Introduction
The family Halcampactinidae Carlgren (Reference Carlgren1921) includes six genera and nine species (Fautin, Reference Fautin2016). Some representatives of this family are characterized by special structural features that are not found or are very rare in the rest of Actiniaria, for example, the atypical location of acontia in Phytocoeteopsis ramunnii Panikkar, Reference Panikkar1936 (Panikkar, Reference Panikkar1936) or the lobed oral disc of Pelocoetes Annandale (Reference Annandale1915) (Annandale, Reference Annandale1915; Panikkar, Reference Panikkar1938; Fautin et al., Reference Fautin, Tan, Yap, Tan, Crowther, Goodwill, Sanpanich and Tay2015). This family combines both brackish-water and marine species representatives even within the same genus. For example, genus Pelocoetes Annandale (Reference Annandale1915) includes the brackish-water species Pelocoetes exul (Annandale, Reference Annandale1907) and the marine Pelocoetes minimus Panikkar (Reference Panikkar1938) (Panikkar, Reference Panikkar1938). Species of this family are distributed in three latitudinal zones, occurring in India, China, Singapore (Tropical zone), in New Zealand (Temperate zone) and in the Arctic (Polar zone) (Carlgren, Reference Carlgren1949; Li, et al., Reference Li, Liu, Liu and Xu2013). Interestingly, the species of each of these three zones differ in several morpho-anatomical features, for example, the presence of cuticle and suckers on the column in the New Zealand's Halcampactis Farquhar (Reference Farquhar1898), the cinclides present in the tropical Phytocoeteopsis ramunnii, Stephensonactis ornata Panikkar (Reference Panikkar1936), Phytocoetes gangeticus Annandale (Reference Annandale1915), P. sinensis Li, Liu & Xu (Reference Li, Liu, Liu and Xu2013), Pelocoetes exul and Pelocoetes minimus and the absence of a strict division of mesenteries into macrocnemes and microcnemes in H. arctica from the Arctic (see Farquhar, Reference Farquhar1898; Carlgren, Reference Carlgren1921; Panikkar, Reference Panikkar1936, Reference Panikkar1938; Li et al., Reference Li, Liu, Liu and Xu2013). For the first time, Panikkar (Reference Panikkar1936, Reference Panikkar1938) drew attention to the dissimilarities in structure of sea anemones from different genera and pointed out the heterogeneity of the family Halcampactinidae.
As noted by Sanamyan et al. (Reference Sanamyan, Sanamyan and Grebelnyi2016), our knowledge about the morphology of many Arctic sea anemones is mainly based on old descriptions. Haliactis arctica is not an exception. No reports have been made since its description by Carlgren (Reference Carlgren1921). Initially, based on materials collected by a number of northern expeditions, Carlgren (Reference Carlgren1921) described two species, Acthelmis schaudinnii Carlgren (Reference Carlgren1921) and H. arctica. However, Carlgren (Reference Carlgren1949) later synonymized them, since the sole difference between these two species was the absence of acontia in A. schaudinnii (see Carlgren, Reference Carlgren1921).
The purpose of this study is to provide a detailed morphological description of Haliactis arctica based on numerous specimens from different localities and also compare this species with other halcampactinids based on the only available morphological data. An additional aim is to clarify the taxonomic status of the families Halcampactinidae Carlgren (Reference Carlgren1921) and Haliactinidae Carlgren (Reference Carlgren1921).
Materials and methods
Specimens examined
The descriptions of the taxon examined in the present study are based on specimens stored at the Laboratory of Marine Researches of the Zoological Institute RAS, Saint Petersburg (ZIN RAS).
Anatomical and micro-anatomical observations
The specimens in the collection were fixed in 4% seawater formaldehyde and then were transferred to 70% ethanol for long-term storage. Ethanol-preserved specimens were examined whole, then dissected. Six specimens from different localities (Franz Josef Land, the White Sea, the Chukchi and East Siberian seas) were embedded in paraffin and cut into histological serial sections 3–7 μm thick, based on the isopropanol-mineral oil method (see Sanamyan et al., Reference Sanamyan, Sanamyan and McDaniel2013). The cnidom was determined from small pieces of tissue from tentacles, column, actinopharynx, filaments and acontia of eight specimens from all localities (two from each of four). For general cnidae terminology, I use a combination of classifications of Weill (Reference Weill1934a, Reference Weillb) and Carlgren (Reference Carlgren1940). Higher classification of Actiniaria follows Carlgren (Reference Carlgren1949). Despite the widespread use of the system for Actiniaria proposed by Rodríguez & Daly in Rodríguez et al. (Reference Rodríguez, Barbeitos, Brugler, Crowley, Grajales, Gusmão, Häussermann, Reft and Daly2014), I adhere to Carlgren's (Reference Carlgren1949) classification, since I do not consider Rodríguez & Daly's system to be exhaustive; in my opinion, the transition to a system based on molecular markers is still premature (see Ivanova, Reference Ivanova2020).
Results
Systematics
Order ACTINIARIA Hertwig, Reference Hertwig1882
Suborder NYNANTHEAE Carlgren, Reference Carlgren1899
Family HALCAMPACTINIDAE Carlgren (Reference Carlgren1921)
Genus Haliactis Carlgren (Reference Carlgren1921)
Diagnosis. (Adapted from Carlgren, Reference Carlgren1921, Reference Carlgren1949; modifications in italics) Halcampactinidae with rounded or flattened aboral end. Column not divisible into regions, smooth. No sphincter. Tentacles rather numerous, short, the inner may be longer than the outer ones. Two weak siphonoglyphs which may not be present and two pairs of directives. Six pairs of perfect, filamented mesenteries with retractors and gametogenic tissue, imperfect mesenteries in three cycles, last cycle only in uppermost part. Mesenteries of the second cycle, as а rule, without retractors but have filaments, acontia and gonads in their middle and distal parts. Mesenteries of the third cycle have only parietal muscles. Retractor muscles are strong and restricted, parietal muscles are elongated. Acontia present on mesenteries of the first and second cycles or sometimes absent, their nematocysts basitrichs and microbasic amastigophors. Cnidom: spirocysts, basitrichs, microbasic amastigophors.
Type species. Haliactis arctica Carlgren (Reference Carlgren1921) by monotypy.
