Introduction
Amphiuridae Ljungman, 1867 is the most diverse of the families of Ophiuroidea (Stöhr et al., Reference Stöhr, O'Hara and Thuy2012) comprising 506 species, distributed across 27 genera (O'Hara et al., Reference O'Hara, Hugall, Thuy, Stöhr and Martynov2017). In Brazil, 48 species belonging to 11 genera have been recorded (Barboza & Borges, Reference Barboza and Borges2012). Amphiurids occur in soft bottoms, rocky shores and many biological substrates such as sponges, algae, coral reefs and seagrass (Hendler et al., Reference Hendler, Miller, Pawson and Kier1995; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005). They are also commonly found extending two to four arms across the sediment surface, with their arm tips slightly raised to capture particles for feeding (Hendler et al., Reference Hendler, Miller, Pawson and Kier1995).
An important morphological difference between the amphiurid genera Amphiodia Verrill, 1899 and Ophiophragmus Lyman, Reference Lyman1865 is that only Ophiophragmus has a fence of papillae at the edge of the disc interradius (H.L. Clark, Reference Clark1918). However, some specimens can cast off their disc as a stress reaction, depending on the sampling method (e.g. dredge and van Veen grab), complicating species recognition. Therefore, the observation and description of other features, such as oral and adoral shields, as well as dorsal and ventral arm plates, is required in order to identify the species (H.L. Clark, Reference Clark1918; Thomas, Reference Thomas1962). In addition to these commonly used characters, others may also be integrated, such as arm microstructure morphology and morphometry of diagnostic structures.
The study of ophiuroid microstructures has provided new information on the identification and classification of modern and fossil species (Martynov, Reference Martynov2010; Thuy & Stöhr, Reference Thuy and Stöhr2011, Reference Thuy and Stöhr2016). Recently, all brittle star families were diagnosed using external morphology and microstructure of ossicles (O'Hara et al., Reference O'Hara, Stöhr, Hugall, Thuy and Martynov2018). The morphometry of diagnostic structures has also been used as a powerful tool for delimiting species, particularly when included in an integrated approach (Arribas et al., Reference Arribas, Andújar, Sánchez-Fernández, Abellán and Millán2013; Alitto et al., Reference Alitto, Amaral, Oliveira, Serrano, Seger, Guilherme, Di Domenico, Christensen, Lourenço, Tavares and Borges2019). This methodology demonstrates how different disciplines can be more informative when treated together (Stöhr, Reference Stöhr2012), providing taxonomy with a more integrated view, and consequently, improving our knowledge.
A total of 36 Amphiodia species are recognized at present (Stöhr et al., Reference Stöhr, O'Hara and Thuy2019) and five are recorded in Brazil (Barboza & Borges, Reference Barboza and Borges2012): A. habilis Albuquerque, Campos-Creasey & Guille, Reference Albuquerque, Campos-Creasey and Guille2001, A. planispina (v. Martens, 1867), A. pulchella (Lyman, 1869), A. riisei (Lütken, Reference Lütken1859), and A. trychna H.L. Clark, Reference Clark1918. At present, 18 Ophiophragmus species are recognized (Stöhr et al., Reference Stöhr, O'Hara and Thuy2019), and seven are recorded in Brazil (Barboza & Borges, Reference Barboza and Borges2012): O. brachyactis H.L. Clark, Reference Clark1915, O. cubanus (A.H. Clark, Reference Clark1917), O. filograneus (Lyman, Reference Lyman1875), O. luetkeni (Ljungman, 1872), O. pulcher H.L. Clark, Reference Clark1918, O. septus (Lütken, Reference Lütken1859), O. wurdemanii (Lyman, 1860).
Our aim is to redescribe the Brazilian species of Amphiodia and Ophiophragmus using external morphology, arm microstructure morphology (arm ossicles) and morphometry. The results are particularly important as they provide additional taxonomic characters and geographic remarks for use in future taxonomic studies and distribution patterns.
Materials and methods
The specimens studied belong to the scientific collections of the Museum of Zoology of the University of Campinas (ZUEC), Museum of Zoology of the University of São Paulo (MZUSP) and Museum of Zoology of the Federal University of Bahia (MZUFBA). All our specimens are preserved in ethanol. The geographic coordinates and main references for each specimen studied are shown in Supplementary Table S1.
The largest and best-preserved specimen of each species was chosen for photography with a camera (ZEISS TK 1270U) mounted on a stereomicroscope. This same individual was used to examine the arm ossicles, which were extracted from a small part of the proximal arm, between the fifth and the tenth segment. The arm segment was immersed in regular household bleach (NaClO) until the soft tissues were removed (Stöhr et al., Reference Stöhr, Conand and Boissin2008). The ossicles were then washed with distilled water, air-dried and prepared for examination with a scanning electron microscope (SEM) (JEOL JSM5800LV). The external morphology as well as the ossicles of Amphiodia habilis were not described since our attempts to locate the type material were unsuccessful.
The photos were organized into plates for each species using Adobe Photoshop. The taxonomy follows the new classification of Ophiuroidea (O'Hara et al., Reference O'Hara, Stöhr, Hugall, Thuy and Martynov2018; Stöhr et al., Reference Stöhr, O'Hara and Thuy2019). The species were described in detail from images, previous notes and appropriate taxonomic resources (Tommasi, Reference Tommasi1970; Borges et al., Reference Borges, Monteiro and Amaral2002; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005; Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018). The terms applied are based on Stöhr et al. (Reference Stöhr, O'Hara and Thuy2012), O'Hara et al. (Reference O'Hara, Stöhr, Hugall, Thuy and Martynov2018) and Hendler (Reference Hendler2018). In relation to the arm ossicles, the terms are based on literature (Hotchkiss et al., Reference Hotchkiss, Prokop and Petr2007; Thuy & Stöhr, Reference Thuy and Stöhr2011, Reference Thuy and Stöhr2016; Hotchkiss & Glass, Reference Hotchkiss, Glass, Kroh and Reich2012; Stöhr et al., Reference Stöhr, O'Hara and Thuy2012) and are illustrated in Supplementary Figure S1. Arm vertebra articular tubercles were classified into types as proposed by Litvinova (Reference Litvinova, David, Guille and Feral1994).
Measurements were taken using an ocular micrometer and through the AxioVision VS program 40.4.8.20 (Carl Zeiss Microscopy, Germany) attached to a ZEISS Discovery V20 stereomicroscope. The following diagnostic characters were measured: disc diameter (dd), number of scales between the centrodorsal plate and the edge of the disc (scales), length (rs_l) and width (rs_w) of radial shields, oral diameter (od), length (os_l) and width (os_w) of oral shield, length (ads_l) and width (ads_w) of adoral shield, length and width of the first (vap1_l, vap1_w) and second ventral arm plate (vap2_l, vap2_w) and length (dap_l) and width (dap_w) of the dorsal arm plate at the third free arm segment (i.e. the third segment not covered by the disc). More information in Supplementary Table S4.
Linear discriminant analysis (LDA) was applied using the R software (R Development Core Team, 2018). To avoid multicollinearity among morphological characters, a correlation matrix was constructed and the variables that were significantly correlated were removed with a threshold value of 0.9. The ‘lda’ function (package MASS) (Venables & Ripley, Reference Venables and Ripley2002) was used to investigate the differences in morphological characters and distinguish the species. The linear discriminant axes were interpreted using their weighting coefficients, and the plots were graphed on the first two axes. Visualization was performed using the package ggplot2 (Wickham, Reference Wickham2009). The LDA was performed with each genus separately, Amphiodia and Ophiophragmus. Amphiodia habilis and O. septus were not used due to the few characters available.
The morphometry was used to show differences or patterns not recognized by classical taxonomy. Therefore, the most important morphometric variables to classify the species indicated by the LDA were additionally included in the taxonomic key as a positive character.
Results
SYSTEMATICS
Order OPHIURIDA Müller & Troschel, 1840
Family AMPHIURIDAE Ljungman, 1867
Genus Amphiodia Verrill, 1899
Type species: Amphiodia violacea (Lütken, Reference Lütken1856).
Species found in Brazil:
Amphiodia habilis Albuquerque et al., Reference Albuquerque, Campos-Creasey and Guille2001
Amphiodia planispina (v. Martens, 1867)
Amphiodia pulchella (Lyman, 1869)
Amphiodia riisei (Lütken, Reference Lütken1859)
Amphiodia trychna H.L. Clark, Reference Clark1918
Diagnosis. Disc completely covered with scales. One pair of infradental papillae on the apex of the jaw. Adoral shield spines: first slightly larger than the second. One or two tentacle scales (Fell, Reference Fell1962; Tommasi, Reference Tommasi1970; Albuquerque, Reference Albuquerque1986).
Amphiodia habilis Albuquerque, Campos-Creasey & Guille, Reference Albuquerque, Campos-Creasey and Guille2001
(Figure 1)
Amphiodia habilis Albuquerque, Campos-Creasey & Guille, Reference Albuquerque, Campos-Creasey and Guille2001: 599.

Fig. 1. Amphiodia habilis Albuquerque, Campos-Creasey & Guille, Reference Albuquerque, Campos-Creasey and Guille2001, figure modified from Albuquerque et al. (Reference Albuquerque, Campos-Creasey and Guille2001) (dd: 3.5 mm): (A) dorsal view; (B) ventral view. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bs: bursal slits; cpp: central primary plate; dap: dorsal arm plate; ds: distal scale; ip: infradental papillae; ma: madreporite; os: oral shields; rpp: radial primary plate; rs: radial shields; ts: tentacle scale; vap: ventral arm plate. Scale bars: 0.5 mm.
Type locality. Doce River – Espírito Santo, Brazil.
Size range (dd). Holotype with 3.5 mm and paratype without disc.
Description
Disc (dd: 3.5 mm): circular, thick, slightly flattened dorsally, covered with large scales, ~5 between the centrodorsal plate and the edge of the disc. A rosette of primary plates: centrodorsal pentagonal and radial plates larger and irregular. On each dorsal interradius, a row of scales larger than the ones surrounding the radial shields; distal most scale semicircular, straight distally and rounded proximally. Radial shields 1.5 times as long as wide, one fifth of dd, contiguous throughout and sometimes separated proximally by one small scale (Figure 1A). Ventral interradius covered by scales smaller than the dorsal surface and imbricated. Bursal slits wide. Oral shields lozenge-shaped, 1.5 times as long as wide. Madreporite larger than other oral shields. Adoral shields triangular, united proximally. Adoral shield spines: first larger than the second and almost seals the oral opening. A pair of robust and rectangular infradental papillae, separated from each other (Figure 1B).
Arms: dorsal arm plates rectangular, almost twice as wide as long and contiguous (Figure 1A). Ventral arm plates pentagonal as long as wide and contiguous, with concave distal and lateral edges, and widely angled proximal edge. Lateral arm plates inconspicuous dorsally. Three arm spines, middle one blunt (almost the length of a segment) and smaller than the dorsal and ventral. Two leaf-like tentacle scales, one attached to the ventral arm plate and the other to the lateral arm plate (Figure 1B).
Taxonomic comments. According to Albuquerque et al. (Reference Albuquerque, Campos-Creasey and Guille2001), Amphiodia habilis is similar to Amphiodia riisei, Amphiodia violacea (Lütken, 1856) and Amphiodia grisea (Ljungman, 1867). However, A. riisei differs from A. habilis in having the dorsal disc slightly elevated; arm spines cylindrical; middle arm spine longer than the dorsal and ventral ones; middle arm spine of the first eight proximal arm segments with a crown of tiny hyaline spines at the apex (Albuquerque et al., Reference Albuquerque, Campos-Creasey and Guille2001); radial shields triangular with straight inner and outer edges. Amphiodia violacea has a protuberance towards the mouth on the first ventral arm plate and each dorsal interradius has a trapezoidal distal-most scale (Nielsen, Reference Nielsen1932; Albuquerque et al., Reference Albuquerque, Campos-Creasey and Guille2001). Amphiodia grisea has a bulge in the shape of a spine on the first arm plate (Koehler, Reference Koehler1926; Albuquerque et al., Reference Albuquerque, Campos-Creasey and Guille2001). Amphiodia habilis is a native species from Brazil, of which the holotype is in the French National Museum of Natural History. The paratype is supposed to be in Brazil, but our attempts to locate it have not been successful.
