INTRODUCTION
Chloeia was established by Lamarck (Reference Lamarck1818) to accommodate Chloeia flava described from the Indian Ocean by Pallas in 1766. The genus was morphologically characterized by having an elliptical body with bipinnate branchiae. This kind of branchiae is shared with the monotypic genera Bathychloeia Horst, Reference Horst1912 and Chloenopsis Fauchald, Reference Fauchald1977. Adults of Chloeia are colourful and have shades of violet, green and yellow pigment impregnated mainly on the dorsum, notopodial cirri, cirrophores and caruncle. Dorsal pigmentation patterns appear to be important species-specific characters, although the maintenance of these patterns in preserved specimens has been questioned (Monro, Reference Monro1933). Other important characters are chaetae, development of parapodial cirri, eyes, caruncle, shape of chaetiger 1, parapodia of mid-body chaetigers, types of noto- and neurochaetae, number and position of noto- and neuroaciculae, distribution and placement of branchiae and degree of development, and type of anal cirri (Kudenov, Reference Kudenov, Blake, Hilbig and Scott1995).
Members of the genus Chloeia have a circumtropical distribution, with many more species in Indian and Pacific than Atlantic waters. Of the 20 valid species of Chloeia recognized by Hartman (Reference Hartman1959), only Chloeia viridis Schmarda, 1961 from Jamaica was originally described from the Atlantic Ocean. Hartman (Reference Hartman1959) recognized four junior synonyms of C. viridis including C. pallida Kinberg 1867 described from Brazil, C. modesta Ehlers, 1887 and C. euglochis Ehlers, 1887 from Florida, and C. candida Kinberg, 1910 from the West Indies. These synonymies should be reevaluated carefully, if possible with a molecular approach.
Moreover, besides C. viridis (Nonato & Luna, Reference Nonato and Luna1970; Gathof, Reference Gathof, Uebelacker and Johnson1984; Amaral & Nonato, Reference Amaral and Nonato1994), C. venusta Quatrefages, 1866 (originally described from the Mediterranean Sea) is the only other species of the genus recorded to date in Atlantic waters (Fauvel, Reference Fauvel1923; Kirkegaard, Reference Kirkegaard2001). Despite the fact that most Chloeia species were described from shallow waters, few of those species have been referred to moderate deep-waters (e.g. C. pinnata Moore, 1911, California, 567 m (Kudenov, Reference Kudenov, Blake, Hilbig and Scott1995); C. venusta Quatrefages, 1866, north-west Africa, 210 m (Kirkegaard, Reference Kirkegaard2001) and C. inermis Quatrefages, 1866, New Zealand, 200 m (Probert & Grove, Reference Probert and Grove1998)).
To date, the only phylogenetic hypothesis regarding relationships within Amphinomida was proposed by Wiklund et al. (Reference Wiklund, Nygren, Pleijel and Sundberg2008), establishing Chloeia as a sister taxa of Archinome and suggesting the abandonment of the family Archinomidae. The morphology of the caruncle, body shape and the characteristic pigmentation patterns on the dorsum give some support to the hypothesis that Chloeia is closely related to Notopygos, the ‘Chloeia–Notopygos’ complex (Kudenov, Reference Kudenov1991), that could include Bathychloeia and Archinome.
In this paper we describe a new deep-water species (750–1045 m) of Chloeia from southern Brazil. The new taxon differs from other described congeners mainly by having extremely long neuropodial cirri on chaetiger 2 and lack of body pigmentation patterns.
MATERIALS AND METHODS
The specimens of Chloeia were collected in 3 stations (Figure 1) in the Campos Basin (southern Brazil—off Rio de Janeiro State, between 21°18′S and 23°00′S), during a deep-sea survey conducted by PETROBRAS (Brazilian Petroleum Company) under the scope of the project ‘Campos Basin Deep-Sea Environmental Project’ coordinated by CENPES/PETROBRAS. The sediment sampled with a box-corer was separated into three vertical strata (0–2 cm, 2–5 cm and 5–10 cm), sieved in a 0.5 mm mesh and fixed in 10% formalin. Type materials were deposited in the polychaete collection of the Zoology Department at the Universidade Federal do Rio de Janeiro, Brazil (IBUFRJ), and in the Museu de História Natural of the Universidade Estadual de Campinas (ZUEC: POL).