Haliactis arctica Carlgren (Reference Carlgren1921)
(Figures 1–12, Table 1)
Haliactis arctica Carlgren (Reference Carlgren1921): 128, Pl. I, fig. 31, textfigs. 153–154.

Fig. 1. Haliactis arctica Carlgren (Reference Carlgren1921), external view and body shape: (A) elongated column and rounded aboral end (ZIN no 12157); (B) spherical column (ZIN no 12138b); (C) cylindrical and extended column (ZIN no 12138a); (D) specimen with extended middle part of the column (ZIN no 12138d). Scale bars: A, C, 5 mm; B, 4 mm; D, 10 mm.

Fig. 2. Haliactis arctica Carlgren (Reference Carlgren1921), external view and body shape: specimen with extended distal part and narrow proximal part of the column (ZIN no 12148c). Scale bar: 3 mm.

Fig. 3. Haliactis arctica Carlgren (Reference Carlgren1921), external view and internal structure: (A) clear mesentery inserts in the case of the absent ectoderm (ZIN no 12155); (B) specimen attached to a stone (ZIN no 12150d); (C) swollen aboral end (ZIN no 12165 l); (D) longitudinal section of the column (ZIN no 12138a); (E) weakly contracted pedal disc (ZIN no 12149); (F) longitudinal section of the column (ZIN no 12140b); (G) strongly contracted pedal disc (ZIN no 12139e). Scale bars: A, C, D, F, G, 5 mm; B, 6 mm; E, 8 mm.
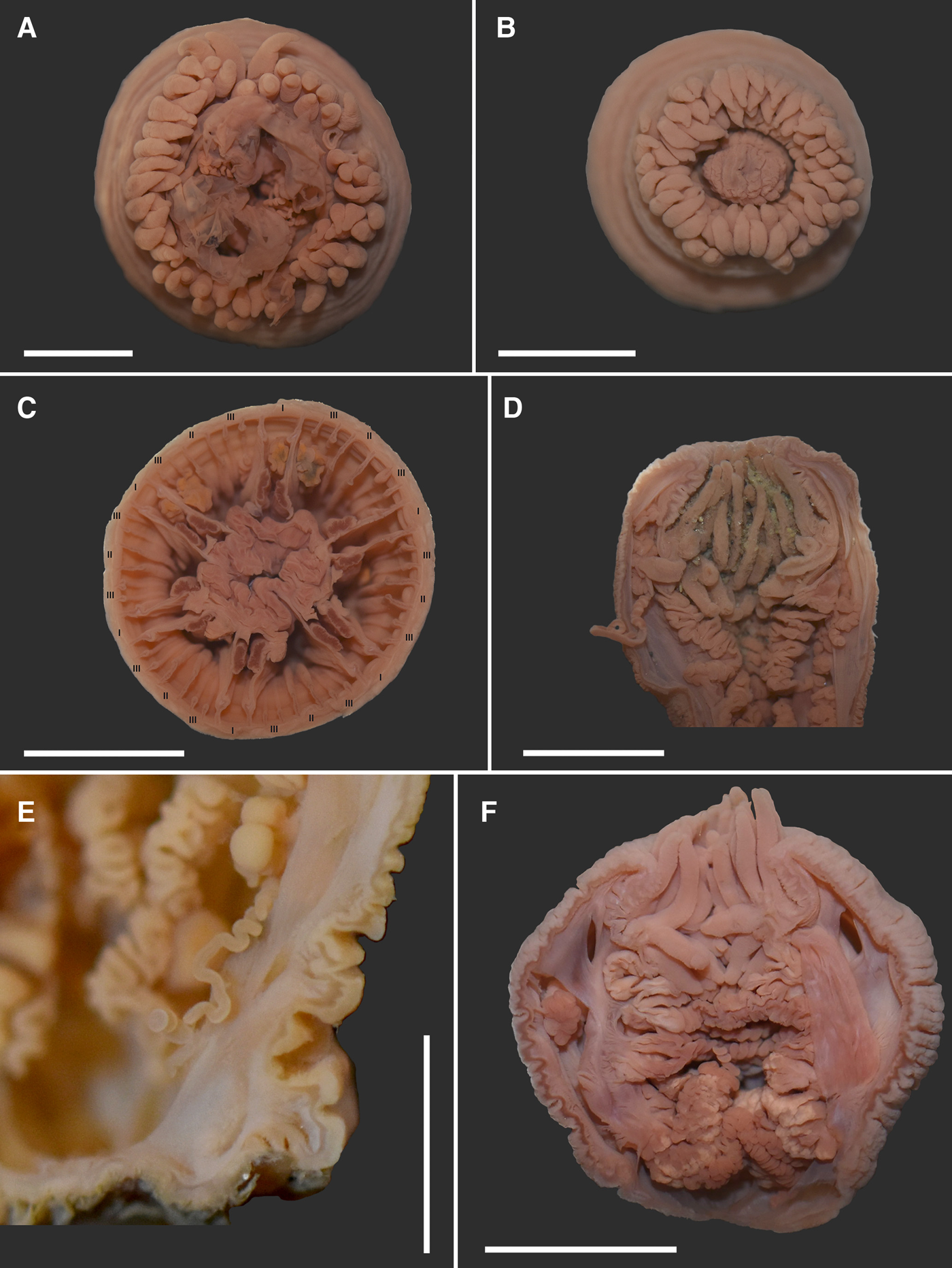
Fig. 4. Haliactis arctica Carlgren (Reference Carlgren1921), external view and internal structure: (A) partially extended oral disc with the everted actinopharynx and protruding acontia; hexamerously arranged tentacles (ZIN no 12139o); (B) tight arrangement of the tentacles covering the oral disc (ZIN no 12139e); (C) cross section of the column at the level of the actinopharynx showing three cycles of hexamerously arranged mesenteries (ZIN no 12139e); (D) bicolour aconitum (ZIN no 12140c); (E) unicolour aconitum (ZIN no 12141c); (F) large marginal stomas (ZIN no 12138b). Scale bars: A–D, 5 mm; E, 2 mm; F, 4 mm.