Remarks. The species was recorded at the opening of the Doce River, where the habitat experiences large quantities of river discharge with substrate composed of organic rich black ooze flowing into the ocean (Albuquerque & Guille, Reference Albuquerque and Guille1991; Albuquerque et al., Reference Albuquerque, Campos-Creasey and Guille2001).
Distribution. Atlantic Ocean: Doce River, Espírito Santo, Brazil. Occurs at 34 m depth (Albuquerque et al., Reference Albuquerque, Campos-Creasey and Guille2001).
Amphiodia planispina (v. Martens, 1867)
(Figure 2)
Amphiura planispina v. Martens, 1867: 347

Fig. 2. Amphiodia planispina (v. Martens, 1867) (ZUEC OPH 424, dd: 13.5 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) detail of dorsal arm; (E) dorsal arm plate – external view; (F) ventral arm plate – external view; (G) lateral arm plate – external view; (H) lateral arm plate – internal view; (I) vertebra – proximal surface; (J) vertebra – distal surface; (K) vertebra – dorsal surface; (L) vertebra – ventral surface. Abbreviations: AdShSp: second adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bs: bursal slits; d: dorsal; dap: dorsal arm plate; ddmf: dorso-distal muscular fossae; di: distal; fas: flattened arm spine; dma: dorsal muscle area; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; rs: radial shields; sa: spine articular tubercle; ts: tentacle scale; v: ventral; vap: ventral arm plate; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: stereomicroscope photographs A–C, 1.0 mm D, 0.2 mm; SEM images F, G, K, L, 100 μm and E, H, I, J 200 μm.
Diamphiodia planispina
Fell, Reference Fell1962: 14; Tommasi, Reference Tommasi1970: 27
Amphiodia planispina H.L. Clark, Reference Clark1915: 248; Thomas, Reference Thomas1962: 648; Parslow & Clark, Reference Parslow and Clark1963: 34; A.M. Clark 1970: 26, 75; Bernasconi & D'Agostino, Reference Bernasconi and D'Agostino1977: 93; Albuquerque, Reference Albuquerque1986: 86, Hendler et al., Reference Hendler, Miller, Pawson and Kier1995; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005: 254
Type locality. Rio de Janeiro, Brazil.
Material examined. 17 specimens (4.6–13.5 mm). See Supplementary Table S1.
Size range (dd). From 4.6 to 13.5 mm (present study).
Description
Disc (dd: 13.5 mm): circular, covered by irregular and imbricating scales, ~18 between the centrodorsal and the edge of the disc. Central primary plate not evident. Radial shields twice as long as wide, one fifth of dd, straight inner edge and curved outer edge, contiguous throughout and separated proximally by one scale (Figure 2A). Ventral interradius covered with scales smaller than the dorsal and imbricated. Bursal slits narrow (Figure 2B). Oral shields lozenge-shaped, proximal edge rounded, and distal edge tapered. Madreporite larger and more whitish than other oral shields. Adoral shields triangular with rounded edges, separated proximally. First and second adoral shield spines equal-sized. A pair of rectangular infradental papillae, usually separated from each other (Figure 2C), not much larger than adjacent lateral papillae.
Arms: dorsal arm plates rectangular, almost three times as wide as long and contiguous (Figure 2D, E). Ventral arm plates (VAP) 1.5 times as wide as long, contiguous (Figure 2B), with pointed proximal angle and straight distal edge in dissociated plate (Figure 2F). Two leaf-like tentacle scales, one inserted on the ventral arm plate and the other on the lateral arm plate (Figure 2C). Three flattened arm spines, half the length of a segment, with wide and blunt edges similar to a paddle in shape (Figure 2D).
Lateral arm plates (Figure 2G, H): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs larger than stereom pores on small part of outer surface. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercles: on same level as remaining outer surface; all similar size; distance between spine articular tubercles increasing dorsalwards. Lobes simply separated; equal-sized; lobes parallel, bent, and oriented nearly horizontal; stereom without perforations. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large central perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 2I). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 2J). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles. (Figure 2K). Zygosphene projecting beyond ventral edge of zygocondyles with projecting part longer than zygocondyles (Figure 2L).
Taxonomic comments. One of the most interesting features of Amphiodia planispina is the paddle shaped arm spine, which may be used as a diagnostic character. Amphiodia planispina is usually described with two adoral shield spines. Borges (Reference Borges2006) described a larger specimen (6.9 mm dd), which according to the author, could be an individual variation. However, all our larger specimens (6.7 to 13.5 mm dd) were atypical, with extra structures in the mouth. Furthermore, two variations were noted between juveniles and adults. First, the central primary plates are evident in specimens up to 6.7 mm dd. Second, larger specimens (from 6.7 to 13.5 mm dd) have all the arm spines flattened, while in the smaller (4.6 to 5.8 mm dd) only the dorsalmost is flattened.
Remarks. Amphiodia planispina can be found on mud flats, shell beds and seagrass (Tommasi, Reference Tommasi1970; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). It may form an association of shallow-water species (<30 m) with Hemipholis cordifera (Bosc, 1802), Ophiactis lymani Ljungman, 1872, and Amphioplus lucyae Tommasi, 1971 along the extreme southern coast of Brazil (Capítoli & Monteiro, Reference Capítoli and Monteiro2000).
Distribution. Atlantic Ocean: From the Antilles to Argentina and from intertidal to 578 m depth (Parslow & A.M. Clark, Reference Parslow and Clark1963; Tommasi, Reference Tommasi1970; Bernasconi & D'Agostino, Reference Bernasconi and D'Agostino1977; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). In Brazil: Maranhão, Ceará, Rio Grande do Norte, Paraíba (Albuquerque, Reference Albuquerque1986; Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a; Gondim et al., Reference Gondim, Dias and Christoffersen2013b), Bahia (Magalhães et al., Reference Magalhães, Martins and Alves2005; Manso et al., Reference Manso, Alves and Martins2008), Espírito Santo – Vitória-Trindade Chain (Albuquerque & Guille, Reference Albuquerque and Guille1991), Rio de Janeiro (Ventura et al., Reference Ventura, Lima, Nobre, Veríssimo, Zama, Lavrado and Ignacio2006; Oliveira et al., Reference Oliveira, Oliveira and Manso2010), São Paulo (Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005; Netto et al., Reference Netto, Hadel and Tiago2005), Paraná (Bueno et al., Reference Bueno, Alitto, Guilherme, Di Domenico and Borges2018) and Rio Grande do Sul (Capítoli & Monteiro, Reference Capítoli and Monteiro2000; Capítoli & Bemvenuti, Reference Capítoli and Bemvenuti2004). Samples of the present study were collected at depths ranging from 25 to 82 m.
Amphiodia pulchella (Lyman, 1869)
(Figure 3)
Amphiura pulchella Lyman, 1869: 337

Fig. 3. Amphiodia pulchella (Lyman, 1869) (ZUEC OPH 104, dd: 5 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) dorsal arm plate – external view; (E) ventral arm plate – internal view; (F) lateral arm plate – external view; (G) lateral arm plate – internal view; (H) vertebra – proximal surface; (I) vertebra – distal surface; (J) vertebra – dorsal surface; (K) vertebra – ventral surface. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bs: bursal slits; d: dorsal; dap: dorsal arm plate; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; rd: ridge; rs: radial shields; sa: spine articular tubercle; ts: tentacle scale; v: ventral; vap: ventral arm plate; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: stereomicroscope photographs A–C, 1.0 mm; SEM images D–K, 100 μm.
Amphiura repens Lyman, Reference Lyman1875: 18
Amphiodia pulchella H.L. Clark, Reference Clark1915: 250; Fell, Reference Fell1962: 5; Thomas, Reference Thomas1962: 641; Parslow & Clark, Reference Parslow and Clark1963: 26; Tommasi, Reference Tommasi1970: 26, Monteiro, Reference Monteiro1987: 41, Hendler et al., Reference Hendler, Miller, Pawson and Kier1995: 153; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005: 256; Manso et al., Reference Manso, Alves and Martins2008: 190; Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018: 31.
Type locality. Florida, USA.
Material examined. 41 specimens (dd: 2.9–5.1 mm). See Supplementary Table S1.
Size range (dd). From 1.7 (Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018) to 5.2 mm (Thomas, Reference Thomas1962).
Description
Disc (dd: 5 mm): circular with interradial depressions, covered with small scales, ~30 between the centrodorsal and the edge of the disc. Central primary plate and radial primary plates not evident. Radial shields three times as long as wide, a tenth of dd, almost contiguous throughout and separated proximally by 2–4 small scales (Figure 3A). Ventral interradius covered by smaller scales than the dorsal. Bursal slits wide (Figure 3B). Oral shields 1.5 times as long as wide, distal edge lobed and distal slightly rounded. Madreporite larger than other oral shields, whitish and with rounded edges. Adoral shields triangular and separated proximally. Adoral shield spines: first larger than the second. One pair of rectangular infradental papillae widely separated from each other, larger than adjacent lateral papillae (Figure 3C).
Arms: dorsal arm plates semi-rectangular, twice as wide as long, proximal edge strongly convex, distal edge straight, contiguous (Figure 3A, D). Ventral arm plates pentagonal, as wide as long, proximal edge pointed and distal edge with a median recess, first 2–3 contiguous, and others separated by lateral arm plates (Figure 3B, E). One operculiform tentacle scale attached to lateral plate. Three arm spines, half the length of a segment, middle one blunt, flattened dorsoventrally, tip with a little tooth (Figure 3A, B).
Lateral arm plates (Figure 3F, G): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs larger than stereom pores on most of outer surface. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercle: on same level as remaining outer surface, sizes all similar; distance between spine articular tubercle equidistant. Lobes simply separated; equal-sized; lobes parallel, straight, and oriented nearly horizontal; stereom compact; each pair of lobes separated by a long ridge (as long as one lobe) with perforations. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large central perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 3H). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 3I). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles (Figure 3J). A deep circular depression in the ventral groove (Figure 3K).
Taxonomic comments. Amphiodia pulchella differs from other Amphiodia species in its single tentacle scale. Primary plates were not observed in specimens larger than 4 mm dd, but they are frequently evident in smaller specimens. The ridges with perforations that were observed between each pair of lobes on the lateral arm plates are a new character, described for the first time in this study. They were not described by Alitto et al. (Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018), but may be phylogenetically informative.
Remarks. Amphiodia pulchella is commonly found with the ophiactids Hemipholis cordifera and Ophiactis lymani and with the amphiurids Amphiodia riisei, Amphipholis januarii Ljungman, 1866, Amphiura kinbergi Ljungman, 1872, Amphiura princeps Koehler, 1907, Microphiopholis subtilis (Ljungman, 1867) and Microphiopholis atra (Stimpson, 1852) (Tommasi, Reference Tommasi1970; Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018). This species can be found on coral reefs, seagrass, muddy, rocky and sandy bottoms (coarse and medium sand), medium silt and rubble bottoms (Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013; Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018).
Distribution. Atlantic Ocean: from USA (Florida) to Argentina (Buenos Aires) and from subtidal to 370 m depth (Tommasi, Reference Tommasi1970; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). In Brazil: Bahia (Manso, Reference Manso2004; Magalhães et al., Reference Magalhães, Martins and Alves2005; Manso et al., Reference Manso, Alves and Martins2008), Rio de Janeiro (Manso & Absalão, Reference Manso and Absalão1988; Manso, Reference Manso1989, Reference Manso1993), São Paulo (Tommasi, Reference Tommasi1970; Monteiro, Reference Monteiro1987; Pires-Vanin et al., Reference Pires-Vanin, Corbisier, Arasaki and Moellmann1997; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005; Netto et al., Reference Netto, Hadel and Tiago2005; Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018), Paraná and Rio Grande do Sul (Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005). Samples of the present study were collected at depths ranging from 15 to 21.6 m.
