Read and Fauchald2009), for a long time the genus Hydroides was regarded to be feminine. However, according to Article 30.1.4.4 of the International Commission of Zoological Nomenclature (1999), ‘genus names ending in…-oides are substantivated adjectives and are masculine’. The new species is named Hydroides dolabrus sp. nov., from the Latin dolabra, a sort of pickaxe that resembles the shape of the verticil spines.
TAXONOMIC REMARKS
Hydroides dolabrus sp. nov. is the 11th species in the genus described from Mexico (Table 1). It is very common throughout the year at Mazatlán port and Mazatlán Marina, where numerous private sailing boats constantly arrive from the USA and Canada. In terms of commercial traffic of agricultural, industrial, fishing, automobile, petroleum and derived products, Mazatlán port is part of a dense connection network comprising many ports in the world.
Among 96 nominal species currently recognized as valid in the genus Hydroides (after Pillai, Reference Qiu and Qian2009; ten Hove & Kupriyanova, Reference ten Hove and Kupriyanova2009), many have verticils with lateral and/or external spinules, others either have a distinct dorsal massive spine (hook) or have the dorsal-most spines longer than the rest, but still similar in shape. Some species have verticils with central spines. The new species belongs to a (likely artificial) group of species in which all verticil spines are nearly identical in size and shape and lack both lateral and external spinules. Within this group where most species have simple verticil spine tips, either sharply pointed or slightly rounded, there is a sub-group of nine species (Hydroides bandaensis Zibrowius, Reference Zibrowius1972, H. bisectus Imajima & ten Hove, Reference Imajima and ten Hove1989, H. diramphus Mörch, Reference Mörch1863, H. microtis Mörch, Reference Mörch1863, H. panamensis Bastida-Zavala & ten Hove, Reference Bastida-Zavala and ten Hove2003, H. recurvispina Rioja, H. sinensis Zibrowius, Reference Zibrowius1972, H. trompi Bastida-Zavala & ten Hove, Reference Bastida-Zavala and ten Hove2003 and H. dolabrus sp. nov.) characterized by various modifications to the tips of their verticil spines.
In H. microtis verticil spinal tips are distinctly globular (Figure 4A), they are globular to blunt in H. bandaensis (Figure 4B), and distinctly T-shaped, having laterally positioned projections, in H. diramphus (Figure 4C). In H. sinensis the tips of verticil spines vary from oval to trapezoidal in shape, but this species is also characterized by large conspicuous internal spines (Figure 4D). While in these four species the verticil tip modifications are symmetrical, the modified tips of verticil spines are distinctly asymmetrical in the rest of the species in this group.
In H. trompi the tips of verticil spines are curved into hooks that are turned clockwise (Figure 4E). In H. bisectus tips of the verticil are modified into projections that are radially positioned, their spines have terminal dark hooks pointing inwards, towards the verticil centre and with long whip-like filaments pointing outwards (Figure 4F). In H. dolabrus sp. nov. these projections are also radially orientated and asymmetrical, both with pointed sides, but the internal sides (inwards verticil centre) are shorter than the external one (outwards verticil centre) (Figures 1E , 3A–C & 4I), which is the most distinct character of the species.
The new species is also similar to H. recurvispina Rioja, Reference Rioja1941a. However, H. recurvispina is distinct in having pointed spine tips with external distal knobs that are absent in H. dolabrus sp. nov. (Figure 4). In addition, H. recurvispina has straight verticil spines with tips turned clockwise, while spines are curved outwards and spine tips are positioned radially, not turned clockwise in H. dolabrus sp. nov. (compare Figure 4G, I).