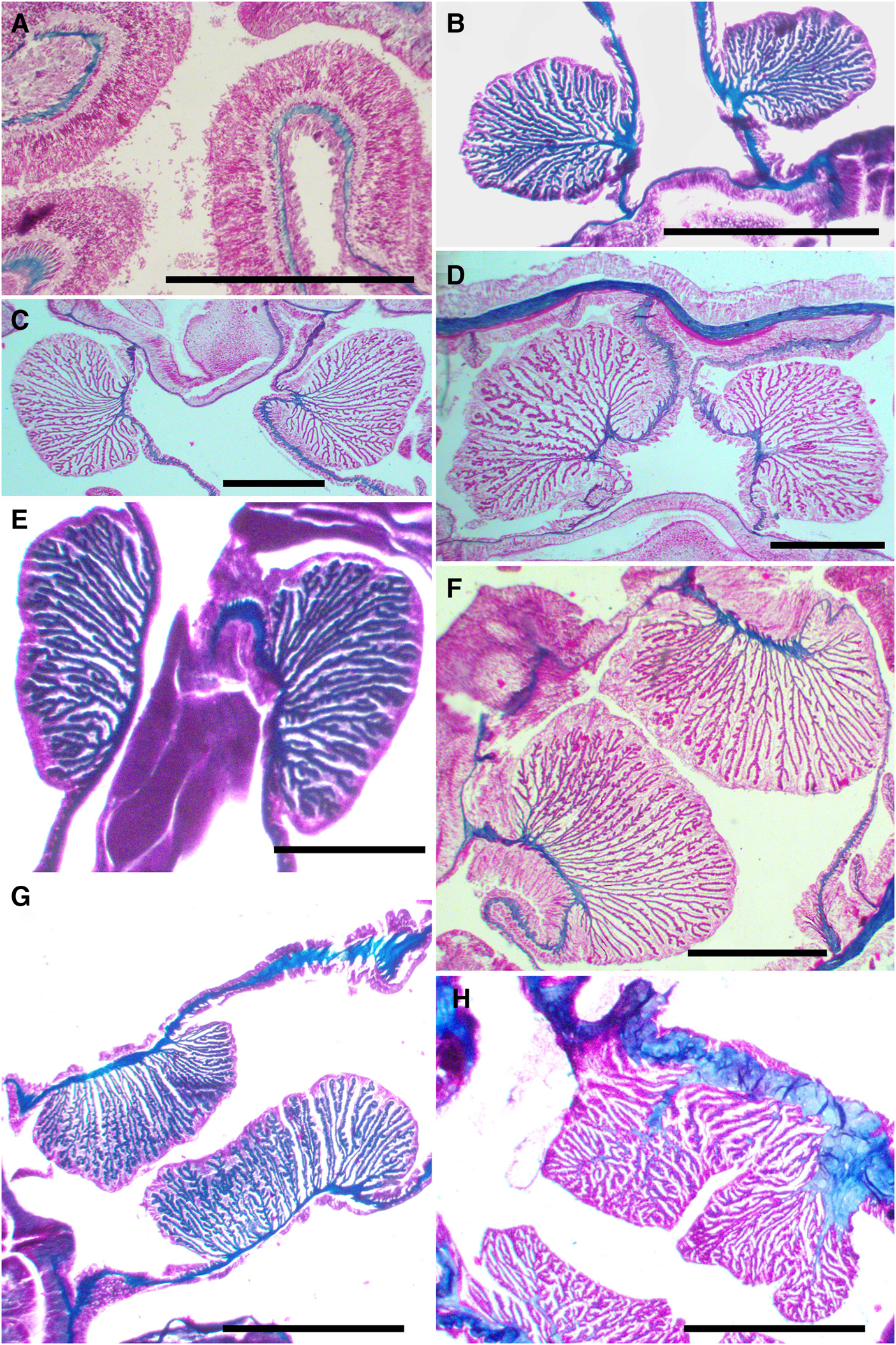
Fig. 5. Haliactis arctica Carlgren (Reference Carlgren1921), musculature of the tentacles and retractor muscles, cross section: (A) cross section of the tentacles showing the ectodermal longitudinal musculature (ZIN no 12149); (B), (C) and (D) retractors of the directive mesenteries in distal part of the column in two specimens (ZIN no 12139b and no 12148); (E) retractors of the directive mesenteries in the reproductive part of the column (ZIN no 12139b); (F) and (G) retractors of the perfect mesenteries in distal part of the column in two specimens (ZIN no 12148 and no 12139b); (H) dissimilar retractor due to very thick mesogloea (ZIN no 12149). Scale bars: A, C–F, H, 0.5 mm; B, G, 1 mm.

Fig. 6. Haliactis arctica Carlgren (Reference Carlgren1921), variability of the parietal muscles of the perfect mesenteries, cross section: (A) weak parietal muscles, represented by rare and rather thick folds in distal part of the column (ZIN no 12149); (B), (D) and (C) two variants of the mesogloeal thickening in the parietal region in distal part of the column (ZIN no 12139b and no 12148); (E) the more developed parietal muscles in proximal part of the column (ZIN no 12149); (F) disappearance of the mesogloeal thickening and stronger parietal muscles in proximal part of the column (ZIN no 12139b). Scale bars: A, C–F, 0.5 mm; B, 1 mm.

Fig. 7. Haliactis arctica Carlgren (Reference Carlgren1921), retractor and parietal muscles of perfect mesenteries in proximal part of the column, cross section: (A) and (B) strong development of parietal muscles and weakening retractors (ZIN no 12139b and no 12149); (C) and (D) highly reduced retractors or almost completely replaced by parietal muscles (ZIN no 12149 and no 12139b). Scale bars: A–D, 0.5 mm.

Fig. 8. Haliactis arctica Carlgren (Reference Carlgren1921), variability of parietal muscles of the secondary and tertiary mesenteries (ZIN no 12139b), cross section: (A) pair of the secondary mesenteries in distal part of the column; (B) and (C) two different pairs of the tertiary mesenteries in one specimen in distal part of the column; (D) mesentery pairs of the second and third cycles in reproductive region of the column; (E) pair of the secondary mesenteries in proximal part of the column. Scale bars: A, C–E, 0.5 mm; B, 0.3 mm.