Amphiodia riisei (Lütken, Reference Lütken1859)
(Figure 4)
Diamphiodia riisei Fell, Reference Fell1962: 14; Tommasi, Reference Tommasi1970: 28
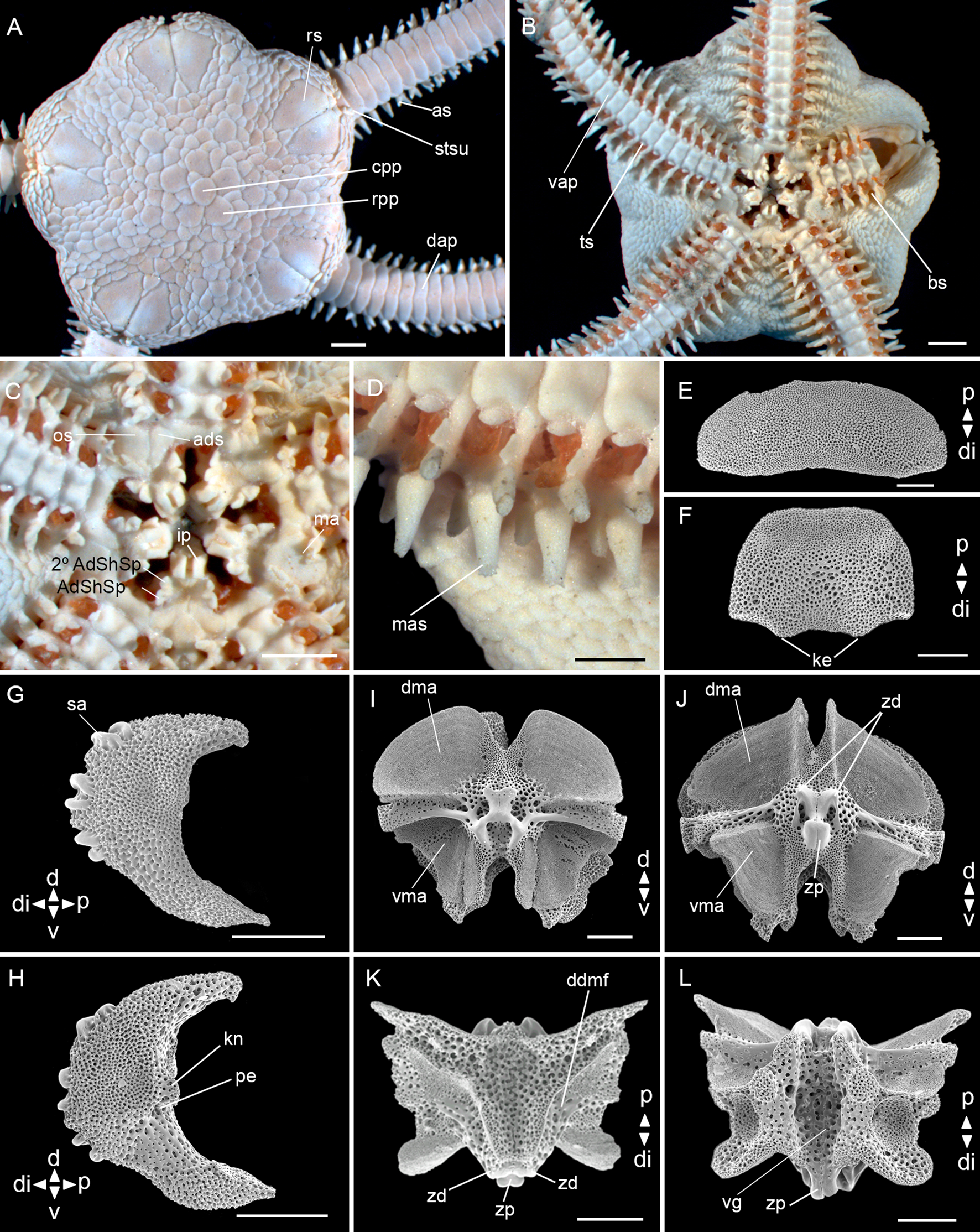
Fig. 4. Amphiodia riisei (Lütken, Reference Lütken1859) (ZUEC OPH 129, dd: 8 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) detail of the middle arm spine; (E) dorsal arm plate – external view; (F) ventral arm plate – internal view; (G) lateral arm plate – external view; (H) lateral arm plate – internal view; (I) vertebra – proximal surface; (J) vertebra – distal surface; (K) vertebra – dorsal surface; (L) vertebra – ventral surface. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bs: bursal slits; cpp: central primary plate; d: dorsal; dap: dorsal arm plate; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; ip: infradental papillae; ke: keel; kn: knob; ma: madreporite; mas: middle arm spine; os: oral shields; p: proximal; pe: perforation; rpp: radial primary plate; rs: radial shields; sa: spine articular tubercle; stsu: scales tending to stick up; ts: tentacle scale; v: ventral; vap: ventral arm plate; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: stereomicroscope photos A–D, 1.0 mm; SEM photos E–L, 200 μm.
Amphipholis riisei Rathbun 1879: 155
Amphiodia riisei H.L. Clark, Reference Clark1915: 249; Parslow & Clark, Reference Parslow and Clark1963: 30; Albuquerque, Reference Albuquerque1986: 91, Monteiro, Reference Monteiro1987: 45; Manso 1991: 33; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005: 257; Manso et al., Reference Manso, Alves and Martins2008: 189; Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a, Reference Gondim, Dias and Christoffersen2013b: 58
Ophiophragmus riisei Hendler et al., Reference Hendler, Miller, Pawson and Kier1995: 175; Alitto et al. 2016: 7
Type locality. Rio de Janeiro, Brazil.
Material examined. 23 specimens (dd: 4.9–10 mm). See Supplementary Table S1.
Size range (dd). From 2.3 (Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018) to 11 mm (Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005).
Description
Disc (dd: 8 mm): pentagonal with interradial depressions, covered by imbricated scales of medium size, ~12 between the central primary plate and the edge of the disc. Primary plates circular and evident, radial primary plates larger than the central plate, touching each other. Radial shields scalene triangular, 1.5 times as long as wide, one fifth of dd, contiguous for almost their entire length, separated proximally by two scales, proximal triangular and larger than the distal. Scales near the distal edge of radial shields tend to stick up (Figure 4A). Ventral interradius covered by scales smaller than the dorsal ones. Bursal slits long and narrow (Figure 4B). Oral shields lozenge-shaped with rounded edges. Madreporite larger than other oral shields and with pores in the centre and distal margin. Adoral shields triangular with rounded edges and touching proximally. Adoral shield spines: first triangular and larger than the second. One pair of rectangular infradental papillae widely separated from each other (Figure 4C).
Arms: dorsal arm plates rectangular with rounded edges, almost three times as wide as long, with a slight concavity at the distal edge and contiguous (Figure 4A, E). Ventral arm plates 1.5 times as wide as long, rounded edges, contiguous (Figure 4B), dissociated plates with two keels at the distal edge (Figure 4F). Two leaf-like tentacle scales smaller than a half length of the ventral arm plate, one attached to the ventral arm plate and the other to the lateral arm plate, not touching each other. Three arm spines shorter than a half segment (Figure 4A, B). Middle arm spine of the first eight proximal arm segments with a crown of tiny hyaline spines at the apex (Figure 4D).
Lateral arm plates (Figure 4G, H): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs larger than stereom pores on most of outer surface. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercles: on same level as remaining outer surface, all similar size, distance between spine articular tubercles increasing dorsalwards. Lobes simply separated, equal-sized; lobes parallel, bent, and oriented nearly horizontal; stereom with perforations. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 4I). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 4J). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles (Figure 4K). Zygosphene projecting beyond ventral edge of zygocondyles with projecting part longer than zygocondyles (Figure 4L).
Taxonomic comments. All specimens of Amphiodia riisei analysed have primary plates touching each other even at different disc diameters, concurring with the descriptions of Borges & Amaral (Reference Borges, Amaral, Amaral, Rizzo and Arruda2005) and Manso et al. (Reference Manso, Alves and Martins2008). Tommasi (Reference Tommasi1970) recorded one specimen with primary plates separated from each other, but all other features agree well with the description provided here. An interesting characteristic observed in our specimens were the scales near the distal edge of the radial shields, which tend to stick up (Figure 4A, Supplementary Figure S2), also described by Koehler (Reference Koehler1914). Albuquerque (Reference Albuquerque1986) and Koehler (Reference Koehler1914) cited a crown of tiny hyaline spines at the apex of the middle arm spine in the first eight or nine arm segments, but this was not mentioned in the original description (Lütken, Reference Lütken1859). This feature was observed in all our specimens analysed, even in the smallest with 4.9 mm of dd. Amphiodia riisei is similar to Amphiodia trychna, but the latest differs in having internally straight and externally curved (half circle) radial shields; the scales near the distal edge of radial shields do not tend to stick up; oral shields with tapered proximal edge and rounded distal edge; ventral arm plates pentagonal without keels at the distal edge, and two large tentacle scales touching each other.
Remarks. This species occurs in silt, muddy and sandy bottoms (Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005; Manso et al., Reference Manso, Alves and Martins2008).
Distribution. Atlantic Ocean: from USA (Florida) to Brazil (Amapá, Pará, Rio de Janeiro, São Paulo and Paraná) (Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005) and from 1 to 311 m depth (Tommasi, Reference Tommasi1970; Hendler et al., Reference Hendler, Miller, Pawson and Kier1995; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005). In Brazil: Pará-Maranhão (Albuquerque, Reference Albuquerque1986), Bahia (Magalhães et al., Reference Magalhães, Martins and Alves2005; Manso et al., Reference Manso, Alves and Martins2008; Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a), Rio de Janeiro (Oliveira et al., Reference Oliveira, Oliveira and Manso2010) and São Paulo (Pires-Vanin et al., Reference Pires-Vanin, Corbisier, Arasaki and Moellmann1997; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005; Netto et al., Reference Netto, Hadel and Tiago2005). Samples of the present study were collected at depths ranging from 10 to 25 m.
Amphiodia trychna Clark, Reference Clark1918
(Figure 5)
Amphiodia tymbara Parslow & Clark, Reference Parslow and Clark1963: 30

Fig. 5. Amphiodia trychna H.L. Clark, Reference Clark1918 (ZUEC OPH 428, dd: 5.4 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) dorsal arm plate – external view; (E) ventral arm plate – internal view; (F) lateral arm plate – external view; (G) lateral arm plate – internal view; (H) vertebra – proximal surface; (I) vertebra – distal surface; (J) vertebra – dorsal surface; (K) vertebra – ventral surface. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bs: bursal slits; cpp: central primary plate; d: dorsal; dap: dorsal arm plate; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; rpp: radial primary plate; rs: radial shields; sa: spine articular tubercle; ssf: small scales forming a fringe; ts: tentacle scale; v: ventral; vap: ventral arm plate; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: stereomicroscope photographs A–C, 1.0 mm; SEM images D–K, 100 μm.
Amphiodia trychna Thomas, Reference Thomas1962: 645; Parslow & Clark, Reference Parslow and Clark1963: 30; Hendler et al., Reference Hendler, Miller, Pawson and Kier1995: 155
Amphiodia riisei Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018: 33
Type locality. Tobago.
Material examined. 10 specimens (dd: 5.3–8.8 mm). See Supplementary Table S1.
Size range (dd). From 4.5 to 8.8 mm (present study).