Fig. 1. Stations where specimens of Chloeia kudenovi sp. nov. were collected.
RESULTS AND DISCUSSION

Fig. 2. Chloeia kudenovi sp. nov. (A) Dorsal view paratype (ZUEC: POL21); (B) anterior region in dorsal view; (C) anterior region in ventral view; (D) anterior parapodium in posterior view.
MATERIAL EXAMINED
Holotype: (IBUFRJ: 634) ovigerous female, 20 mm long, 6 mm wide (without chaetae), 24 chaetigers; 20 November 2002; 22°19′50″S 40°00′35″W, 775 m; paratypes: (IBUFRJ: 636, 8 specimens) 14 mm long, 5 mm wide, 22 chaetigers; 8 mm long, 2.5 mm wide, 22 chaetigers; 7 mm long, 2.7 mm wide, 20 chaetigers; 6.7 mm long, 2.2 mm wide, 19 chaetigers; 11 mm long, 3 mm wide, 24 chaetigers; 10 mm long, 3 mm wide, 22 chaetigers; 7.5 mm long, 3.1 mm wide, 21 chaetigers; 6 mm long, 2.3 mm wide, 17 chaetigers; 20 November 2002, 22°40′57″S 40°16′30″W, 1045 m. (ZUEC: POL21, 1 specimen) 9 mm long, 3 mm wide, 21 chaetigers; 20 November 2002, 22°10′27″S 39°54′46″W, 745 m.
DESCRIPTION OF THE HOLOTYPE
The holotype lacks dorsal pigmentation pattern. Anterior lobe of prostomium rounded. Posterior lobe of prostomium with two lateral cirriform antennae which are smaller than the palps. Two pairs of eyes the anterior pair being larger. Median antennae arising from anterior margin of caruncle are longer than lateral antennae and palps. Palps slender, cirriform. Palps fused, converging mid-ventrally into a longitudinal groove leading to mouth. Caruncle extends posteriorly to end of chaetiger 3 fused to dorsum on chaetigers 1–2 and free thereafter (Figure 2A, B). Mouth located between palps and posterior lip formed by chaetiger 2 (Figure 2C). Parapodia well developed with widely separated rami in all chaetigers (Figure 2D).
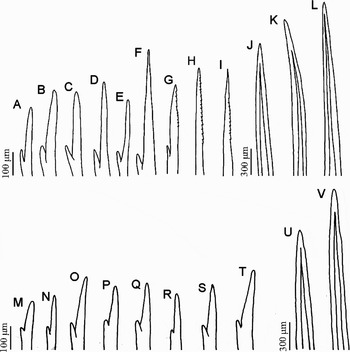
Notopodial chaetae of five types: (1) bifurcate chaetae (Figure 3A–F); (2) bifurcate harpoon chaetae with denticulations offset from small prong (Figure 3G) in all fascicles posterior to first third of body; (3) harpoon notochaetae (Figure 3H, I); (4) spinose notochaetae (Figure 3J), 8–12 per notopodial fascicle, arrayed in a row in superior region of fascicles; and (5) spinose notoaciculae (Figure 3K, L), 3–4 per fascicle, arrayed in front of notopodiol cirrus. Neurochaetae longer than notochaetae and arrayed in denser tufts, being of two types: (1) bifurcate chaetae (Figure 3M–T); and (2) spinose neuroaciculae, numbering 10–12 per fascicle, arrayed in a row along the most ventral region of fascicle (Figure 3U, V).