Fig. 9. Haliactis arctica Carlgren (Reference Carlgren1921), variability of parietal muscles of the secondary and tertiary mesenteries (ZIN no 12149), cross section: (A) the secondary mesentery in distal part of the column; (B) the tertiary mesentery in distal part of the column; (C) pair of the secondary mesenteries in proximal part of the column. Scale bars: A–C, 0.5 mm.

Fig. 10. Haliactis arctica Carlgren (Reference Carlgren1921), variability of retractor muscles of the secondary mesenteries (ZIN no 12148), cross section: (A) distinct retractor muscle on the secondary mesentery in distal part of the column; (B) the absence of retractor muscle on the secondary mesentery in lower part of the actinopharynx. Scale bars: A, 0.5 mm; B, 0.1 mm.

Fig. 11. Cnidom of Haliactis arctica Carlgren (Reference Carlgren1921): (A), (D), (E) and (G), microbasic amastigophore; (B) and (F), basitrich; (C) spirocyst.

Fig. 12. Distribution of Haliactis arctica Carlgren (Reference Carlgren1921). Abbreviations: black circles, data of collection ZIN RAS, white circles, literature data.
Table 1. Size ranges of the cnidae of Haliactis arctica Carlgren (Reference Carlgren1921)

X, mean; SD, standard deviation; N, total number of capsules measured; F, frequency; +++, very numerous; ++, numerous; +, common.
Letters correspond with images in Figure 11.
Acthelmis schaudinnii Carlgren (Reference Carlgren1921): 95, textfigs. 122–124.
Examined material (128 individuals)
ZIN 12142, 1 individual, 15.09.1908, White Sea, 65°3′N 38°48′E, Station 38; ZIN 12145, 5 individuals, RV ‘Krasnoarmeez’, 12.08.1933, Chukchi Sea, 67°35′N 171°27′W, Station 39, 47 m depth, silt; ZIN 12144, 2 individuals, RV ‘Dalnevostochnik’, 17.08.1932, Chukchi Sea, 67°27′N 167°38′W, Station 28, 49 m depth, silt; ZIN 12148, 3 individuals, 12.08.1932, Chukchi Sea, Wrangle Island, 71°15′N 180°W, Station 63, sand; ZIN 12141, 19 individuals, 16.08.1960, White Sea, Station 731; ZIN 12150, 3 individuals, White Sea Biological Station, RV ‘Pr. Zenkevich’, 02.07.1974, White Sea, Station 84, 140–155 m depth; ZIN 12149, 1 individual, White Sea Biological Station, RV ‘Pr. Zenkevich’, 02.07.1974, White Sea, Station 83, 100 m depth; ZIN 12143, 1 individual, White Sea Biological Station, RV ‘Pr. Zenkevich’, 03.07.1974, White Sea, Station 90, 130 m depth; ZIN 12147, 1 individual, White Sea Biological Station, RV ‘Pr. Zenkevich’, 04.07.1974, White Sea, Station 96, 105 m depth; ZIN 12151, 1 individual, White Sea Biological Station, RV ‘Pr. Zenkevich’, 31.07.1974, White Sea, Station 136, 90 m depth; ZIN 12146, 1 individual, White Sea Biological Station, 11.08.1975, White Sea, 66°39.12′N 33°37.24′E, Station 305, 170 m depth; ZIN 12139, 14 individual, RV ‘Ak. Korolyov’, 47 cruise, 12.08.1988, Chukchi Sea, 68°44′N 171°20′W, Station 57, 43 m depth; ZIN 12140, 6 individuals, RV ‘Ak. Korolyov’, 47 cruise, 14.08.1988, Chukchi Sea, 66°54.5′N 169°54.4′W, Station 70, 44 m depth; ZIN 12138, 7 individuals, RV ‘Ak. Korolyov’, 47 cruise, 09.08.1988, Chukchi Sea, 68°16′N 170°53′W, Station 47, 46 m depth; ZIN 12153, 8 individuals, 13.08.1989, Chukchi Sea, 68°N 172°W, 50 m depth; ZIN 12154, 3 individuals, 05.09.1989, Chukchi Sea, 68°18.0′N 172°50.6′W, 52 m depth; ZIN 12242, 2 individuals, ‘Alpha-Helix’–1995, 28.08.95, East Siberian Sea, 69°40′N 177°10′E, Station 16(A1), 19 m depth, silt, pebbles, gravel, sand; ZIN 12157, 1 individual, ‘Alpha-Helix’–1995, 31.08.95, Chukchi Sea, 68°N 173°W, Station 30(K4), 47 m depth, silt; ZIN 12160, 5 individuals, ‘Alpha-Helix’–1995, 31.08.95, Chukchi Sea, 68°N 173°W, Station 30(K4), 47 m depth, silt; ZIN 12163, 1 individual, ‘Alpha-Helix’–1995, 31.08.95, Chukchi Sea, 68°40′N 172°00′W, Station 29(K6), 51 m depth, silt; ZIN 12156, 1 individual, ‘Alpha-Helix’–1995, 31.08.95, Chukchi Sea, 68°40′N 172°00′W, Station 29(K6), 51 m depth, silt; ZIN 12161, 2 individuals, ‘Alpha-Helix’–1995, 31.08.95, Chukchi Sea, 68°N 173°W, Station 30(K4), 47 m depth, silt; ZIN 12158, 1 individual, ‘Alpha-Helix’–1995, 01.09.95, Chukchi Sea, 67°12′N 171°48′W, Station 32(KL2), 44 m depth, silt; ZIN 12159, 1 individual, ‘Alpha-Helix’–1995, 01.09.95, Chukchi Sea, 66°40′N 170°00′W, Station 33(L1), 45 m depth, sand and silt; ZIN 12162, 5 individuals, ‘Alpha-Helix’–1995, 02.09.95, Chukchi Sea, 67°20′N 169°00′W, Station 34(L3), 48 m depth, silt; ZIN 12236, 1 individual, 07.09.2004, East Siberian Sea, 69°10.50′N 169°52.87′E, Station 85, 15 m depth; ZIN 12237, 1 individual, 07.09.2004, East Siberian Sea, 69°20.90′N 170°09.37′E, Station 86, 15 m depth; ZIN 12238, 2 individuals, 09.09.2004, East Siberian Sea, 70°44.36′N 178°39.61′E, Station 101, 30 m depth; ZIN 12239, 1 individual, 10.09.2004, East Siberian Sea, 70°29.03′N 173°07.82′E, Station 105, 37 m depth; ZIN 12240, 1 individual, 09.09.2004, East Siberian Sea, 70°44.36′N 178°39.61′E, Station 101, 30 m depth; ZIN 12241, 4 individuals, 10.09.2004, East Siberian Sea, 70°29.03′N 173°07.82′E, Station 105, 37 m depth; ZIN 12243, 1 individual, 07.09.2004, East Siberian Sea, 69°20.90′N 170°09.37′E, Station 86, 15 m depth; ZIN 12152, 2 individuals, 23.08.2013, Franz Josef Land, Wilton Island, Markham Sound, 80°34′N 54°19′E, Station 31, 18–28 m depth; ZIN 12164, 4 individuals, Transarctica–2019, RV ‘Pr. Multanovsky’, 16.08.19, Chukchi Sea, 67°10′N 170°18′W, Station 5 Sample 1, 46 m depth, brown sediment, black and green silt; ZIN 12165, 13 individuals, Transarctica–2019, RV ‘Pr. Multanovsky’, 16.08.19, Chukchi Sea, 67°16′N 170°29′W, Station 5 Sample 2, 46 m depth, brown sediment, black and green silt; ZIN 12167, 2 individuals, Transarctica–2019, RV ‘Pr. Multanovsky’, 16.08.19, Chukchi Sea, 67°24′N 169°04′W, Station 7 Sample 1, 49 m depth, brown sediment, black and green silt; ZIN 12166, 1 individual, Transarctica – 2019, RV ‘Pr. Multanovsky’, 16.08.19, Chukchi Sea, 67°24′N 169°04′W, Station 7 Sample 2, 49 m depth, brown sediment, black and green silt.