Description
Disc (dd: 5.4 mm): circular with radial incisions above the arms, covered by irregular scales of medium size, ~10 between the central primary plate and the edge of the disc. Central primary plates circular and evident, radial primary plates irregular and larger than the central plate, not touching each other. Small scales at the edge of the disc forming a line. Radial shields twice as long as wide, one fifth of dd, internally straight and externally curved (half circle), contiguous for almost their entire length, separated proximally by one triangular scale (Figure 5A). Ventral interradius covered by smaller scales than the dorsal and imbricated. Bursal slits long and narrow (Figure 5B). Oral shields 1.5 times as long as wide, proximal edge tapered, and distal edge rounded. Madreporite slightly larger than the oral shields. Adoral shields triangular, united proximally. Adoral shield spines: first triangular and larger than the second. One pair of rectangular infradental papillae widely separated from each other (Figure 5C).
Arms: dorsal arm plates broadly oval, twice as wide as long and contiguous (Figure 5A, D). Ventral arm plates as wide as long, contiguous (Figure 5B) and in dissociated plates pentagonal, with a slight concavity at the distal edge (5B and 5E). Two subequal tentacle scales, larger (almost the length of the ventral arm plate), one attached to the ventral arm plate and the other to the lateral arm plate. Three short (shorter than a half segment) and blunt arm spines (Figure 5B).
Lateral arm plates (Figure 5F, G): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs larger than stereom pores on most of outer surface. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercles: on same level as remaining outer surface, all similar size, distance between spine articular tubercles increasing dorsalwards. Lobes simply separated, equal-sized; lobes parallel, bent, and oriented nearly horizontal; stereom with perforations. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 5H). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 5I). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles. (Figure 5J). Zygosphene projecting beyond ventral edge of zygocondyles with projecting part longer than zygocondyles (Figure 5K).
Taxonomic comments. All specimens of Amphiodia trychna analysed have separated primary plates at both small (5.3 mm) and large (8.8 mm) disc diameters. Two important characters observed in our specimens were the shape of the oral shields, which are sharply pointed as mentioned in the original description (H.L. Clark, Reference Clark1918) and small scales at the edge of the disc forming a fringe (Borges & Amaral, Reference Borges and Amaral2007). Amphiodia trychna is similar to Amphiodia riisei, but the latter differs in having a larger size, oral and adoral shields with rounded edges, small tentacle scales and arm spines less blunt (Borges & Amaral, Reference Borges and Amaral2007). In addition to these features, we observed that A. riisei has triangular radial shields with straight edges; scales near the distal edge of radial shields tending to stick up; and ventral arm plates pentagonal with keels at the distal edge. It is important to highlight that the specimens identified as A. riisei by Alitto et al. (Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018) were studied and agree well with the description of A. trychna provided here, suggesting the identification might have been incorrect.
Remarks. Amphiodia trychna can be found in seagrass, mangroves, sandy and rubble bottoms (Borges & Amaral, Reference Borges and Amaral2007; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013).
Distribution. Atlantic Ocean: Trinidad and Tobago, USA (Sandy Point) to Brazil (São Paulo) and from 1 to 160 m depth (H.L. Clark, Reference Clark1918; Borges & Amaral, Reference Borges and Amaral2007; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). In Brazil: Bahia (Manso et al., Reference Manso, Alves and Martins2008), Rio de Janeiro (Oliveira et al., Reference Oliveira, Oliveira and Manso2010) and São Paulo (Borges & Amaral, Reference Borges and Amaral2007). Samples of the present study were collected at depths ranging from 9.2 to 15.6 m.
Genus Ophiophragmus Lyman, Reference Lyman1865
Type species: Ophiophragmus wurdemanii (Lyman, 1860)
Species included in Brazil:
Ophiophragmus brachyactis H.L. Clark, Reference Clark1915
Ophiophragmus cubanus (A.H. Clark, Reference Clark1917)
Ophiophragmus luetkeni (Ljungman, 1872)
Ophiophragmus pulcher H.L. Clark, Reference Clark1918
Ophiophragmus septus (Lütken, Reference Lütken1859)
Ophiophragmus wurdemanii (Lyman, 1860)
Diagnosis. Disc covered with scales dorsally and ventrally. Radial shields contiguous for almost their entire length or separated proximally by up to five scales. Fence of papillae at the edge of the disc interradius. Adoral shield spines: first larger than the second. One to three tentacle scales. Three arm spines (Lyman, Reference Lyman1865; H.L. Clark, Reference Clark1918; Thomas, Reference Thomas1962; Tommasi, Reference Tommasi1970; Albuquerque, Reference Albuquerque1986).
Ophiophragmus brachyactis H.L. Clark, Reference Clark1915
(Figure 6)
Ophiophragmus brachyactis H.L. Clark, Reference Clark1915: 238, Reference Clark1918: 278; Thomas, Reference Thomas1962: 666; Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a, Reference Gondim, Dias and Christoffersen2013b: 65; Manso, Reference Manso1988: 967

Fig. 6. Ophiophragmus brachyactis Clark, Reference Clark1915 (ZUEC OPH 48, dd: 6.08 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) dorsal arm plate – external view; (E) ventral arm plate – external view; (F) lateral arm plate – external view; (G) lateral arm plate – internal view; (H) vertebra – proximal surface; (I) vertebra – distal surface; (J) vertebra – dorsal surface; (K) vertebra – ventral surface. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bs: bursal slits; d: dorsal; dap: dorsal arm plate; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; ep: end processes; fp: fence of papillae; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; rs: radial shields; sa: spine articular tubercle; ts: tentacle scale; v: ventral; vap: ventral arm plate; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: stereomicroscope photographs A–C, 1.0 mm; SEM images D–K, 100 μm.
Type locality. Florida, USA.
Material examined. 1 specimen. See Supplementary Table S1.
Size range (dd). From 4.45 (Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a) to 8.8 mm (Thomas, Reference Thomas1962).
Description
Disc (dd: 6.08 mm): Circular, covered by numerous small and imbricating scales, ~25 between the centrodorsal and the edge of the disc. Fence of papillae at the edge of the disc interradius. Radial shields almost as long as wide, a tenth of dd, separated proximally by two scales (Figure 6A). Ventral interradius covered with scales smaller than the dorsal. Bursal slits broad (Figure 6B). Oral shields lozenge-shaped with rounded edges. Madreporite larger than other oral shields and with pores in the centre. Adoral shields triangular, wider distally and united proximally. Adoral shield spines: first triangular and slightly larger than the second. One pair of rectangular infradental papillae widely separated from each other (Figure 6C).
Arms: dorsal arm plates rectangular with rounded edges, twice as wide as long and contiguous (Figure 6A, D). Ventral arm plates 1.5 times as wide as long, contiguous (Figure 6B), in dissociated plates with projected proximal angle and with a slight concavity at the distal edge (Figure 6E). Two leaf-like tentacle scales, one attached to ventral plate and one to lateral plate. Three thick and blunt arm spines, half the length of a segment, dorsalmost smallest (Figure 6B, C).
Lateral arm plates (Figure 6F, G): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs larger than stereom pores on small part. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercles: on same level as remaining outer surface, all similar size, distance between spine articular tubercles increasing dorsalwards. Lobes simply separated, equal-sized; lobes parallel, bent, and oriented nearly horizontal; stereom without perforations. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 6H). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 6I). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles and dorsal groove divided into two separate end processes (Figure 6J). Zygosphene projecting beyond ventral edge of zygocondyles with projecting part longer than zygocondyles (Figure 6K).
Taxonomic comments. A.H. Clark (Reference Clark1915) described Ophiophragmus brachyactis with diamond-shaped oral shields with rounded edges; blunt arm spines and dorsalmost smallest. Thomas (Reference Thomas1962) described and illustrated the oral shields with an acute proximal angle and a long, rounded distal end; middle arm spine with two or three short, acute teeth. We reevaluated the identification of the specimen sampled from Brazil, which agrees well with the original description of O. brachyactis (A.H. Clark, Reference Clark1915). Therefore, a detailed taxonomic analysis is required to verify if the specimen identified by Thomas (Reference Thomas1962) shows intraspecific variations of O. brachyactis or belongs to a different species.
Remarks. This species occurs in sandy bottoms with bryozoans (Manso, Reference Manso1988).
Distribution. Atlantic Ocean: USA (Florida), the Antilles, Gulf of Mexico and Brazil (A.H. Clark, Reference Clark1915; Manso, Reference Manso1988; Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a). In Brazil: Paraíba (Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a), Bahia (Manso, Reference Manso2004), Espírito Santo, Rio de Janeiro (Manso, Reference Manso1988, Reference Manso1993; Oliveira et al., Reference Oliveira, Oliveira and Manso2010), São Paulo (present study). From 12–87 m depth (Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a). Sample of the present study was collected at 12 m depth.
Ophiophragmus cubanus (A.H. Clark, Reference Clark1917)
(Figures 7 & 8)
Ophiocnida cubana A.H. Clark, Reference Clark1917: 69; H.L. Clark 1933: 55
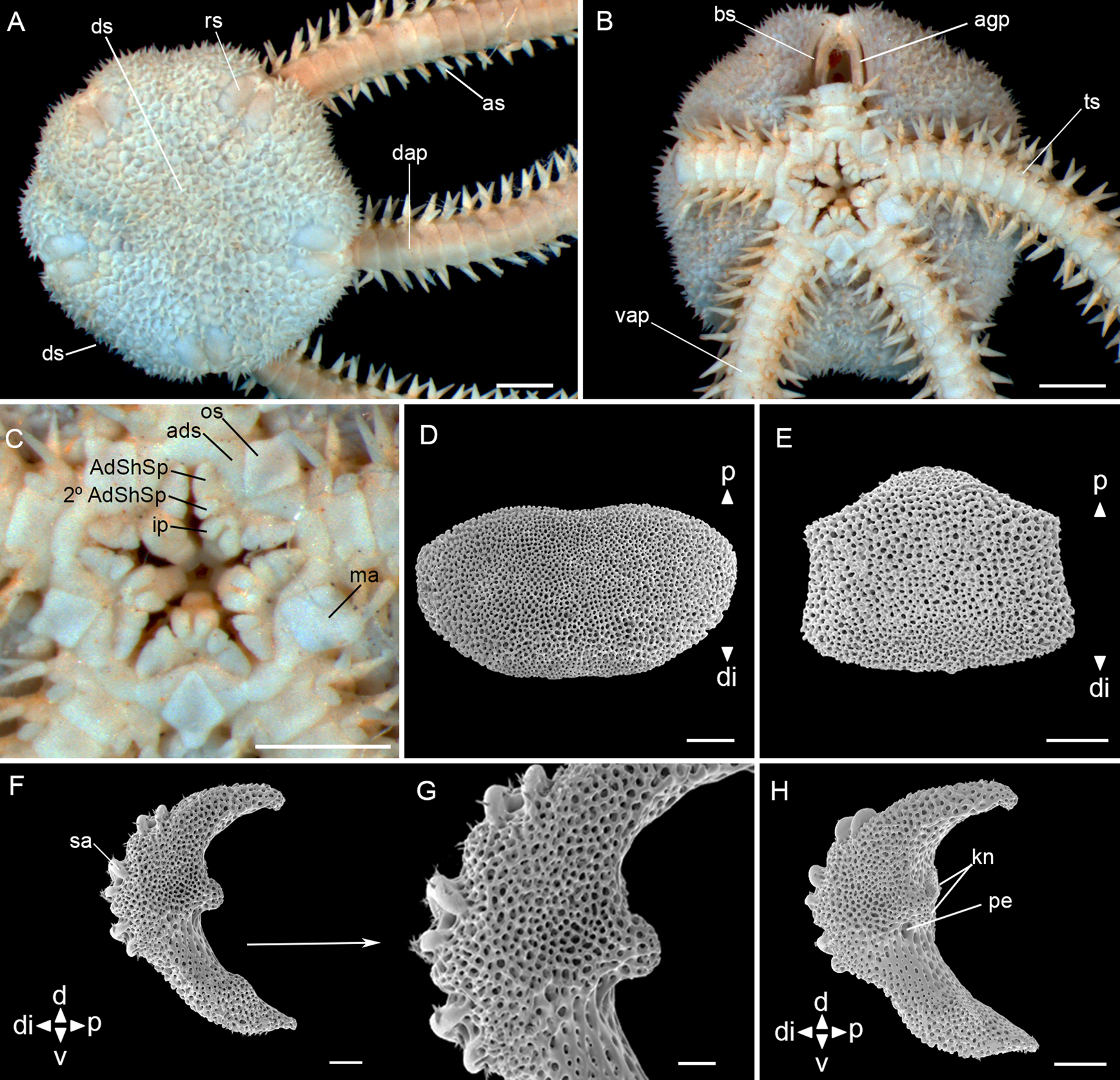
Fig. 7. Ophiophragmus cubanus H.L. Clark, Reference Clark1917 (MZUSP 1624, dd: 5.5 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) dorsal arm plate – external view; (E) ventral arm plate – external view; (F) lateral arm plate – external view; (G) lateral arm plate – detail of external view; (H) lateral arm plate – internal view. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; agp: abradial genital plate; as: arm spine; bs: bursal slits; d: dorsal; ds: disc spines; dap: dorsal arm plate; di: distal; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; rs: radial shields; sa: spine articular tubercle; ts: tentacle scale; v: ventral; vap: ventral arm plate. Scale bar: stereomicroscope photographs A–C, 1.0 mm; SEM images D–H, 100 μm.