Fig. 3. (A–F) Bifurcate notochaetae; (G) bifurcate harpoon notochaetae; (H, I) harpoon notochaetae; (J) spinose notochaetae; (K, L) notoacicula; (M–T) bifurcate neurochaetae; (U, V) neuroaciculae.
Parapodial cirri present in all chaetigers. Chaetigers 1–3 include branchial, notopodial and neuropodial cirri. Branchial cirri are lacking cirrophores. Notopodial cirri cirriform with cirrophores. The cirrostyle slender is about 3 × longer than the cirrophores. Neuropodial cirrophores are smaller than notopodial ones. Notopodial and neuropodial cirri of similar size throughout the body, except for neuropodial cirri of the 2nd chaetiger, which are three times longer than dorsal (Figure 2A–C).
Branchiae are bipinnate from chaetiger 4 to the end of body, with 8–12 alternating branches arising from the primary axis each terminating in smaller alternating terminal filaments. Branchiae best developed in mid-chaetigers, decreasing in size in posterior chaetigers (Figure 2A, D).
Pygidium is terminal opening between a pair of thick, digitiform anal cirri (Figure 2A).
REMARKS
Regarding the recognition of valid species of Chloeia, three studies have presented a list based on literature surveys (Horst, Reference Horst1910; Hartman, Reference Hartman1959; Baird, Reference Baird1968), with, respectively, 13, 11 and 20 valid species.
The most referred and better described species are presented in Table 1, with the main morphological characters, current distribution and references. Some of these species are superficially described. Information regarding number and position of aciculae as well as shape and distribution of chaetae along the body is not given clearly in many descriptions.
Table 1. Most referred Chloeia species and Chloeia Kudenovi sp. nov. Character variation (number of chaetigers and size (length × width); 1st branchiate chaetiger; types of notopodiol chaetoe; types of neuropodiol chaetae; pigmentation pattern on dorsum); distribution and main references.

However, Chloeia kudenovi and C. violacea differ from all other known species of the genus by the length of the neuropodial cirrus on the second chaetiger. Chloeia kudenovi differs from C. violacea in lacking a specific pattern of dorsal pigmentation, having shorter median and lateral antennae (not reaching the end of the caruncle), and having both noto- and neuropodial spines.
Furthermore, C. violacea was described from the Malay Archipelago (Indo-Pacific Ocean) in shallower depths. Regarding body pigmentation, C. kudenovi sp. nov. is similar to C. inermis Quatrefages, 1866, C. pinnata Moore, 1911, C. entypa Chamberlin, 1919 and C. inermis Quatrefages, 1866 in lacking pigmentation, but differs from these species in having a long neuropodial cirri on chaetiger 2.
This is the second species of Chloeia that has been reported from Brazilian waters and the third from the Atlantic Ocean. Until now, only Chloeia viridis (described from Jamaica) was previously reported for the Brazilian coast (Nonato & Luna, Reference Nonato and Luna1970; Amaral & Nonato, Reference Amaral and Nonato1994) and C. venusta from the western South Atlantic (Kirkegaard, Reference Kirkegaard2001). The low diversity of the genus in the Atlantic Ocean when compared to Indian-Pacific waters could be explained by the restricted distribution of coral reefs in this ocean, an environment which usually bears a great diversity and prevalence of Chloeia species.
Feeding
Large amounts of foraminifera were found in the digestive tract of two specimens.
Reproduction
One collected specimen (holotype) presented oocytes in its coelomic cavity, with diameter ranging between 0.60 and 0.95 µm (X = 75.15; SD = 10.93; N = 30).
ETYMOLOGY
The species is named after Jerry Kudenov for his essential contribution to the knowledge of taxonomy and biology of amphinomids.
ACKNOWLEDGEMENTS
We thank the CENPES/PETROBRAS for providing the studied samples. Gabriel Monteiro scanned and sent essential literature, Vasily Radashevsky, Joana Zanol and three anonymous referees provided valuable comments and suggestons. R.B. is a PhD student at Museu Nacional (UFRJ) and has a scholarship from FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro). P.C.P. is supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).