Description
External anatomy. Body shape elongated, spherical, compressed in the oral-aboral direction, many specimens with extended middle and narrow proximal part (Figures 1A–E, 2, 3A–C), 3–25 mm in height and 5–15 mm in diameter. Column not divisible into regions. It is smooth, devoid of cuticles, warts, papillae or other specialized formations, covered with numerous annular wrinkles (Figures 1A–E, 2, 3B–C). Mesenterial inserts often visible (Figures 1A, 2, 3A). Many specimens have a fully retracted pedal disc, some specimens with expanded and flat disc, which is sometimes attached to the stone (Figures 2 & 3B, D–G), but sometimes the aboral end is rounded (Figures 1A & 3C). Deep fossa well-developed. Oral disc small, rounded and often retracted (Figures 1A–C, 3F, 4D, F), completely hidden by tentacles when expanded (Figure 4A, B). Tentacles numerous, 60–87 in number, but 24–50 in number in juvenile individuals and some sexually mature. Tentacles arranged hexamerously, conical, smooth, not longitudinally or transversally sulcated, without acrospheres. Tentacles tightly pressed together (Figure 4A, B). Length of outer and inner tentacles varies at intra- and inter-individual level. Tentacles nearly equal in length (2–5 mm in length) or often the outer tentacles are longer than the inner ones (3–6 mm and 1.5–5 mm in length, respectively), in this case inner tentacles thicker than the outer ones.
Internal anatomy and microanatomy. Actinopharynx of ordinary length, strongly folded; two siphonoglyphs without aboral prolongations. Siphonoglyphs sometimes absent (Figure 3D), in many cases they could not be identified due to poor preservation of the material. Marginal sphincter muscle absent. Longitudinal muscles of tentacles and radial muscles of oral disc ectodermal (Figure 5A). Two pairs of directive mesenteries. Mesenteries hexamerously arranged in three cycles (6 + 6 + 12), ranging from base to margin (Figure 4C), incomplete fourth cycle distally. Mesenteries of first cycle perfect, with retractors and parietal muscles, filaments, gametogenic tissue and acontia; with large marginal stomas (Figures 3D & 4F). Mesenteries of second cycle may have filaments, gametogenic tissue and acontia at the middle and/or distal part of the body (Figure 3D). Mesenteries of third and fourth cycles are microcnemes, although some mesenteries of the third cycle rarely present weak filaments at distal part of the body. Acontia long, rich in nematocysts. They are unicolour, beige, like the rest of the internal structures in beige or sand coloured specimens (Figure 4E), or sometimes bicolour, divided into pinkish and white stripes in dark coloured specimens (Figure 4D). Number and occurrence of acontia varies among different specimens. Most often few acontia (1–3) developed only on some mesenteries of first and second cycles, or only of first or second cycle, rarely in mesenteries of the third cycle; rarely 6–8 acontia. In several individuals, acontia are completely absent. Longitudinal retractors muscles of directives strong, reniform and almost circumscribed in distal column (Figure 5B–D), but elongated proximally and their processes branch off from the main mesogloeal plate of the mesentery (Figure 5E). Retractors muscles of some individuals with 1–2 large mesogloeal lamellae or 1 strong thickening (varies even within one individual), giving numerous branching folds (Figure 5B–D). Retractor muscles of other perfect mesenteries strong, restricted, but elongated; their numerous ramifying folds branch off from the main mesogloeal plate (Figure 5F, G). One of the studied specimens has a very thick mesoglea, so the appearance of its retractors is dissimilar (Figure 5H). Parietal muscles on cross sections elongated, do not spread over the column, on the side of the longitudinal muscles pass into retractor; weak in the distal and strong in proximal part. In some specimens, they are represented by rare and rather thick folds (Figure 6A), sometimes a thickening of the mesoglea is formed near the accretion of mesentery to the wall of the column (Figures 4С & 6B–D). Basilar muscles absent. Gonochoric (Figures 3D, F & 4E, F): oocytes large, diameter up to about 1.5 mm. In gastric cavity of some individuals, remains and whole specimens of amphipods, presumably belonging to the genus Metopa Boeck, Reference Boeck1871 (Stenothoidae Boeck, Reference Boeck1871), were found.
Cnidom. Spirocysts, basitrichs, and microbasic amastigophors. See Figure 11 and Table 1 for size and distribution.
Colour. Preserved material grey with pink, beige, brownish and terracotta.