Fig. 8. Ophiophragmus cubanus H.L. Clark, Reference Clark1917 (MZUSP 1624, dd: 5.5 mm): (A) vertebra – proximal surface; (B) vertebra – distal surface; (C) vertebra – dorsal surface; (D) vertebra – ventral surface. Abbreviations: d: dorsal; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; ep: end processes; p: proximal; v: ventral; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: SEM images, 100 μm.
Ophiophragmus cubanus Thomas, Reference Thomas1963: 218
Type locality. Santa Rosa Cove (West of Cuba).
Material examined. 5 specimens (dd: 3.3–5.3 mm). See Supplementary Table S1.
Size range (dd). From 3.3 (present study) to 14 mm (Thomas, Reference Thomas1963).
Description – juvenile
Disc (dd: 5.3 mm): circular with shallow interradial incisions, covered by numerous small, imbricating scales, ~16 between the centrodorsal and the edge of the disc. Some spines sparsely distributed on the dorsal surface. Radial shields twice as long as wide, an eighth of dd, separated proximally by 2–5 scales (Figure 7A). Ventral interradius covered with scales and spinulose papillae similar to the dorsal. Bursal slits narrow and long. Abradial genital plate bar-like (Figure 7B). Oral shields spearhead-shaped, distally rounded, proximal angle acute, with latero-posterior indentations. Madreporite larger and more rounded than other oral shields. Adoral shields triangular wider distally and united proximally. Adoral shield spines: first larger than the second. A pair of infradental papillae, well developed and semi-rectangular, separated from each other (Figure 7C).
Arms: dorsal arm plates oval, twice as wide as long and contiguous (Figure 7A, D). Ventral arm plates almost as wide as long, lateral edges with incisions for tentacles openings, contiguous (Figure 7B), and in dissociated plates trapezoid and slightly convex distally (Figure 7E). Two leaf-like tentacle scales, one attached to ventral plate and one to lateral plate. Three pointed arm spines, half the length of a segment (Figure 7A, B).
Lateral arm plates (Figure 7F, G, H): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs approximately the same size as stereom pores. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercles: on same level as remaining outer surface, all similar size, distance between spine articular tubercles increasing dorsalwards. Lobes simply separated, equal-sized; lobes parallel, bent, and oriented nearly horizontal.
Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 8A). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 8B). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles and dorsal groove divided into two separate end processes (Figure 8C). Zygosphene projecting beyond ventral edge of zygocondyles with projecting part longer than zygocondyles (Figure 8D).
Taxonomic comments. Ophiophragmus cubanus was first described as Ophiocnida cubanus (by A.H. Clark, Reference Clark1917) because of the conical spines on the dorsal surface of a juvenile specimen with 4.3 mm dd, similar to our specimens. Thomas (Reference Thomas1963) redescribed the species based on larger specimens up to 14 mm dd. He suggested the genus Ophiophragmus instead of Ophiocnida, due to a well-defined interradial fence of papillae, particularly in the larger specimens. We observed spinelets on the surfaces of the lateral arm plates and vertebrae (Figures 7, G, 8A, B, C and D). Initially, these characters were considered as unusual structures on these plates, similar to those observed by Martynov & Litvinova (Reference Martynov and Litvinova2008) on the dorsal arm plates of Ophiocamax patersoni. However, after detailed studies of our SEM images and comparison with those from the previous authors, it was considered that they are artifacts in the case of O. cubanus, possibly caused by contamination at the time of preparation of the material, since they do not present a size nor distribution pattern over the plates and vertebrae. Furthermore, they do not appear to be ‘continuations of stereoma’ as observed in O. patersoni.
Remarks. This species occurs in seagrass, mangroves and muddy bottoms (Albuquerque, Reference Albuquerque1986; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013).
Distribution. Atlantic Ocean: USA (Florida), Cuba, West Indies and Brazil (A.H. Clark, Reference Clark1917; Thomas, Reference Thomas1963; Albuquerque, Reference Albuquerque1986). In Brazil: Pará-Maranhão (Albuquerque, Reference Albuquerque1986), Bahia (Manso, Reference Manso2004; Magalhães et al., Reference Magalhães, Martins and Alves2005; Oliveira et al., Reference Oliveira, Oliveira and Manso2010). Samples were collected at depths ranging from 30 to 103 m.
Ophiophragmus luetkeni (Ljungman, 1872)
(Figure 9)
Amphipholis lutkeni Ljungman, 1871: 636.

Fig. 9. Ophiophragmus luetkeni (Ljungman, 1872) (ZUEC OPH 340, dd: 5.4 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) dorsal arm plate – internal view; (E) ventral arm plate – external view; (F) lateral arm plate – external view; (G) lateral arm plate – internal view; (H) vertebra – proximal surface; (I) vertebra – distal surface; (J) vertebra – dorsal surface; (K) vertebra – ventral surface. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bs: bursal slits; d: dorsal; dap: dorsal arm plate; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; fp: fence of papillae; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; rs: radial shields; sa: spine articular tubercle; ts: tentacle scale; v: ventral; vap: ventral arm plate; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: stereomicroscope photographs A–C, 1.0 mm; SEM images D–K, 100 μm.
Ophiophragmus lutkeni Thomas, Reference Thomas1962: 666; Tommasi, Reference Tommasi1965: 7; 1970: 31, Monteiro, Reference Monteiro1987: 88; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005: 269; Manso et al., Reference Manso, Alves and Martins2008: 193; Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018: 24
Type locality. Tortola, British Virgin Islands.
Material examined. 26 specimens (dd: 3.9–8.8 mm). See Supplementary Table S1.
Size range (dd). From 3.25 (Tommasi, Reference Tommasi1970) to 9 mm (Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005).
Description
Disc (dd: 5.4 mm): circular, covered by numerous small and imbricating scales, ~17 between the centrodorsal and the edge of the disc. Radial shields twice as long as wide, a seventh of dd, separated proximally by one to three scales. Fence of blunt papillae at the edge of the disc interradius (Figure 9A). Ventral interradius covered with scales smaller than the dorsal and strongly imbricated. Bursal slits long and narrow (Figure 9B). Oral shields spearhead-shaped, distally rounded, proximal angle acute, with latero-posterior indentations. Madreporite larger and more whitish than other oral shields. Adoral shields triangular, wider distally and united proximally. Adoral shield spines: first larger than the second. A pair of rectangular infradental papillae separated from each other (Figure 9C).
Arms: dorsal arm plates rectangular with rounded edges, almost three times as wide as long, some fragmented and contiguous (Figure 9A, D). Ventral arm plates 1.5 times as wide as long, contiguous (Figure 9B), and distal edge incised in dissociated plates (Figure 9E). Two operculiform tentacle scales, one attached to ventral plate and larger than the one to the lateral plate. Three pointed arm spines, almost the length of a segment (Figure 9A, B).
Lateral arm plates (Figure 9F, G): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs larger than stereom pores on small part. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercles: on same level as remaining outer surface, all similar size, distance between spine articular tubercles increasing dorsalwards. Lobes simply separated, equal-sized; lobes parallel, bent, and oriented nearly horizontal; stereom without perforations. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 9H). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 9I). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles (Figure 9J). Zygosphene projecting beyond ventral edge of zygocondyles with projecting part longer than zygocondyles (Figure 9J, K).
Taxonomic comments. Ophiophragmus luetkeni is frequently sampled with a regenerating disc or without the disc. In these cases, the shape of oral and adoral shields as well as the dorsal and ventral arm plates are important to observe. Borges (Reference Borges2006) described the adoral shields united or separated proximally as an intraspecific variation. Moreover, we also observed operculiform or leaf-like tentacle scales; and oval semi-circular or rectangular dorsal arm plates.
Remarks. Ophiophragmus luetkeni is often found in low densities, possibly indicating that it is not gregarious. It occurs in muddy, sandy (very fine) and silt bottoms (H.L. Clark, Reference Clark1918; Tommasi, Reference Tommasi1970; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005; Manso et al., Reference Manso, Alves and Martins2008; Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018).
Distribution. Atlantic Ocean: British Virgin Islands, Trinidad and Tobago, USA (Virgin Islands) and Brazil (Tommasi, Reference Tommasi1970; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). In Brazil: Pará-Maranhão Basin (present study), Bahia (Manso et al., Reference Manso, Alves and Martins2008), Rio de Janeiro (Manso & Absalão, Reference Manso and Absalão1988; Oliveira et al., Reference Oliveira, Oliveira and Manso2010), São Paulo (Tommasi, Reference Tommasi1965; Pires-Vanin et al., Reference Pires-Vanin, Corbisier, Arasaki and Moellmann1997; Borges & Amaral, Reference Borges, Amaral, Amaral, Rizzo and Arruda2005; Netto et al., Reference Netto, Hadel and Tiago2005; Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018), Paraná (Barboza, Reference Barboza2010; Bueno et al., Reference Bueno, Alitto, Guilherme, Di Domenico and Borges2018). From intertidal to 50 metres depth (Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). Samples of the present study were collected from the intertidal to 50 m depth.
Ophiophragmus pulcher H.L. Clark, Reference Clark1918
(Figure 10)
Amphiodia rhabdota H.L. Clark, Reference Clark1918: 288

Fig. 10. Ophiophragmus pulcher H.L. Clark, Reference Clark1918 (ZUEC OPH REF 124, dd: 3.6 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) dorsal arm plate-external view; (E) ventral arm plate – internal view; (F) lateral arm plate – external view; (G) lateral arm plate – internal view; (H) vertebra – proximal surface; (I) vertebra – distal surface; (J) vertebra – dorsal surface; (K) vertebra – ventral surface. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bp: bent papilla; bs: bursal slits; d: dorsal; dap: dorsal arm plate; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; fp: fence of papillae; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; rs: radial shields; sa: spine articular tubercle; ts: tentacle scale; v: ventral; vap: ventral arm plate; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: stereomicroscope photographs A–C, 1.0 mm; SEM images D–K, 100 μm.
Ophiophragmus pulcher H.L. Clark, Reference Clark1918: 274; Tommasi, Reference Tommasi1974: 12
Type locality. Florida, USA.
Material examined. 14 specimens (dd: 1.9–3.7 mm). See Supplementary Table S1.
Size range (dd). From 1.9 to 3.6 mm (present study).