Hydroides dolabrus sp. nov. is more similar to H. panamensis than to the rest of the species to the extent that these two species can be easily confused, but there are clear differences between them. In H. panamensis dorsal spines along with their distal radially positioned projections are slightly longer than remaining spines (Figure 5A, E), whereas in H. dolabrus sp. nov. all spines and projections are of equal length (Figures 1E, 3A–C, 4I & 5F). Each verticil spine in H. panamensis has one distal radially positioned projection (Figures 4H & 5E). However, distal projections of dorsal and ventral spines in H. panamensis are different, as in the former the distal projections are nearly triangular or arrow head-shaped when seen from the top (Figure 5A, C, E), while in the latter distal radial projections are with both symmetrical pointed ends, having a pickaxe-like appearance (Figure 5A, C, E). In contrast, all distal projections of verticil spines in H. dolabrus sp. nov. are identical, having an asymmetrical pickaxe-like appearance (external projection being longer than internal) (Figures 3A–C & 5B, D, F). Basal internal spinules on verticil spines are long in H. panamensis (Figure 5C), but relatively short in H. dolabrus sp. nov. (Figures 3C, 4I & 5D). Tips of radii in the opercular funnel are rounded in H. dolabrus sp. nov. (Figures 3A, B & 5F), but are pointed in H. panamensis (Figure 5C, E).
MOLECULAR RESULTS AND DISCUSSION
The results of a Bayesian analysis based on the combined dataset of 18S, cyt b and COI of 17 Hydroides spp. (Table 2) are shown in Figure 6. The partially resolved tree supports H. dolabrus sp. nov. as a separate species within the genus. Hydroides dolabrus sp. nov. and H. panamensis were recovered as a well-supported (pp 1.0) sister group, supporting the morphology-based hypothesis of close phylogenetic relationship between these species. The clade H. brachyacanthus – H. dolabrus – H. panamensis – H. sanctaecrucis is also well supported (pp 0.98).

Fig. 6. Bayesian 50% majority-rule consensus phylogram based combined dataset of 18S, cyt b and COI. Bayesian posterior probabilities above 0.9 are indicated near the nodes. Asterisk indicates posterior probability 1.0.
Three species morphologically similar to H. dolabrus (see Figure 4), H. diramphus, H. panamensis and H. recurvispina, were included in the analysis. Hydroides diramphus is recovered as a sister group to the H. brachyacanthus – H. dolabrus – H. panamensis – H. sanctaecrucis clade with relatively low branch support (pp < 0.9). In contrast to the group of species with similar opercular morphology, H. recurvispina is genetically distinct from H. dolabrus sp. nov., recovered as a sister group to a clade consisting of eight other species in this study. As the support value of this group is low (pp < 0.9), the relationship between H. recurvispina and other Hydroides species is still uncertain. Hydroides recurvispina was barcoded by Sun et al. (Reference Sun, Kupriyanova and Qiu2012) and recovered as a sister group to H. sanctaecrucis. However, based on opercular morphology of the specimen (AM W46941), the ‘Hydroides recurvispina’ used in their study is H. dolabrus sp. nov.
Genetic data, especially those of the standard barcoding gene COI, have been used to help with the species delimitation within a limited number of Hydroides species (Kupriyanova et al., Reference Kupriyanova, Bastida-Zavala, Halt, Lee and Rouse2008, Sun et al., Reference Sun, Kupriyanova and Qiu2012). Our molecular results indicate a clear distinction among H. dolabrus sp. nov., H. panamensis, H. diramphus and H. recurvispina. However, we were not able to include another five species belonging to the morphologically similar subgroup (Figure 4) because of the lack of available DNA sequences. A better understanding of speciation in the genus Hydroides will result from a large-scale study using molecular data to address phylogeny and barcoding of the genus. Such a project is being carried out as part of Yanan Sun's PhD thesis.
ACKNOWLEDGEMENTS
Thanks are due to Jaime Ruiz for allow us access and sampling at Marina Mazatlán. Silvia Espinosa Matías (Facultad de Ciencias, UNAM) helped us with processing SEM photographs. We want to extend our thanks to curators and collections managers for loans of the comparative material: Joke Bleeker (Naturalis), Hironori Komatsu (NSMT) and Luis F. Carrera Parra (ECOSUR). Roger Blanco and María Martha Chavarría provided collecting facilities and support to TFVG in Guanacaste, Costa Rica. Eunice Wong helped with taking colour photos for Figure 4. The study greatly benefited from critical remarks of Rolando Bastida-Zavala who reviewed an early version of the manuscript, as well as Ángel de León-González and two anonymous reviewers.
FINANCIAL SUPPORT
This study was partially funded by Australian Biological Resource Survey (ABRS) grant RF213-19 to EKK.