Distribution. Haliactis arctica was originally described from Greenland; West Greenland, Nordre Stromfiord, 375–380 m depth; Spitsbergen, King Charles Land 78°50′N 29°39′E, 60–70 m depth; Norway, Barents Sea, Bear Island; Siberia, Arctic Ocean, 2 miles north of the winter station of the Vega (Vega Expedition) (Carlgren, Reference Carlgren1921). Synonymized Acthelmis schaudinnii was originally described from East Spitsbergen, Great Fiord, Cape Blanck 77°49′N 20°3′E, 65 m depth and Russia, Novaya Zemlya, Besimennaja Bay, 7–9 m depth (Carlgren, Reference Carlgren1921). Here I extend the distribution of the species in the Arctic to include Franz Josef Land, Wilton Island, 18–28 m depth, the White Sea, 90–170 m depth, East Siberian Sea, 15–37 m depth, and increase the number of localities in the Chukchi Sea, between 43–50 m depth (Figure 12).
Remarks
The original description of Haliactis arctica is based on a small number of specimens from Greenland, Spitsbergen, Norway and Chukchi Sea collected at depths from about 7–9 to 380 m. The specimens described here are from the Chukchi Sea, White Sea and Franz Josef Land. Both external and internal features agree well with the original description of this species (Carlgren, Reference Carlgren1921). Body measurements in preserved condition indicated by Carlgren (Reference Carlgren1921) are 8–20 mm in height and 5–10 mm in diameter. The examined specimens slightly expand this range. One of the largest specimens is 25 mm in height, 13 mm in diameter in the widest part and 5 mm in diameter in the narrowest proximal part. The smallest specimen is 3 mm in height and the diameter of its pedal disc is 4 mm. The height of other specimens varies from 5–23 mm. Their width is also quite variable (5–15 mm) due to their different body shapes. Many of the examined specimens look identical to the specimens from Greenland, which were described and figured by Carlgren (Reference Carlgren1921, Pl. I, figure 3). In addition, they were similarly preserved in a contracted position with a wider distal or middle part; the surface of their column is similarly covered with numerous annular wrinkles. Like Carlgren (Reference Carlgren1921), I also found specimens whose proximal end is rounded. However, most of our polyps have a well-developed pedal disc, very often retracted. There are several differences in the number and size of the tentacles. Carlgren (Reference Carlgren1921) found 80–90 tentacles, but among the examined material the range is 40–87 due to the study of both adult sexually mature and juvenile specimens. In addition, I found a variation in the size of the tentacles, which is probably associated with a different degree of expansion when the sea anemones were fixed. Both inner and outer tentacles can be of approximately equal length. In addition, in specimens with fully retracted tentacles, the outer ones are usually longer and thinner, the inner ones are usually shorter and thicker. When the tentacles are extended, the length of both types can vary. It also cannot be said strictly that, for example, all the outer (or inner) tentacles are long, some of them will be short and vice versa.
The number of the cycles of the mesenteries and their arrangement correspond to Carlgren's (Reference Carlgren1921) description. Nevertheless, several juveniles have been found only with two cycles of the mesenteries, which may be complete or not. In addition, it was found that acontia and filaments can also be developed on mesenteries of the second cycle at the level of the actinopharynx and below its border. In the proximal part, they are usually absent. In rare cases, particular mesenteries of the third cycle have weak filaments, as well as acontia, a feature not mentioned by Carlgren (Reference Carlgren1921).
Mesenteric musculature often differs in appearance between individuals due to dissimilar degrees of contraction (compare Figure 5F–H). The mesenteric plate of some polyps has a thicker mesoglea. It gives large branches both in the area of the retractors and in the parietal part. A similar phenomenon is described by Carlgren (Reference Carlgren1921: 131). The structure of the retractors of the studied polyps is somewhat different from that described by Carlgren (Reference Carlgren1921). He described retractors in diagnosis (1921: 129) as ‘strong, furnished with high folds and rather richly ramificated, especially in the inner and outer parts’. In specimens examined, the entire retractor is characterized by branching high folds, and not only its outer and inner parts. In addition, on histological preparations, it is shown that the longitudinal retractor muscles of the directives and other perfect mesenteries often belong to different types (e.g. Figure 5D, F). Retractor muscles of the directives reniform and almost circumscribed in distal column, but below about the lower end of the actinopharynx, however, they become more elongated. Retractor muscles of other perfect mesenteries elongated throughout the column. Some polyps, however, do not have such a clear difference (Figure 4C). This was not recorded by Сarlgren (Reference Carlgren1921). His remark (1921: 131, textfig. 153) that the folds are very high and more branching in the reproductive area than in the lower part of the actinopharynx is not confirmed by my observations. The present research shows that towards the aboral end, on the contrary, the retractors are reduced more and more, almost completely being replaced by parietal muscles in the pedal disc area (Figure 7C, D). More branching folds can be observed in the reproductive region only in the retractors of the directive mesenteries. It should be noted, only one specimen shows the presence of weak retractors in the mesenteries of the second cycle in the distal part of the column (Figure 10A), but below about the middle of the actinopharynx, the retractors begin to decrease in some of the mesenteries, and the mesogloeal plate of the mesentery was completely occupied by clear parietal muscles (Figure 10B).
The parietal muscles of the specimens examined correspond to the description of Carlgren (Reference Carlgren1921): however, in a number of individuals, a mesogloeal thickening was found in the region of the mesenteric insertion. This thickening produces well-defined folds (thick and short or long and thin) on the side of the retractor, weak or absent on the opposite side. The folds are shorter between this thickening and the column wall (Figures 4С & 6B–D). Closer to the proximal part, the parietal muscles develop more strongly. Their folds become longer, thicker and more frequent. Mesogloeal thickening presenting in some specimens in the distal part disappears at this point (Figure 6E, F). Retractors are significantly reduced in size (Figure 7A, B). In the base area, parietal muscles displace retractors and almost completely occupy the mesenteric plate (Figure 7C, D). Of course, it should be noted that the changes in the musculature vary in different mesenteries. Nevertheless, the general trend is associated with a gradual reduction of the retractors and, on the other hand, the increasing development of the parietal muscles towards the aboral end. Mesenteries of the second and third cycle have well-developed parietal muscles with numerous folds, which can be thin or thick (Figures 8, 9 & 10). In addition, as in the case of retractors, the parietal muscles of the younger cycles have a slightly different structure in the distal and proximal parts of the column (Figures 8A, D, E & 9). The degree of development is variable both in different specimens and within one specimen (Figure 8B, C).