Description
Disc (dd: 3.6 mm): circular, covered by numerous small, irregular, and imbricating scales, ~10 between the centrodorsal and the edge of the disc. Central primary plate circular and evident. Radial shields 1.5 times as long as wide, one sixth of dd, contiguous throughout and separated proximally by two scales, proximal circular and larger than the distal. Fence of blunt papillae at the edge of the disc interradius. One bent papilla at the distal tip of each radial shield (Figure 10A). Ventral interradius covered with scales smaller than the dorsal and strongly imbricated. Bursal slits long and narrow (Figure 10B). Oral shields lozenge-shaped, twice as long as wide. Madreporite larger than other oral shields. Adoral shields triangular, wider distally and united proximally. Adoral shield spines: first slightly larger than the second. A pair of infradental papillae, small and separated from each other (Figure 10C).
Arms: dorsal arm plates rectangular, twice as wide as long, proximal edge convex, distal edge straight and contiguous (Figure 10A, D). Ventral arm plates as wide as long and contiguous (Figure 10B), and pentagonal in dissociated plates (Figure 10E). Two operculiform tentacle scales, one attached to the ventral arm plate and the other to the lateral arm plate. Three flattened and subequal arm spines, half the length of a segment (Figure 10B, C).
Lateral arm plates (Figure 10F, G): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs larger than stereom pores on most part. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercles: on same level as remaining outer surface, all similar size, similar distance between spine articular tubercles. Lobes simply separated, dorsal larger than the ventral; lobes parallel, bent, and oriented nearly horizontal; stereom without perforations. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 10H). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 10I). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles (Figure 10J). Zygosphene projecting beyond ventral edge of zygocondyles with projecting part longer than zygocondyles (Figure 10J, K).
Taxonomic comments. Ophiophragmus pulcher has a bent papilla at the distal tip of each radial shield, which was not recorded by H.L. Clark (Reference Clark1918) but it was described by Thomas (Reference Thomas1962). This feature could be used to distinguish O. pulcher from other Ophiophragmus species. Ophiophragmus pulcher greatly resembles O. septus, but the latter differs in having longer radial shields, blunt and shorter arm spines (shorter than one segment) (Thomas, Reference Thomas1962).
Remarks. It is considered one of the most beautiful brittle stars from Tortugas (Gulf of Mexico) and Biscayne Bay region (Florida, USA) due to the variety of colours observed in living specimens, e.g. white, green and orange-red (H.L. Clark, Reference Clark1918; Thomas, Reference Thomas1962). It occurs in high densities in the alga Halimeda and seagrass Thalassia along with other brittle stars such as Amphipholis januarii (Thomas, Reference Thomas1962). It is also found on coral reefs and muddy bottoms (Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013).
Distribution. Atlantic Ocean: USA (Florida), Mexico, Belize, Panama, Venezuela, Cuba, and Puerto Rico (H.L. Clark, Reference Clark1918; Thomas, Reference Thomas1962; Tommasi, Reference Tommasi1974; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). In Brazil, it has only been recorded in the North-east (Bahia) (Tommasi, Reference Tommasi1974). From 0.5 to 47 m depth (Tommasi, Reference Tommasi1974; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). Samples from the present study were collected at 47 m depth.
Ophiophragmus septus (Lütken, Reference Lütken1859)
(Figures 11 and 12)
Amphiura septa Lütken, Reference Lütken1859: 120
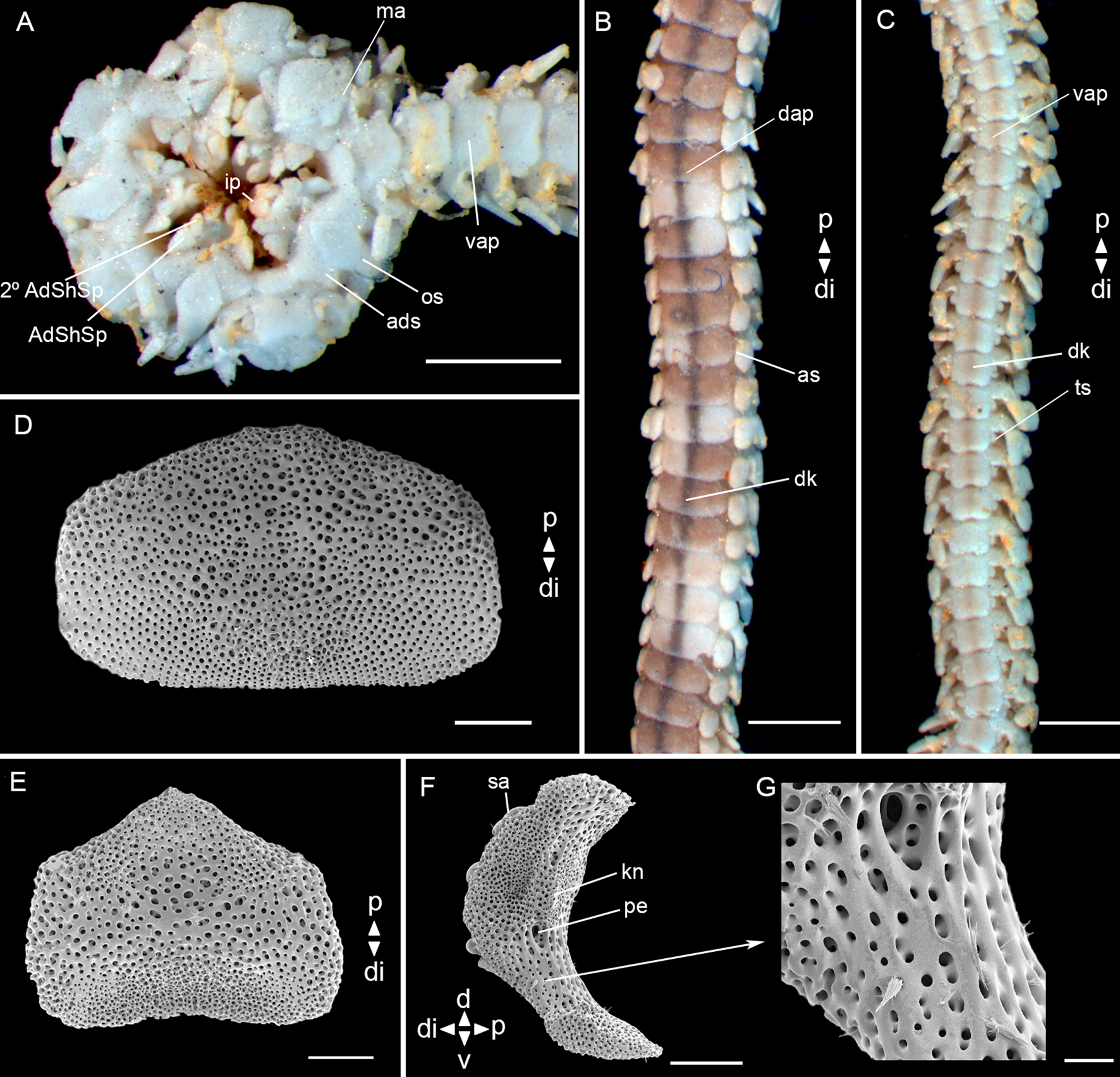
Fig. 11. Ophiophragmus septus (Lütken, Reference Lütken1859) (MZUFBA 01145, dd: 3.8 mm): (A) detail of the oral view; (B) detail of dorsal arm; (C) detail of ventral arm; (D) dorsal arm plate – internal view; (E) ventral arm plate – internal view; (F) lateral arm plate – internal view; (G) lateral arm plate – detail of internal view. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; dap: dorsal arm plate; di: distal; dk: dark line; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; sa: spine articular tubercle; ts: tentacle scale; vap: ventral arm plate. Scale bar: stereomicroscope photographs A–C, 1.0 mm; SEM images D–F, 100 μm, G, 20 μm.

Fig. 12. Ophiophragmus septus (Lütken, Reference Lütken1859) (MZUFBA 01145, dd: 3.8 mm): (A) vertebra – proximal surface; (B) vertebra – distal surface; (C) vertebra – dorsal surface; (D) vertebra – ventral surface. Abbreviations: d: dorsal; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; p: proximal; v: ventral; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: SEM images, 100 μm.
Ophiophragmus septus Lyman, Reference Lyman1865: 132; H.L. Clark, Reference Clark1915: 239, Reference Clark1918: 275, 1933: 48; Thomas, Reference Thomas1962: 669
Amphipholis septa Lütken 1872: pl. 2
Amphiodia erecta Koehler, Reference Koehler1914: 67
Type locality. St. Thomas, Caribbean.
Material examined. 1 specimen (dd: 3.8 mm). See Supplementary Table S1.
Size range (dd). From 3.8 (present study) to 9 mm (H.L. Clark, Reference Clark1918).
Description
Disc (dd: 3.8 mm) according to Thomas (Reference Thomas1962) (Figure 15A, B): pentagonal, covered by numerous small and imbricating scales, ~22 between the centrodorsal and the edge of the disc. Radial shields acute proximally, twice as long as wide, continuous throughout and separated proximally by two scales, distal triangular and smaller than the proximal. Fence of blunt papillae at the edge of the disc. Ventral interradius covered with scales similar to the dorsal. Bursal slits long and narrow. Oral shields lozenge-shaped, distally rounded, proximal angle acute. Madreporite larger than other oral shields. Adoral shields triangular, equilateral and united proximally. Adoral shield spines: first triangular and larger than the second. A pair of infradental papillae separated from each other (Figure 11A).
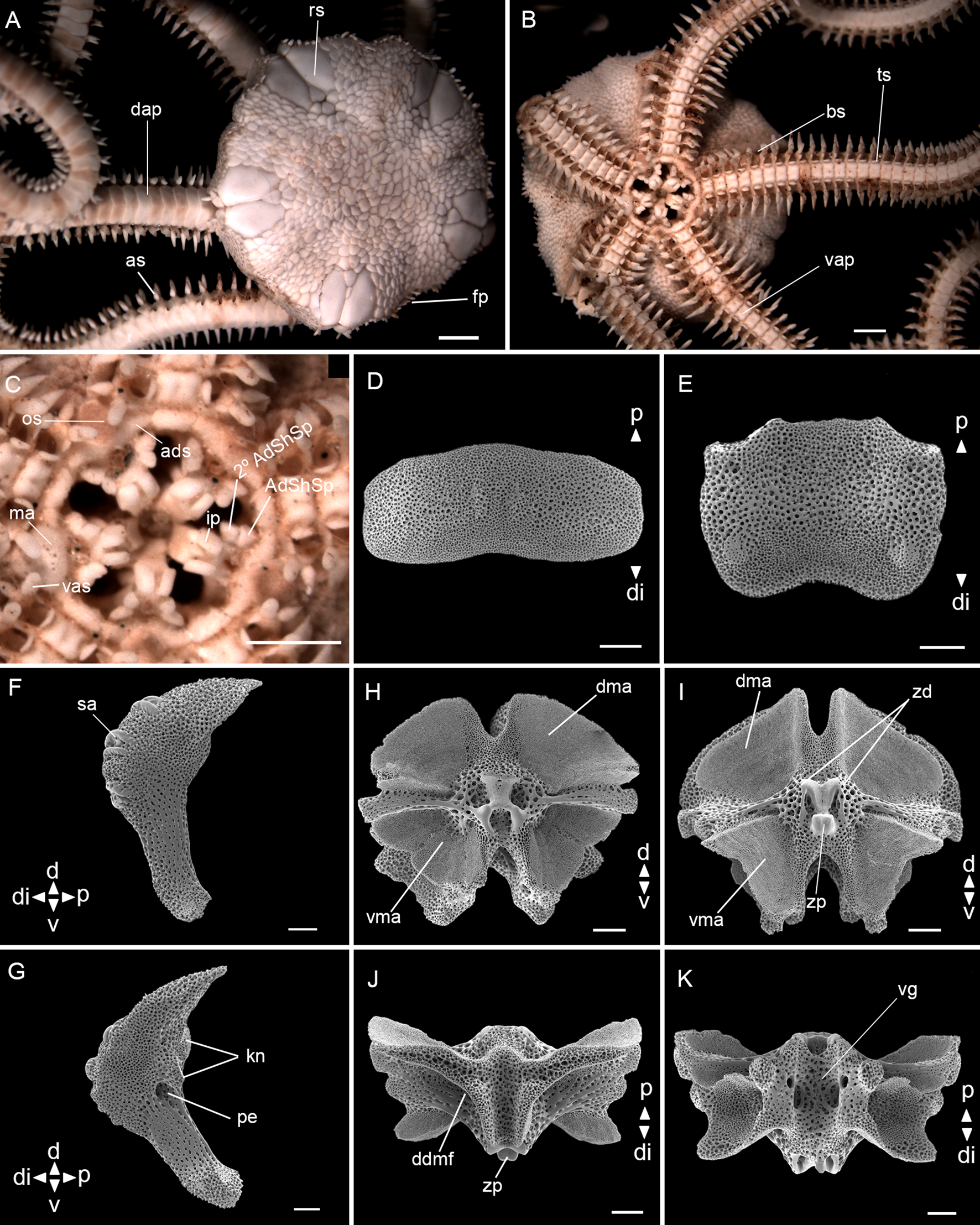
Fig. 13. Ophiophragmus wurdemanii Lyman, 1860 (ZUEC OPH 1906, dd: 6.2 mm): (A) dorsal view; (B) ventral view; (C) detail of the oral view; (D) dorsal arm plate – internal view; (E) ventral arm plate – internal view; (F) lateral arm plate – external view; (G) lateral arm plate – internal view; (H) vertebra – proximal surface; (I) vertebra – distal surface; (J) vertebra – dorsal surface; (K) vertebra – ventral surface. Abbreviations: AdShSp: adoral shield spine; 2° AdShSp: second adoral shield spine; ads: adoral shields; as: arm spine; bs: bursal slits; d: dorsal; dap: dorsal arm plate; ddmf: dorso-distal muscular fossae; di: distal; dma: dorsal muscle area; fp: fence of papillae; ip: infradental papillae; kn: knob; ma: madreporite; os: oral shields; p: proximal; pe: perforation; rs: radial shields; sa: spine articular tubercle; ts: tentacle scale; v: ventral; vap: ventral arm plate; vas: ventralmost arm spine; vg: ventral groove; vma: ventral muscle area; zd: zygocondyle; zp: zygosphene. Scale bar: stereomicroscope photographs A–C, 1.0 mm; SEM images D–K, 100 μm.