With respect to the acontia, Carlgren (Reference Carlgren1921) noted their abundance. The examined material exhibits that the number of acontia varies in H. arctica. Acontia are numerous (6–8) only in a few specimens. As a rule, acontia are few in number and most polyps have 1–3 acontia. Some specimens lack acontia that is also noted by Carlgren (Reference Carlgren1921) for the anemones, described by him as Acthelmis schaudinnii. The absence of acontia is also known for deep-sea Bathyphellia margaritacea (Danielssen, Reference Danielssen1890) (Bathyphelliidae Carlgren, Reference Carlgren1932) and Seepactis galkini Sanamyan & Sanamyan, Reference Sanamyan and Sanamyan2007 (Kadosactinidae Riemann-Zürneck, Reference Riemann-Zürneck1991) (see Riemann-Zürneck, Reference Riemann-Zürneck1997; Sanamyan & Sanamyan, Reference Sanamyan and Sanamyan2007; Sanamyan et al., Reference Sanamyan, Cherniaev and Sanamyan2009). An explanation of this phenomenon was given by Riemann-Zürneck (Reference Riemann-Zürneck1997) and Sanamyan & Sanamyan (Reference Sanamyan and Sanamyan2007). According to the assumptions of these researchers, acontia can be destroyed during the collection of samples from great depths. According to Stephenson (Reference Stephenson1920, Reference Stephenson1935), acontia could be repeatedly reduced in the course of evolution during the origin of Mesomyaria Stephenson, Reference Stephenson1921 from the representatives of Acontiaria Stephenson (Reference Stephenson1935).
Nematocysts were examined in specimens from all localities (in two from Franz Josef Land, in two from the White Sea, the Chukchi and East Siberian seas). Cnidom includes spirocysts, basitrichs and microbasic amastigophors (see Table 1 and Figure 11). Since the original description of Carlgren (Reference Carlgren1921) does not include data on filaments and acontia nematocysts, and, according to him, the material was in poor condition, I compared my material mainly with the clearer and more complete description of Carlgren (Reference Carlgren1940: 28). This comparison showed that the microbasic amastigophors of сolumn in the examined specimens have a smaller size, namely, 24–32 × 5–6 μm vs 25–36 × 5–6.5 μm (however, they exceed the size of the a- and b-nematocysts of the material in Carlgren (Reference Carlgren1921)). The size range of basitrichs of the tentacles turns out to be larger, namely, 15–26 × 2–3 μm vs 22–24 × 2.5 μm in Carlgren (Reference Carlgren1940). The size of spirocysts is similar to ones indicated by Carlgren (Reference Carlgren1921). The sizes of nematocysts in actinopharynx, filaments and acontia closely agrees with those in Carlgren (Reference Carlgren1940). Spirocysts from tentacles are numerous in all examined specimens. Basitrichs are smaller in number, and in some specimens they are rather rare, which is also noted for microbasic amastigophors in column. Microbasic amastigophors of the actinopharynx and filaments are of almost the same size, but the nematocysts of the filaments are more frequent. The frequency of nematocysts of the acontia is high, especially of microbasic amastigophors, and it is similar in all examined specimens. Several spirocysts are sometimes found in the ectoderm of column.
Thus, this material corresponds to the original description of Haliactis arctica given by Carlgren (Reference Carlgren1921), but shows wider variability in several characters.
Discussion
Comparison of morpho-anatomical characters of H. arctica and other representatives of Halcampactinidae shows that perhaps this family is heterogeneous, as Panikkar (Reference Panikkar1936, Reference Panikkar1938) noted earlier. Representatives of the five genera Halcampactinidae, in contrast to Haliactis, are characterized by a worm-like body, the length of which significantly exceeds the width (Farquhar, Reference Farquhar1898; Stuckey, Reference Stuckey1909; Panikkar, Reference Panikkar1936, Reference Panikkar1937, Reference Panikkar1938; Li et al., Reference Li, Liu, Liu and Xu2013; Fautin et al., Reference Fautin, Tan, Yap, Tan, Crowther, Goodwill, Sanpanich and Tay2015). For example, the column height of Pelocoetes minimus Panikkar (Reference Panikkar1938) is 30–35 mm, the diameter reaches 3–5 mm in the widest part. The specimens of H. arctica studied by both Carlgren (Reference Carlgren1921) and myself are not so thin and long. One of the largest specimens from the examined material is 25 mm in height and 13 mm in diameter. Most importantly, the study of numerous specimens of H. arctica shows that this sea anemone has a well-developed pedal disc, which, depending on the degree of contraction and fixation, can be retracted or swollen, giving the aboral end of the body a rounded appearance (Figures 1 & 3). Other Halcampactinidae do not have a true pedal disc and are burrowing. Whether H. arctica has burrowing behaviour remains unknown. Some of the studied specimens are attached to stones; in some of the polyps collected from sandy and silty substrates when the oral and pedal discs are fully retracted, both the gastric cavity and the space between the tentacles are filled with sand; some specimens are covered with grains of sand (Figures 3B, F, 4D). Other characters that distinguish tropical Halcampactinidae (Pelocoetes, Phytocoeteopsis, Phytocoetes, and Stephensonactis) include the lines of cinclides, as well as stinging warts in some species (Annandale, Reference Annandale1907, Reference Annandale1915; Panikkar, Reference Panikkar1936, Reference Panikkar1937, Reference Panikkar1938; Li et al., Reference Li, Liu, Liu and Xu2013; Fautin et al., Reference Fautin, Tan, Yap, Tan, Crowther, Goodwill, Sanpanich and Tay2015). Farquhar (Reference Farquhar1898) notes the presence of exceedingly minute suckers and cuticles in the New Zealand Halcampactis mirabilis Farquhar (Reference Farquhar1898). In contrast, the body wall of H. arctica does not carry any specialized structure. Among the anatomical features, a clear difference lies in the absence of a strict division of mesenteries into macro- and microcnemes in H. arctica. As indicated above, the mesenteries of the second and, in rare cases, the third cycle carry filaments and acontia. The rest of the Halcampactinidae are characterized by a very clear division