Arms: a single dark line extending the entire length of the dorsal surface (Figure 11B). Dorsal arm plates twice as wide as long, contiguous (Figure 11B), in dissociated plates distal edge straight, proximal edge concave (Figure 11D). Ventral arm plates as wide as long, contiguous (Figure 11C), in dissociated plates pentagonal, distal edge straight, proximal edge tapered (Figure 11E). Two operculiform tentacle scales, one attached to the ventral arm plate and the other to the lateral arm plate. Three blunt and short arm spines (shorter than half the length of a segment), dorsalmost thicker (Figure 11B, C).
Lateral arm plates (Figure 11F): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: Stereom with small pores approximately the same size on ventral and dorsal edges. Outer proximal edge: without spurs; central part not protruding. Spine articular tubercles: on same level as remaining outer surface, all similar size, distance between spine articular tubercles equidistant. Lobes simply separated, equal-sized; lobes parallel, bent, and oriented nearly horizontal. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 12A). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 12B). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles (Figure 12C). Zygosphene not projecting beyond ventral edge of zygocondyles (Figure 12D).
Taxonomic comments. Our description of the disc surface of Ophiophragmus septus was based on previously published illustrations (Thomas, Reference Thomas1962; Hendler et al., Reference Hendler, Miller, Pawson and Kier1995) since our specimen had no disc. The most distinctive feature of O. septus is the single dark line extending the entire length of the dorsal surface of the arms, and sometimes more clearly on the ventral surface (H.L. Clark, Reference Clark1918; Thomas, Reference Thomas1962). Ophiophragmus septus greatly resembles O. pulcher, but the latest differs in having shorter radial shields, flattened and longer arm spines (longer than one segment) (Thomas, Reference Thomas1962). We observed spinelets on the surfaces of the lateral arm plates and vertebrae (Figures 11F, G, 12A–D). Initially, these characters were considered as unusual structures on these plates, similar to those observed by Martynov & Litvinova (Reference Martynov and Litvinova2008) on the dorsal arm plates of Ophiocamax patersoni. However, after detailed studies of our SEM images and comparison with those from the previous authors, it was considered that they are artifacts in the case of O. septus, possibly caused by contamination at the time of preparation of the material, since they do not present a size nor distribution pattern over the plates and vertebrae. Furthermore, they do not appear to be ‘continuations of stereoma’ as observed in O. patersoni.
Remarks. This species occurs in coral reefs, mangroves, muddy and sandy bottoms (Thomas, Reference Thomas1962; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013).
Distribution. Atlantic Ocean: USA (Florida), Mexico, Belize, Panama, Trinidad and Tobago, Cuba, Puerto Rico, Colombia, Venezuela (Thomas, Reference Thomas1962; Magalhães et al., Reference Magalhães, Martins and Alves2005; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). In Brazil: Bahia (Magalhães et al., Reference Magalhães, Martins and Alves2005). From intertidal to 116 m depth (Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). Samples of the present study were collected at 26 m depth.
Ophiophragmus wurdemanii (Lyman, 1860)
(Figure 13)
Amphiura wurdemanii Lyman, 1860: 169; Ljungman 1871: 648

Fig. 14. Axes 1 and 2 from linear discriminant analysis (LDA) based on 15 brittle star characters with 54 specimens (Supplementary Table S2). See Materials and methods section for definitions of the morphological characters used for the morphometric analysis. Size of each dot was scaled based in the disc diameter of the specimens.
Ophiophragmus wurdemanii Lyman, Reference Lyman1865: 42, Reference Lyman1875: 21, 1882: 159; H.L. Clark, Reference Clark1915: 239, Reference Clark1918: 273, 1933: 47
Type locality. Florida, USA.
Material examined. 2 specimens (dd: 6.2–7.4 mm). See Supplementary Table S1.
Size range (dd). From 6.2 (present study) to 9.5 mm (Lyman, Reference Lyman1865).
Description
Disc (dd: 6.2 mm): circular with interradial recesses, covered by numerous small and imbricating scales, ~20 between the centrodorsal and the edge of the disc. Fence of blunt papillae at the edge of the disc interradius. Radial shields almost twice as long as wide, one fifth of dd, acute proximally, continuous throughout and separated proximally by one triangular scale. Three subequal and larger scales at the proximal edge of radial shields (Figure 13A). Ventral interradius covered with scales smaller than the dorsal and strongly imbricated. Bursal slits long and narrow (Figure 13B). Oral shields spearhead-shaped, distally rounded, proximal angle acute, with latero-posterior indentations. Madreporite larger than other oral shields, whitish and with pores proximally. Adoral shields narrow and long, placed horizontally and touching both the first ventral arm plates, establishing a continuous border encircling the mouth opening and jaws – forming a ring. Adoral shield spines: first slightly larger than the second. A pair of infradental papillae, well developed, rectangular and separated from each other (Figure 13C).
Arms: dorsal arm plates rectangular with rounded edges, almost three times as wide as long, proximal edge convex, middle portion of distal edge concave, contiguous (Figure 13A, D). Ventral arm plates as wide as long, contiguous (Figure 13B), in dissociated plates with projected proximal angle and with a slight concavity at the distal edge (Figure 13E). Two leaf-like tentacle scales, one attached to ventral plate and one to lateral plate, completely separated by a gap. Three arm spines, almost half the length of a segment, ventralmost blunt and flattened, others pointed (Figure 13B, C).
Lateral arm plates (Figure 13F, G): general outline: ventral portion projecting ventro-proximalwards; ventro-distal tip not projecting ventralwards. Outer surface features: trabecular intersections protruding to form knobs approximately the same size than stereom pores. Outer proximal edge: surface lined by discernible band of different stereom structure, restricted to central part; without spurs; central part not protruding; surface without horizontal striation. Spine articular tubercles: on same level as remaining outer surface, all similar size, distance between spine articular tubercles equidistant. Lobes simply separated, equal-sized; lobes parallel, bent, and oriented nearly horizontal; stereom with perforations. Inner side, ridges and knobs: inner side dominated by two separate central knobs; without additional dorsal structure on inner side; single large perforation on inner side.
Vertebrae: zygospondylous of universal type. Proximal side of vertebrae dorsally without large groove on the dorsal-distal muscular fossae (Figure 13H). Zygocondyles dorsalwards converging and zygosphene fused with pair of zygocondyles (Figure 13I). Dorso-distal muscular fossae positioned before the distal edge of zygocondyles (Figure 13J). Zygosphene projecting beyond ventral edge of zygocondyles with projecting part longer than zygocondyles (Figure 13J, K).
Taxonomic comments. Three features differentiate Ophiophragmus wurdemanii from other congeneric species: (i) the radial shields are acute proximally and with a row of three subequal and larger scales at their proximal edge; (ii) the adoral shields are wider distally, touching the first ventral arm plates, establishing a continuous border encircling the oral aperture and jaws; and (iii) the ventralmost arm spine is blunt and flatted while others are pointed. Ophiophragmus wurdemanii is similar to O. filograneus, but the latter differs in having papilliform granules on the ventral disc and teeth on the proximal second arm spines (Thomas, Reference Thomas1962).
Remarks. Ophiophragmus wurdemanii occurs on coral reefs and sandy bottoms (Thomas, Reference Thomas1962; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). The specimen from south-eastern Brazil was sampled under rocks.
Distribution. Atlantic Ocean: USA (North Carolina and Florida), Gulf of Mexico, Venezuela (Thomas, Reference Thomas1962; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). In Brazil: Pernambuco (Lima & Fernandes, Reference Lima and Fernandes2009), Bahia (Magalhães et al., Reference Magalhães, Martins and Alves2005) and São Paulo (Tommasi et al., Reference Tommasi, Castro and Sousa1988). From intertidal to 45 m depth (Tommasi et al., Reference Tommasi, Castro and Sousa1988; Alvarado & Solís-Marín, Reference Alvarado and Solís-Marín2013). Samples of the present study were collected in the intertidal.
ADDITIONAL REMARKS
Records of Ophiophragmus filograneus from Brazil were invalidated after the comparison with specimens from the type locality (Florida, USA). The 29 Floridian specimens were deposited in ZUEC and labelled as ZUEC OPH 1904, ZUEC OPH 2681 and ZUEC OPH 2682. These specimens were studied using specialized literature (Thomas, Reference Thomas1962; Tommasi, Reference Tommasi1970; Lyman, Reference Lyman1875; Bueno et al., Reference Bueno, Alitto, Guilherme, Di Domenico and Borges2018) and comparative material (syntype of Ophiocnida loveni from the Museum of Comparative Zoology OPH-1498). This study found that O. filograneus and O. loveni have been confused in the past. Therefore, a brief comparison between both is presented.
Ophiophragmus filograneus (dd: 3.5 mm) is covered by small scales, ~17 between the centrodorsal and the edge of the disc; fence of blunt papillae at the edge of the disc interradius; oral shields lozenge-shaped; dorsal arm plates almost three times as wide as long; ventral arm plates hexagonal. Ophiocnida loveni (dd: 3.2 mm) is covered by large scales, ~9–10 between the centrodorsal and the edge of the disc; short spinules around the disc and covering the ventral interradius; oral shields distally rounded and proximal angle acute; dorsal arm plates 1.5 times as wide as long; ventral arm plates pentagonal. The Brazilian specimens identified by Albuquerque (Reference Albuquerque1986) and Paim et al. (Reference Paim, Guerrazzi and Borges2015) were reassessed and reidentified as Ophiocnida loveni. Other specimens identified as O. filograneus in Brazil should be re-evaluated since their identifications may be based on the description by Albuquerque (Reference Albuquerque1986).
MORPHOMETRY
Amphiodia spp.
A total of 54 specimens was used: Amphiodia planispina = 10; Amphiodia pulchella = 20; Amphiodia riisei = 14; Amphiodia trychna = 10.
The LDA using all 15 morphological characters was effective in discriminating between the four Amphiodia species. The first and second linear discriminant axes represent 89.17% and 8.46% of the total dispersion in morphological characters among Amphiodia (Figure 14).

Fig. 15. Axes 1 and 2 from linear discriminant analysis (LDA) based on 15 brittle star characters and with 40 specimens (Supplementary Table S3). See Supplementary Table S4 for definitions of the morphological characters used for the morphometric analysis. Size of each dot was scaled based on the disc diameter of the specimens.
The first discriminant vector (LD1) identified the length and the width of the adoral shield (ads_l and ads_w, respectively) as the morphological characters with the highest positive coefficients. The width of the radial shield (rs_w) and length of the oral shield (os_l) had the highest negative coefficients. Discriminates mainly Amphiodia pulchella from Amphiodia riisei and Amphiodia trychna.
Alternatively, the second discriminant vector (LD2) provides a good distinction between Amphiodia riisei with bigger adoral shield (ads_l) and second ventral arm plate (vap2_l) than other Amphiodia species. On the other hand, the width of the second ventral arm plate (vap2_w) and the length of the dorsal arm plate (dap_l) were associated with Amphiodia pulchella, Amphiodia planispina and Amphiodia trychna.
Ophiophragmus spp.
A total of 40 specimens were used: Ophiophragmus brachyactis = 1; Ophiophragmus cubanus = 4; Ophiophragmus luetkeni = 19; Ophiophragmus pulcher = 14; Ophiophragmus wurdemanii = 2.
The LDA using all 15 morphological characters was effective in discriminating between the three Ophiophragmus species, with some degree of overlap. The first and second linear discriminant axes described 75.11% and 13.52% of the variation in morphological characters, respectively (Figure 15).
The first discriminant vector (LD1) discriminates three groups, (1) Ophiophragmus cubanus and Ophiophragmus pulcher; (2) Ophiophragmus brachyactis and Ophiophragmus luetkeni and (3) Ophiophragmus wurdermanii. The separation of groups is based mainly on the length of the oral shield (os_l) and the width of the first ventral arm plate (vap1_w) as the morphological characters with the highest positive coefficients, and the length of the adoral shield (ads_l) and the width of the radial shield (rs_w) were the highest negative coefficients.
Alternatively, the second discriminant vector (LD2) sorts only Ophiophragmus wurdemanii with bigger oral diameter (od) and large first ventral arm plate (vap1_l) than Ophiophragmus luetkeni with larger oral shield (os_w) and the bigger dorsal arm plate (dap_l).
Key to the genera Ophiophragmus and Amphiodia in Brazil
1. Fence of papillae at the edge of the disc interradi ………Ophiophragmus………2
Disc without papillae at the disc edge………Amphiodia………7
2. Dorsal surface of the arms with a single dark line extending the entire length………Ophiophragmus septus
Dorsal surface of the arms without a single dark line ……… 3
3. Spines scattered on the dorsal and ventral surface, oral shields as long as wide ……… Ophiophragmus cubanus
Without spines on the dorsal and ventral surface, oral shields more than 1.5 times as long as wide ………4
4. One bent papilla at the distal tip of each radial shield ………Ophiophragmus pulcher
Without a bent papilla at the distal tip of each radial shield ……… 5
5. Adoral shields placed horizontally touching the first ventral arm plates, establishing a continuous border encircling the oral aperture and jaws – forming a ring; the ventralmost arm spine is blunt and flatted while others are pointed ……… Ophiophragmus wurdemanii
Adoral shields placed vertically touching both the first ventral arm plate, but not establishing a continuous border, and consequently, not forming a ring; arm spines all pointed………6
6. Radial shields almost as long as wide; oral shields lozenge-shaped with rounded edges; dorsal arm plates twice as wide as long ……… Ophiophragmus brachyactis
Radial shields twice as long as wide; oral shields spearhead-shaped, distally rounded, proximal angle acute and with latero-posterior indentations; dorsal arm plates almost three times as wide as long ……… Ophiophragmus luetkeni
7. One tentacle scale; three arm spines, middle larger, flattened and hatchet-shaped, proportion between ads_l and ads_w higher than 0.7 ….……..…. Amphiodia pulchella
Two tentacle scales; three arm spines, middle not hatchet-shaped, proportion between ads_l and ads_w lower than 0.7 ……… 8
8. Three arm spines with wide and blunt edges similar to a paddle in shape ……… Amphiodia planispina
Three arm spines ……… 9
9. A rosette of primary plates: central pentagonal and radial plates larger and irregular………Amphiodia habilis
Without a rosette of primary plates ……… 10
10. Radial shields triangular with straight edges; scales near the distal edge of radial shields tending to stick up; oral shields with rounded edges; ventral arm plates pentagonal with keels at the distal edge ……… Amphiodia riisei
Radial shields internally straight and externally curved (half circle); scales near the distal edge of radial shields do not tend to stick up; oral shields with proximal edge tapered and distal edge rounded; ventral arm plates pentagonal without keels at the distal edge ………Amphiodia trychna
Discussion
Our taxonomic study provided detailed descriptions of five Amphiodia spp. and six Ophiophragmus spp. recorded in Brazil, using external morphology, arm microstructures and morphometry. The examinations found new diagnostic characters, offering more data for comparative studies of closely related species with similar morphology. All the information presented could be used in taxonomic, ecological and phylogenetic studies, helping to fill gaps that still exist in our knowledge of the biodiversity, ecology and evolution of Ophiuroidea.
The study of arm ossicles, particularly lateral arm plates and vertebrae, found new features that should be taken into account in future studies. Amphiodia pulchella has lateral arm plates with ridges between each pair of lobes. This attribute was not described by Alitto et al. (Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018) but it can be observed in their figures. Amphiodia riisei has ventral arm plates with two keels at the distal edge, only visible through SEM.
Amphiodia and Ophiophragmus did not present significant differences in relation to the dorsal, ventral, lateral arm plates and vertebrae, but some details attracted attention. Most species have rectangular dorsal arm plates (DAP) with rounded edges, while A. trychna and O. cubanus have oval DAP and A. pulchella and O. septus have semi-rectangular DAP with straight distal edges and convex proximal edges. Ventral arm plates are commonly pentagonal, semi-pentagonal or rectangular. The general outline of the lateral arm plates (LAP) is similar, except in A. pulchella where it is strongly concave with a ‘C’ shape. The two knobs of the inner side of the LAPs are more protruding in A. pulchella and O. cubanus than in other species. The dorsal surface of the vertebrae is similar among most of the here examined species, except in A. pulchella which has a narrow dorsal groove on the dorsal-distal muscular fossae (DDMF) whereas O. brachyactis and O. cubanus have very large, projecting DDMF divided into two end processes.
A detailed comparison between Amphiodia riisei and Amphiodia trychna is provided, because they previously had overlapping diagnoses. Differences were observed related to: (i) triangular radial shields with straight edges in A. riisei and half circle with straight adradial edge and curved abradial edge in A. trychna; (ii) radial primary plates touching each other in A. riisei and completely separated in A. trychna (at all sizes); (iii) scales near the distal edge of radial shields tending to stick up only in A. riisei; (iv) oral shields with rounded edges in A. riisei and with tapered proximal edge and rounded distal edge in A. trychna and (v) keels on the ventral arm plates visible by SEM only in A. riisei. Specimens from Araçá Bay, State of São Paulo, Brazil (Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018), previously identified as A. riisei were reevaluated and reidentified as A. trychna.
Amphiodia habilis was collected at the mouth of the Doce River (State of Espírito Santo, Brazil) and described by Albuquerque et al. (Reference Albuquerque, Campos-Creasey and Guille2001). Since then, A. habilis has not been reported, probably because few studies have been conducted at its type locality. However, due to the detailed description (Albuquerque et al., Reference Albuquerque, Campos-Creasey and Guille2001), it was possible to compare A. habilis with its congeners. Amphiodia habilis differs from other Amphiodia recorded in Brazil in its rosette of primary plates: central pentagonal and radial plates are larger than the disc scales and irregular in shape.
Ophiophragmus brachyactis and Amphiodia riisei are very similar, but certainly cannot be considered the same species, because of the fence of papillae on the edge of the disc of O. brachyactis. According to H.L. Clark (Reference Clark1918), this fence is one of the most important characters of the genus Ophiophragmus. In addition to the fence of papillae, we highlighted other morphological differences in the LAP, DAP and ventral arm plates (VAP). The dorsal portion of the LAP is more projecting proximalwards in A. riisei than in O. brachyactis, and A. riisei has a slight concavity at the distal edge of the DAP, unlike O. brachyactis.
The morphometric analysis of the species in the genera Amphiodia and Ophiophragmus was useful in detecting patterns in their morphological characters. Measurements of oral and adoral shields are highly indicative in the separation of Amphiodia species, while measurements of oral shields and ventral arm plates are significant in the separation of Ophiophragmus species. The size of the specimens wasn't important for the classification of species in the models, demonstrating that young specimens of these species maintain their morphological characters. This demonstrates the power of morphometry for taxonomic studies and highlights the characters that should be considered in diagnoses. We emphasize the use of morphometry as a powerful tool particularly when applied with an integrative approach (Arribas et al., Reference Arribas, Andújar, Sánchez-Fernández, Abellán and Millán2013; Alitto et al., Reference Alitto, Amaral, Oliveira, Serrano, Seger, Guilherme, Di Domenico, Christensen, Lourenço, Tavares and Borges2019). Probably the species not well separated in the LDA are related to the low number of specimens due to the difficulties with collecting them.
Two species of Ophiophragmus are new records for some localities in Brazil: O. brachyactis was registered in the north-east (Manso, Reference Manso2004; Gondim et al., Reference Gondim, Alonso, Dias, Manso and Christoffersen2013a), east (Manso, Reference Manso1988, Reference Manso1993; Oliveira et al., Reference Oliveira, Oliveira and Manso2010), and in the south-east; O. luetkeni was recorded from the north-east to the south-east (Alitto et al., Reference Alitto, Bueno, Guilherme, Di Domenico, Christensen and Borges2018), and in the north (Pará-Maranhão Basin).
Ethics statements
Specimens data were registered at the National System for the Management of Genetic Heritage and Associated Traditional Knowledge–SisGen, according to Brazilian legislation Law number 13.123/2015 and Decree 8772/2016. Approval ID for this study was AB511BE and AD395C0.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315420000521.
Acknowledgements
The authors would like to thank Espaço da Escrita – Pró-Reitoria de Pesquisa – UNICAMP – for the language services provided. We are grateful to Letícia Oliveira, Cecilia Damiano and Giovanna Aranha for their support with morphometry; to Maristela Bueno for collecting the Paranaguá specimens and to Professor Richard Turner from the Florida Institute of Technology for the specimens of Ophiophragmus filograneus provided. We also thank MZUSP and MZUFBA for specimen loans and MCZ Harvard, particularly Adam Baldinger, for the excellent photos provided of Ophiocnida loveni. Additionally, we show great gratitude to Adriane Sprogis and Stella de Ferraz for their help with the scanning electron microscopy at UNICAMP. Finally, many thanks to the reviewers whose valuable comments were greatly appreciated.
Financial support
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 and the São Paulo Research Foundation (FAPESP) (grant number 2011/50317-5, 2018/10313-0). Additionally, this work was supported by FAPESP RASA grant 2012/11773-8, MB grant 2011/50724-0, HS grant 2016/08869-4, GG grant 2017/09987-3.

