of the mesenteries: only perfect mesenteries of the first cycle carry retractors, filaments, gonads and acontia (Farquhar, Reference Farquhar1898; Panikkar, Reference Panikkar1936, Reference Panikkar1937, Reference Panikkar1938; Li et al., Reference Li, Liu, Liu and Xu2013; Fautin et al., Reference Fautin, Tan, Yap, Tan, Crowther, Goodwill, Sanpanich and Tay2015). The number of cycles of the mesenteries is similar in H. arctica, P. ramunnii, Stephensonactis ornata Panikkar (Reference Panikkar1936), Phytocoetes sinensis Li, Liu & Xu (Reference Li, Liu, Liu and Xu2013) and Pelocoetes exul (Annandale, 1907). All these species have four cycles of the mesenteries, but only three are located throughout the column; the youngest cycle is present only in the most distal part of body (Panikkar, Reference Panikkar1936, Reference Panikkar1937, Reference Panikkar1938; Li et al., Reference Li, Liu, Liu and Xu2013; Fautin et al., Reference Fautin, Tan, Yap, Tan, Crowther, Goodwill, Sanpanich and Tay2015). Pelocoetes minimus Panikkar (Reference Panikkar1938) and Phytocoetes gangeticus Annandale (Reference Annandale1915) differ from H. arctica in that they have only three cycles of the mesenteries, but in these species, the last cycle also confined only to the most distal part of the column (Panikkar, Reference Panikkar1937, Reference Panikkar1938). Halcampactis mirabilis has only two mesenteric cycles: six macrocnemes and six microcnemes (Farquhar, Reference Farquhar1898). The anatomy of Halcampactis dubia Stuckey (Reference Stuckey1909) remains unknown (Stuckey, Reference Stuckey1909). The retractors of all Halcampactinidae are strong, well-developed with numerous branching folds, with the exception of P. gangeticus, in which individual folds are not much ramified and represent a simple appearance (Panikkar, Reference Panikkar1936, Reference Panikkar1937, Reference Panikkar1938; Li et al., Reference Li, Liu, Liu and Xu2013). The parietal muscles of H. arctica, however, differ significantly from the ones of other Halcampactinidae. In this sea anemone, these muscles are very elongated, on the longitudinal muscle-side passing into the retractor. Considering the whole complex of the listed differences as well as habitat in the Arctic, Haliactis stands apart from the five other genera of the family Halcampactinidae (Halcampactis Farquhar, Reference Farquhar1898, Pelocoetes Annandale, Reference Annandale1915, Phytocoeteopsis Panikkar, Reference Panikkar1936, Phytocoetes Annandale, Reference Annandale1915, Stephensonactis Panikkar, Reference Panikkar1936). Unfortunately, I do not have material suitable for carrying out molecular genetic studies, since almost all studied specimens were collected before the use of molecular markers in taxonomy. The GenBank database (www.ncbi.nlm.nih.gov/genbank/) also does not contain information to compare all species of this family. Therefore, at this time, I cannot confidently place Haliactis in a separate family, this will be a future study.
In addition, the taxonomic status of Halcampactinidae Carlgren (Reference Carlgren1921) and Haliactinidae Carlgren (Reference Carlgren1921) should be discussed. Halcampactis was the first species described of the six listed genera of this family, but Farquhar (Reference Farquhar1898: 528) noted that ‘by its strange combination of characters it forms a link between the two families Sagartidae and Halcampidae’ and did not establish its position in the system. Сarlgren (Reference Carlgren1921: 128) created the family Halcampactiidae, in which he assigned two genera: Halcampactis and Haliactis. The name Halcampactiidae is available. It is derived from the name of a valid genus (Halcampactis) and conforms to the requirements of the International Code of Zoological Nomenclature, its right spelling is Halcampactinidae (from Greek ‘ακτίς’ – ‘ray’, its genitive singular is ‘ακτίνος’ so the stem for formation of the family name is ‘ακτίν-’, i.e. ‘actin-’). It is important to pay attention to Carlgren's (Reference Carlgren1921: 128) remark that ‘If Halcampactis has to be removed from the family, it will be necessary to give it a new name, Haliactiidae’, that is, Carlgren created another family and considered it a junior synonym for Halcampactinidae. The name Haliactiidae is also available according to the ICZN (Articles 11.5 and 11.6.1) and its correct spelling is Haliactinidae (from Greek ‘ακτίς’–”ray”, its genitive singular is ‘ακτίνος’ so the stem for formation of the family name is ‘ακτίν-’, i.e. ‘actin-’). Thus, the main, valid, name of the family is Halcampactinidae Carlgren (Reference Carlgren1921), but Haliactinidae Carlgren (Reference Carlgren1921) is a junior synonym. Therefore, the widespread use of Haliactinidae Carlgren (Reference Carlgren1949) is incorrect. Carlgren (Reference Carlgren1949: 36) notes that ‘I prefer to use Haliactiidae instead of Halcampactiidae as the genus Halcampactis is imperfectly known’. However, this change of names has not been agreed with the ICZN. According to the Principle of the First Reviser (Article 24.2.1), Carlgren (Reference Carlgren1921) already established the priority of names. Thus, the valid name of the family is Halcampactinidae Carlgren (Reference Carlgren1921).
Acknowledgements
I would like to express my deep gratitude to Dr N. Sanamyan and Dr K. Sanamyan (Kamchatka Branch of Pacific Geographical Institute of Far-Eastern Branch of the Russian Academy of Sciences) for their recommendations, explanations and advice on photographing and making histological preparations as well as for their clarifications on nomenclature issues. I am grateful to Dr E. Soldatenko (Zoological Institute, Russian Academy of Sciences) for the preliminary identification of amphipods and for her ongoing work on these crustaceans. I would like to thank Dr E. Markhaseva (Zoological Institute, Russian Academy of Sciences) for her valuable comments on the initial version of the manuscript. I am grateful to the reviewers for their helpful and substantial comments.
Financial support
The study was funded by the Russian Foundation for Basic Research (grant numbers 19-34-90083, 18-05-60157). The study used the collection materials of the Zoological Institute RAS (http://www.ckp-rf.ru/usu/73561/).















